Adding Nano-TiO2 to Water and Paraffin to Enhance Total Efficiency of a Photovoltaic Thermal PV/T System Subjected to Harsh Weathers
Abstract
:1. Introduction
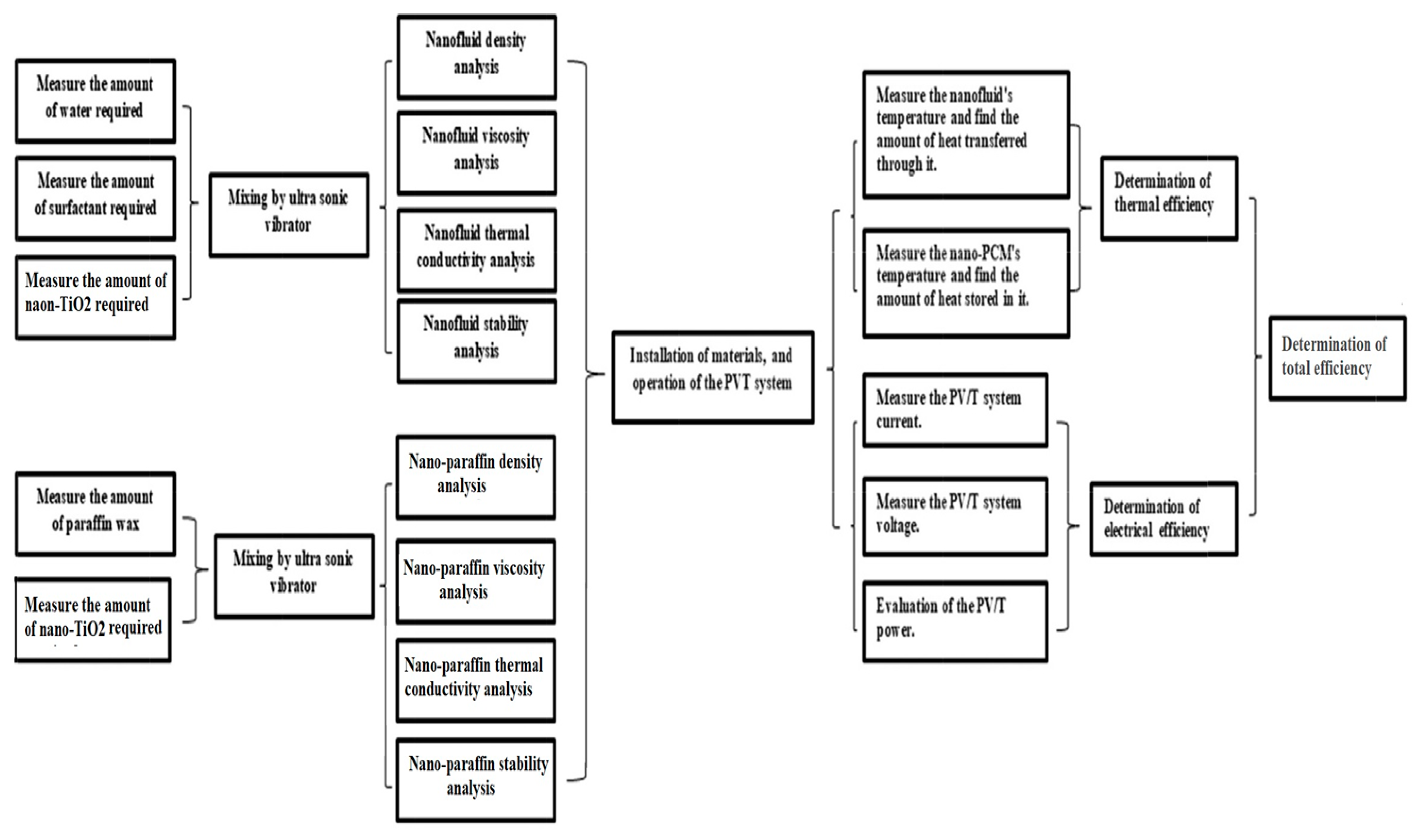
2. Materials and Methods
2.1. PV/T System
- Collected thermal energy:where Qu is the useful thermal energy gained from the water or nanofluid, Cp is the water or nanofluid specific heat (J/kg K), and To and Ti are the outlet and inlet temperatures, respectively.
- The PV/T system thermal efficiency:where is the thermal efficiency, Is is the solar radiation intensity, and Aco is the collector surface area.
