Recent Progress in Lanthanide-Doped Inorganic Perovskite Nanocrystals and Nanoheterostructures: A Future Vision of Bioimaging
Abstract
:1. Introduction
2. Metal Halide Perovskite Nanocrystals
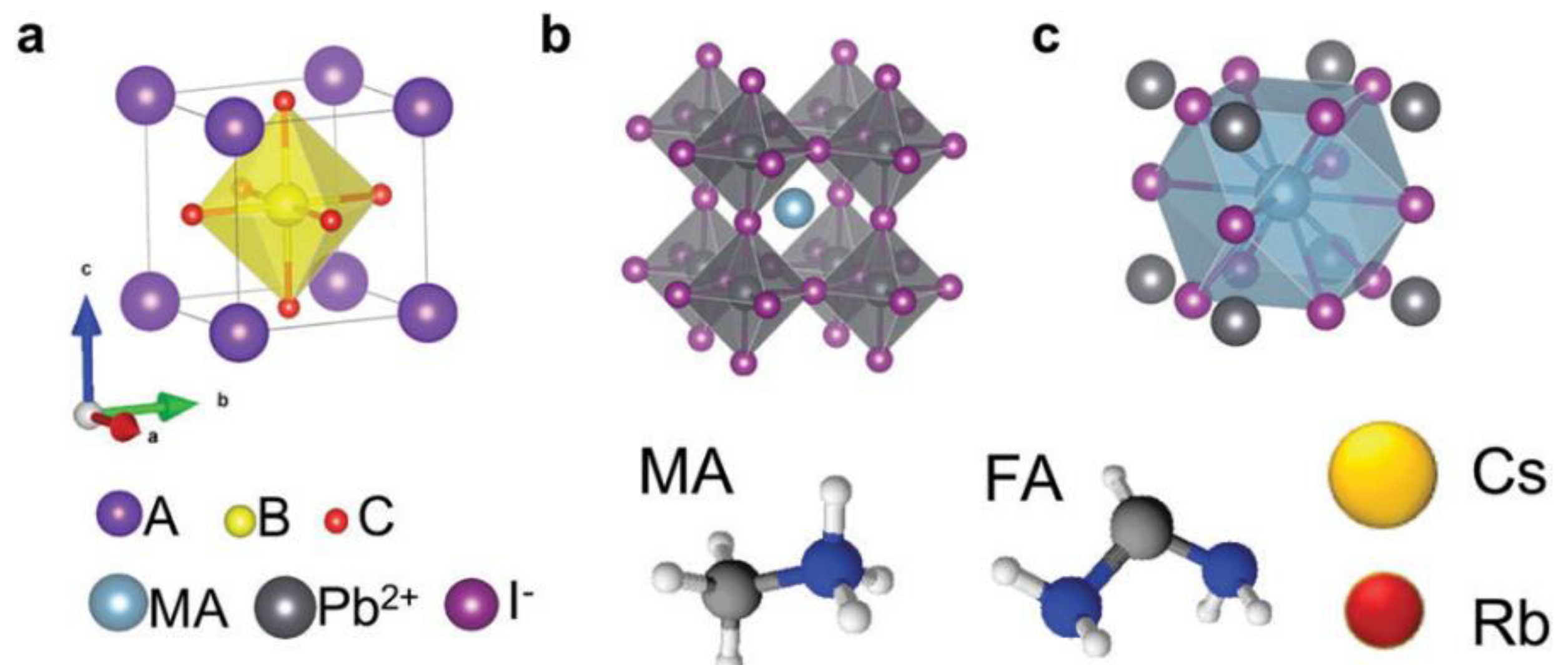
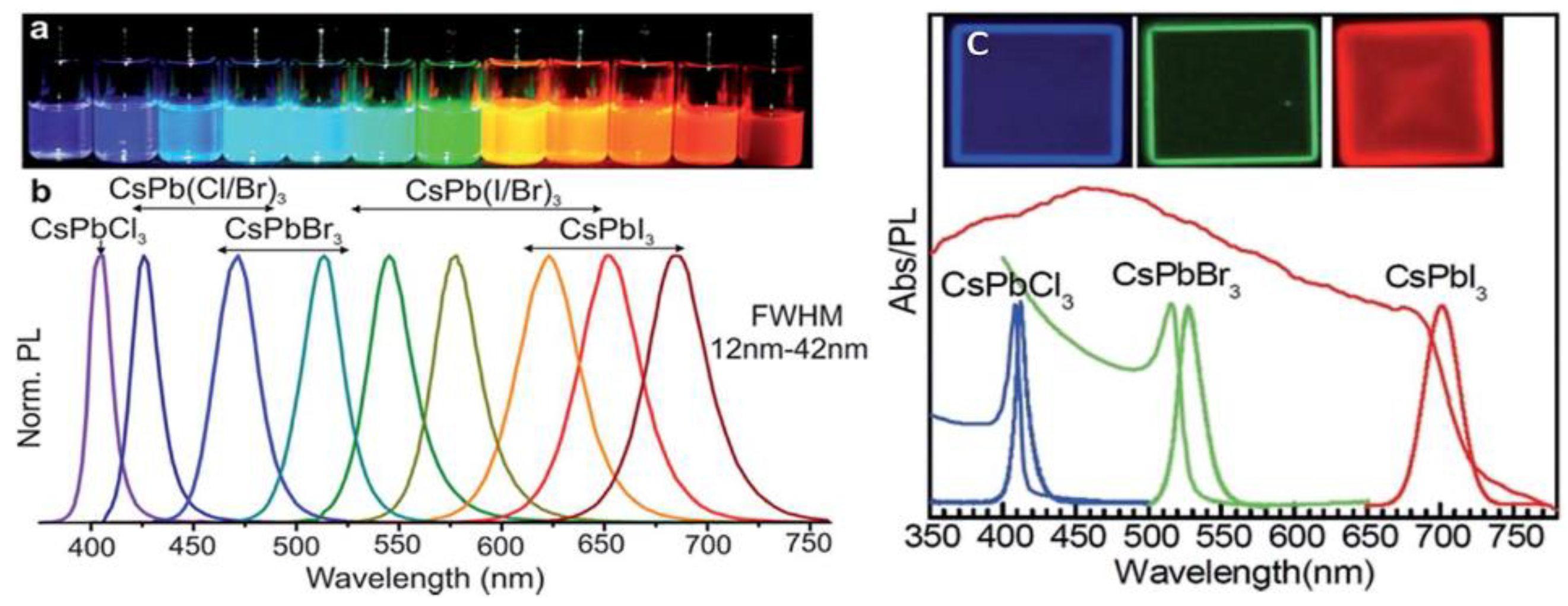
2.1. All-Inorganic Halide Perovskites (IHP)
2.2. Ln-Doped Inorganic Halide Perovskites (LnIHPs)
3. Lanthanide-Doped Matrices (NaLnF4 Matrices, Ln NPs)
4. Nanoheterostructures Based on IHP NCs and Ln NPs
5. Other UC Luminescence Materials
6. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.-G.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly Luminescent and Color-Tunable Colloidal CH3NH3PbX3 (X = Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bodnarchuk, M.I.; Kershaw, S.V.; Kovalenko, M.V.; Rogach, A.L. Lead Halide Perovskite Nanocrystals in the Research Spotlight: Stability and Defect Tolerance. ACS Energy Lett. 2017, 2, 2071–2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Yakunin, S.; Protesescu, L.; Krieg, F.; Bodnarchuk, M.I.; Nedelcu, G.; Humer, M.; De Luca, G.; Fiebig, M.; Heiss, W.; Kovalenko, M.V. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of caesium lead halide perovskites. Nat. Commun. 2015, 6, 8056. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Kanatzidis, M.G. The Renaissance of Halide Perovskites and Their Evolution as Emerging Semiconductors. Acc. Chem. Res. 2015, 48, 2791–2802. [Google Scholar] [CrossRef]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef]
- Dey, A.; Ye, J.; De, A.; Debroye, E.; Ha, S.K.; Bladt, E.; Kshirsagar, A.S.; Wang, Z.; Yin, J.; Wang, Y.; et al. State of the Art and Prospects for Halide Perovskite Nanocrystals. ACS Nano 2021, 15, 10775–10981. [Google Scholar] [CrossRef]
- Ma, J.; McLeod, J.A.; Chang, L.-Y.; Pao, C.-W.; Lin, B.-H.; Li, X.-Y.; Wang, Z.; Chen, J.; Sham, T.-K.; Liu, L. Increasing photoluminescence yield of CsPbCl3 nanocrystals by heterovalent doping with Pr3+. Mater. Res. Bull. 2020, 129, 110907. [Google Scholar] [CrossRef]
- Yuan, L.; Zhou, L.; Xiang, W.; Liang, X. Enhanced stability of red-emitting CsPbI3:Yb3+nanocrystal glasses: A potential luminescent material. J. Non-Cryst. Solids 2020, 545, 120232. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, C.; Ding, L.; Liu, J.; Xiang, W.; Liang, X. Novel B-site Cd2+ doped CsPbBr3 quantum dot glass toward strong fluorescence and high stability for wLED. Opt. Mater. 2020, 107, 110046. [Google Scholar] [CrossRef]
- Fu, P.; Hu, S.; Tang, J.; Xiao, Z. Material exploration via designing spatial arrangement of octahedral units: A case study of lead halide perovskites. Front. Optoelectron. 2021, 14, 252–259. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, P.; Tu, D.; Ma, E.; Zhu, H.; Chen, X. Lanthanide-doped upconversion nano-bioprobes: Electronic structures, optical properties, and biodetection. Chem. Soc. Rev. 2015, 44, 1379–1415. [Google Scholar] [CrossRef]
- Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2004, 104, 139–174. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Chen, W. Nanoparticle Fluorescence Based Technology for Biological Applications. J. Nanosci. Nanotechnol. 2008, 8, 1019–1051. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Huang, D.; Chen, G. Recent advances of lanthanide-doped upconversion nanoparticles for biological applications. Nanotechnology 2019, 31, 072001. [Google Scholar] [CrossRef]
