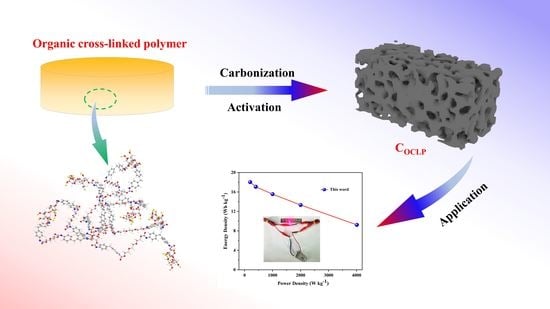
Organic Crosslinked Polymer-Derived N/O-Doped Porous Carbons for High-Performance Supercapacitor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
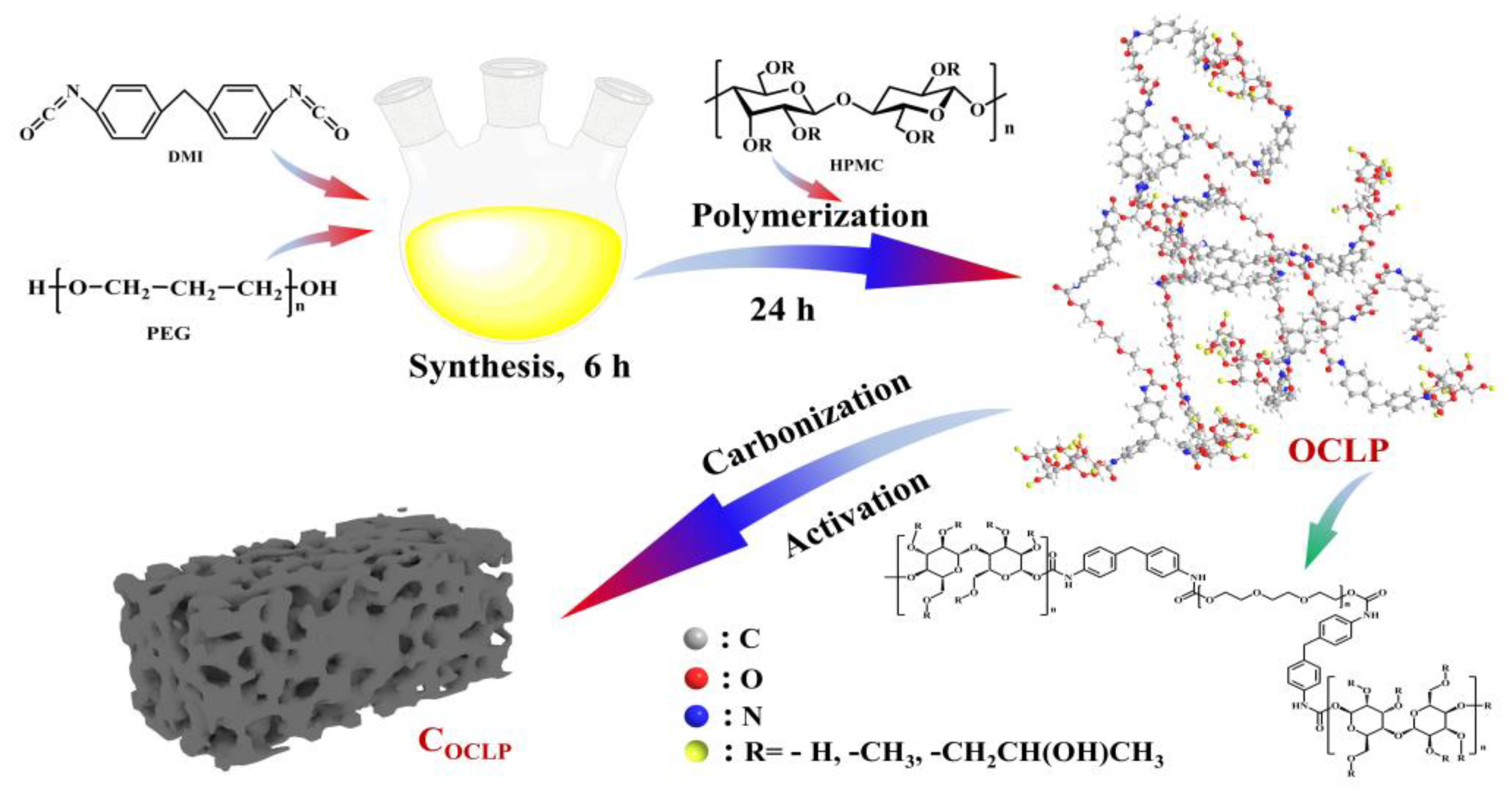
2.2. Synthesis of Organic Crosslinked Polymers
2.3. Preparation of Porous Carbon Materials
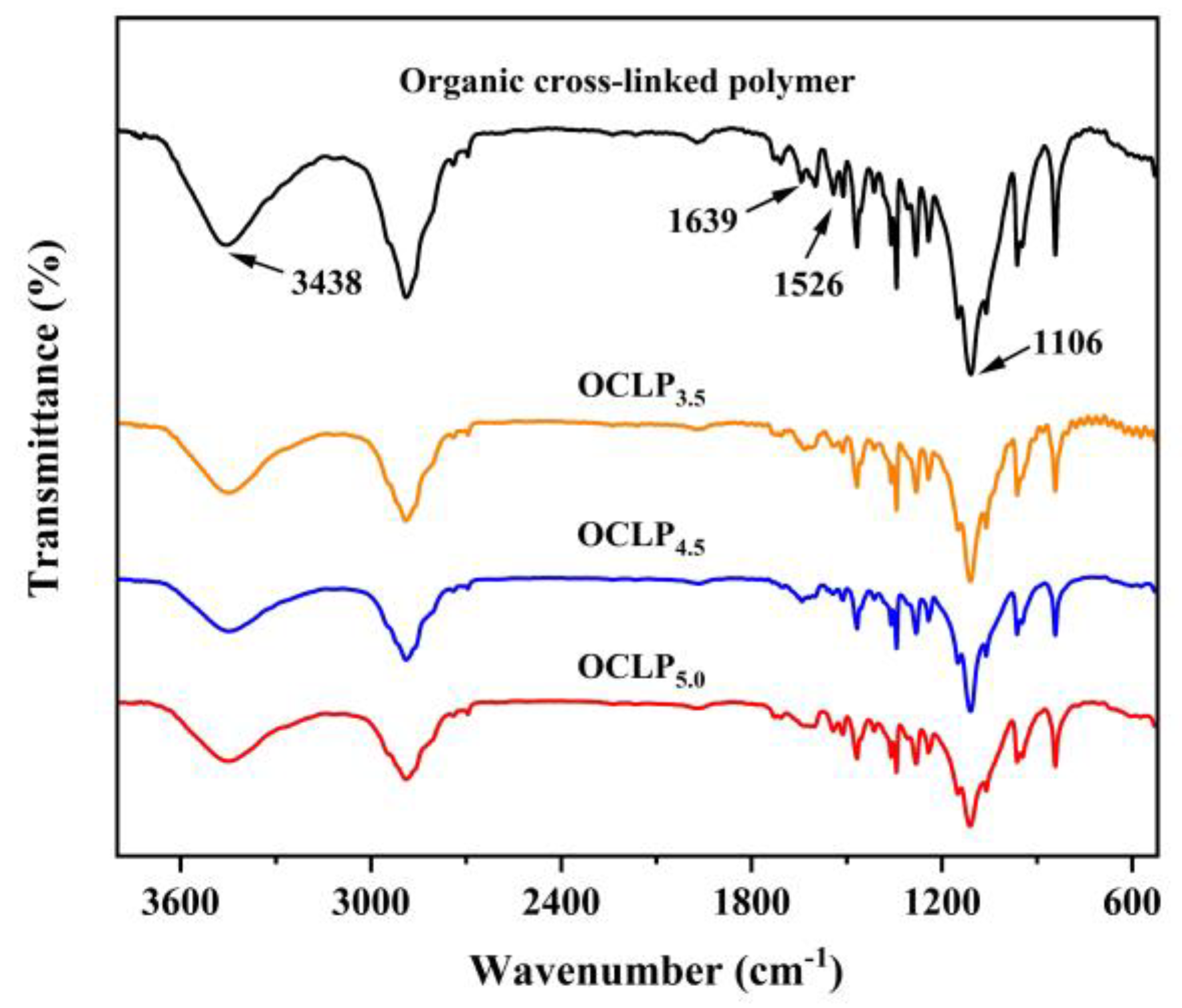
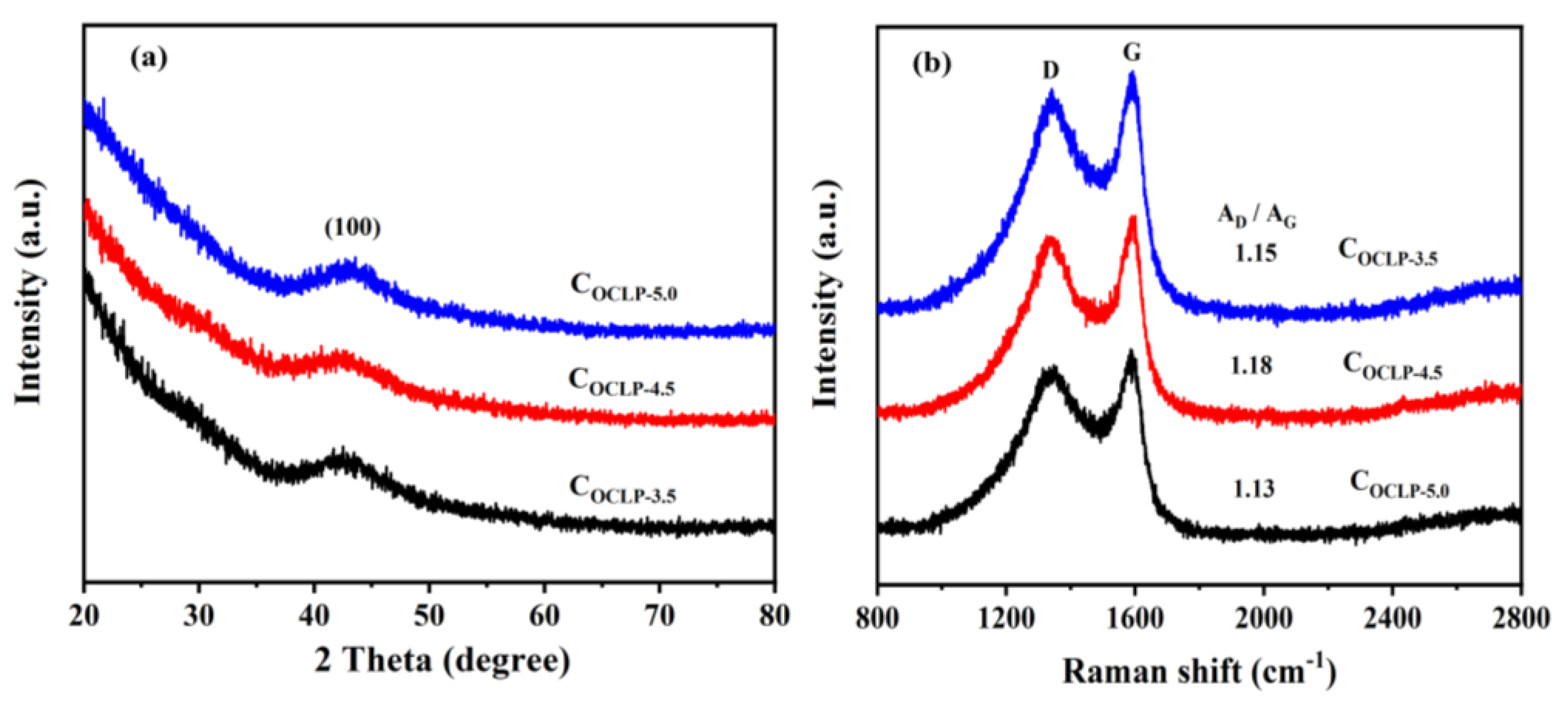
2.4. Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
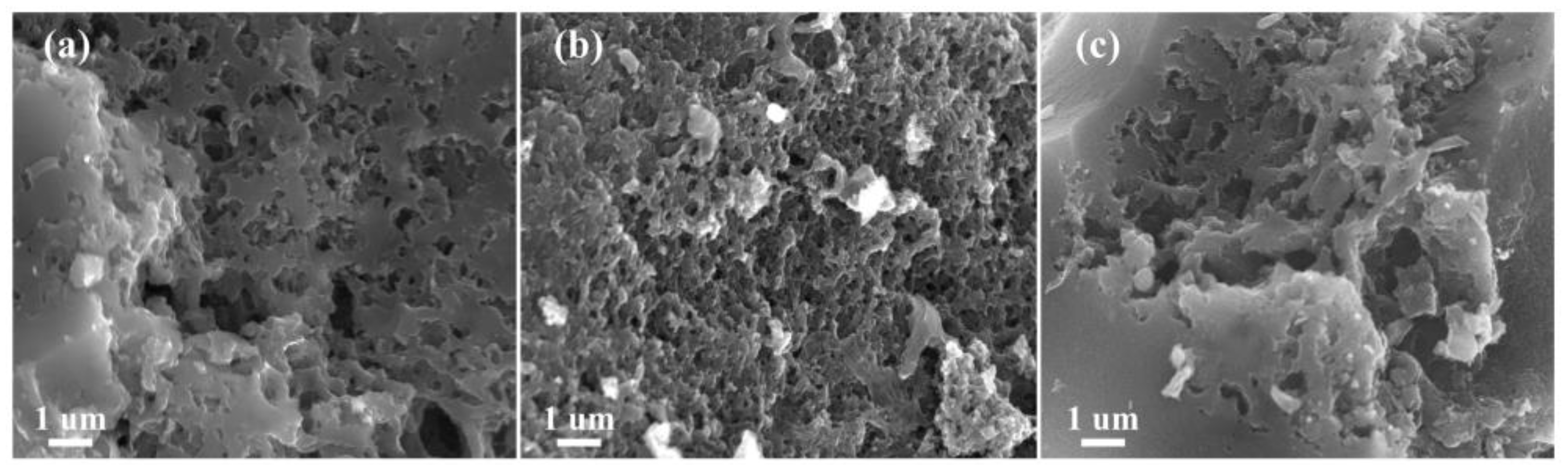
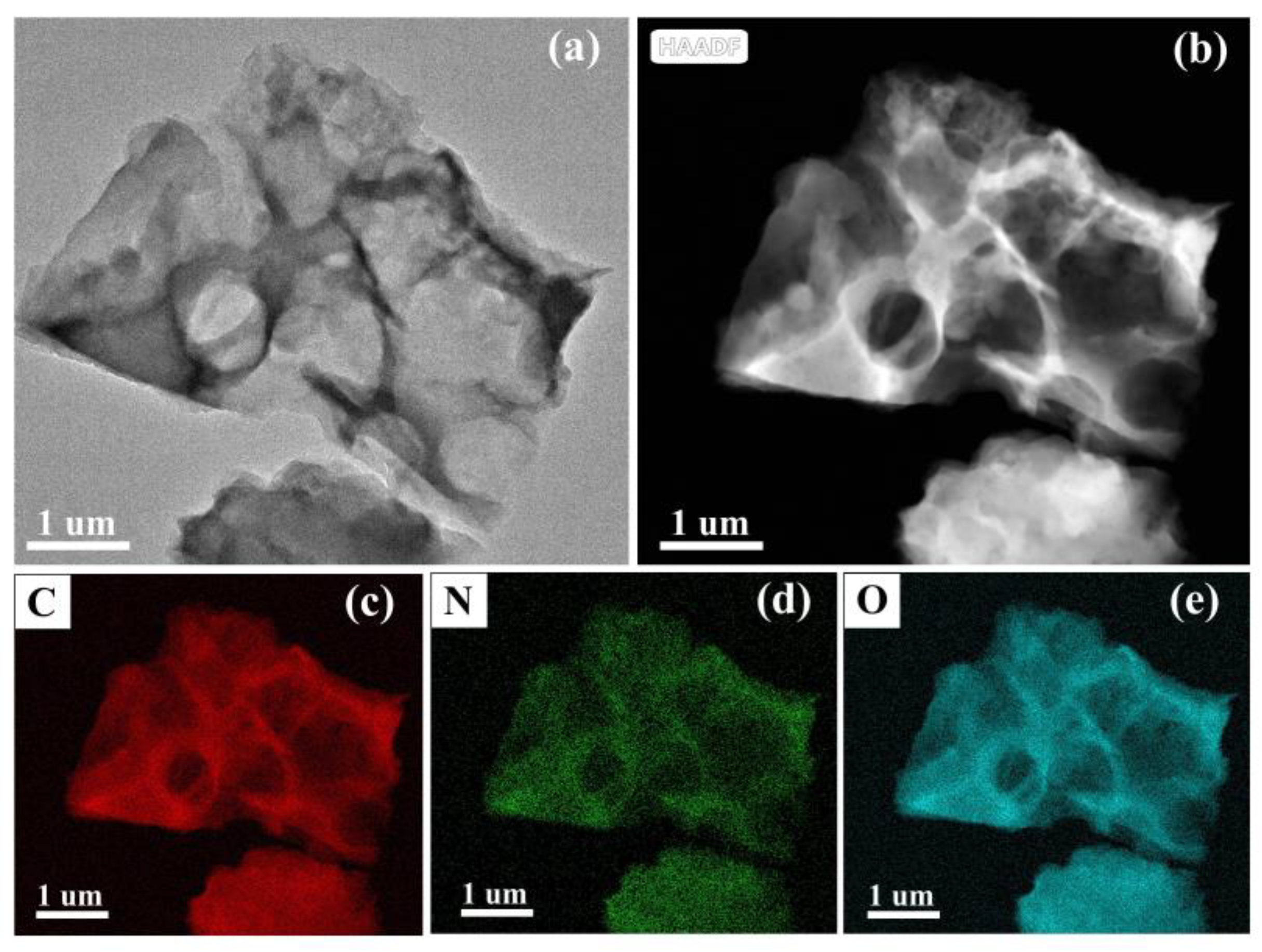
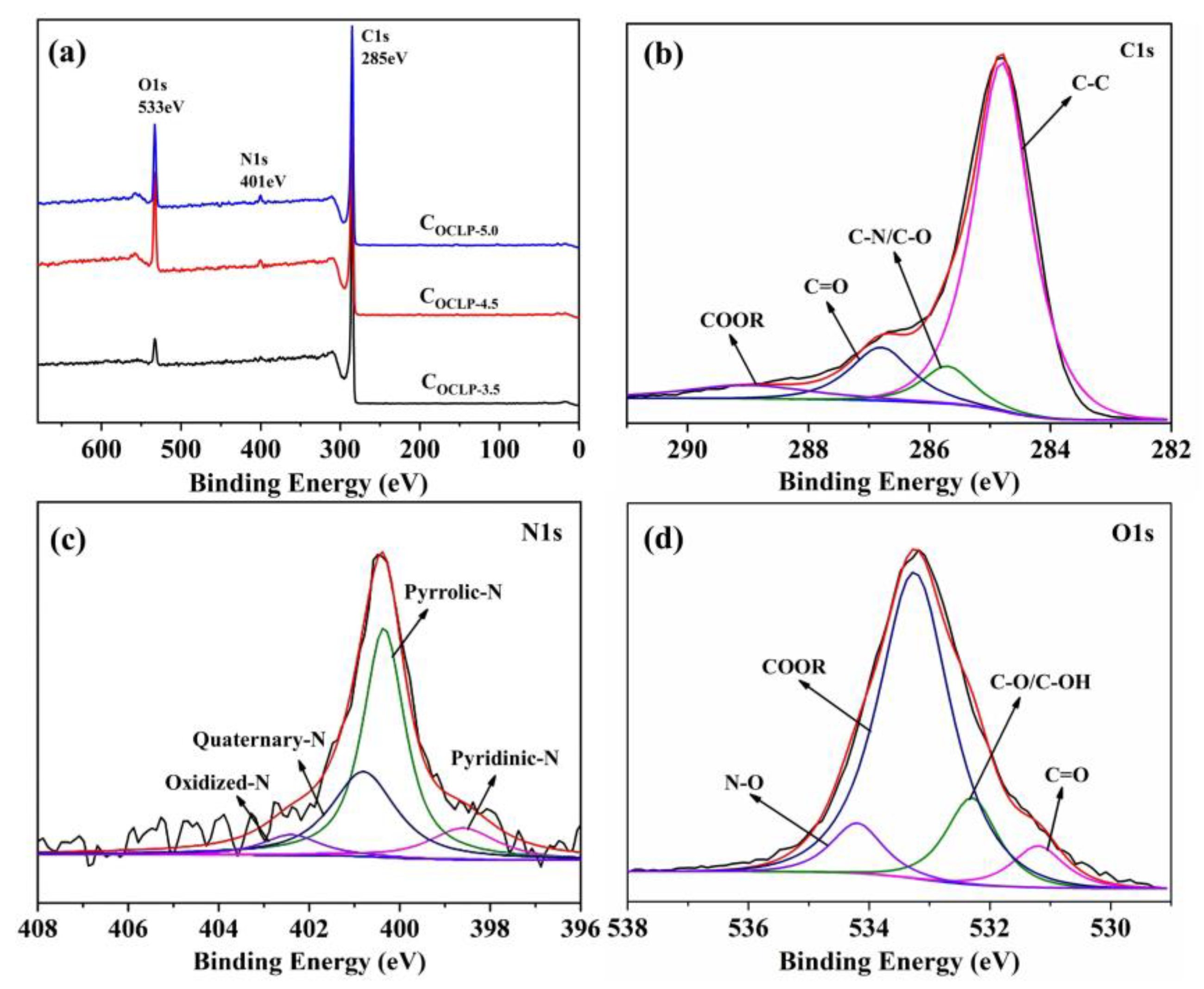
3.1. Structural and Morphological Characterization
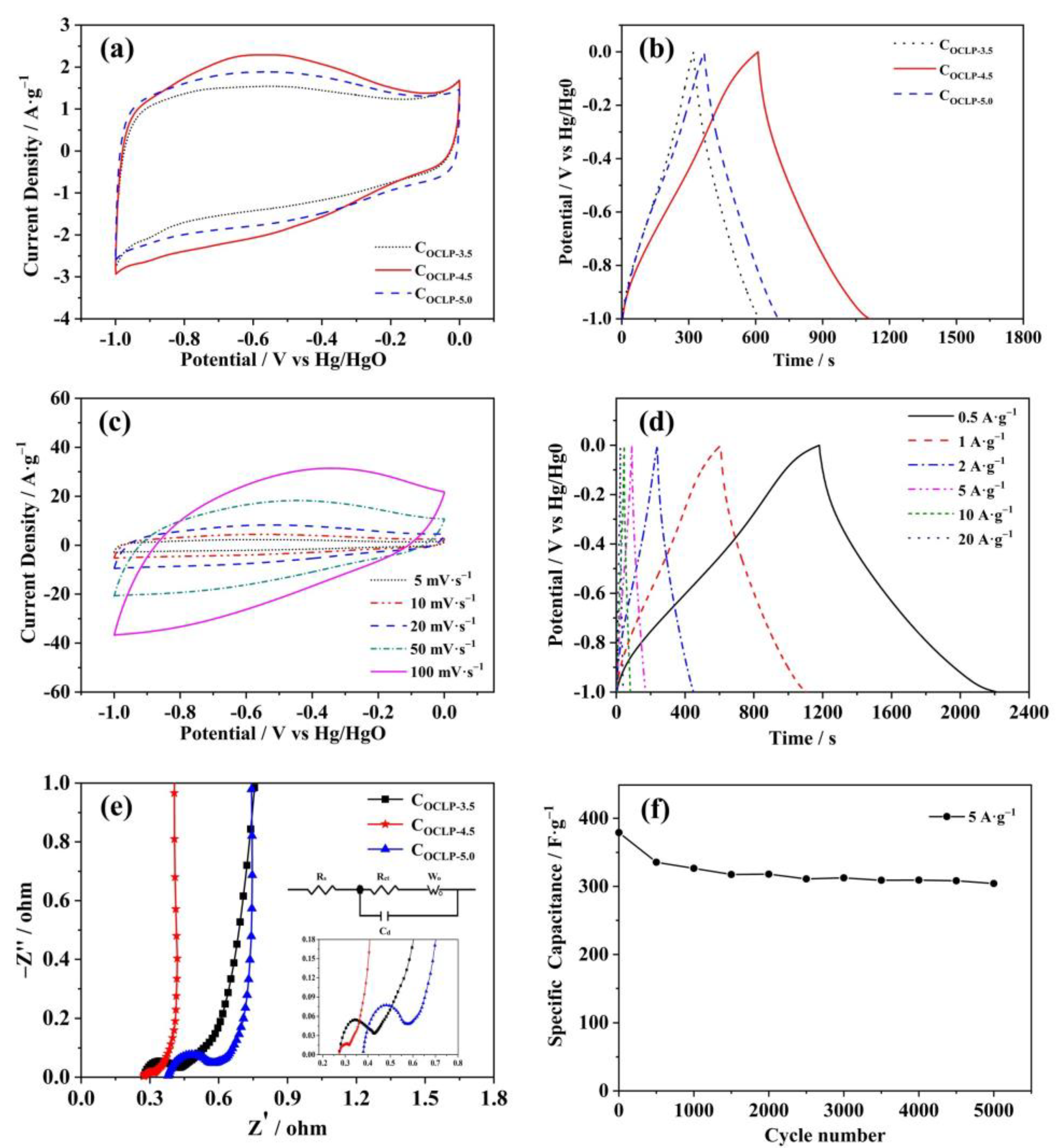
3.2. Electrochemistry Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, J.; Cao, C.; Idrees, F.; Ma, X. Hierarchical Porous Nitrogen-Doped Carbon Nanosheets Derived from Silk for Ultrahigh-Capacity Battery Anodes and Supercapacitors. ACS Nano 2015, 9, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Deng, Y.; Chen, J.; Qian, Y.; Yang, Y.; Li, Y.; Chen, G. Hierarchically porous nitrogen-doped carbon derived from the activation of agriculture waste by potassium hydroxide and urea for high-performance supercapacitors. J. Power Sources 2018, 378, 579–588. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Dash, R.; Gogotsi, Y. Effect of pore size and surface area of carbide derived carbons on specific capacitance. J. Power Sources 2006, 158, 765–772. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, J.; Zhao, X.; Zhuang, Q.; Zhou, Z.; Huang, Y.; Wei, X. High-performance electrode material for electric double-layer capacitor based on hydrothermal pre-treatment of lignin by ZnCl2. Appl. Surf. Sci. 2020, 508, 144536. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Amirkhiz, B.S.; Tan, X.; Xu, Z.; Wang, H.; Olsen, B.C.; Holt, C.M.B.; Mitlin, D. Carbonized Chicken Eggshell Membranes with 3D Architectures as High-Performance Electrode Materials for Supercapacitors. Adv. Energy Mater. 