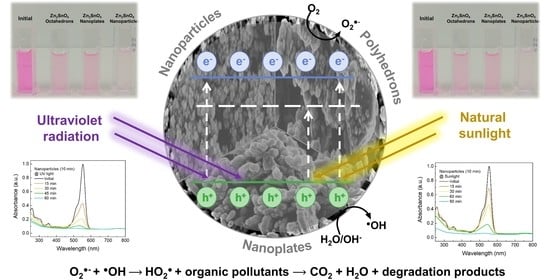
Microwave-Assisted Synthesis of Zn2SnO4 Nanostructures for Photodegradation of Rhodamine B under UV and Sunlight
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Zn2SnO4 Nanostructures
2.2. Structural, Morphological, and Optical Characterization of Zn2SnO4 Nanostructures
2.3. Evaluation of the Zn2SnO4 Nanostructures Photocatalytic Performance
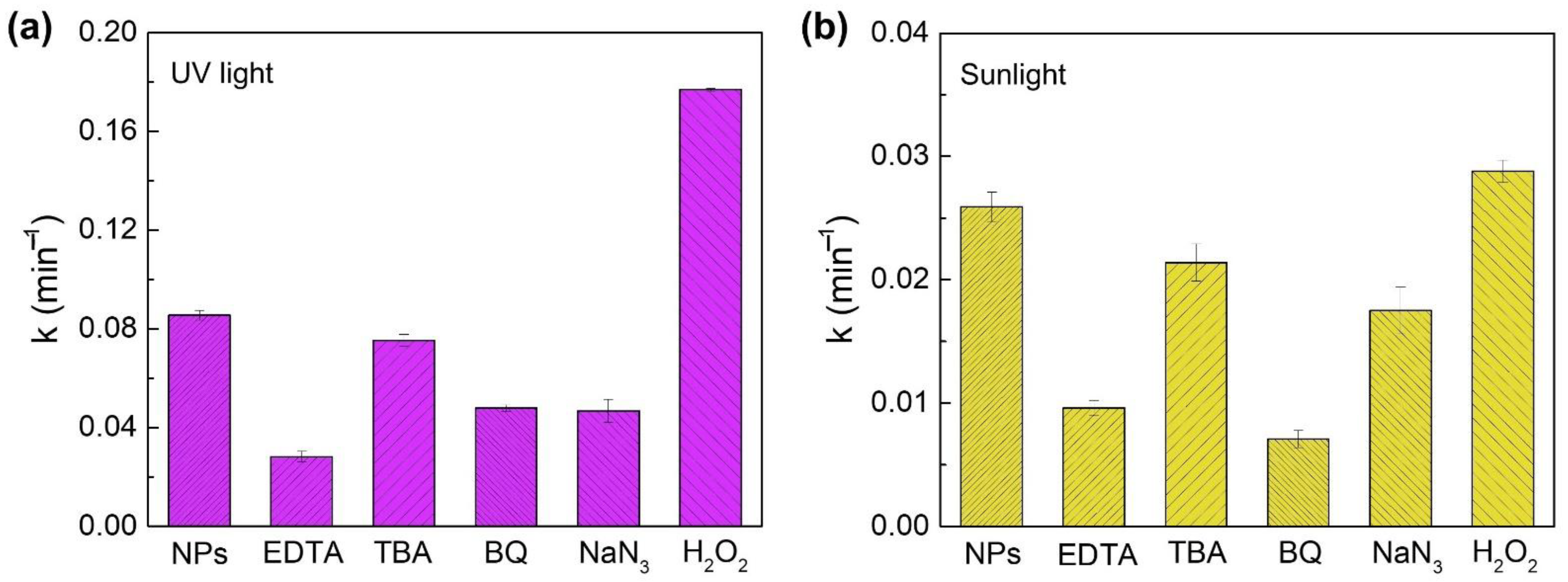
2.4. Investigation of the Reactive Oxygen Species (ROS) Involved in the Photocatalytic Degradation of RhB by Zn2SnO4 Nanostructures
3. Results and Discussion
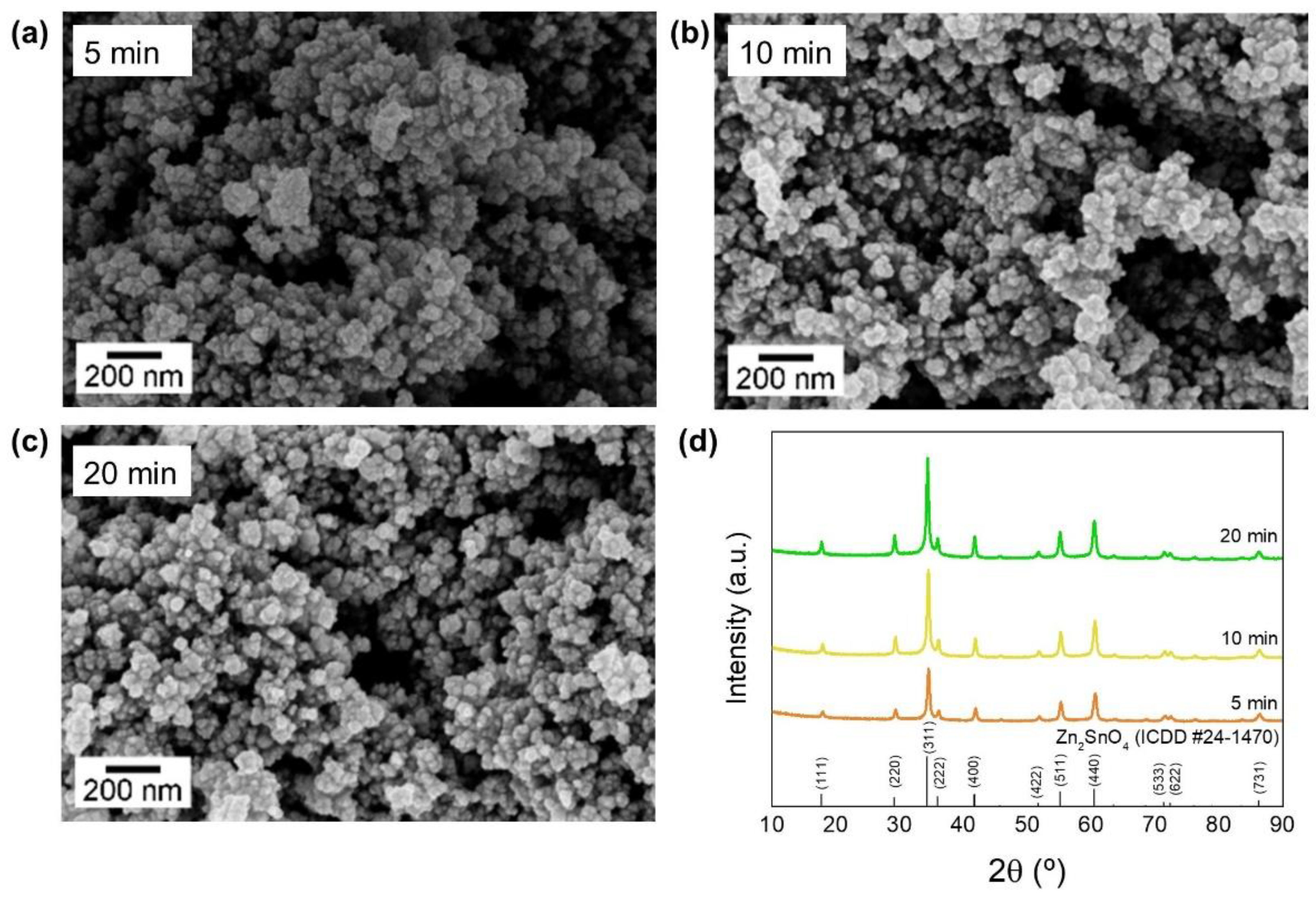
3.1. Synthesis of Zn2SnO4 Nanostructures
3.1.1. Zn2SnO4 Polyhedrons and Nanoplates
3.1.2. Zn2SnO4 Nanoparticles
3.2. Optical Properties of Zn2SnO4 Nanostructures
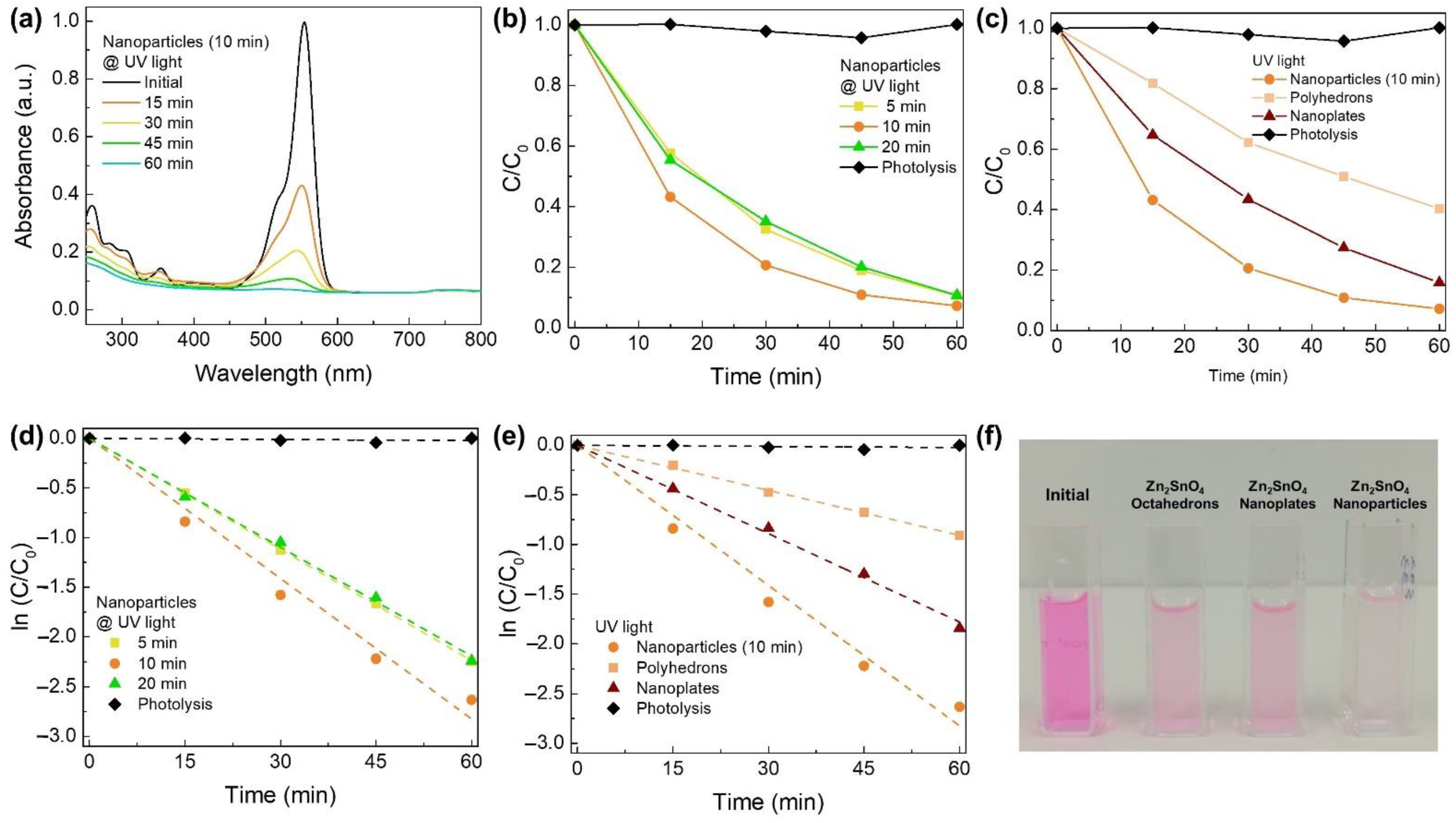
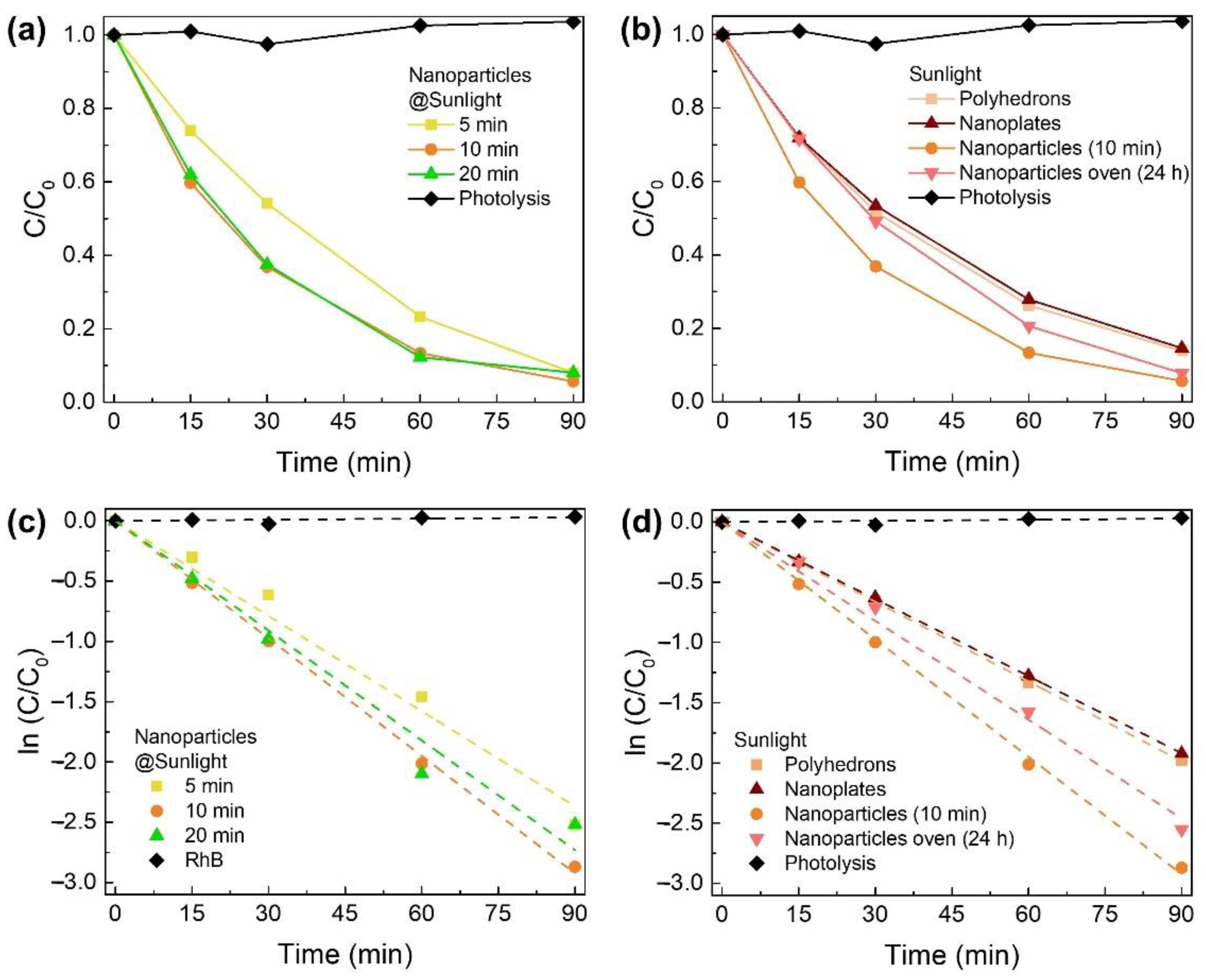
3.3. Photocatalytic Activity of Zn2SnO4 Nanostructures under UV Light
3.4. Photocatalytic Activity of Zn2SnO4 Nanostructures under Natural Sunlight
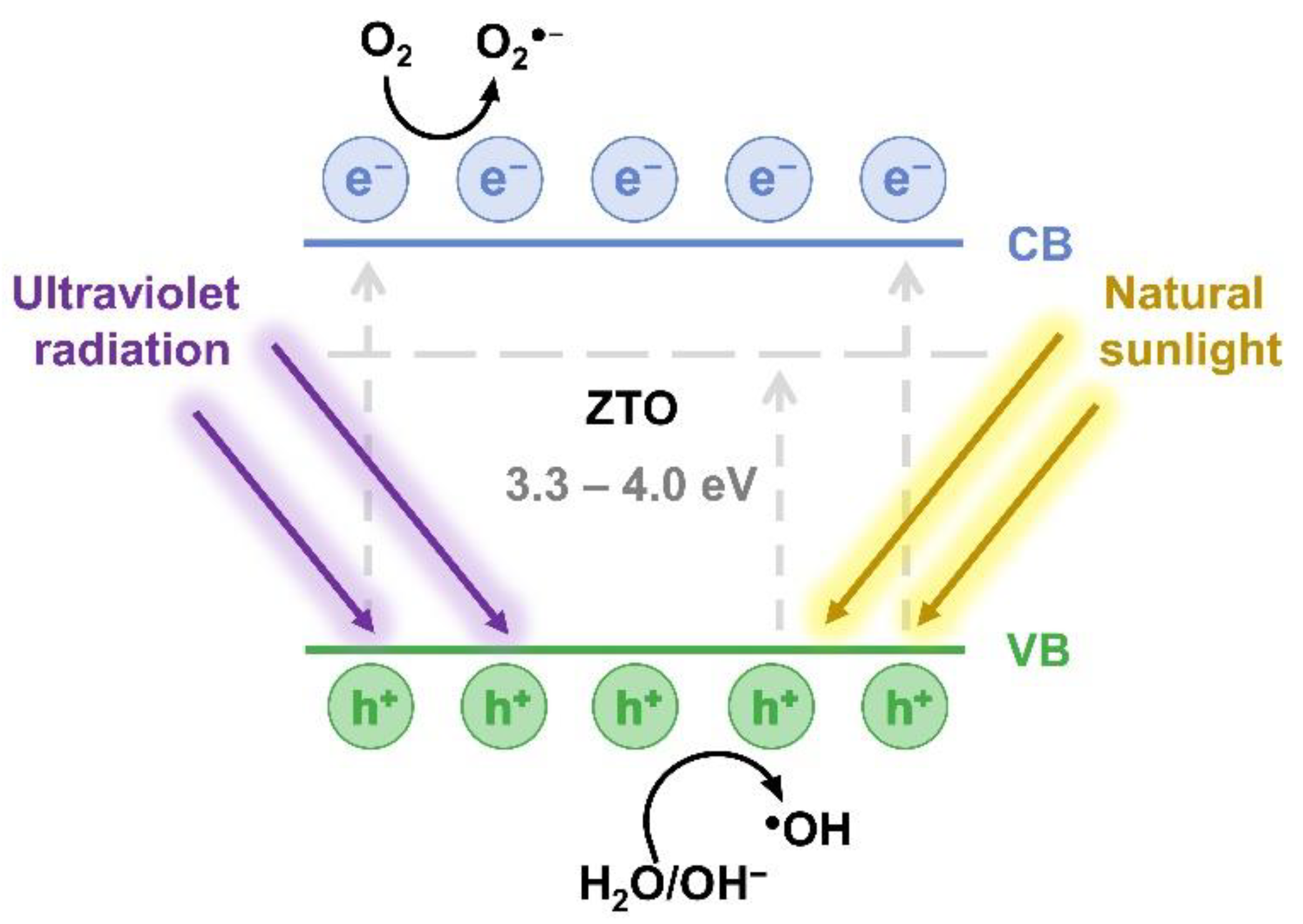
3.5. Photodegradation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, S.H.; Morais, M.; Nunes, D.; Oliveira, M.J.; Rovisco, A.; Pimentel, A.; Águas, H.; Fortunato, E.; Martins, R. High UV and Sunlight Photocatalytic Performance of Porous ZnO Nanostructures Synthesized by a Facile and Fast Microwave Hydrothermal Method. Materials 2021, 14, 2385. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Zinc stannate nanostructures: Hydrothermal synthesis. Sci. Technol. Adv. Mater. 2011, 12, 013004. [Google Scholar] [CrossRef] [PubMed]
- Barrocas, B.; Sério, S.; Melo Jorge, M.E. Hierarchically Grown CaMn3O6 Nanorods by RF Magnetron Sputtering for Enhanced Visible-Light-Driven Photocatalysis. J. Phys. Chem. C 2014, 118, 24127–24135. [Google Scholar] [CrossRef]
- Nunes, D.; Fragoso, A.R.; Freire, T.; Matias, M.; Marques, A.C.; de Paiva Martins, R.F.; Fortunato, E.; Pimentel, A. Ultrafast Microwave Synthesis of WO3 Nanostructured Films for Solar Photocatalysis. Phys. Status Solidi-Rapid Res. Lett. 2021, 15, 2100196. [Google Scholar] [CrossRef]
- Michelin, C.; Hoffmann, N. Photosensitization and Photocatalysis—Perspectives in Organic Synthesis. ACS Catal. 2018, 8, 12046–12055. [Google Scholar] [CrossRef]
- Firooz, A.A.; Mahjoub, A.R.; Khodadadi, A.A.; Movahedi, M. High photocatalytic activity of Zn2SnO4 among various nanostructures of Zn2xSn1−xO2 prepared by a hydrothermal method. Chem. Eng. J. 2010, 165, 735–739. [Google Scholar] [CrossRef]
- Kampouri, S.; Stylianou, K.C. Dual-Functional Photocatalysis for Simultaneous Hydrogen Production and Oxidation of Organic Substances. ACS Catal. 2019, 9, 4247–4270. [Google Scholar] [CrossRef]
