Zirconia-Doped Methylated Silica Membranes via Sol-Gel Process: Microstructure and Hydrogen Permselectivity
Abstract
:1. Introduction
2. Materials and Methods
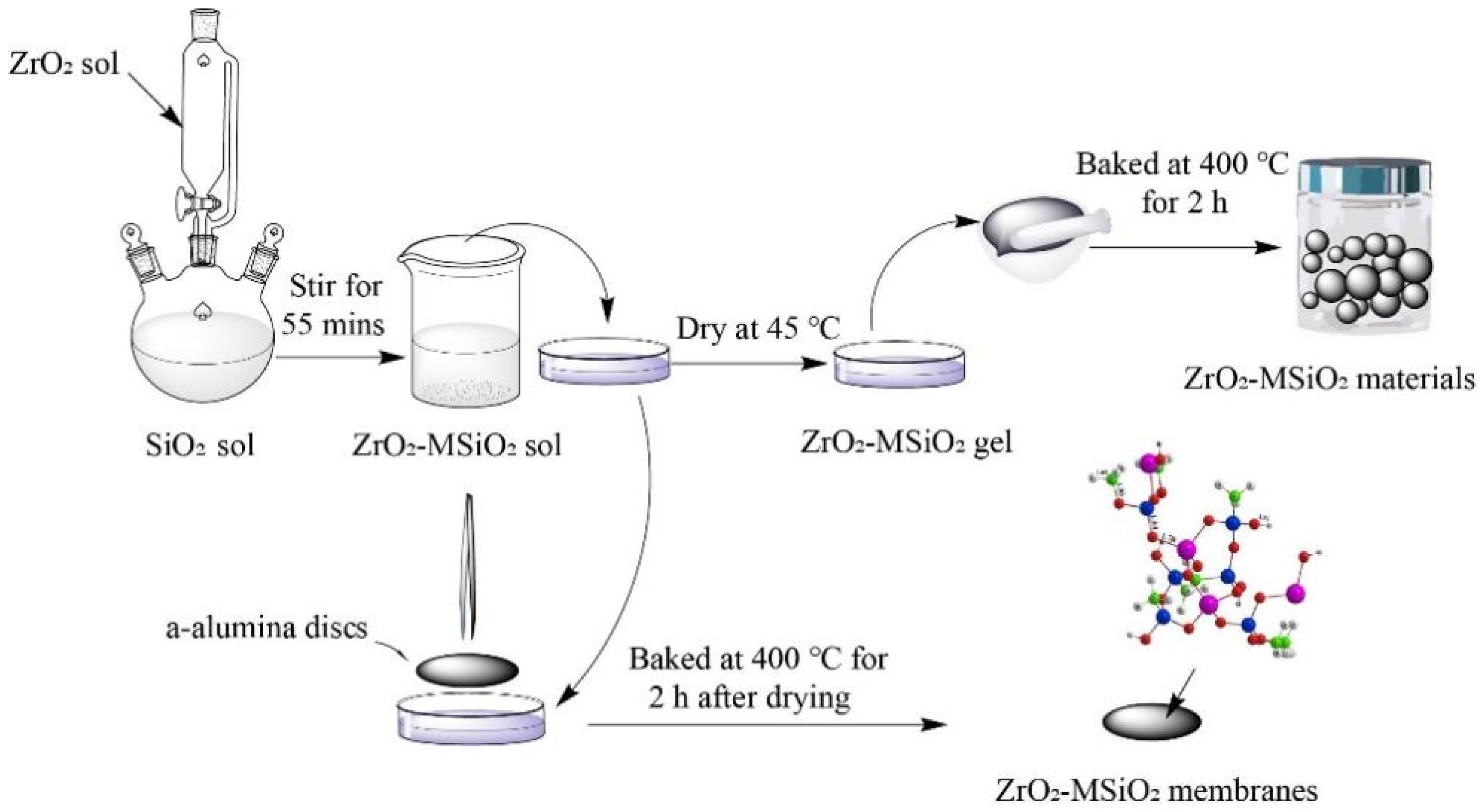
2.1. Preparation of MSiO2 Sols
2.2. Preparation of ZrO2 Sols
2.3. Preparation of ZrO2-MSiO2 Sols
2.4. Preparation of ZrO2-MSiO2 Materials
2.5. Preparation of ZrO2-MSiO2 Membranes
2.6. Steam Treatment and Regeneration of ZrO2-MSiO2 Membranes
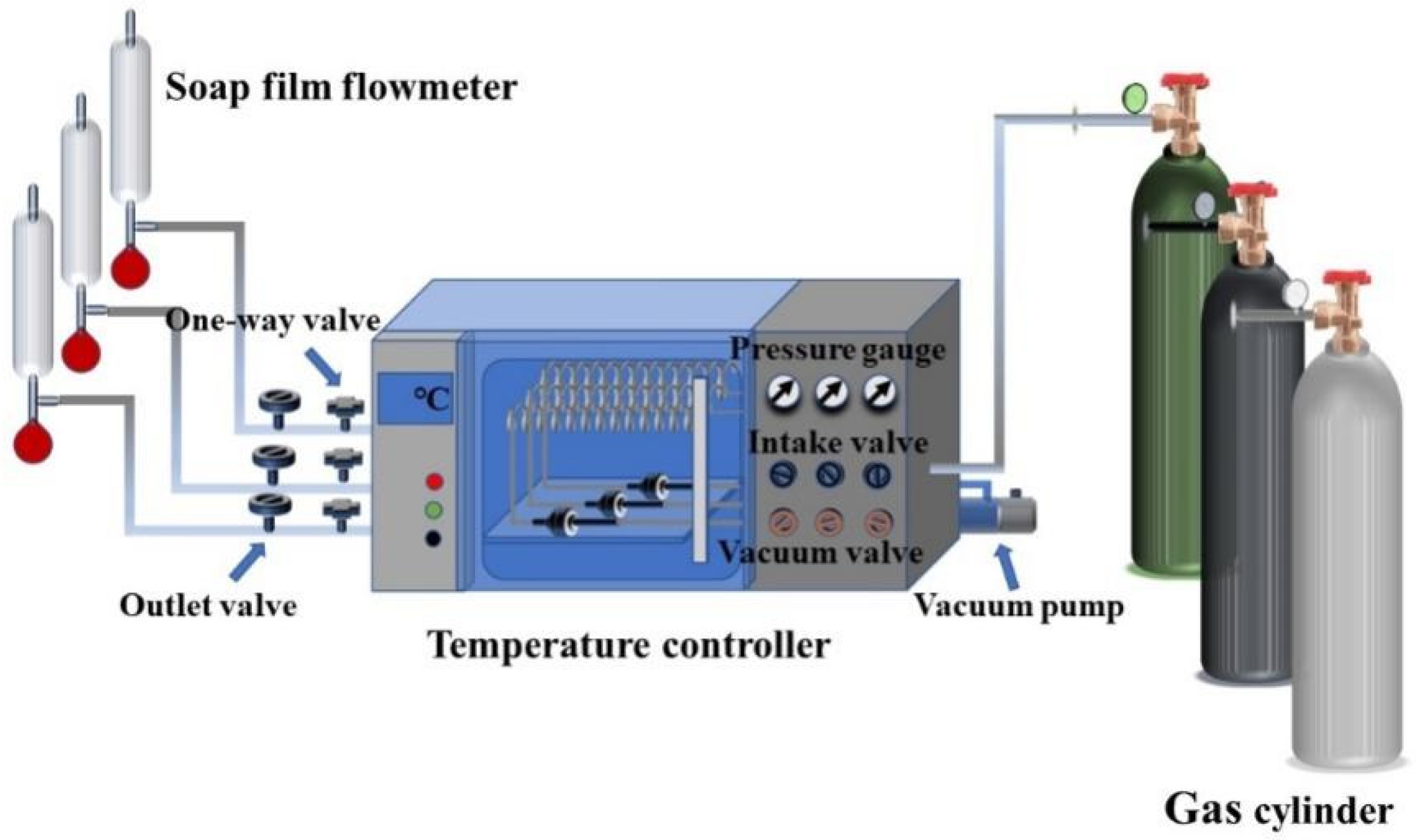
2.7. Characterizations
3. Results
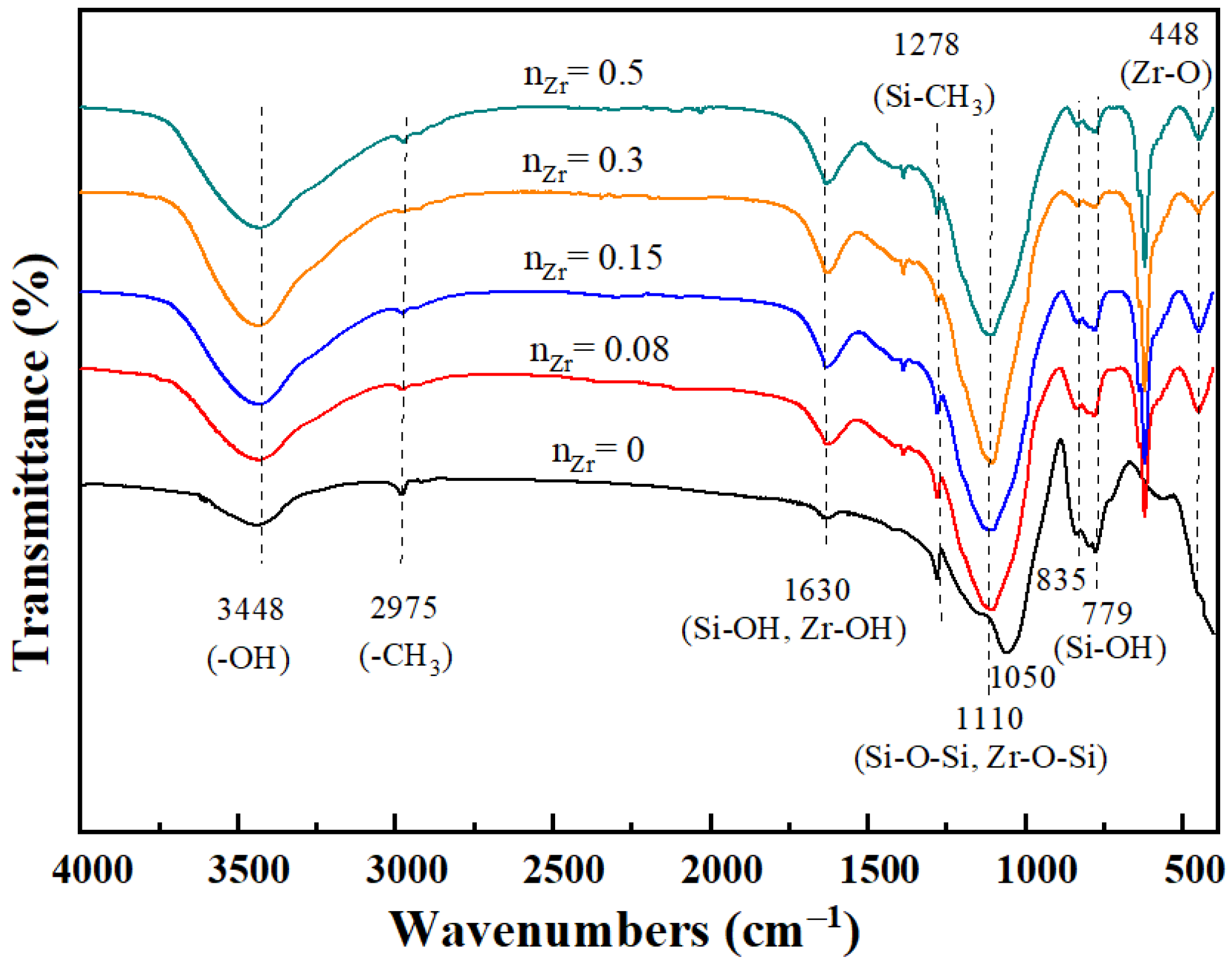
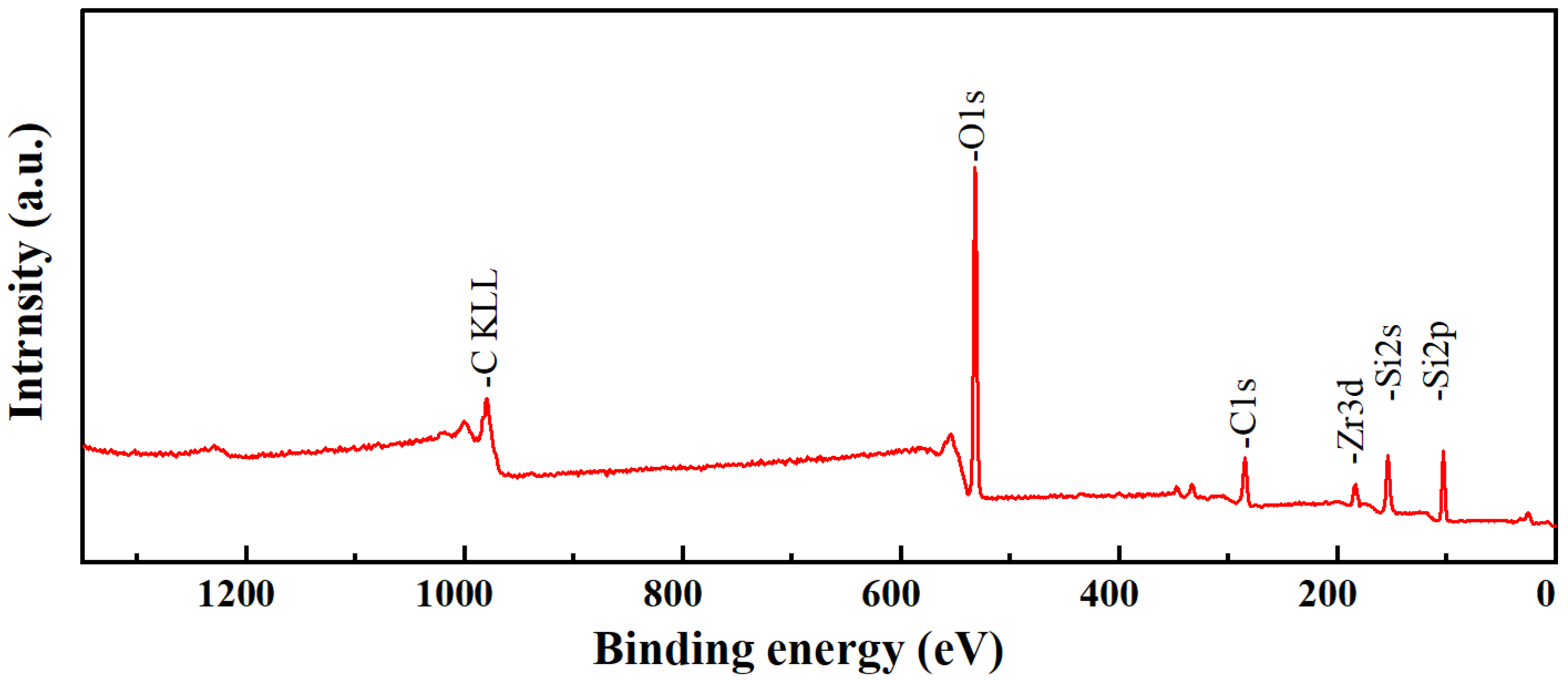
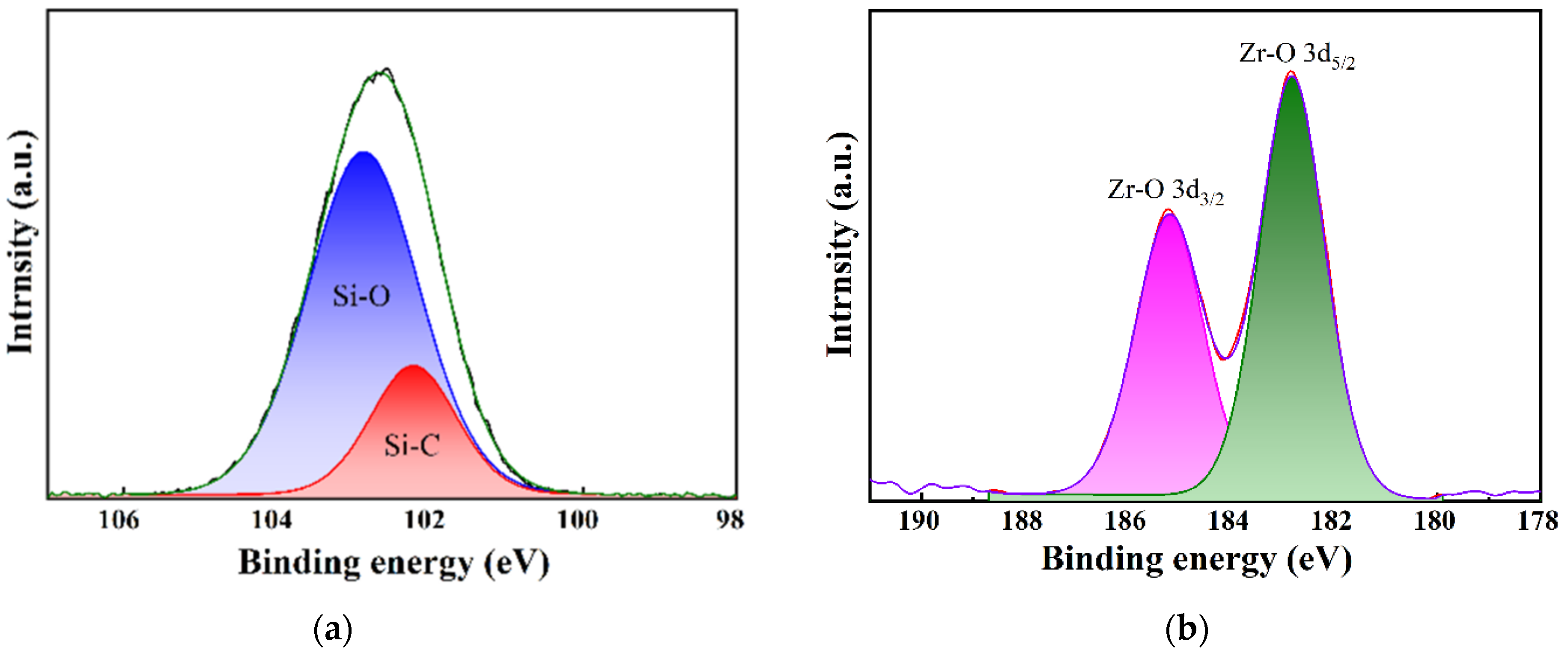
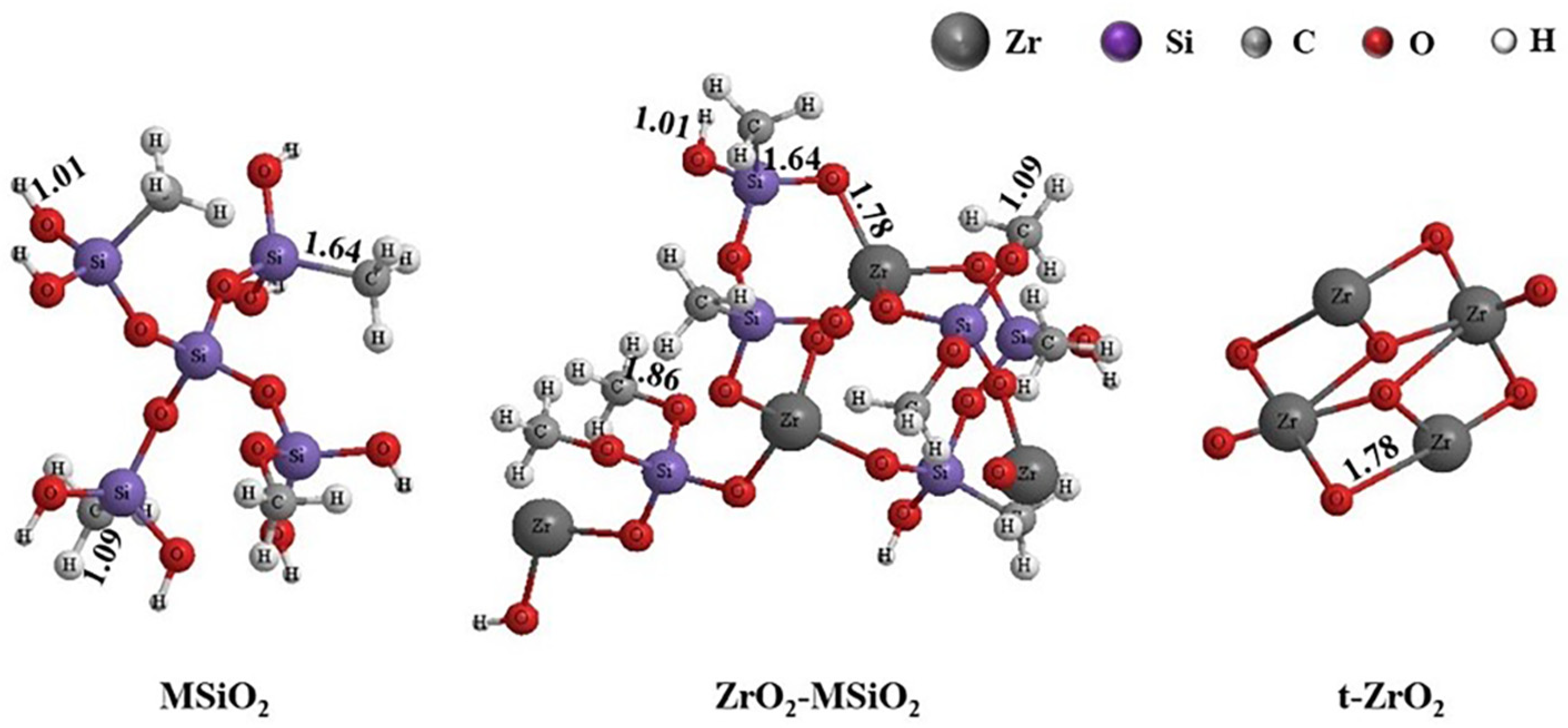
3.1. Chemical Structure Analysis
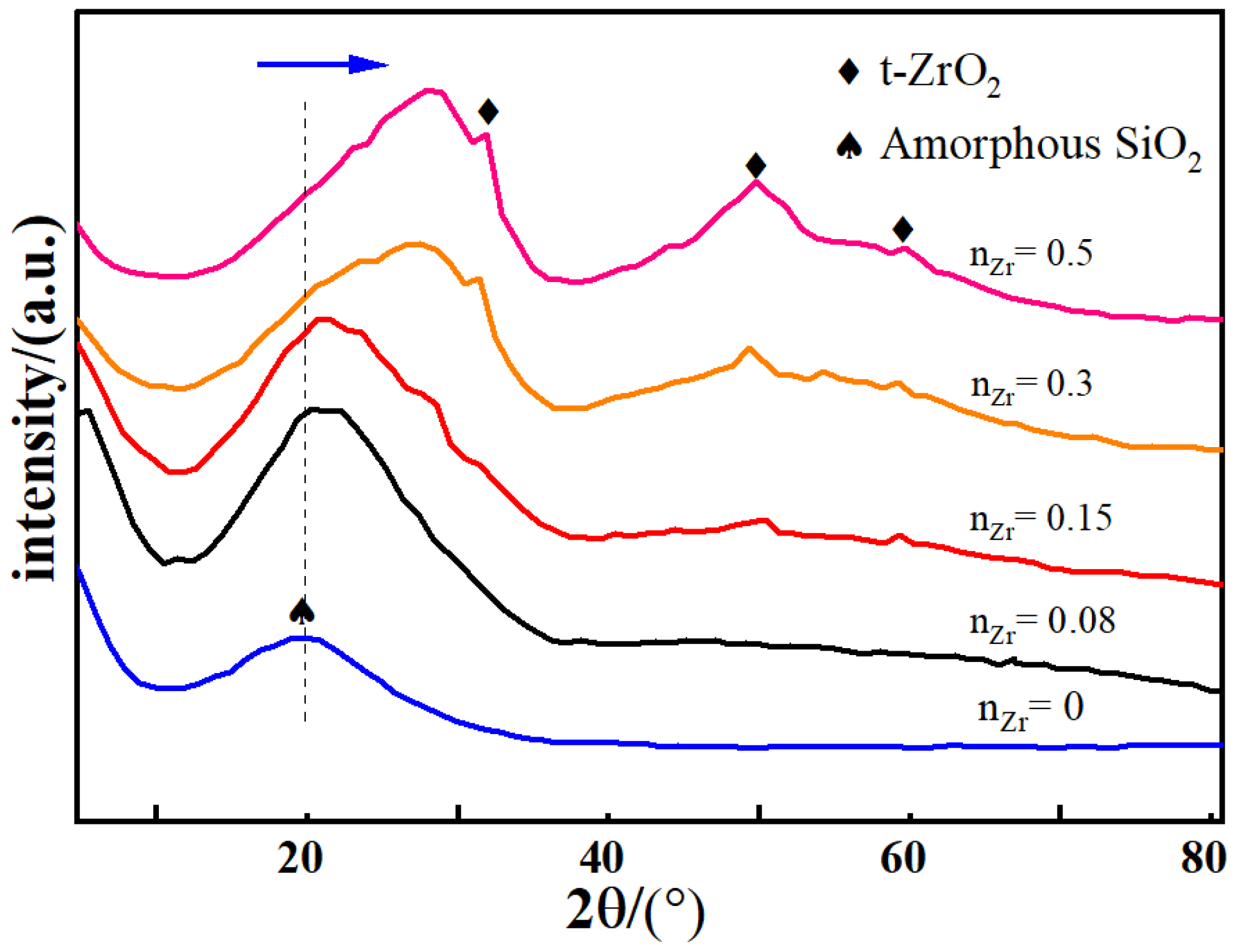
3.2. Phase Structure Analysis
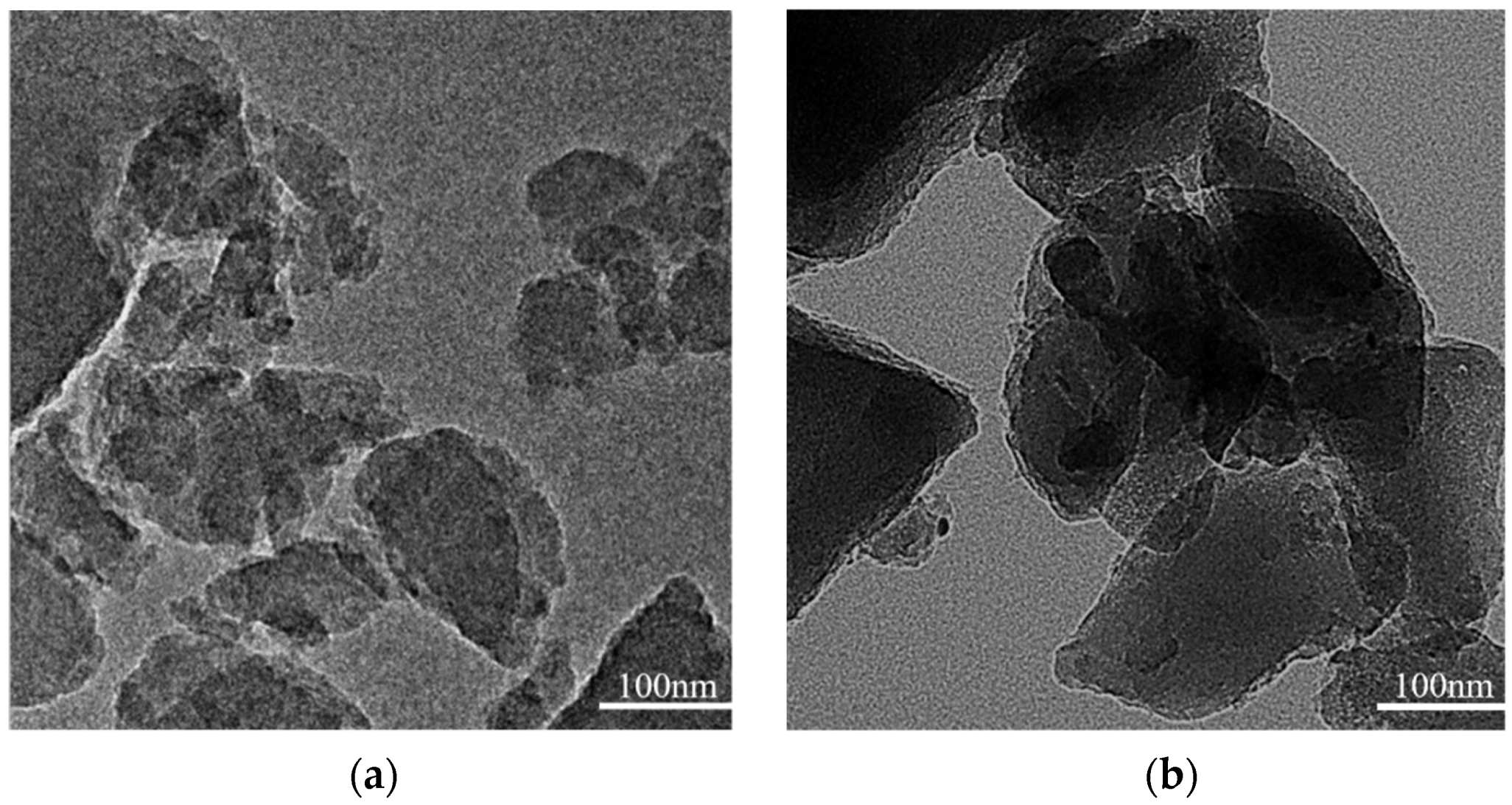
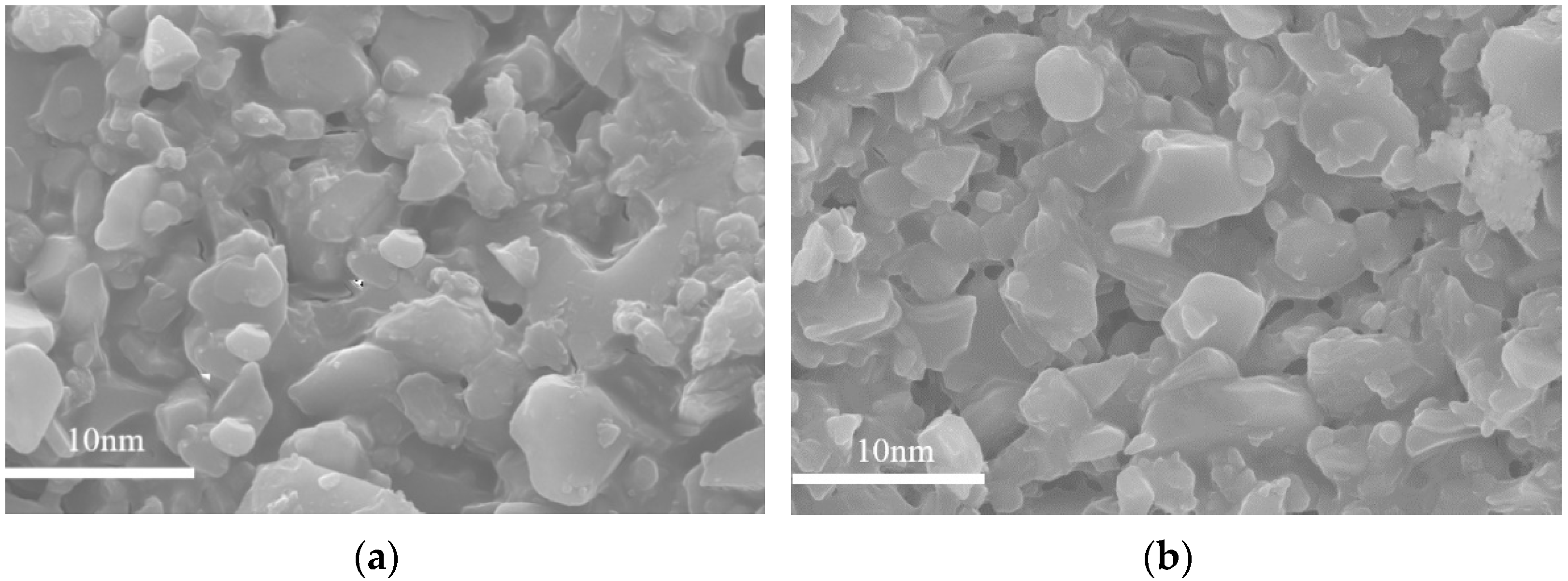
3.3. TEM Analysis
3.4. Pore Structure Analysis
3.5. Gas Permselectivity Analysis
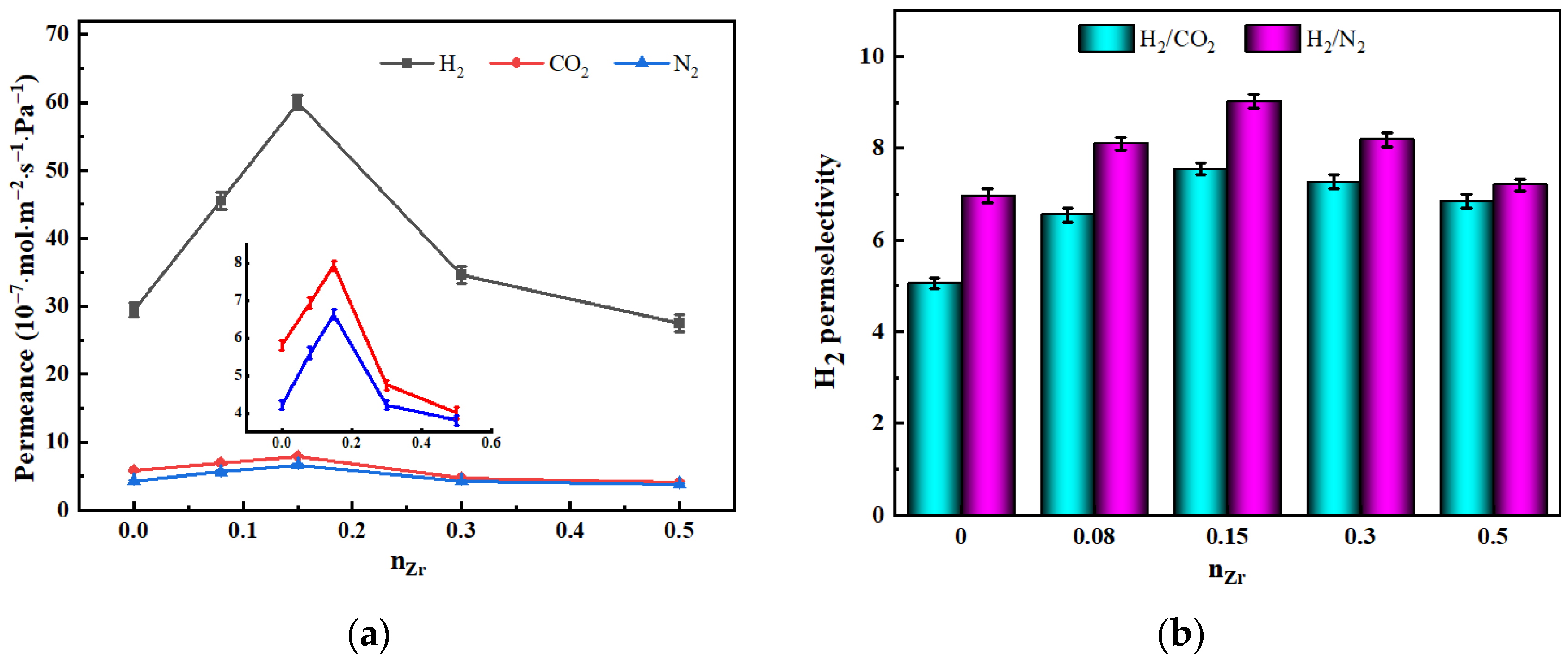
3.5.1. The Influence of nZr
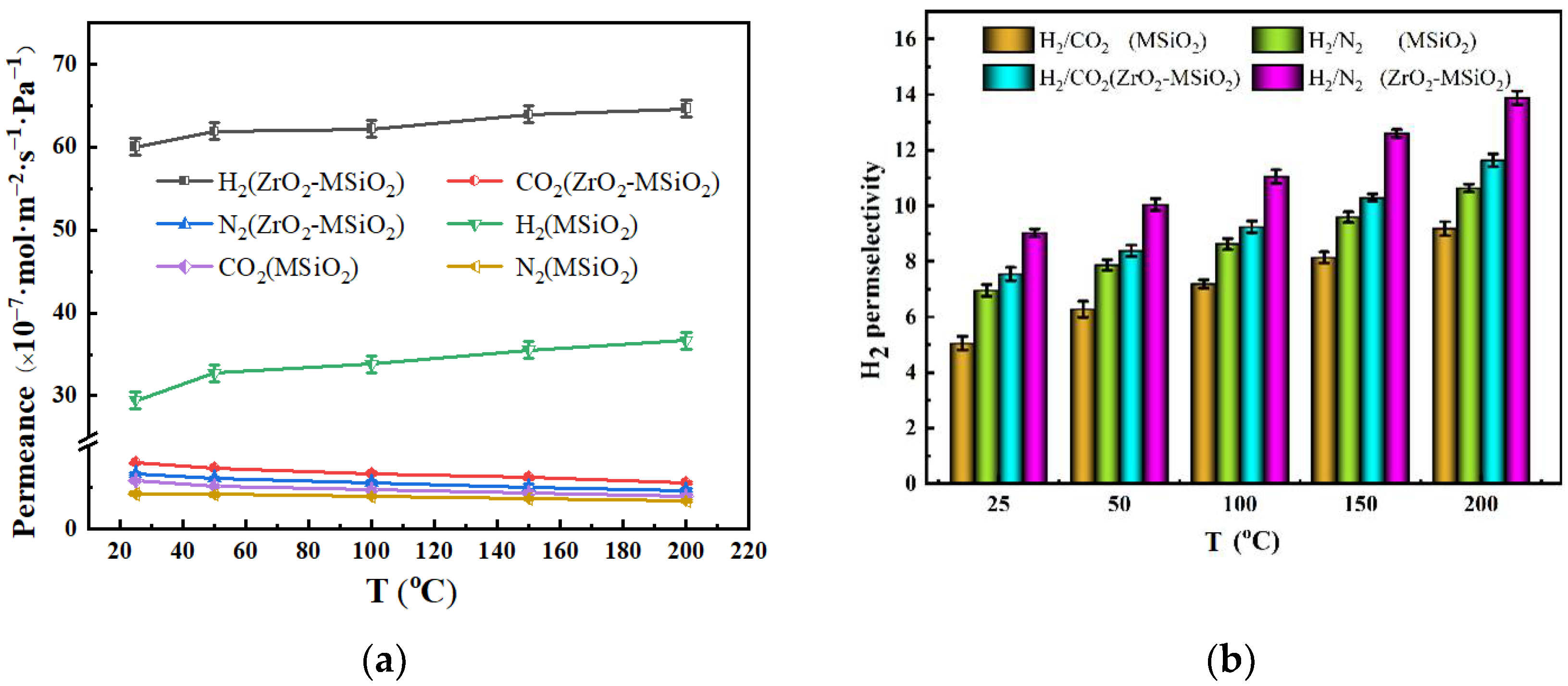
3.5.2. The Influence of Temperature
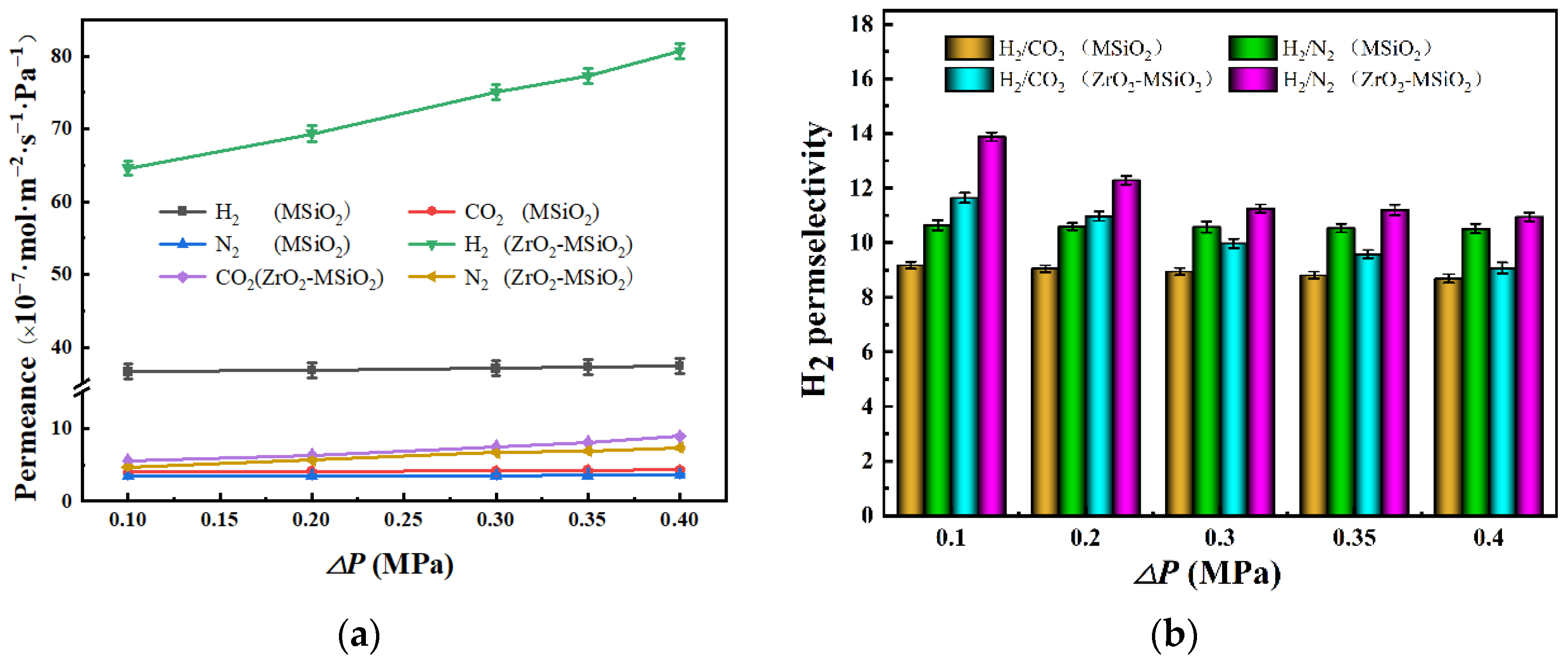
3.5.3. The Influence of Pressure Difference
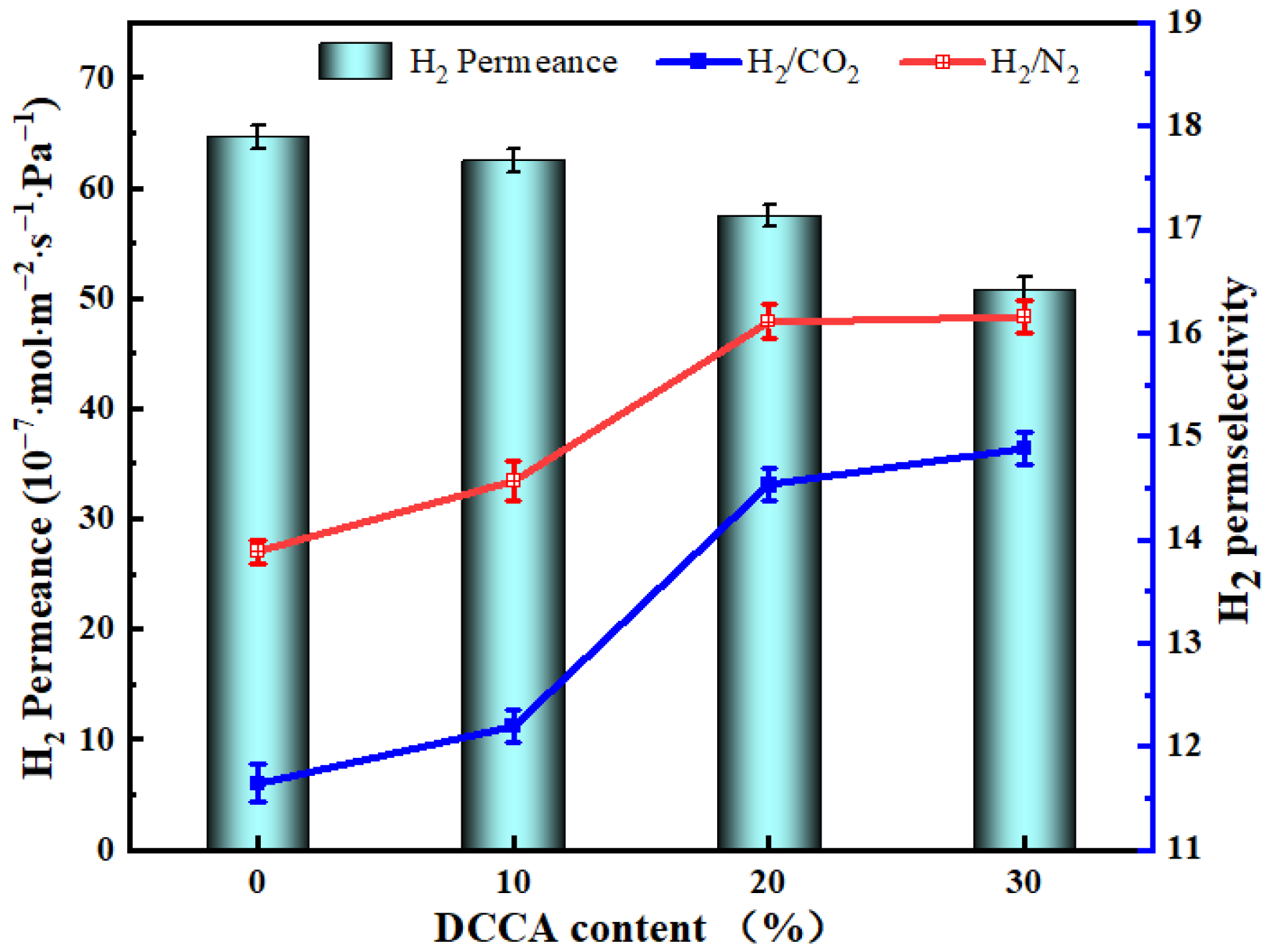
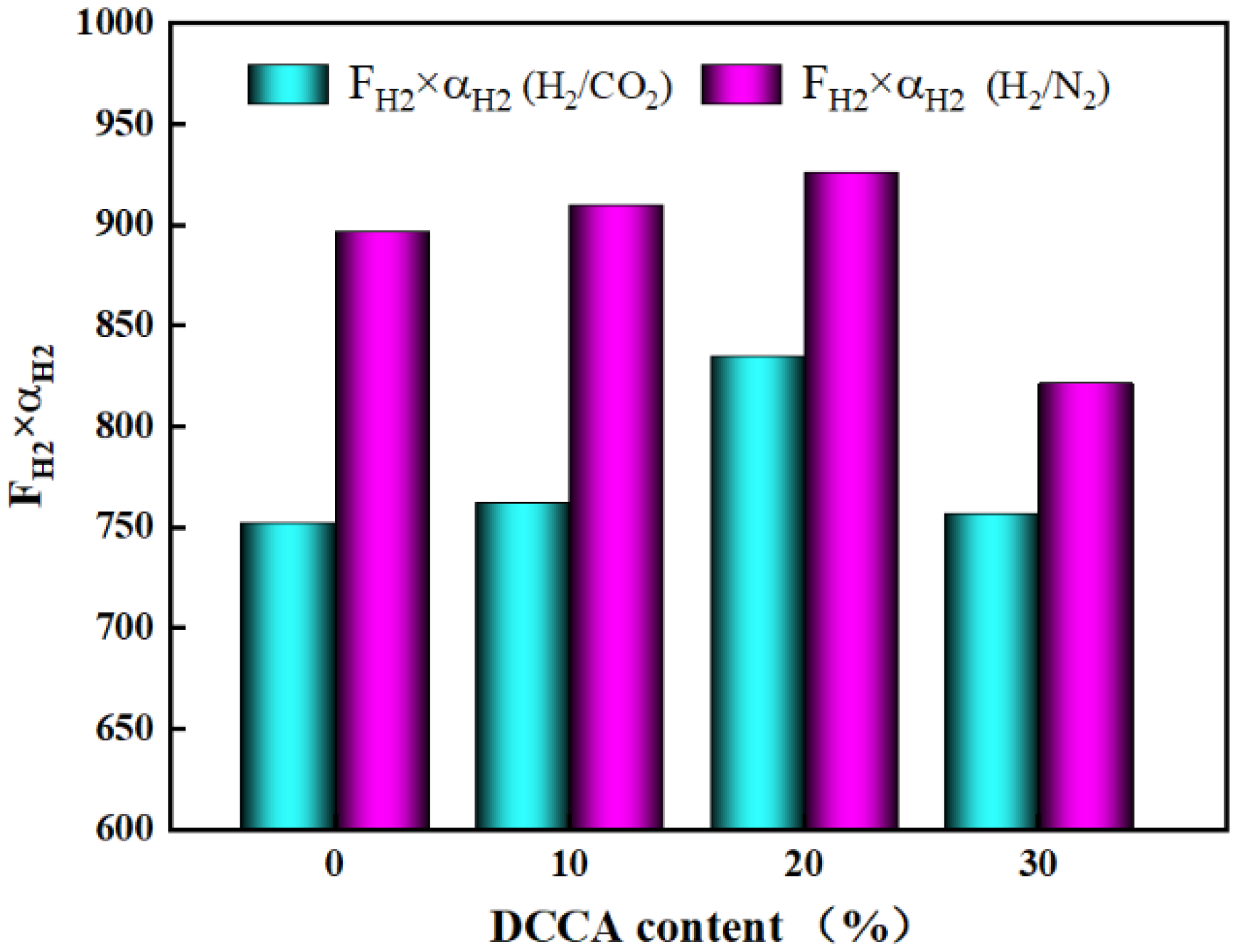
3.5.4. The Influence of the DCCA
3.5.5. Steam Treatment and Regeneration Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nazir, H.; Louis, C.; Jose, S.; Prakash, J.; Muthuswamy, N.; Buan, M.E.; Flox, C.; Chavan, S.; Shi, X.; Kauranen, P.; et al. Is the H2 economy realizable in the foreseeable future? Part I: H2 production methods. Int. J. Hydrogen Energy 2020, 45, 13777–13788. [Google Scholar] [CrossRef]
- Nordio, M.; Wassie, S.A.; Van Sint Annaland, M.; Tanaka, D.A.P.; Sole, J.L.V.; Gallucci, F. Techno-economic evaluation on a hybrid technology for low hydrogen concentration separation and purification from natural gas grid. Int. J. Hydrogen Energy 2020, 46, 23417–23435. [Google Scholar] [CrossRef]