2012, 2, 431–437. [Google Scholar] [CrossRef]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Kohandehghan, A.; Li, Z.; Cui, K.; Tan, X.; Stephenson, T.J.; King ondu, C.K.; Holt, C.M.B.; Olsen, B.C.; et al. Interconnected Carbon Nanosheets Derived from Hemp for Ultrafast Supercapacitors with High Energy. ACS Nano 2013, 7, 5131–5141. [Google Scholar] [CrossRef]
- Chang, Y.; Shi, H.; Yan, X.; Zhang, G.; Chen, L. A ternary B, N, P-Doped carbon material with suppressed water splitting activity for high-energy aqueous supercapacitors. Carbon 2020, 170, 127–136. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Li, Z.; Su, Q.; Wei, S.; Pang, S.; Zhao, X.; Liang, L.; Kang, L.; Cao, S. Preparation of Boron/Sulfur-Codoped Porous Carbon Derived from Biological Wastes and Its Application in a Supercapacitor. Nanomaterials 2022, 12, 1182. [Google Scholar] [CrossRef]
- Wang, P.; Ding, X.; Zhe, R.; Zhu, T.; Qing, C.; Liu, Y.; Wang, H. Synchronous Defect and Interface Engineering of NiMoO4 Nanowire Arrays for High-Performance Supercapacitors. Nanomaterials 2022, 12, 1094. [Google Scholar] [CrossRef]
- Han, X.; Tao, K.; Wang, D.; Han, L. Design of a porous cobalt sulfide nanosheet array on Ni foam from zeolitic imidazolate frameworks as an advanced electrode for supercapacitors. Nanoscale 2018, 10, 2735–2741. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Alkhedher, M.; Raza, R.; Ahmad, M.A.; Majid, A.; Din, E.M.T.E. Electrochemical Investigation of PANI:PPy/AC and PANI:PEDOT/AC Composites as Electrode Materials in Supercapacitors. Polymers 2022, 14, 1976. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Shi, L.; Ye, J.; Lu, H.; Wang, G.; Lv, J.; Ning, G. Flexible all-solid-state supercapacitors based on boron and nitrogen-doped carbon network anchored on carbon fiber cloth. Chem. Eng. J. 2021, 410, 128365. [Google Scholar] [CrossRef]
- Wu, D.; Cheng, J.; Wang, T.; Liu, P.; Yang, L.; Jia, D. A Novel Porous N- and S-Self-Doped Carbon Derived from Chinese Rice Wine Lees as High-Performance Electrode Materials in a Supercapacitor. ACS Sustain. Chem. Eng. 2019, 7, 12138–12147. [Google Scholar] [CrossRef]