- Barrocas, B.; Sério, S.; Rovisco, A.; Melo Jorge, M.E. Visible-Light Photocatalysis in Ca0.6Ho0.4MnO3 Films Deposited by RF-Magnetron Sputtering Using Nanosized Powder Compacted Target. J. Phys. Chem. C 2014, 118, 590–597. [Google Scholar] [CrossRef]
- Najam Khan, M.; Al-Hinai, M.; Al-Hinai, A.; Dutta, J. Visible light photocatalysis of mixed phase zinc stannate/zinc oxide nanostructures precipitated at room temperature in aqueous media. Ceram. Int. 2014, 40, 8743–8752. [Google Scholar] [CrossRef]
- Hezam, A.; Namratha, K.; Ponnamma, D.; Drmosh, Q.A.; Saeed, A.M.N.; Sadasivuni, K.K.; Byrappa, K. Sunlight-Driven Combustion Synthesis of Defective Metal Oxide Nanostructures with Enhanced Photocatalytic Activity. ACS Omega 2019, 4, 20595–20605. [Google Scholar] [CrossRef] [Green Version]
- Nunes, D.; Pimentel, A.; Pinto, J.V.; Calmeiro, T.R.; Nandy, S.; Barquinha, P.; Pereira, L.; Carvalho, P.A.; Fortunato, E.; Martins, R. Photocatalytic behavior of TiO2 films synthesized by microwave irradiation. Catal. Today 2016, 278, 262–270. [Google Scholar] [CrossRef]
- Pimentel, A.; Rodrigues, J.; Duarte, P.; Nunes, D.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Effect of solvents on ZnO nanostructures synthesized by solvothermal method assisted by microwave radiation: A photocatalytic study. J. Mater. Sci. 2015, 50, 5777–5787. [Google Scholar] [CrossRef]
- Barrocas, B.; Monteiro, O.C.; Jorge, M.E.M.; Sério, S. Photocatalytic activity and reusability study of nanocrystalline TiO2 films prepared by sputtering technique. Appl. Surf. Sci. 2013, 264, 111–116. [Google Scholar] [CrossRef]
- Pimentel, A.; Nunes, D.; Duarte, P.; Rodrigues, J.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Synthesis of Long ZnO Nanorods under Microwave Irradiation or Conventional Heating. J. Phys. Chem. C 2014, 118, 14629–14639. [Google Scholar] [CrossRef]
- Khan, M.N.; Jaisai, M.; Dutta, J. Photocatalytic Inactivation of Escherichia Coli Using Zinc Stannate Nanostructures under Visible Light. Adv. Mater. Res. 2015, 1131, 203–209. [Google Scholar] [CrossRef]
- Najam Khan, M.; Dutta, J. Comparison of photocatalytic activity of zinc stannate particles and zinc stannate/zinc oxide composites for the removal of phenol from water, and a study on the effect of pH on photocatalytic efficiency. Mater. Sci. Semicond. Process. 2015, 36, 124–133. [Google Scholar] [CrossRef]
- Barrocas, B.; Sério, S.; Rovisco, A.; Nunes, Y.; Jorge, M.E.E.M. Removal of rhodamine 6G dye contaminant by visible light driven immobilized Ca1-xLnxMnO3 (Ln = Sm, Ho; 0.1 ≤ x ≤ 0.4) photocatalysts. Appl. Surf. Sci. 2015, 360, 798–806. [Google Scholar] [CrossRef]
- Liu, X.; Gu, S.; Zhao, Y.; Zhou, G.; Li, W. BiVO4, Bi2WO6 and Bi2MoO6 photocatalysis: A brief review. J. Mater. Sci. Technol. 2020, 56, 45–68. [Google Scholar] [CrossRef]