- Cai, L.; Cao, Z.; Zhu, X.; Yang, W. Improved hydrogen separation performance of asymmetric oxygen transport membranes by grooving in the porous support layer. Green Chem. Eng. 2020, 2, 96–103. [Google Scholar] [CrossRef]
- Lv, B.; Luo, Z.; Deng, X.; Chen, J.; Fang, C.; Zhu, X. Study on dry separation technology of a continuous gas-solid separation fluidized bed with a moving scraper (И)–Separation performance. Powder Technol. 2021, 377, 565–574. [Google Scholar] [CrossRef]
- Koutsonikolas, D.E.; Pantoleontos, G.; Karagiannakis, G.; Konstandopoulos, A.G. Development of H2 selective silica membranes: Performance evaluation through single gas permeation and gas separation tests. Sep. Purif. Technol. 2021, 264, 118432. [Google Scholar] [CrossRef]
- Wu, R.; Yue, W.; Li, Y.; Huang, A. Ultra-thin and high hydrogen permeable carbon molecular sieve membrane prepared by using polydopamine as carbon precursor. Mater. Lett. 2021, 295, 129863. [Google Scholar] [CrossRef]
- Singla, S.; Shetti, N.P.; Basu, S.; Mondal, K.; Aminabhavi, T.M. Hydrogen production technologies—Membrane based separation, storage and challenges. J. Environ. Manag. 2022, 302, 113963. [Google Scholar] [CrossRef]
- Yan, E.; Huang, H.; Sun, S.; Zou, Y.; Chu, H.; Sun, L. Development of Nb-Ti-Co alloy for high-performance hydrogen separating membrane. J. Membr. Sci. 2018, 565, 411–424. [Google Scholar] [CrossRef]
- Farina, L.; Santucci, A.; Tosti, S. Plasma Enhancement Gases separation via ceramic porous membranes for plasma exhaust processing system of DEMO. Fusion Eng. Des. 2021, 169, 112484. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Zhou, X.; Song, Y.; Jiang, Y.; Lin, B. Phase stability and hydrogen permeation performance of BaCo0·4Fe0·4Zr0·1Y0·1O3-δ ceramic membranes. Ceram. Int. 2021, 48, 9946–9954. [Google Scholar] [CrossRef]
- Bakoglidis, K.D.; Palisaitis, J.; Dos Santos, R.B.; Rivelino, R.; Persson, P.O.; Gueorguiev, G.K.; Hultman, L. Self-Healing in Carbon Nitride Evidenced As Material Inflation and Superlubric Behavior. ACS Appl. Mater. Interfaces 2018, 10, 16238–16243. [Google Scholar] [CrossRef] [Green Version]