- Lei, W.; Yang, B.; Sun, Y.; Xiao, L.; Tang, D.; Chen, K.; Sun, J.; Ke, J.; Zhuang, Y. Self-sacrificial template synthesis of heteroatom doped porous biochar for enhanced electrochemical energy storage. J. Power Sources 2021, 488, 229455. [Google Scholar] [CrossRef]
- Jiang, W.; Cai, J.; Pan, J.; Guo, S.; Sun, Y.; Li, L.; Liu, X. Nitrogen-doped hierarchically ellipsoidal porous carbon derived from Al-based metal-organic framework with enhanced specific capacitance and rate capability for high performance supercapacitors. J. Power Sources 2019, 432, 102–111. [Google Scholar] [CrossRef]
- Lima, R.M.A.P.; Dos, R.G.S.; Thyrel, M.; AlcarazEspinoza, J.J.; Larsson, S.H.; de Oliveira, H.P. Facile Synthesis of Sustainable Biomass-Derived Porous Biochars as Promising Electrode Materials for High-Performance Supercapacitor Applications. Nanomaterials 2022, 12, 866. [Google Scholar] [CrossRef]
- Alabadi, A.; Yang, X.; Dong, Z.; Li, Z.; Tan, B. Nitrogen-doped activated carbons derived from a co-polymer for high supercapacitor performance. J. Mater. Chem. A 2014, 2, 11697–11705. [Google Scholar] [CrossRef]
- Park, M.; Ryu, J.; Kim, Y.; Cho, J. Corn protein-derived nitrogen-doped carbon materials with oxygen-rich functional groups: A highly efficient electrocatalyst for all-vanadium redox flow batteries. Energy Environ. Sci. 2014, 7, 3727–3735. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A.; Antonietti, M. A detailed view on the polycondensation of ionic liquid monomers towards nitrogen doped carbon materials. J. Mater. Chem. 2010, 20, 6746. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Bai, J.; Xu, M.; Luo, C.; Yang, L.; Bai, L.; Wei, D.; Wang, W.; Yang, H. N-doped hierarchically porous carbon derived from grape marcs for high-performance supercapacitors. J. Alloys Compd. 2021, 854, 157207. [Google Scholar] [CrossRef]