- Jalani, G.; Naccache, R.; Rosenzweig, D.H.; Haglund, L.; Vetrone, F.; Cerruti, M. Photocleavable Hydrogel-Coated Upconverting Nanoparticles: A Multifunctional Theranostic Platform for NIR Imaging and On-Demand Macromolecular Delivery. J. Am. Chem. Soc. 2016, 138, 1078–1083. [Google Scholar] [CrossRef] [Green Version]
- Jayakumar, M.K.G.; Idris, N.M.; Zhang, Y. Remote activation of biomolecules in deep tissues using near-infrared-to-UV upconversion nanotransducers. Proc. Natl. Acad. Sci. USA 2012, 109, 8483–8488. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, X.; Ying, Q.; Wang, C.; Jia, W.; Xing, X.; Yin, L.; Lu, Z.; Zhang, K.; Pan, Y.; et al. Self-Assembly of Perovskite CsPbBr3 Quantum Dots Driven by a Photo-Induced Alkynyl Homocoupling Reaction. Angew. Chem. Int. Ed. 2020, 59, 17207–17213. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, D.; Gao, M.; Lai, M.; Lin, J.; Lei, T.; Lin, Z.; Quan, L.N.; Yang, P. Quantitative imaging of anion exchange kinetics in halide perovskites. Proc. Natl. Acad. Sci. USA 2019, 116, 12648–12653. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Yin, Y. All-Inorganic Metal Halide Perovskite Nanocrystals: Opportunities and Challenges. ACS Cent. Sci. 2018, 4, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Patibandla, S.; Gao, Y.; Gates, K.; Ray, P.C. Water Triggered Synthesis of Highly Stable and Biocompatible 1D Nanowire, 2D Nanoplatelet, and 3D Nanocube CsPbBr3 Perovskites for Multicolor Two-Photon Cell Imaging. JACS Au 2021, 1, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, G.; Wang, R.; Shao, H.; Wang, H.; Xu, W.; Cui, H.; Song, H. Considerably enhanced exciton emission of CsPbCl3 perovskite quantum dots by the introduction of potassium and lanthanide ions. Nanoscale 2018, 10, 14067–14072. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xu, W.; Pan, G.; Liu, Y.; Yang, M.; Hua, S.; Chen, X.; Peng, H.; Song, H. Enhancing the exciton emission of CsPbCl3 perovskite quantum dots by incorporation of Rb+ ions. Mater. Res. Bull. 2019, 112, 142–146. [Google Scholar] [CrossRef]
- Shin, S.G.; Hur, J.; Choi, H.W. A Study on the Characteristics of Perovskite/ZnO-Based Ultraviolet Sensors. J. Nanosci. Nanotechnol. 2021, 21, 4336–4340. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yang, C.; Huang, P.; Wang, S.; Liu, M.; Jiang, N.; Chen, D. Photoluminescence tuning from glass-stabilized CsPbX3 (X = Cl, Br, I) perovskite nanocrystals triggered by upconverting Tm: KYb2F7 nanoparticles for high-level anti-counterfeiting. Chem. Eng. J. 2020, 395, 125214. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Abate, A.; Saliba, M.; Tress, W.; Jesper Jacobsson, T.; Grätzel, M.; Hagfeldt, A. The rapid evolution of highly efficient perovskite solar cells. Energy Environ. Sci. 2017, 10, 710–727. [Google Scholar] [CrossRef]
- Liu, P.; Han, N.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. High-Quality Ruddlesden–Popper Perovskite Film Formation for High-Performance Perovskite Solar Cells. Adv. Mater. 2021, 33, 2002582. [Google Scholar] [CrossRef]