- Kakanakova-Georgieva, A.; Gueorguiev, G.; Sangiovanni, D.G.; Suwannaharn, N.; Ivanov, I.G.; Cora, I.; Pécz, B.; Nicotra, G.; Giannazzo, F. Nanoscale phenomena ruling deposition and intercalation of AlN at the graphene/SiC interface. Nanoscale 2020, 12, 19470–19476. [Google Scholar] [CrossRef] [PubMed]
- Thirumal, V.; Yuvakkumar, R.; Kumar, P.S.; Ravi, G.; Keerthana, S.P.; Velauthapillai, D. Facile single-step synthesis of [emailprotected] hybrid nanocomposite by CVD method to remove hazardous pollutants. Chemosphere 2021, 286, 131733. [Google Scholar] [CrossRef] [PubMed]
- Kanezashi, M.; Asaeda, M. Hydrogen permeation characteristics and stability of Ni-doped silica membranes in steam at high temperature. J. Membr. Sci. 2006, 271, 86–93. [Google Scholar] [CrossRef]
- Rosli, A.; Ahmad, A.L.; Low, S.C. Anti-wetting polyvinylidene fluoride membrane incorporated with hydrophobic polyethylene-functionalized-silica to improve CO2 removal in membrane gas absorption. Sep. Purif. Technol. 2019, 221, 275–285. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J. Hydrophobic modification and silver doping of silica membranes for H2/CO2 separation. J. CO2 Util. 2013, 3–4, 21–29. [Google Scholar] [CrossRef]
- Wei, Q.; Ding, Y.L.; Nie, Z.R.; Liu, X.G.; Li, Q.Y. Wettability, pore structure and performance of perfluorodecyl-modified silica membranes. J. Membr. Sci. 2014, 466, 114–122. [Google Scholar] [CrossRef]
- Mukherjee, D.; Kar, S.; Mandal, A.; Ghosh, S.; Majumdar, S. Immobilization of tannery industrial sludge in ceramic membrane preparation and hydrophobic surface modification for application in atrazine remediation from water. J. Eur. Ceram. Soc. 2019, 39, 3235–3246. [Google Scholar] [CrossRef]
- Karimiab, S.; Mortazavia, Y.; Khodadadia, A.A.; Holmgrenb, A.; Korelskiyc, D.; Hedlund, J. Functionalization of silica membranes for CO2 separation. Sep. Purif. Technol. 2020, 235, 116207. [Google Scholar] [CrossRef]