- Rovisco, A.; Branquinho, R.; Deuermeier, J.; Freire, T.; Fortunato, E.; Martins, R.; Barquinha, P. Shape Effect of Zinc-Tin Oxide Nanostructures on Photodegradation of Methylene Blue and Rhodamine B under UV and Visible Light. ACS Appl. Nano Mater. 2021, 4, 1149–1161. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.; Janes, D.B.; Ju, S. Interface studies of N2 plasma-treated ZnSnO nanowire transistors using low-frequency noise measurements. Nanotechnology 2013, 24, 305201. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Liang, S. Morphological zinc stannate: Synthesis, fundamental properties and applications. J. Mater. Chem. A 2017, 5, 20534–20560. [Google Scholar] [CrossRef]
- Lei, M.; Sheng, Y.; Wan, L.; Bi, K.; Huang, K.; Jia, R.; Liu, J.; Wang, Y. A novel self-catalytic route to zinc stannate nanowires and cathodoluminescence and electrical transport properties of a single nanowire. J. Alloys Compd. 2016, 657, 394–399. [Google Scholar] [CrossRef]
- Nawaz, A.; Goudarzi, S.; Saravanan, P.; Zarrin, H. Z-scheme induced g-C3N4/WS2 heterojunction photocatalyst with improved electron mobility for enhanced solar photocatalysis. Sol. Energy 2021, 228, 53–67. [Google Scholar] [CrossRef]
- Nandy, S.; Fortunato, E.; Martins, R. Green economy and waste management: An inevitable plan for materials science. Prog. Nat. Sci. Mater. Int. 2022, 32, 1–9. [Google Scholar] [CrossRef]
- Wu, J.M.; Xu, C.; Zhang, Y.; Wang, Z.L. Lead-free nanogenerator made from single ZnSnO3 microbelt. ACS Nano 2012, 6, 4335–4340. [Google Scholar] [CrossRef]
- Wu, J.M.; Chen, C.-Y.; Zhang, Y.; Chen, K.-H.; Yang, Y.; Hu, Y.; He, J.-H.; Wang, Z.L. Ultrahigh Sensitive Piezotronic Strain Sensors Based on a ZnSnO3 Nanowire/Microwire. ACS Nano 2012, 6, 4369–4374. [Google Scholar] [CrossRef]
- Wu, J.M.; Xu, C.; Zhang, Y.; Yang, Y.; Zhou, Y.; Wang, Z.L. Flexible and transparent nanogenerators based on a composite of lead-free ZnSnO3 triangular-belts. Adv. Mater. 2012, 24, 6094–6099. [Google Scholar] [CrossRef]
- Guo, R.; Guo, Y.; Duan, H.; Li, H.; Liu, H. Synthesis of Orthorhombic Perovskite-Type ZnSnO3 Single-Crystal Nanoplates and Their Application in Energy Harvesting. ACS Appl. Mater. Interfaces 2017, 9, 8271–8279. [Google Scholar] [CrossRef]
- Rovisco, A.; dos Santos, A.; Cramer, T.; Martins, J.; Branquinho, R.; Águas, H.; Fraboni, B.; Fortunato, E.; Martins, R.; Igreja, R.; et al. Piezoelectricity Enhancement of Nanogenerators Based on PDMS and ZnSnO3 Nanowires through Microstructuration. ACS Appl. Mater. Interfaces 2020, 12, 18421–18430. [Google Scholar] [CrossRef]