- Xiang, H.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Towards highly stable and efficient planar perovskite solar cells: Materials development, defect control and interfacial engineering. Chem. Eng. J. 2021, 420, 127599. [Google Scholar] [CrossRef]
- Liu, P.; Chen, Y.; Xiang, H.; Yang, X.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Benefitting from Synergistic Effect of Anion and Cation in Antimony Acetate for Stable CH3NH3PbI3-Based Perovskite Solar Cell with Efficiency Beyond 21%. Small 2021, 17, 2102186. [Google Scholar] [CrossRef]
- Wang, W.; Xu, M.; Xu, X.; Zhou, W.; Shao, Z. Perovskite Oxide Based Electrodes for High-Performance Photoelectrochemical Water Splitting. Angew. Chem. Int. Ed. 2020, 59, 136–152. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Ruddlesden–Popper Perovskite Oxides for Photocatalysis-Based Water Splitting and Wastewater Treatment. Energy Fuels 2020, 34, 9208–9221. [Google Scholar] [CrossRef]
- Han, X.; Liu, P.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Non-metal fluorine doping in Ruddlesden–Popper perovskite oxide enables high-efficiency photocatalytic water splitting for hydrogen production. Mater. Today Energy 2022, 23, 100896. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Enhancing the photocatalytic activity of Ruddlesden-Popper Sr2TiO4 for hydrogen evolution through synergistic silver doping and moderate reducing pretreatment. Mater. Today Energy 2022, 23, 100899. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Liu, P.; Xiang, H.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Simultaneous Power Conversion Efficiency and Stability Enhancement of Cs2AgBiBr6 Lead-Free Inorganic Perovskite Solar Cell through Adopting a Multifunctional Dye Interlayer. Adv. Funct. Mater. 2020, 30, 2001557. [Google Scholar] [CrossRef]
- Yang, X.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Recent Advances in Cs2AgBiBr6-Based Halide Double Perovskites as Lead-Free and Inorganic Light Absorbers for Perovskite Solar Cells. Energy Fuels 2020, 34, 10513–10528. [Google Scholar] [CrossRef]
- Yang, X.; Xie, A.; Xiang, H.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. First investigation of additive engineering for highly efficient Cs2AgBiBr6-based lead-free inorganic perovskite solar cells. Appl. Phys. Rev. 2021, 8, 041402. [Google Scholar] [CrossRef]
- Liu, P.; Yang, X.; Chen, Y.; Xiang, H.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Promoting the Efficiency and Stability of CsPbIBr2-Based All-Inorganic Perovskite Solar Cells through a Functional Cu2+ Doping Strategy. ACS Appl. Mater. Interfaces 2020, 12, 23984–23994. [Google Scholar] [CrossRef]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef]
- Tsai, H.; Nie, W.; Blancon, J.-C.; Stoumpos, C.C.; Asadpour, R.; Harutyunyan, B.; Neukirch, A.J.; Verduzco, R.; Crochet, J.J.; Tretiak, S.; et al. High-efficiency two-dimensional Ruddlesden-Popper perovskite solar cells. Nature 2016, 536, 312–316. [Google Scholar] [CrossRef]