- Mohd Abdah, M.A.A.; Azman, N.H.N.; Kulandaivalu, S.; Abdul Rahman, N.; Abdullah, A.H.; Sulaiman, Y. Potentiostatic deposition of poly (3, 4-ethylenedioxythiophene) and manganese oxide on porous functionalised carbon fibers as an advanced electrode for asymmetric supercapacitor. J. Power Sources 2019, 444, 227324. [Google Scholar] [CrossRef]
- Zou, W.; Zhang, S.; Abbas, Y.; Liu, W.; Zhang, Y.; Wu, Z.; Xu, B. Structurally designed heterochain polymer derived porous carbons with high surface area for high-performance supercapacitors. Appl. Surf. Sci. 2020, 530, 147296. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, F.; Qiu, F.; Lu, C.; Kang, J.; Zhao, D.; Han, S.; Zhuang, X. Cobalt-Doped Porous Carbon Nanosheets Derived from 2D Hypercrosslinked Polymer with CoN4 for High Performance Electrochemical Capacitors. Polymers 2018, 10, 1339. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Xu, F.; Sun, L.; Xia, Y.; Wei, S.; Zhang, H. Preparation and thermal property of PEG based composite phase change material. New Chenical Mater. 2020, 48, 75–79. [Google Scholar]
- Chen, C.; Liu, W.; Wang, H.; Peng, K. Synthesis and performances of novel solid–solid phase change materials with hexahydroxy compounds for thermal energy storage. Appl. Energy 2015, 152, 198–206. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Dai, Y.; Wang, H.; Zhang, H.; Wang, Q.; Hou, W.; Yan, H.; Li, W.; Zheng, J. Nitrogen and phosphorus co-doped silkworm-cocoon-based self-activated porous carbon for high performance supercapacitors. J. Power Sources 2019, 438, 227045. [Google Scholar] [CrossRef]
- Zhipeng, Q.; Yesheng, W.; Xu, B.; Tong, Z.; Jin, Z.; Jinping, Z.; Zhichao, M.; Weiming, Y.; Peng, F.; Shuping, Z. Biochar-based carbons with hierarchical micro-meso-macro porosity for high rate and long cycle life supercapacitors. J. Power Sources 2018, 376, 82–90. [Google Scholar]
- Kong, L.N.; Yang, W.; Su, L.; Hao, S.G.; Shao, G.J.; Qin, X.J. Nitrogen-doped 3D web-like interconnected porous carbon prepared by a simple method for supercapacitors. Ionics 2019, 25, 4333–4340. [Google Scholar] [CrossRef]