- Hwang, J.K.; Cho, S.; Dang, J.M.; Kwak, E.B.; Song, K.; Moon, J.; Sung, M.M. Direct nanoprinting by liquid-bridge-mediated nanotransfer moulding. Nat. Nanotechnol. 2010, 5, 742–748. [Google Scholar] [CrossRef]
- Lim, T.; Kim, H.; Meyyappan, M.; Ju, S. Photostable Zn2SnO4 Nanowire Transistors for Transparent Displays. ACS Nano 2012, 6, 4912–4920. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, G.U.; Rehman, M.M.; Choi, K.H. Enhanced resistive switching in all-printed, hybrid and flexible memory device based on perovskite ZnSnO3 via PVOH polymer. Polymer 2016, 100, 102–110. [Google Scholar] [CrossRef]
- Yang, Y.J.; Rehman, M.M.; Siddiqui, G.U.; Na, K.H.; Choi, K.H. Effect of adding a polymer and varying device size on the resistive switching characteristics of perovskite nanocubes heterojunction. Curr. Appl. Phys. 2017, 17, 1733–1741. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, M.; Hu, C. Novel Zn2SnO4 hierarchical nanostructures and their gas sensing properties toward ethanol. J. Phys. Chem. C 2011, 115, 5522–5529. [Google Scholar] [CrossRef]
- Singh, R.; Yadav, A.K.; Gautam, C. Synthesis and Humidity Sensing Investigations of Nanostructured ZnSnO3. J. Sens. Technol. 2011, 01, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Tharsika, T.; Haseeb, A.S.M.A.; Akbar, S.A.; Sabri, M.F.M.; Wong, Y.H. Gas sensing properties of zinc stannate (Zn2SnO4) nanowires prepared by carbon assisted thermal evaporation process. J. Alloys Compd. 2015, 618, 455–462. [Google Scholar] [CrossRef]
- Zeng, J.; Xin, M.; Li, K.; Wang, H.; Yan, H.; Zhang, W. Transformation Process and Photocatalytic Activities of Hydrothermally Synthesized Zn2 SnO4 Nanocrystals. J. Phys. Chem. C 2008, 112, 4159–4167. [Google Scholar] [CrossRef]
- Fu, X.; Wang, X.; Ding, Z.; Leung, D.Y.C.; Zhang, Z.; Long, J.; Zhang, W.; Li, Z.; Fu, X. Hydroxide ZnSn(OH)6: A promising new photocatalyst for benzene degradation. Appl. Catal. B Environ. 2009, 91, 67–72. [Google Scholar] [CrossRef]
- Fu, X.; Wang, X.; Long, J.; Ding, Z.; Yan, T.; Zhang, G.; Zhang, Z.; Lin, H.; Fu, X. Hydrothermal synthesis, characterization, and photocatalytic properties of Zn2SnO4. J. Solid State Chem. 2009, 182, 517–524. [Google Scholar] [CrossRef]
- Lo, M.-K.; Lee, S.-Y.; Chang, K.-S. Study of ZnSnO3-Nanowire Piezophotocatalyst Using Two-Step Hydrothermal Synthesis. J. Phys. Chem. C 2015, 119, 5218–5224. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Chang, K.-S. Piezopotential-Induced Schottky Behavior of Zn1−xSnO3 Nanowire Arrays and Piezophotocatalytic Applications. J. Am. Ceram. Soc. 2016, 99, 2593–2600. [Google Scholar] [CrossRef]
- Biswas, A.; Saha, S.; Jana, N.R. ZnSnO3 Nanoparticle-Based Piezocatalysts for Ultrasound-Assisted Degradation of Organic Pollutants. ACS Appl. Nano Mater. 2019, 2, 1120–1128. [Google Scholar] [CrossRef]