- Zhang, Q.; Ha, S.T.; Liu, X.; Sum, T.C.; Xiong, Q. Room-Temperature Near-Infrared High-Q Perovskite Whispering-Gallery Planar Nanolasers. Nano Lett. 2014, 14, 5995–6001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X.Y. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef]
- Xing, J.; Yan, F.; Zhao, Y.; Chen, S.; Yu, H.; Zhang, Q.; Zeng, R.; Demir, H.V.; Sun, X.; Huan, A.; et al. High-Efficiency Light-Emitting Diodes of Organometal Halide Perovskite Amorphous Nanoparticles. ACS Nano 2016, 10, 6623–6630. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Quan, L.N.; Comin, R.; Walters, G.; Sabatini, R.; Voznyy, O.; Hoogland, S.; Zhao, Y.; Beauregard, E.M.; Kanjanaboos, P.; et al. Perovskite energy funnels for efficient light-emitting diodes. Nat. Nanotechnol. 2016, 11, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Gurudayal; Sabba, D.; Kumar, M.H.; Wong, L.H.; Barber, J.; Grätzel, M.; Mathews, N. Perovskite–Hematite Tandem Cells for Efficient Overall Solar Driven Water Splitting. Nano Lett. 2015, 15, 3833–3839. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Im, J.-H.; Mayer, M.T.; Schreier, M.; Nazeeruddin, M.K.; Park, N.-G.; Tilley, S.D.; Fan, H.J.; Grätzel, M. Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 2014, 345, 1593–1596. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- MØLler, C.K. Crystal Structure and Photoconductivity of Cæsium Plumbohalides. Nature 1958, 182, 1436. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, R.; Liu, X.; Xing, J.; Sum, T.C.; Xiong, Q. High-Quality Whispering-Gallery-Mode Lasing from Cesium Lead Halide Perovskite Nanoplatelets. Adv. Funct. Mater. 2016, 26, 6238–6245. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Bünzli, J.-C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [Green Version]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Hinklin, T.R.; Rand, S.C.; Laine, R.M. Transparent, Polycrystalline Upconverting Nanoceramics: Towards 3-D Displays. Adv. Mater. 2008, 20, 1270–1273. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Sun, L.-D.; Yan, C.-H. Luminescent rare earth nanomaterials for bioprobe applications. Dalton Trans. 2008, 42, 5687–5697. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Liu, Z. Upconversion nanoparticles and their composite nanostructures for biomedical imaging and cancer therapy. Nanoscale 2013, 5, 23–37. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Tu, D.; Chen, Z.; Huang, M.; Zhu, H.; Ma, E.; Chen, X. Amine-Functionalized Lanthanide-Doped Zirconia Nanoparticles: Optical Spectroscopy, Time-Resolved Fluorescence Resonance Energy Transfer Biodetection, and Targeted Imaging. J. Am. Chem. Soc. 2012, 134, 15083–15090. [Google Scholar] [CrossRef]
- Mir, W.J.; Sheikh, T.; Arfin, H.; Xia, Z.; Nag, A. Lanthanide doping in metal halide perovskite nanocrystals: Spectral shifting, quantum cutting and optoelectronic applications. NPG Asia Mater. 2020, 12, 9. [Google Scholar] [CrossRef]
- Reinhard, C.; Valiente, R.; Güdel, H.U. Exchange-Induced Upconversion in Rb2MnCl4:Yb3+. J. Phys. Chem. B 2002, 106, 10051–10057. [Google Scholar] [CrossRef] [Green Version]
- Beurer, E.; Grimm, J.; Gerner, P.; Güdel, H.U. Absorption, Light Emission, and Upconversion Properties of Tm2+-Doped CsCaI3 and RbCaI3. Inorg. Chem. 2006, 45, 9901–9906. [Google Scholar] [CrossRef]