- Boujibar, O.; Ghamouss, F.; Ghosh, A.; Achak, O.; Chafik, T. Activated carbon with exceptionally high surface area and tailored nanoporosity obtained from natural anthracite and its use in supercapacitors. J. Power Sources 2019, 436, 226882. [Google Scholar] [CrossRef]
- Hu, J.; He, W.; Qiu, S.; Xu, W.; Mai, Y.; Guo, F. Nitrogen-doped hierarchical porous carbons prepared via freeze-drying assisted carbonization for high-performance supercapacitors. Appl. Surf. Sci. 2019, 496, 143643. [Google Scholar] [CrossRef]
- Yiju, L.; Guiling, W.; Tong, W.; Zhuangjun, F.; Peng, Y. Nitrogen and sulfur co-doped porous carbon nanosheets derived from willow catkin for supercapacitors. Nano Energy 2016, 19, 165–175. [Google Scholar]
- Jia, H.; Zhang, H.; Wan, S.; Sun, J.; Xie, X.; Sun, L. Preparation of nitrogen-doped porous carbon via adsorption-doping for highly efficient energy storage. J. Power Sources 2019, 433, 226712. [Google Scholar] [CrossRef]
- Javaid, A.; Irfan, M. Multifunctional structural supercapacitors based on graphene nanoplatelets/carbon aerogel composite coated carbon fiber electrodes. Mater. Res. Express 2018, 6, 16310. [Google Scholar] [CrossRef]
- Li, C.; Wu, W.; Wang, P.; Zhou, W.; Wang, J.; Chen, Y.; Fu, L.; Zhu, Y.; Wu, Y.; Huang, W. Fabricating an Aqueous Symmetric Supercapacitor with a Stable High Working Voltage of 2 V by Using an Alkaline-Acidic Electrolyte. Adv. Sci. 2019, 6, 1801665. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Fan, Z. Nitrogen-doped carbon mesh from pyrolysis of cotton in ammonia as binder-free electrodes of supercapacitors. Microporous Mesoporous Mater. 2019, 274, 313–317. [Google Scholar] [CrossRef]