- Tobergte, D.R.; Curtis, S. Formation of Self-Assembled Defect-Free Zn2SnO4 Nanostructures from Binary Oxides without the Kirkendall Effect. J. Chem. Inf. Model. 2013, 53, 1689–1699. [Google Scholar]
- Tian, Z.; Liang, C.; Liu, J.; Zhang, H.; Zhang, L.-D. Zinc Stannate Nanocubes and Nanourchins with High Photocatalytic Activity for Methyl Orange and 2,5-DCP Degradation. J. Mater. Chem. 2012, 22, 17210–17214. [Google Scholar] [CrossRef]
- Ivetić, T.B.; Finčur, N.L.; Đačanin, L.R.; Abramović, B.F.; Lukić-Petrović, S.R. Ternary and coupled binary zinc tin oxide nanopowders: Synthesis, characterization, and potential application in photocatalytic processes. Mater. Res. Bull. 2015, 62, 114–121. [Google Scholar] [CrossRef]
- Jia, T.; Liu, M.; Yu, D.; Long, F.; Mo, S.; Deng, Z.; Wang, W. A Facile Approach for the Synthesis of Zn2SnO4/BiOBr Hybrid Nanocomposites with Improved Visible-Light Photocatalytic Performance. Nanomaterials 2018, 8, 313. [Google Scholar] [CrossRef] [Green Version]
- Ben Ali, M.; Barka-Bouaifel, F.; Elhouichet, H.; Sieber, B.; Addad, A.; Boussekey, L.; Férid, M.; Boukherroub, R. Hydrothermal synthesis, phase structure, optical and photocatalytic properties of Zn2SnO4 nanoparticles. J. Colloid Interface Sci. 2015, 457, 360–369. [Google Scholar] [CrossRef]
- Jain, S.; Shah, A.P.; Shimpi, N.G. An efficient photocatalytic degradation of organic dyes under visible light using zinc stannate (Zn2SnO4) nanorods prepared by microwave irradiation. Nano-Struct. Nano-Objects 2020, 21, 100410. [Google Scholar] [CrossRef]
- Bulut, N.; Baytar, O.; Şahin, Ö.; Horoz, S. Synthesis zto nanoparticles and study of their photocatalytic properties. J. Ovonic Res. 2019, 15, 143–150. [Google Scholar]
- Ayesha, B.; Jabeen, U.; Naeem, A.; Kasi, P.; Malghani, M.N.K.; Khan, S.U.; Akhtar, J.; Aamir, M. Synthesis of zinc stannate nanoparticles by sol-gel method for photocatalysis of commercial dyes. Results Chem. 2020, 2, 100023. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Peter, A.; Al-Najar, B.; Vijaya, J.; Bououdina, M. Photocatalysis: Activity of Nanomaterials. Nanotechnol. Environ. Sci. 2018, 1–2, 209–292. [Google Scholar]
- Akir, S.; Barras, A.; Coffinier, Y.; Bououdina, M.; Boukherroub, R.; Omrani, A.D. Eco-friendly synthesis of ZnO nanoparticles with different morphologies and their visible light photocatalytic performance for the degradation of Rhodamine B. Ceram. Int. 2016, 42, 10259–10265. [Google Scholar] [CrossRef]
- Huang, N.; Shu, J.; Wang, Z.; Chen, M.; Ren, C.; Zhang, W. One-step pyrolytic synthesis of ZnO nanorods with enhanced photocatalytic activity and high photostability under visible light and UV light irradiation. J. Alloys Compd. 2015, 648, 919–929. [Google Scholar] [CrossRef]