- Gong, S.; Li, M.; Ren, Z.; Yang, X.; Li, X.; Shen, G.; Han, G. Polarization-Modified Upconversion Luminescence in Er-Doped Single-Crystal Perovskite PbTiO3 Nanofibers. J. Phys. Chem. C 2015, 119, 17326–17333. [Google Scholar] [CrossRef]
- Wu, M.; Song, E.H.; Chen, Z.T.; Ding, S.; Ye, S.; Zhou, J.J.; Xu, S.Q.; Zhang, Q.Y. Single-band red upconversion luminescence of Yb3+–Er3+via nonequivalent substitution in perovskite KMgF3 nanocrystals. J. Mater. Chem. C 2016, 4, 1675–1684. [Google Scholar] [CrossRef]
- Ge, Y.-Y.; Zhao, Y.-J.; Yuan, X.-Y.; Sun, S.-Y.; Zhao, Y.-Z.; Zhou, H.-P. Synthesis of Er3+-doped perovskite nanorods with outstanding UC PL behavior. RSC Adv. 2016, 6, 67353–67360. [Google Scholar] [CrossRef]
- Arumugam, G.M.; Xu, C.; Karunakaran, S.K.; Shi, Z.; Zhu, C.; Wei, M.; Feifei, Q. Efficient red up-conversion emission from Er3+-Yb3+ co-doped rubidium lead iodide perovskite nanowires with surface plasmons. Appl. Phys. Lett. 2018, 112, 054104. [Google Scholar] [CrossRef]
- Liu, C.; Gao, Z.; Zeng, J.; Hou, Y.; Fang, F.; Li, Y.; Qiao, R.; Shen, L.; Lei, H.; Yang, W.; et al. Magnetic/Upconversion Fluorescent NaGdF4:Yb,Er Nanoparticle-Based Dual-Modal Molecular Probes for Imaging Tiny Tumors in Vivo. ACS Nano 2013, 7, 7227–7240. [Google Scholar] [CrossRef]
- Brgoch, J.; Lehner, A.J.; Chabinyc, M.; Seshadri, R. Ab Initio Calculations of Band Gaps and Absolute Band Positions of Polymorphs of RbPbI3 and CsPbI3: Implications for Main-Group Halide Perovskite Photovoltaics. J. Phys. Chem. C 2014, 118, 27721–27727. [Google Scholar] [CrossRef]
- Wang, F.; Banerjee, D.; Liu, Y.; Chen, X.; Liu, X. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst 2010, 135, 1839–1854. [Google Scholar] [CrossRef]
- Rinkel, T.; Raj, A.N.; Dühnen, S.; Haase, M. Synthesis of 10 nm β-NaYF4:Yb,Er/NaYF4 Core/Shell Upconversion Nanocrystals with 5 nm Particle Cores. Angew. Chem. Int. Ed. 2016, 55, 1164–1167. [Google Scholar] [CrossRef]
- Yin, W.; Zhao, L.; Zhou, L.; Gu, Z.; Liu, X.; Tian, G.; Jin, S.; Yan, L.; Ren, W.; Xing, G.; et al. Enhanced Red Emission from GdF3:Yb3+,Er3+ Upconversion Nanocrystals by Li+ Doping and Their Application for Bioimaging. Chem.—Eur. J. 2012, 18, 9239–9245. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion Luminescent Materials: Advances and Applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef]
- Xiang, J.; Xu, L.; Gong, H.; Zhu, W.; Wang, C.; Xu, J.; Feng, L.; Cheng, L.; Peng, R.; Liu, Z. Antigen-Loaded Upconversion Nanoparticles for Dendritic Cell Stimulation, Tracking, and Vaccination in Dendritic Cell-Based Immunotherapy. ACS Nano 2015, 9, 6401–6411. [Google Scholar] [CrossRef]
- Hesse, J.; Klier, D.T.; Sgarzi, M.; Nsubuga, A.; Bauer, C.; Grenzer, J.; Hübner, R.; Wislicenus, M.; Joshi, T.; Kumke, M.U.; et al. Rapid Synthesis of Sub-10 nm Hexagonal NaYF4-Based Upconverting Nanoparticles using Therminol® 66. ChemistryOpen 2018, 7, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Kostiv, U.; Engstová, H.; Krajnik, B.; Šlouf, M.; Proks, V.; Podhorodecki, A.; Ježek, P.; Horák, D. Monodisperse Core-Shell NaYF4:Yb3+/Er3+@NaYF4:Nd3+-PEG-GGGRGDSGGGY-NH2 Nanoparticles Excitable at 808 and 980 nm: Design, Surface Engineering, and Application in Life Sciences. Front. Chem. 2020, 8, 497. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, P.; Gong, Z.; Tu, D.; Xu, J.; Zou, Q.; Li, R.; You, W.; Bünzli, J.-C.G.; Chen, X. Near-infrared-triggered photon upconversion tuning in all-inorganic cesium lead halide perovskite quantum dots. Nat. Commun. 2018, 9, 3462. [Google Scholar] [CrossRef] [Green Version]