- Méndez-Morales, T.; Ganfoud, N.; Li, Z.; Haefele, M.; Rotenberg, B.; Salanne, M. Performance of microporous carbon electrodes for supercapacitors: Comparing graphene with disordered materials. Energy Storage Mater. 2019, 17, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Huo, S.; Liu, M.; Wu, L.; Liu, M.; Xu, M.; Ni, W.; Yan, Y. Synthesis of ultrathin and hierarchically porous carbon nanosheets based on interlayer-confined inorganic/organic coordination for high performance supercapacitors. J. Power Sources 2019, 414, 383–392. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, J.; Deng, S.; He, Q.; Deng, S.; Hu, Y.; Wang, D. Hypercrosslinked polymers enabled micropore-dominant N, S Co-Doped porous carbon for ultrafast electron/ion transport supercapacitors. Nano Energy 2019, 65, 103993. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, S.; Sun, G.; Kang, K.; Zhu, M.; Geng, Z. Comparison of activated carbons prepared by one-step and two-step chemical activation process based on cotton stalk for supercapacitors application. Energy 2021, 215, 119144. [Google Scholar] [CrossRef]
- Wang, M.; Han, K.; Qi, J.; Teng, Z.; Zhang, J.; Li, M. Study on performance and charging dynamics of N/O codoped layered porous carbons derived from L-tyrosine for supercapacitors. Appl. Surf. Sci. 2022, 578, 151888. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, S.; Zhang, Z.; Chi, Y.; Yang, C.; Wang, C.; Zhen, Y.; Wang, D.; Fu, F.; Chi, R. Pore structure regulation of hierarchical porous carbon derived from coal tar pitch via pre-oxidation strategy for high-performance supercapacitor. J. Colloid Interface Sci. 2022, 614, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Tolendra, K.; Duy, T.T.; Dinh, C.N.; Nam, H.K.; Kin-tak, L.; Joong, H.L. Ternary graphene-carbon nanofibers-carbon nanotubes structure for hybrid supercapacitor. Chem. Eng. J. 2020, 380, 122543. [Google Scholar]
- Zhang, F.; Zong, S.; Zhang, Y.; Lv, H.; Liu, X.; Du, J.; Chen, A. Preparation of hollow mesoporous carbon spheres by pyrolysis-deposition using surfactant as carbon precursor. J. Power Sources 2021, 484, 229274. [Google Scholar] [CrossRef]
- Juan, D.; Yue, Z.; Haixia, W.; Senlin, H.; Aibing, C. N-doped hollow mesoporous carbon spheres by improved dissolution-capture for supercapacitor. Carbon 2020, 156, 523–528. [Google Scholar]