- Khan, A.A.; Maitlo, H.A.; Khan, I.A.; Lim, D.; Zhang, M.; Kim, K.-H.; Lee, J.; Kim, J.-O. Metal oxide and carbon nanomaterial based membranes for reverse osmosis and membrane distillation: A comparative review. Environ. Res. 2021, 202, 111716. [Google Scholar] [CrossRef]
- Kurt, T.; Topuz, B. Sol-gel Control on Mixed Network Silica Membranes for Gas Separation. Sep. Purif. Technol. 2020, 255, 117654. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, B.; Mardkhe, M.K.; Woodfield, B.F. Thermal and hydrothermal stability of pure and silica-doped mesoporous aluminas. Microporous Mesoporous Mater. 2019, 284, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Hacarlioglu, P.; Oyama, S.T. Hydrothermally stable silica–alumina composite membranes for hydrogen separation. J. Membr. Sci. 2008, 310, 28–37. [Google Scholar] [CrossRef]
- Teo, H.T.; Siah, W.R.; Yuliati, L. Enhanced adsorption of acetylsalicylic acid over hydrothermally synthesized iron oxide-mesoporous silica MCM-41 composites. J. Taiwan Inst. Chem. Eng. 2016, 65, 591–598. [Google Scholar] [CrossRef]
- Uhlmann, D.; Smart, S.; da Costa, J.C.D. H2S stability and separation performance of cobalt oxide silica membranes. J. Membr. Sci. 2011, 380, 48–54. [Google Scholar] [CrossRef]
- Chang, C.H.; Gopalan, R.; Lin, Y.S. A comparative study on thermal and hydrothermal stability of alumina, titania and zirconia membranes. J. Membr. Sci. 1994, 91, 27–45. [Google Scholar] [CrossRef]
- Yoshida, K.; Hirano, Y.; Fujii, H.; Tsuru, T.; Asaeda, M. Hydrothermal stability and performance of silica-zirconia membranes for hydrogen separation in hydrothermal conditions. J. Chem. Eng. Jpn. 2001, 34, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Díez, B.; Roldán, N.; Martín, A.; Sotto, A.; Perdigón-Melón, J.; Arsuaga, J.; Rosal, R. Fouling and biofouling resistance of metal-doped mesostructured silica/polyethersulfone ultrafiltration membranes. J. Membr. Sci. 2017, 526, 252–263. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Mu, R.; Guo, Y.; Hou, H. Sol-Gel Processed Cobalt-Doped Methylated Silica Membranes Calcined under N2 Atmosphere: Microstructure and Hydrogen Perm-Selectivity. Materials 2021, 14, 4188. [Google Scholar] [CrossRef]