- Ruan, L.; Zhang, Y. NIR-excitable heterostructured upconversion perovskite nanodots with improved stability. Nat. Commun. 2021, 12, 219. [Google Scholar] [CrossRef]
- Shao, L.; Liu, D.; Lyu, J.; Zhou, D.; Ding, N.; Sun, R.; Xu, W.; Wang, N.; Xu, S.; Dong, B.; et al. Near-infrared-pumped photon upconversion in CsPbI3 and CaF2:Yb3+/Ho3+ nanocomposites for bio-imaging application. Mater. Today Phys. 2021, 21, 100495. [Google Scholar] [CrossRef]
- Talianov, P.M.; Peltek, O.O.; Masharin, M.; Khubezhov, S.; Baranov, M.A.; Drabavičius, A.; Timin, A.S.; Zelenkov, L.E.; Pushkarev, A.P.; Makarov, S.V.; et al. Halide Perovskite Nanocrystals with Enhanced Water Stability for Upconversion Imaging in a Living Cell. J. Phys. Chem. Lett. 2021, 12, 8991–8998. [Google Scholar] [CrossRef]
- Estebanez, N.; Cortés-Villena, A.; Ferrera-González, J.; González-Béjar, M.; Galian, R.E.; González-Carrero, S.; Pérez-Prieto, J. Linear Coassembly of Upconversion and Perovskite Nanoparticles: Sensitized Upconversion Emission of Perovskites by Lanthanide-Doped Nanoparticles. Adv. Funct. Mater. 2020, 30, 2003766. [Google Scholar] [CrossRef]
- Pan, G.; Bai, X.; Yang, D.; Chen, X.; Jing, P.; Qu, S.; Zhang, L.; Zhou, D.; Zhu, J.; Xu, W.; et al. Doping Lanthanide into Perovskite Nanocrystals: Highly Improved and Expanded Optical Properties. Nano Lett. 2017, 17, 8005–8011. [Google Scholar] [CrossRef]
- Shao, H.; Bai, X.; Cui, H.; Pan, G.; Jing, P.; Qu, S.; Zhu, J.; Zhai, Y.; Dong, B.; Song, H. White light emission in Bi3+/Mn2+ ion co-doped CsPbCl3 perovskite nanocrystals. Nanoscale 2018, 10, 1023–1029. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Zhang, X.; Yin, W.; Wang, Y.; Wang, H.; Lu, M.; Li, Z.; Gu, Z.; Yu, W.W. Yb3+ and Yb3+/Er3+ doping for near-infrared emission and improved stability of CsPbCl3 nanocrystals. J. Mater. Chem. C 2018, 6, 10101–10105. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wei, Y.; Jiang, F.; Liu, Y.; Hong, M. Photon upconversion of all-inorganic CsPbX3 quantum dots based on fluorescence resonance energy transfer in hetero-structured perovskite/upconversion nanocomposites. J. Lumin. 2022, 242, 118565. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, J.; Li, X.; Xu, X.; Huang, J.; Ji, X.; Yang, G.; Pan, G. Stable and Efficient Upconversion Single Red Emission from CsPbI3 Perovskite Quantum Dots Triggered by Upconversion Nanoparticles. Inorg. Chem. 2021, 60, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zhao, M.; Yu, M.; Zhu, M.; Yan, W.; Song, J.; Qu, J. Mechanistic Investigation of Upconversion Photoluminescence in All-Inorganic Perovskite CsPbBrI2 Nanocrystals. J. Phys. Chem. C 2018, 122, 3152–3156. [Google Scholar] [CrossRef]