- Hongmei, H.; Li, M.; Shenna, F.; Mengyu, G.; Liangqing, H.; Huanhuan, Z.; Fei, X.; Minghang, J. Fabrication of 3D ordered honeycomb-like nitrogen-doped carbon/PANI composite for high-performance supercapacitors. Appl. Surf. Sci. 2019, 484, 1288–1296. [Google Scholar]
- Zhou, Z.; Cao, J.; Wu, Y.; Zhuang, Q.; Zhao, X.; Wei, Y.; Bai, H. Waste sugar solution polymer-derived N-doped carbon spheres with an ultrahigh specific surface area for superior performance supercapacitors. Int. J. Hydrogen Energy 2021, 46, 22735–22746. [Google Scholar] [CrossRef]
- He, W.; Haitao, N.; Hongjie, W.; Wenyu, W.; Xin, J.; Hongxia, W.; Hua, Z.; Tong, L. Micro-meso porous structured carbon nanofibers with ultra-high surface area and large supercapacitor electrode capacitance. J. Power Sources 2021, 482, 228986. [Google Scholar]
- Man, W.; Juan, Y.; Siyu, L.; Muzi, L.; Chao, H.; Jieshan, Q. Nitrogen-doped hierarchically porous carbon nanosheets derived from polymer/graphene oxide hydrogels for high-performance supercapacitors. J. Colloid Interf. Sci. 2020, 560, 69–76. [Google Scholar]
- Li, J.; Zou, Y.; Xiang, C.; Xu, F.; Sun, L.; Li, B.; Zhang, J. Osmanthus fragrans-derived N-doped porous carbon for supercapacitor applications. J. Energy Storage 2021, 42, 103017. [Google Scholar] [CrossRef]
- Liu, H.; Song, H.; Hou, W.; Chang, Y.; Zhang, Y.; Li, Y.; Zhao, Y.; Han, G. Coal tar pitch-based hierarchical porous carbons prepared in molten salt for supercapacitors. Mater. Chem Phys. 2021, 265, 124491. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Dai, S.; Liu, L.; Zhang, H.; Shen, W.; Sun, W.; Ming, L.C. Rationally tuning ratio of micro- to meso-pores of biomass-derived ultrathin carbon sheets toward supercapacitors with high energy and high power density. J. Colloid Interf. Sci. 2022, 606, 817–825. [Google Scholar] [CrossRef] [PubMed]
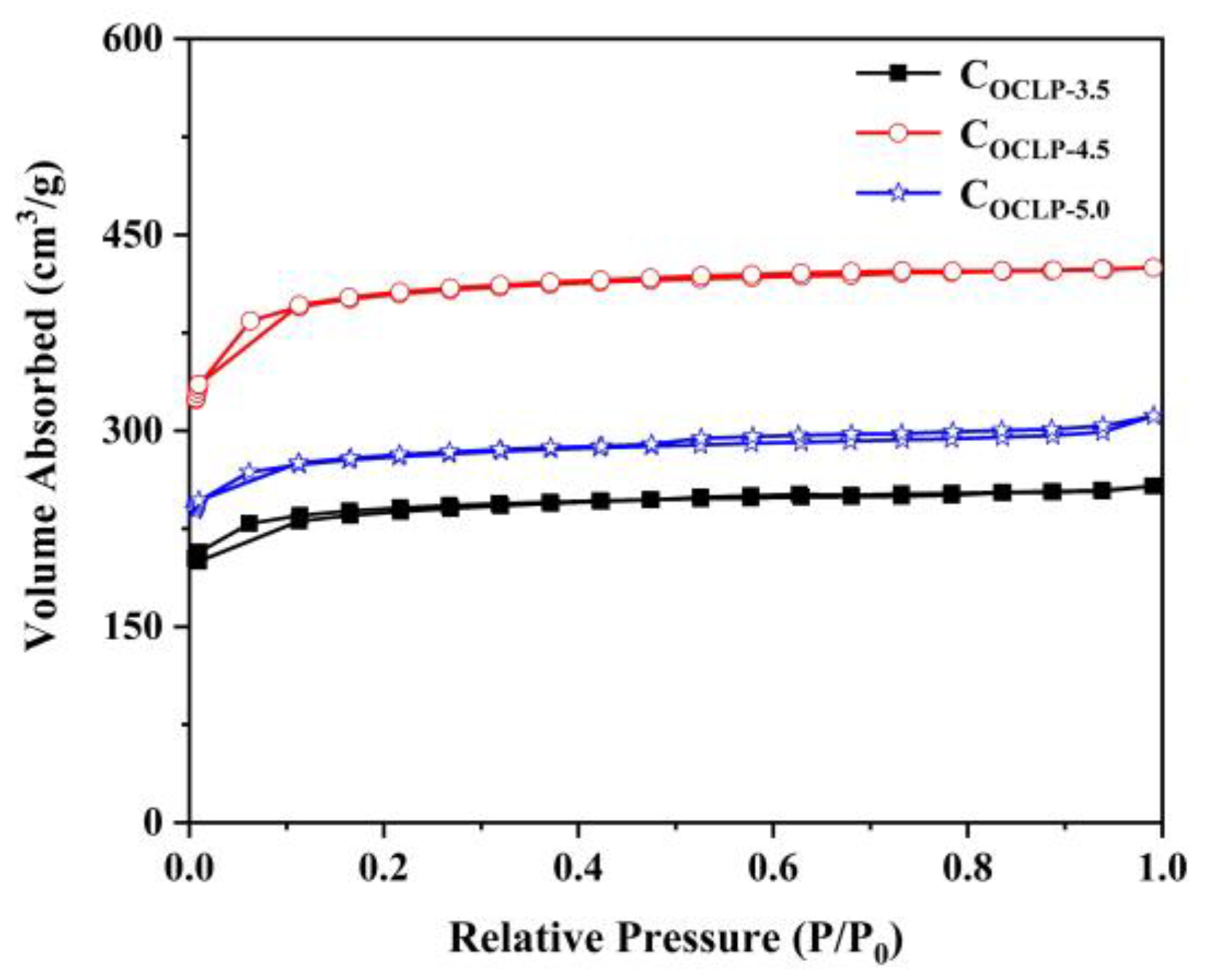


| Samples | Specific Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | ||||