- Li, L.; Hong, Q. Gas separation using sol–gel derived microporous zirconia membranes with high hydrothermal stability. Chin. J. Chem. Eng. 2015, 23, 1300–1306. [Google Scholar] [CrossRef]
- Gu, Y.; Kusakabe, K.; Morooka, S. Sulfuric acid-modified zirconia membrane for use in hydrogen separation. Sep. Purif. Technol. 2001, 24, 489–495. [Google Scholar] [CrossRef]
- Van Gestel, T.; Velterop, F.; Meulenberg, W.A. Zirconia-supported hybrid organosilica microporous membranes for CO2 separation and pervaporation. Sep. Purif. Technol. 2020, 259, 118114. [Google Scholar] [CrossRef]
- Ahn, S.J.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. Permeation properties of silica-zirconia composite membranes supported on porous alumina substrates. J. Membr. Sci. 2017, 526, 409–416. [Google Scholar] [CrossRef]
- Hove, M.T.; Luiten-Olieman, M.; Huiskes, C.; Nijmeijer, A.; Winnubst, L. Hydrothermal stability of silica, hybrid silica and Zr-doped hybrid silica membranes. Purif. Technol. 2017, 189, 48–53. [Google Scholar] [CrossRef]
- Goswami, K.P.; Pugazhenthi, G. Effect of binder concentration on properties of low-cost fly ash-based tubular ceramic membrane and its application in separation of glycerol from biodiesel. J. Clean. Prod. 2021, 319, 128679. [Google Scholar] [CrossRef]
- Pan, G.S.; Gu, Z.H.; Yan, Z.; Li, T.; Hua, G.; Yan, L. Preparation of silane modified SiO2 abrasive particles and their Chemical Mechanical Polishing (CMP) performances. Wear 2011, 273, 100–104. [Google Scholar] [CrossRef]
- Azmiyawati, C.; Niami, S.S.; Darmawan, A. Synthesis of silica gel from glass waste for adsorption of Mg2+, Cu2+, and Ag+ metal ions. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012028. [Google Scholar] [CrossRef]