- Ruan, L.; Zhang, Y. Upconversion Perovskite Nanocrystal Heterostructures with Enhanced Luminescence and Stability by Lattice Matching. ACS Appl. Mater. Interfaces 2021, 13, 51362–51372. [Google Scholar] [CrossRef]
- Del Águila, A.G.; Do, T.T.H.; Xing, J.; Jee, W.J.; Khurgin, J.B.; Xiong, Q. Efficient up-conversion photoluminescence in all-inorganic lead halide perovskite nanocrystals. Nano Res. 2020, 13, 1962–1969. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, F.; Yan, J.; Zhang, Y.; Chen, X.; Chen, S.; Wu, D.; Li, X.; Zhang, Y.; Shan, C. Robust frequency-upconversion lasing operated at 400 K from inorganic perovskites microcavity. Nano Res. 2022, 15, 492–501. [Google Scholar] [CrossRef]
- Li, Z.; Qiao, X.; He, G.; Sun, X.; Feng, D.; Hu, L.; Xu, H.; Xu, H.-B.; Ma, S.; Tian, J. Core-satellite metal-organic framework@upconversion nanoparticle superstructures via electrostatic self-assembly for efficient photodynamic theranostics. Nano Res. 2020, 13, 3377–3386. [Google Scholar] [CrossRef]
- Sun, X.; Sun, J.; Dong, B.; Huang, G.; Zhang, L.; Zhou, W.; Lv, J.; Zhang, X.; Liu, M.; Xu, L.; et al. Noninvasive temperature monitoring for dual-modal tumor therapy based on lanthanide-doped up-conversion nanocomposites. Biomaterials 2019, 201, 42–52. [Google Scholar] [CrossRef]
- Francés-Soriano, L.; Estebanez, N.; Pérez-Prieto, J.; Hildebrandt, N. DNA-Coated Upconversion Nanoparticles for Sensitive Nucleic Acid FRET Biosensing. Adv. Funct. Mater. 2022, 2201541. [Google Scholar] [CrossRef]
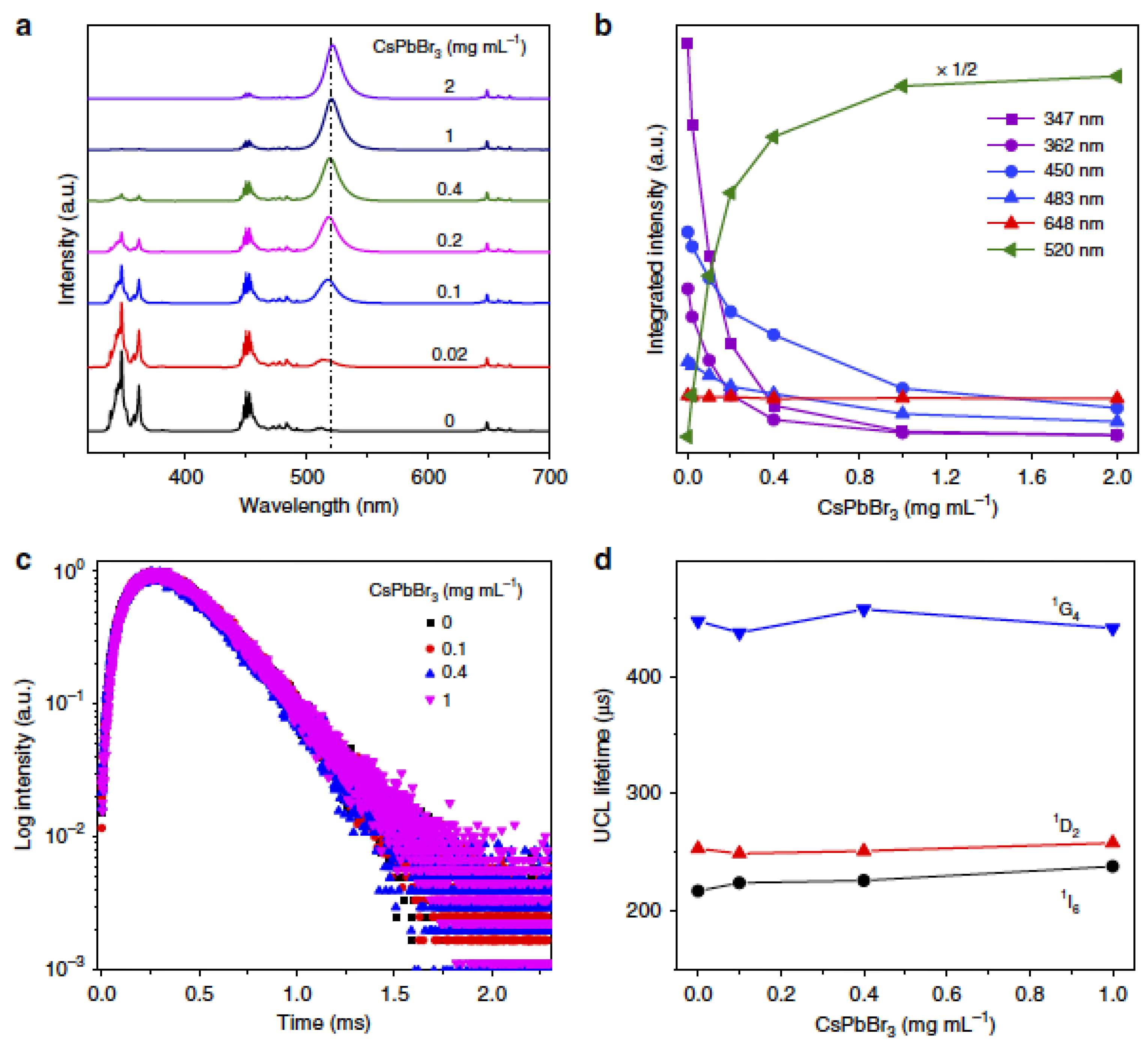
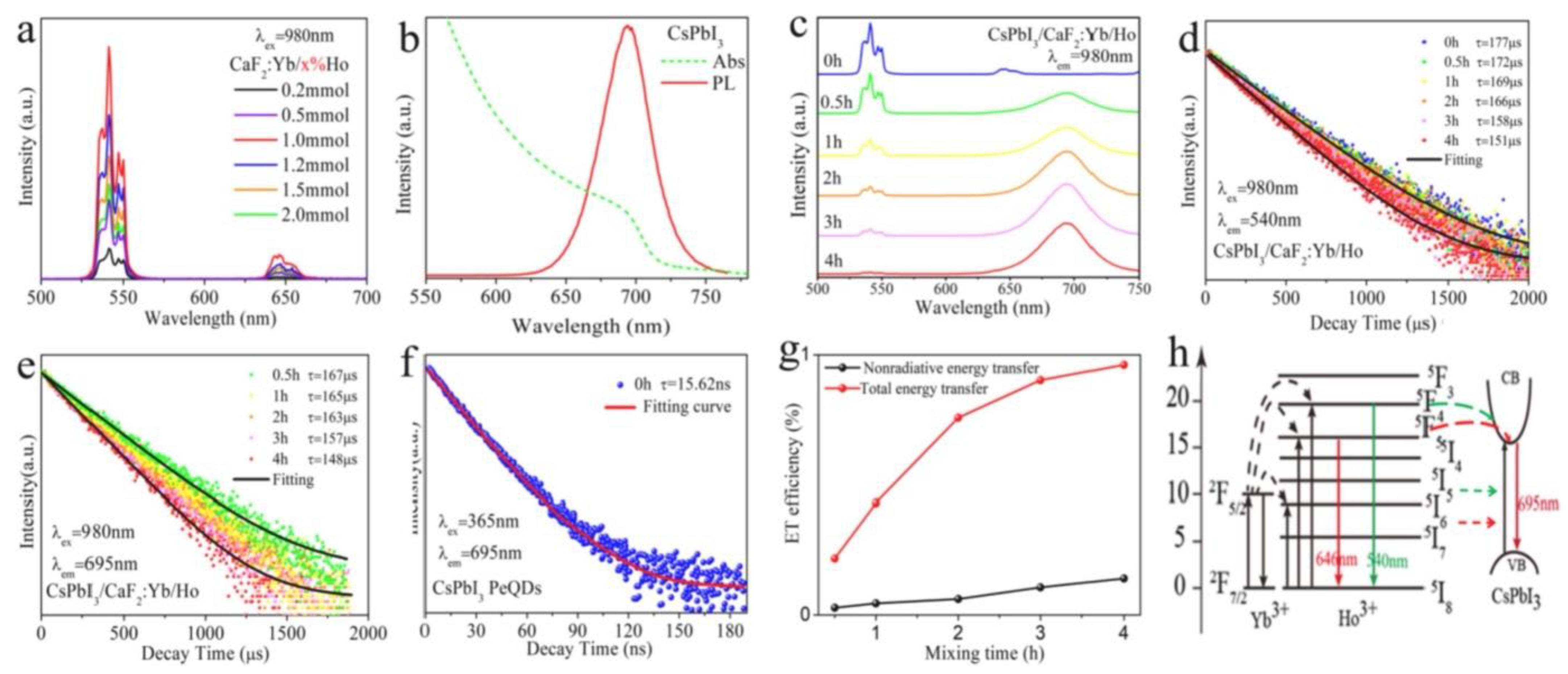

| S.No | Perovskites | Lanthanide Ions | Description | Application | Ref. |
|---|---|---|---|---|---|
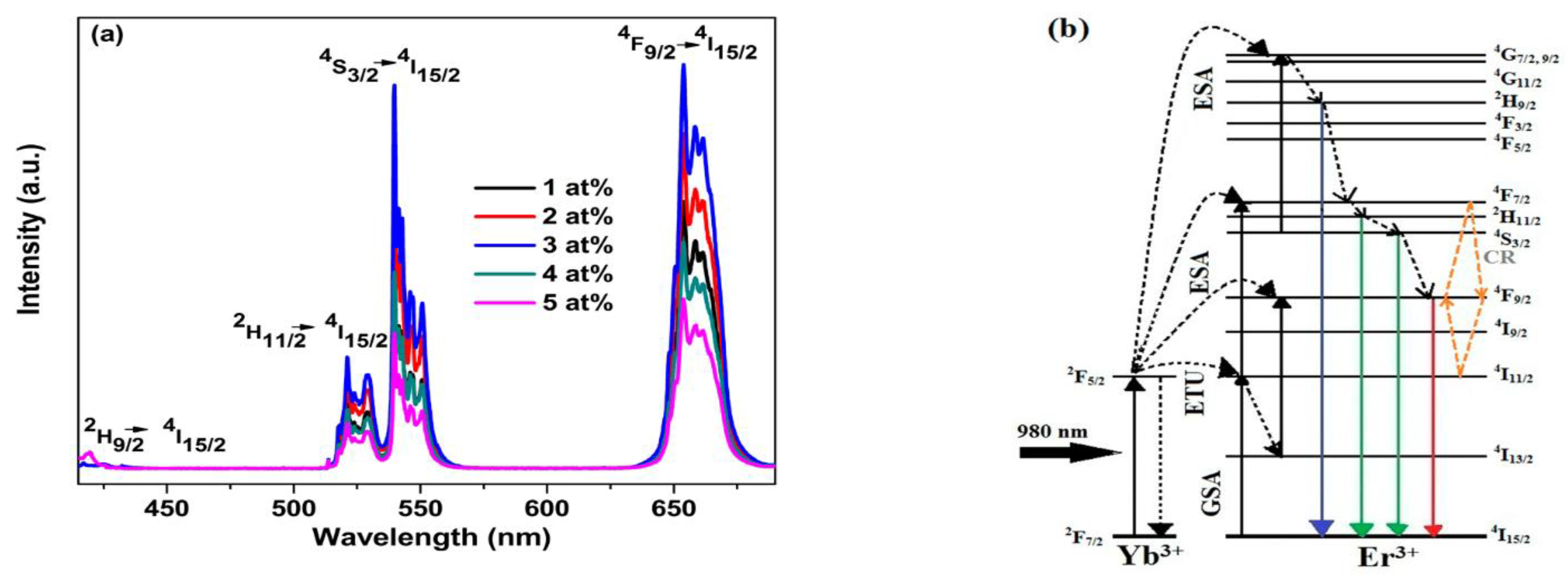
| 1 | CsPbCl3 NCs | Ce3+, Sm3+, Eu3+, Tb3+, Dy3+ and Er3+ | The introduction of lanthanide ions can considerably improve the PLQY of CsPbCl3 NCs and can provide visible light emissions and even NIR emissions. | Light emitting and other photoelectronic devices | [79] |
| 2 | CsPbCl3 | Bi3+/Mn2+ | The co-doped perovskite exhibits tuneable emissions spanning the wide range of correlated colour temperature (CCT) from 19,000 K to 4250 K under UV excitation. This interesting spectroscopic behaviour benefits from efficient energy transfer from the perovskite NCs to intrinsic energy levels of Bi3+ or Mn2+ doping ions. | Lighting and displays | [80] |
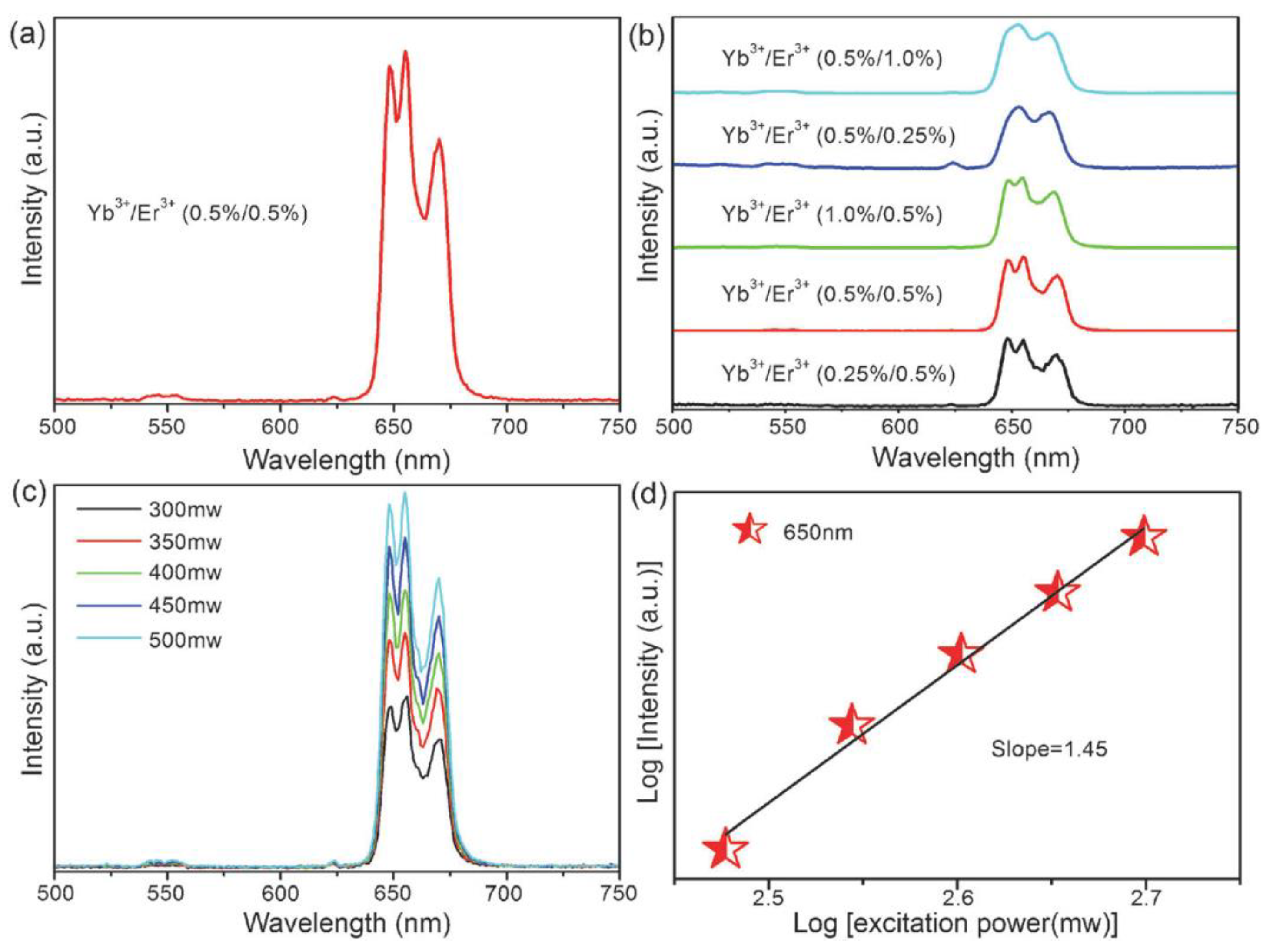
| 3 | CsPbCl3 NCs | Yb3+ and Yb3+/Er3+ | The Yb3+-doped CsPbCl3 NCs emit strong NIR light at 986 nm, whereas the Yb3+/Er3+ co-doped CsPbCl3 NCs emit at 1533 nm. The total PLQY of the CsPbCl3 NCs changes from 5.0% to 127.8% upon incorporating 2.0% Yb3+, resulting in a 25.6 enhancement factor. | Diode lasers and photo-communications | [81] |
| 4 | CsPbX3 NCs | CaF2: Ln (Ln = Yb3+/Er3+, Yb3+/Ho3+ and Yb3+/Tm3+) | Owing to extremely high fluorescence resonance energy transfer (FRET) efficiency (~99.7%), excitonic UCL from CsPbX3 is performed under a low-power density of 980 nm diode laser irradiation. | Opto-electronics and photovoltaics | [82] |