|---|---|---|---|---|---|---|
| Total | Microporous | Mesoporous | Total | Microporous | Mesoporous | |
| COCLP-3.5 | 942 | 894 | 48 | 0.399 | 0.353 | 0.046 |
| COCLP-4.5 | 1589 | 1509 | 80 | 0.657 | 0.592 | 0.065 |
| COCLP-5.0 | 1102 | 1040 | 62 | 0.482 | 0.407 | 0.075 |
| Samples | Element Content | ||
|---|---|---|---|
| Carbon (%) | Nitrogen (%) | Oxygen (%) | |
| COCLP-3.5 | 92.83 | 1.98 | 5.19 |
| COCLP-4.5 | 85.75 | 1.68 | 12.75 |
| COCLP-5.0 | 84.51 | 2.65 | 12.84 |
| Material | Electrolyte | Current Density (A·g−1) | Capacitance (F·g−1) | Reference |
|---|---|---|---|---|
| Grape marc | 6 M KOH | 0.5 | 446 | [23] |
| Polyphosphazene | 6 M KOH | 0.5 | 438 | [25] |
| Polypyrrole/Polythiophene | KOH | 0.5 | 455 | [41] |
| Cotton stalk | 1 M H2SO4 | 0.2 | 338 | [42] |
| L-tyrosine | KOH | 0.3 | 512 | [43] |
| Coal tar pitch | 6 M KOH | 0.5 | 298 | [44] |
| CNTs@Gr-CNF | 6 M KOH | 0.25 | 521 | [45] |
| CTAB | 6 M KOH | 1.0 | 241 | [46] |
| 3-aminophenol-formaldehyde resin | 6 M KOH | 0.5 | 381 | [47] |
| Organic crosslinked polymer | 6 M KOH | 0.5 | 522 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lao, J.; Lu, Y.; Fang, S.; Xu, F.; Sun, L.; Wang, Y.; Zhou, T.; Liao, L.; Guan, Y.; Wei, X.; et al. Organic Crosslinked Polymer-Derived N/O-Doped Porous Carbons for High-Performance Supercapacitor. Nanomaterials 2022, 12, 2186. https://doi.org/10.3390/nano12132186
Lao J, Lu Y, Fang S, Xu F, Sun L, Wang Y, Zhou T, Liao L, Guan Y, Wei X, et al. Organic Crosslinked Polymer-Derived N/O-Doped Porous Carbons for High-Performance Supercapacitor. Nanomaterials. 2022; 12(13):2186. https://doi.org/10.3390/nano12132186
Chicago/Turabian StyleLao, Jianhao, Yao Lu, Songwen Fang, Fen Xu, Lixian Sun, Yu Wang, Tianhao Zhou, Lumin Liao, Yanxun Guan, Xueying Wei, and et al. 2022. "Organic Crosslinked Polymer-Derived N/O-Doped Porous Carbons for High-Performance Supercapacitor" Nanomaterials 12, no. 13: 2186. https://doi.org/10.3390/nano12132186