- Sumanjit; Rani, S.; Mahajan, R.K. Equilibrium, kinetics and thermodynamic parameters for adsorptive removal of dye Basic Blue 9 by ground nut shells and Eichhornia. Arab. J. Chem. 2016, 9, S1464–S1477. [Google Scholar] [CrossRef] [Green Version]
- Xiong, R.; Li, X.; Ji, H.; Sun, X.; He, J. Thermal stability of ZrO2–SiO2 aerogel modified by Fe(III) ion. J. Sol-Gel Sci. Technol. 2014, 72, 496–501. [Google Scholar] [CrossRef]
- Del Monte, F.; Larsen, W.; Mackenzie, J.D. Chemical Interactions Promoting the ZrO2 Tetragonal Stabilization in ZrO2–SiO2 Binary Oxides. J. Am. Ceram. Soc. 2010, 83, 1506–1512. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, J.; Wei, D.; Wan, H.; Zheng, S.; Xu, Z.; Zhu, D. ZrO2-functionalized magnetic mesoporous SiO2 as effective phosphate adsorbent. J. Colloid Interface Sci. 2013, 407, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Musić, S.; Filipović-Vinceković, N.; Sekovanić, L. Precipitation of amorphous SiO2 particles and their properties. Braz. J. Chem. Eng. 2011, 28, 89–94. [Google Scholar] [CrossRef]
- Zheng, W.; Bowen, K.H.; Li, J.; Dabkowska, I.; Gutowski, M. Electronic structure differences in ZrO2 vs. HfO2. J. Phys. Chem. A 2005, 109, 11521–11525. [Google Scholar] [CrossRef] [PubMed]
- Kazunari, K.; Jyunichi, I.; Hideaki, M.; Satoshi, F. Evaluation of hydrogen permeation rate through zirconium pipe. Nucl. Mater. Energy 2018, 16, 12–18. [Google Scholar]
- Lin, R.B.; Xiang, S.; Xing, H.; Zhou, W.; Chen, B. Exploration of porous metal–organic frameworks for gas separation and purification. Coord. Chem. Rev. 2017, 378, 87–103. [Google Scholar] [CrossRef]
- Hong, Q.; Chen, H.; Li, L.; Zhu, G.; Xu, N. Effect of Nb content on hydrothermal stability of a novel ethylene-bridged silsesquioxane molecular sieving membrane for H2/CO2 separation. J. Membr. Sci. 2012, S421–S422, 190–200. [Google Scholar]
- Boffa, V.; Blank, D.; Elshof, J. Hydrothermal stability of microporous silica and niobia–silica membranes. J. Membr. Sci. 2008, 319, 256–263. [Google Scholar] [CrossRef]