- Isabel Bento Rovisco, A.; Branquinho, R.; Vaz Pinto, J.; Martins, R.; Fortunato, E.; Barquinha, P. Hydrothermal Synthesis of Zinc Tin Oxide Nanostructures for Photocatalysis, Energy Harvesting and Electronics. In Novel Nanomaterials; IntechOpen: London, UK, 2021. [Google Scholar]
- Henriques Ferreira, S.; Rovisco, A.; dos Santos, A.; Águas, H.; Igreja, R.; Barquinha, P.; Fortunato, E.; Martins, R. Porous ZnO Nanostructures Synthesized by Microwave Hydrothermal Method for Energy Harvesting Applications. In Nanopores; Ameen, S., Akhtar, M.S., Shin, H.-S., Eds.; IntechOpen: London, UK, 2021. [Google Scholar]
- Chen, D.; Wang, Q.; Shen, G.; Wang, R. Ternary oxide nanostructured materials for supercapacitors: A review. J. Mater. Chem. A Mater. Energy Sustain. 2015, 3, 10158–10173. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Effect of Microwave Radiation Power on the Size of Aggregates of ZnO NPs Prepared Using Microwave Solvothermal Synthesis. Nanomaterials 2018, 8, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehnen, T.; Zopes, D.; Mathur, S. Phase-selective microwave synthesis and inkjet printing applications of Zn2SnO4 (ZTO) quantum dots. J. Mater. Chem. 2012, 22, 17732. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef]
- Nehru, L.C.; Sanjeeviraja, C. Processing Research Controllable growth of Zn2SnO4 nanostructures by urea assisted microwave-assisted solution combustion process. J. Ceram. Process. Res. 2013, 14, 606–609. [Google Scholar]
- Reyes, O.; Pal, M.; Escorcia-García, J.; Sánchez-Albores, R.; Sebastian, P.J. Microwave-assisted chemical synthesis of Zn2SnO4 nanoparticles. Mater. Sci. Semicond. Process. 2020, 108, 104878. [Google Scholar] [CrossRef]
- Foletto, E.L.; Simões, J.M.; Mazutti, M.A.; Jahn, S.L.; Muller, E.I.; Pereira, L.S.F.; Flores, E.M.D.M. Application of Zn2SnO4 photocatalyst prepared by microwave-assisted hydrothermal route in the degradation of organic pollutant under sunlight. Ceram. Int. 2013, 39, 4569–4574. [Google Scholar] [CrossRef]
- Rovisco, A.; Branquinho, R.; Martins, J.; Oliveira, M.J.; Nunes, D.; Fortunato, E.; Martins, R.; Barquinha, P. Seed-layer free zinc tin oxide tailored nanostructures for nanoelectronic applications: Effect of chemical parameters. ACS Appl. Nano Mater. 2018, 1, 3986–3997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovisco, A.; Branquinho, R.; Martins, R.; Fortunato, E.; Barquinha, P. Microwave-Assisted Hydrothermal Synthesis of Zn2SnO4 Nanostructures for Photocatalytic Dye Degradation. Mater. Proc. 2021, 4, 92. [Google Scholar]
- Annamalai, A.; Carvalho, D.; Wilson, K.C.; Lee, M.-J. Properties of hydrothermally synthesized Zn2SnO4 nanoparticles using Na2CO3 as a novel mineralizer. Mater. Charact. 2010, 61, 873–881. [Google Scholar] [CrossRef]