| 5 | CsPbI3 | NaYF4:Yb/Tm @NaYF4 | An efficient single red band UC emission of CsPbI3 perovskite quantum dots (PQDs) was observed. In addition, the emission was easily regulated from 705 to 625 nm by introducing an appropriate proportion of Br ions, which is very difficult to achieve for traditional UCNPs. Moreover, benefiting from the efficient downshifting (DS) red emission of CsPbI3 PQDs, the composites displayed dual-wavelength excitation characteristics. | Dual-mode anticounterfeiting application | [83] |
| 6 | CsPbBrI2 | w/o lanthanides | When photons only excite electrons in shallow trap states, some excited photons are absorbed by the shallow trap state, thus producing single-photon UCPL while the remaining photons are absorbed by the valence band, resulting in electron transfer from the valence band to the conduction band. Hence, the UC process is gradually dominated by a two-photon process as the energy of the incident photons decreases. | Optoelectronics | [84] |
| 7 | CsPbBr1 × 2 PQDs | NaYF4 Ln NPs | To improve the lattice matching between UCNPs and PQDs by replacing Y instead of Gd, the heterostructured CsPbBr3-NaGdF4:Yb,Tm NCs are obtained. They exhibit enhanced luminescence as well as stability at high temperatures, in polar solvents and under continuous UV excitation when compared with CsPbBr3-NaYF4:Yb,Tm nanocrystals and pure PQDs. | Optoelectronics | [85] |
| 8 | CsPbA3 (A = Cl, Br and I) | w/o lanthanides | An efficient UCPL with a striking phonon-assisted energy gain of ~8 kBT is obtained with high-quality, all-inorganic CsPbA3 perovskite NCs. In non-equilibrium conditions, the acoustic phonon UC recycles the population of optical modes and boosts the efficiency of photon UC. | Optoelectronics | [86] |
| 9 | CsPbBr3 | w/o lanthanides | Vapor-phase epitaxial CsPbBr3 microplatelets are obtained with high crystallinity; self-formed high-quality microcavities; and great thermal stability, low-threshold and high-quality factor whispering-gallery mode lasing under one, two and three-photon excitation, and the lasing action is very stable under continuous pulsed laser irradiation (~3.6 Å~107 laser shots). | Lasing | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arumugam, G.M.; Karunakaran, S.K.; Galian, R.E.; Pérez-Prieto, J. Recent Progress in Lanthanide-Doped Inorganic Perovskite Nanocrystals and Nanoheterostructures: A Future Vision of Bioimaging. Nanomaterials 2022, 12, 2130. https://doi.org/10.3390/nano12132130
Arumugam GM, Karunakaran SK, Galian RE, Pérez-Prieto J. Recent Progress in Lanthanide-Doped Inorganic Perovskite Nanocrystals and Nanoheterostructures: A Future Vision of Bioimaging. Nanomaterials. 2022; 12(13):2130. https://doi.org/10.3390/nano12132130
Chicago/Turabian StyleArumugam, Gowri Manohari, Santhosh Kumar Karunakaran, Raquel E. Galian, and Julia Pérez-Prieto. 2022. "Recent Progress in Lanthanide-Doped Inorganic Perovskite Nanocrystals and Nanoheterostructures: A Future Vision of Bioimaging" Nanomaterials 12, no. 13: 2130. https://doi.org/10.3390/nano12132130
APA StyleArumugam, G. M., Karunakaran, S. K., Galian, R. E., & Pérez-Prieto, J. (2022). Recent Progress in Lanthanide-Doped Inorganic Perovskite Nanocrystals and Nanoheterostructures: A Future Vision of Bioimaging. Nanomaterials, 12(13), 2130. https://doi.org/10.3390/nano12132130