- Liu, L.; Wang, D.K.; Martens, D.L.; Smart, S.; da Costa, J.C.D. Binary gas mixture and hydrothermal stability investigation of cobalt silica membranes. J. Membr. Sci. 2015, 493, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, H.F.; Nijmeijer, A.; Winnubst, L. Influence of sol–gel process parameters on the micro-structure and performance of hybrid silica membranes. J. Membr. Sci. 2013, 446, 19–25. [Google Scholar] [CrossRef]
- He, D.; Zhang, H.; Ren, Y.; Qi, H. Fabrication of a novel microporous membrane based on ZIF-7 doped 1,2-bis(triethoxysilyl)ethane for H2/CO2 separation. Microporous Mesoporous Mater. 2022, 331, 111674. [Google Scholar] [CrossRef]
- Song, H.; Zhao, S.; Lei, J.; Wang, C.; Qi, H. Pd-doped organosilica membrane with enhanced gas permeability and hydrothermal stability for gas separation. J. Mater. Sci. 2016, 51, 6275–6286. [Google Scholar] [CrossRef]
- Beyler, A.P.; Boye, D.M.; Hoffman, K.R.; Silversmith, A.J. Fluorescence enhancement in rare earth doped sol-gel glass by N,N dimethylformamide as a drying control chemical additive. Phys. Procedia 2011, 13, 4–8. [Google Scholar] [CrossRef] [Green Version]
- García, M.G.; Marchese, J.; Ochoa, N.A. Aliphatic–aromatic polyimide blends for H2 separation. Int. J. Hydrogen Energy 2010, 35, 8983–8992. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Tanczyk, M.; Warmuzinski, K.; Jaschik, M.; Sandru, M.; Dahl, P.I.; Genua, A.; Lois, S.; Sheridan, E.; et al. The performance of affordable and stable cellulose-based poly-ionic membranes in CO2/N2 and CO2/CH4 gas separation. J. Membr. Sci. 2018, 564, 552–561. [Google Scholar] [CrossRef]
- Chen, F.; Ji, Z.; Qi, Q. Effect of liquid surface tension on the filtration performance of coalescing filters. Sep. Purif. Technol. 2019, 209, 881–891. [Google Scholar] [CrossRef]



| nZr | BET Surface Area (m2·g−1) | Average Pore Size (nm) | Vtotal (STP) (cm3·g−1) | Vmicro (STP) (cm3·g−1) | Vmicro/Vtotal (%) |
|---|---|---|---|---|---|
| 0 | 389.38 | 1.75 | 0.23 | 0.15 | 65.22 |
| 0.08 | 579.96 | 2.08 | 0.37 | 0.13 | 35.14 |
| 0.15 | 616.77 | 2.19 | 0.43 | 0.12 | 27.91 |