- Mishra, V.; Warshi, M.K.; Sati, A.; Kumar, A.; Mishra, V.; Sagdeo, A.; Kumar, R.; Sagdeo, P.R. Diffuse reflectance spectroscopy: An effective tool to probe the defect states in wide band gap semiconducting materials. Mater. Sci. Semicond. Process. 2018, 86, 151–156. [Google Scholar] [CrossRef]
- Viezbicke, B.D.; Patel, S.; Davis, B.E.; Birnie, D.P. Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system. Phys. Status Solidi 2015, 252, 1700–1710. [Google Scholar] [CrossRef]
- Kamiya, T.; Nomura, K.; Hosono, H. Electronic structure of the amorphous oxide semiconductor a-InGaZnO4-x: Tauc-Lorentz optical model and origins of subgap states. Phys. Status Solidi 2009, 206, 860–867. [Google Scholar] [CrossRef]
- Ji, X.; Huang, X.; Liu, J.; Jiang, J.; Li, X.; Ding, R.; Hu, Y.; Wu, F.; Li, Q. Hydrothermal synthesis of novel Zn2SnO4 octahedron microstructures assembled with hexagon nanoplates. J. Alloys Compd. 2010, 503, L21–L25. [Google Scholar] [CrossRef]
- Zhao, Q.; Ju, D.; Song, X.; Deng, X.; Ding, M.; Xu, X.; Zeng, H. Polyhedral Zn2SnO4: Synthesis, enhanced gas sensing and photocatalytic performance. Sens. Actuators B Chem. 2016, 229, 627–634. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Liu, X.; Ren, C.; Miao, X.; Li, X. Engineering of Gd/Er/Lu-triple-doped Bi2MoO6 to synergistically boost the photocatalytic performance in three different aspects: Oxidizability, light absorption and charge separation. Appl. Surf. Sci. 2019, 463, 556–565. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Branquinho, R.; Fortunato, E.; Martins, R. Metal oxide-based photocatalytic paper: A green alternative for environmental remediation. Catalysts 2021, 11, 504. [Google Scholar] [CrossRef]

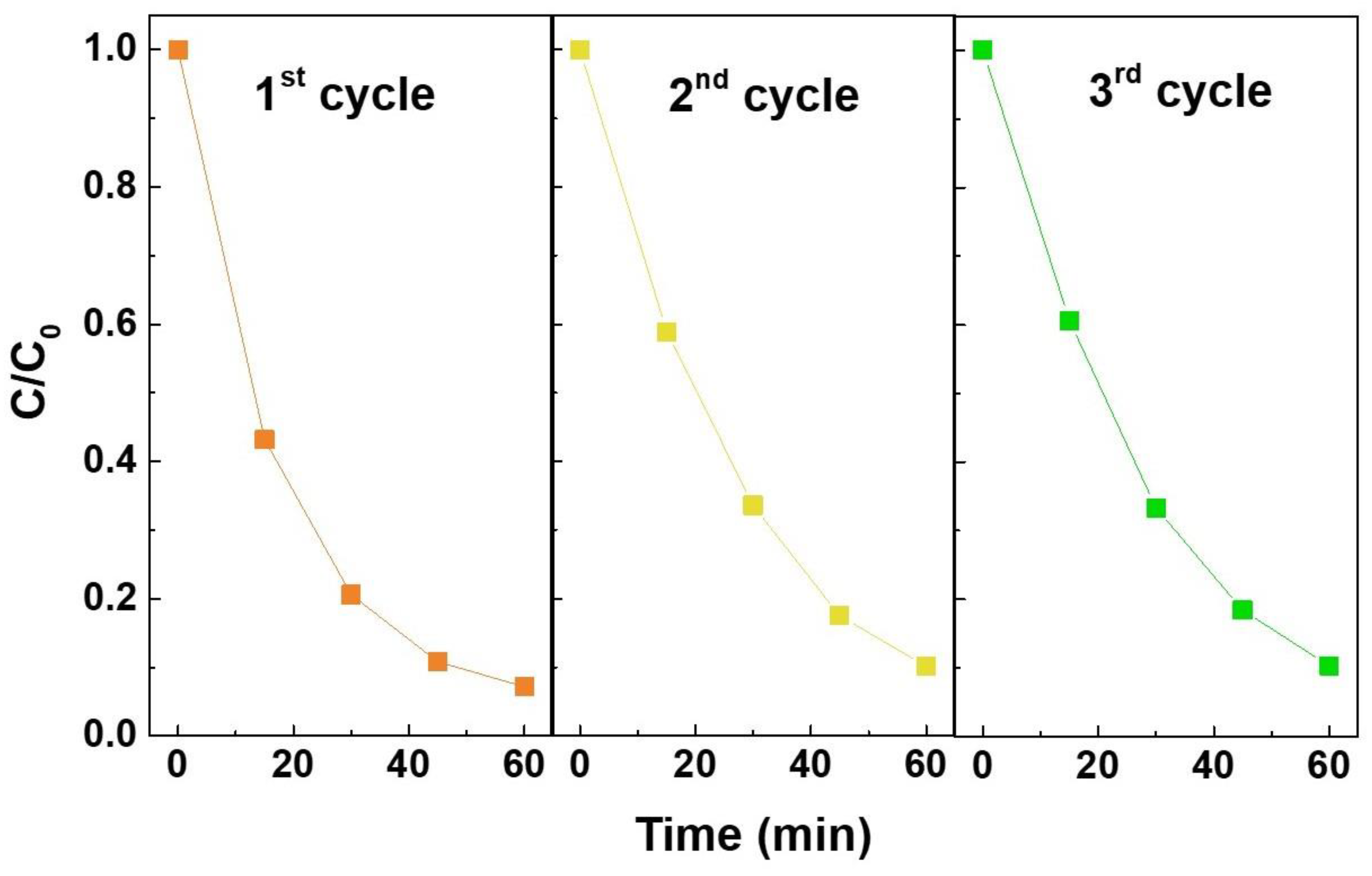

| ZTO Nanostructure | Dye | Catalyst Concentration | Radiation | Adsorption-Desorption | Photodegradation Rate (min−1) | Ref. |
|---|---|---|---|---|---|---|
| Zn2SnO4 Nanoparticles | MB (20 mg in 1 L of DW) | 0.3 mg/mL | UV lamp | 30 min | 0.2638 | [49] |
| Zn2SnO4 Nanocubes | MO (10 µM) | 2.5 mg/mL (ethanolic solution drop-casted onto glass) | UV lamp | 1 h | 0.0326 | [50] |
| MB (10 µM) | 0.0326 | |||||
| Zn2SnO4 Nanoparticles | RhB (5 µM) | 0.2 g/L | Visible light (λ > 420 nm, 0.5 W cm−2) | 1 h | 0.0249 | [47] |
| Phenol (5 µM) | 0.5 mg/mL | UV (λ = 365 nm) | N.A. | N.A. | ||
| Zn2SnO4 Nanorods | RhB (10 mg/L) | 1 × 10−5 M | Visible light (λ ≥ 420 nm, 350 W) | 60 min | 0.00264 | [48] |
| MO (10 mg/L) | 0.00141 | |||||
| Zn2SnO4 Nanoparticles | RhB (5 mg/L) | 0.8 mg/mL | Visible light (100 mW·cm−2) | 30 min | 10.1 × 10−4 | [19] |
| UV (λ = 254 nm, 35 W/m2) | 0.0340 | |||||
| MB (1.5 mg/L) | 0.8 mg/mL | UV (λ = 254 nm, 35 W/m2) | 30 min | 0.0273 | ||
| Zn2SnO4 Polyhedrons | RhB (5 mg/L) | 0.8 mg/mL | Visible light (100 mW·cm−2) | 30 min | 4.7 × 10−4 | [19] |
| UV (λ = 254 nm, 35 W/m2) | 0.0132 | |||||
| MB (1.5 mg/L) | 0.8 mg/mL | UV (λ = 254 nm, 35 W/m2) | 30 min | 0.0164 | ||
| ZnSnO3 Nanowires | RhB (5 mg/L) | 0.8 mg/mL | Visible light (100 mW·cm−2) | 30 min | 8.1 × 10−4 | [19] |
| UV (λ = 254 nm, 35 W/m2) | 0.0330 | |||||
| MB (1.5 mg/L) | 0.8 mg/mL | UV (λ = 254 nm, 35 W/m2) | 30 min | 0.0381 |
| Sample | Diameter (nm) | Optical Band Gap (eV) | Urbach Energy, EU (eV) |
|---|---|---|---|
| Nanoparticles (5 min) | 23 ± 2 | 4.25 | 0.07 |
| Nanoparticles (10 min) | 31 ± 4 | 4.18 | 0.11 |
| Nanoparticles (20 min) | 28 ± 3 | 4.20 | 0.18 |
| Nanoparticles (24 h @oven) | 18 ± 3 1 | 3.95 1 | 0.17 1 |
| Polyhedrons (60 min) | 313 ± 99 | 3.98 | 0.06 |
| Nanoplates (30 min) | 244 ± 27 | 3.84 | 0.07 |
| Sample | Degradation Rate (min−1) |
|---|---|
| Nanoparticles (5 min) | 0.0374 ± 0.0001 |
| Nanoparticles (10 min) | 0.0471 ± 0.0002 |
| Nanoparticles (20 min) | 0.0366 ± 0.0005 |
| Nanoparticles (24 h @oven) | 0.0340 ± 0.0016 1 |
| Polyhedrons (60 min) | 0.0151 ± 0.0002 |
| Nanoplates (30 min) | 0.0297 ± 0.0006 |
| Sample | Degradation Rate (min−1) |
|---|---|
| Nanoparticles (5 min) | 0.0263 ± 0.0012 |
| Nanoparticles (10 min) | 0.0325 ± 0.0004 |
| Nanoparticles (20 min) | 0.0304 ± 0.0016 |
| Nanoparticles (24 h@oven) | 0.0273 ± 0.0007 |
| Polyhedrons (60 min) | 0.0221 ± 0.0001 |
| Nanoplates (30 min) | 0.0213 ± 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovisco, A.; Morais, M.; Branquinho, R.; Fortunato, E.; Martins, R.; Barquinha, P. Microwave-Assisted Synthesis of Zn2SnO4 Nanostructures for Photodegradation of Rhodamine B under UV and Sunlight. Nanomaterials 2022, 12, 2119. https://doi.org/10.3390/nano12122119
Rovisco A, Morais M, Branquinho R, Fortunato E, Martins R, Barquinha P. Microwave-Assisted Synthesis of Zn2SnO4 Nanostructures for Photodegradation of Rhodamine B under UV and Sunlight. Nanomaterials. 2022; 12(12):2119. https://doi.org/10.3390/nano12122119
Chicago/Turabian StyleRovisco, Ana, Maria Morais, Rita Branquinho, Elvira Fortunato, Rodrigo Martins, and Pedro Barquinha. 2022. "Microwave-Assisted Synthesis of Zn2SnO4 Nanostructures for Photodegradation of Rhodamine B under UV and Sunlight" Nanomaterials 12, no. 12: 2119. https://doi.org/10.3390/nano12122119
APA StyleRovisco, A., Morais, M., Branquinho, R., Fortunato, E., Martins, R., & Barquinha, P. (2022). Microwave-Assisted Synthesis of Zn2SnO4 Nanostructures for Photodegradation of Rhodamine B under UV and Sunlight. Nanomaterials, 12(12), 2119. https://doi.org/10.3390/nano12122119