| 0.3 | 606.35 | 2.35 | 0.41 | 0.09 | 21.95 |
| 0.5 | 545.32 | 3.58 | 0.38 | 0.08 | 21.05 |
| Membrane | Gases | Ea (kJ·mol −1) | Qst (kJ·mol−1) | Em (kJ·mol−1) |
|---|---|---|---|---|
| MSiO2 | H2 | 2.32 | 6.00 | 8.32 |
| CO2 | −1.53 | 24.00 | 22.47 | |
| N2 | −1.48 | 18.00 | 16.52 | |
| ZrO2-MSiO2 | H2 | 2.10 | 6.00 | 8.10 |
| CO2 | −1.64 | 24.00 | 22.36 | |
| N2 | −1.94 | 18.00 | 16.06 |
| Type | Temperature/Pressure | Ea of H2 (kJ·mol−1) | Pore Diameter (nm) | H2 Permeance (mol·m−2·s−1·Pa−1) | H2 Permselectivities | |
|---|---|---|---|---|---|---|
| H2/CO2 | H2/N2 | |||||
| SiO2 [49] | 200 °C, 2 bar | - | 0.30–0.54 | 4.62 × 10−7 | 3.7 | 10.5 |
| ZIF-7-SiO2 [50] | 200 °C | - | 5 | 8 × 10−7 | 8.78 | 11.8 |
| Pd-SiO2 [51] | 200 °C, 0.3 MPa | - | 0.57 | 7.26 × 10−7 | 4.3 | 14 |
| ZrO2 [52] | 350 °C | - | 4.95 | 5.3 × 10−8 | 14.3 | 3.1 |
| ZrO2-SiO2 [27] | 550 °C | 7.0 | 0.165 | 1.8 × 10−7 | - | - |
| BTDA-DDS polyimide [53] | 30 °C, 5 MPa | - | - | 2.52 × 10−9 | 5.16 | 193.21 |
| Cellulose acetate [54] | 25 °C, 1 bar | - | - | 3.55 × 10−9 | - | 30.3 |
| ZrO2-MSiO2 * | 200 °C, 0.1 MPa | 2.10 | 2.19 | 6.46 × 10−6 | 11.64 | 13.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Yang, J. Zirconia-Doped Methylated Silica Membranes via Sol-Gel Process: Microstructure and Hydrogen Permselectivity. Nanomaterials 2022, 12, 2159. https://doi.org/10.3390/nano12132159
Wang L, Yang J. Zirconia-Doped Methylated Silica Membranes via Sol-Gel Process: Microstructure and Hydrogen Permselectivity. Nanomaterials. 2022; 12(13):2159. https://doi.org/10.3390/nano12132159
Chicago/Turabian StyleWang, Lintao, and Jing Yang. 2022. "Zirconia-Doped Methylated Silica Membranes via Sol-Gel Process: Microstructure and Hydrogen Permselectivity" Nanomaterials 12, no. 13: 2159. https://doi.org/10.3390/nano12132159
APA StyleWang, L., & Yang, J. (2022). Zirconia-Doped Methylated Silica Membranes via Sol-Gel Process: Microstructure and Hydrogen Permselectivity. Nanomaterials, 12(13), 2159. https://doi.org/10.3390/nano12132159