- Electrical power (W):where P is the electrical power, I is the electrical current, and V is the voltage.
- Electrical efficiency:where is the electrical efficiency, P is the power, Is is the solar radiation intensity, and Ap is the PV panel area.
- The total efficiency:where is the total efficiency.
2.2. Materials
2.3. Preparation of Nano-Paraffin
2.4. Preparation of Nanofluid
2.5. Uncertainty Analysis
3. Results and Discussion
3.1. Thermal Conductivity
3.2. Density
3.3. Viscosity
3.4. Nanofluid Stability
3.5. Nano-Paraffin Stability
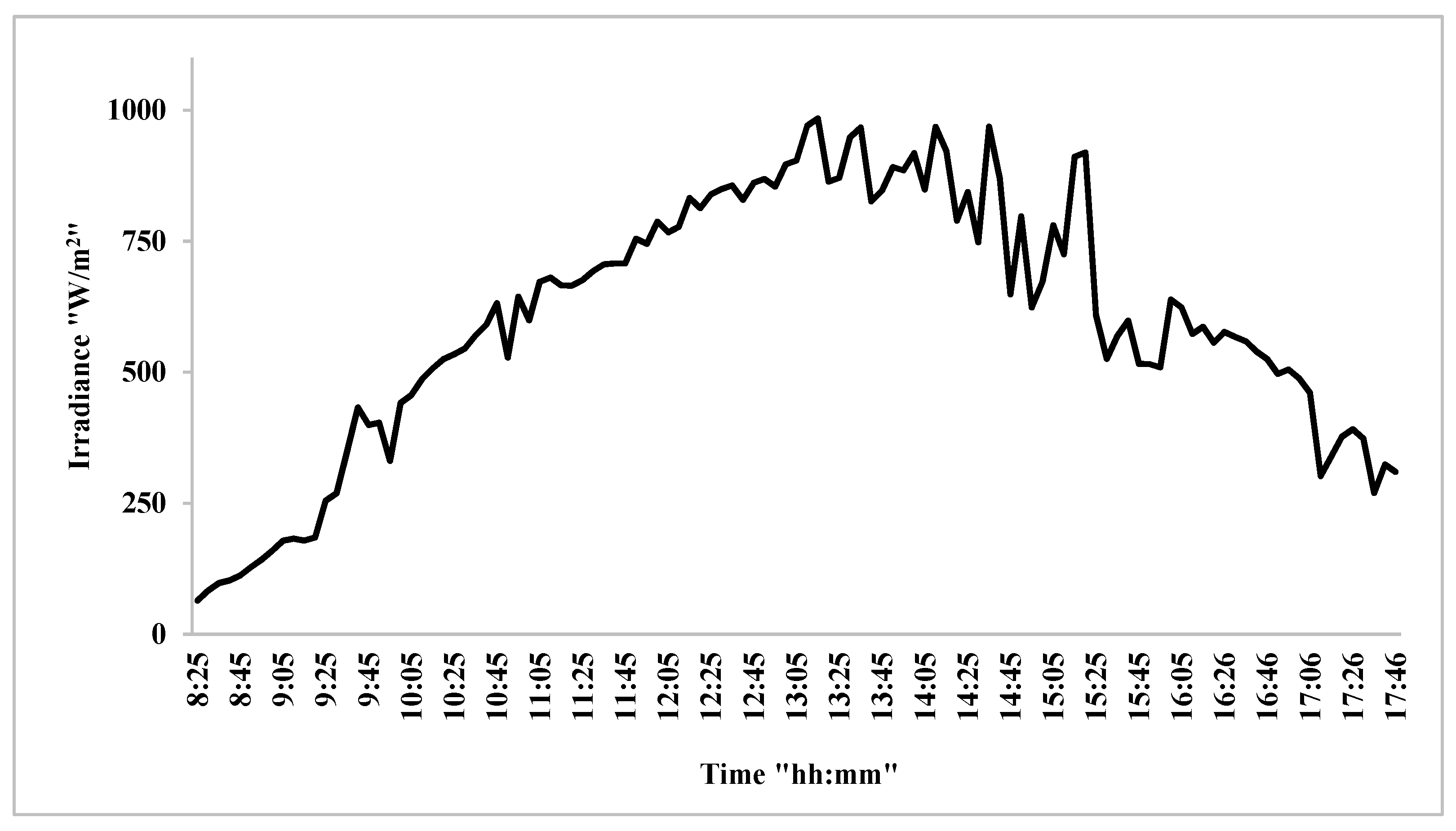
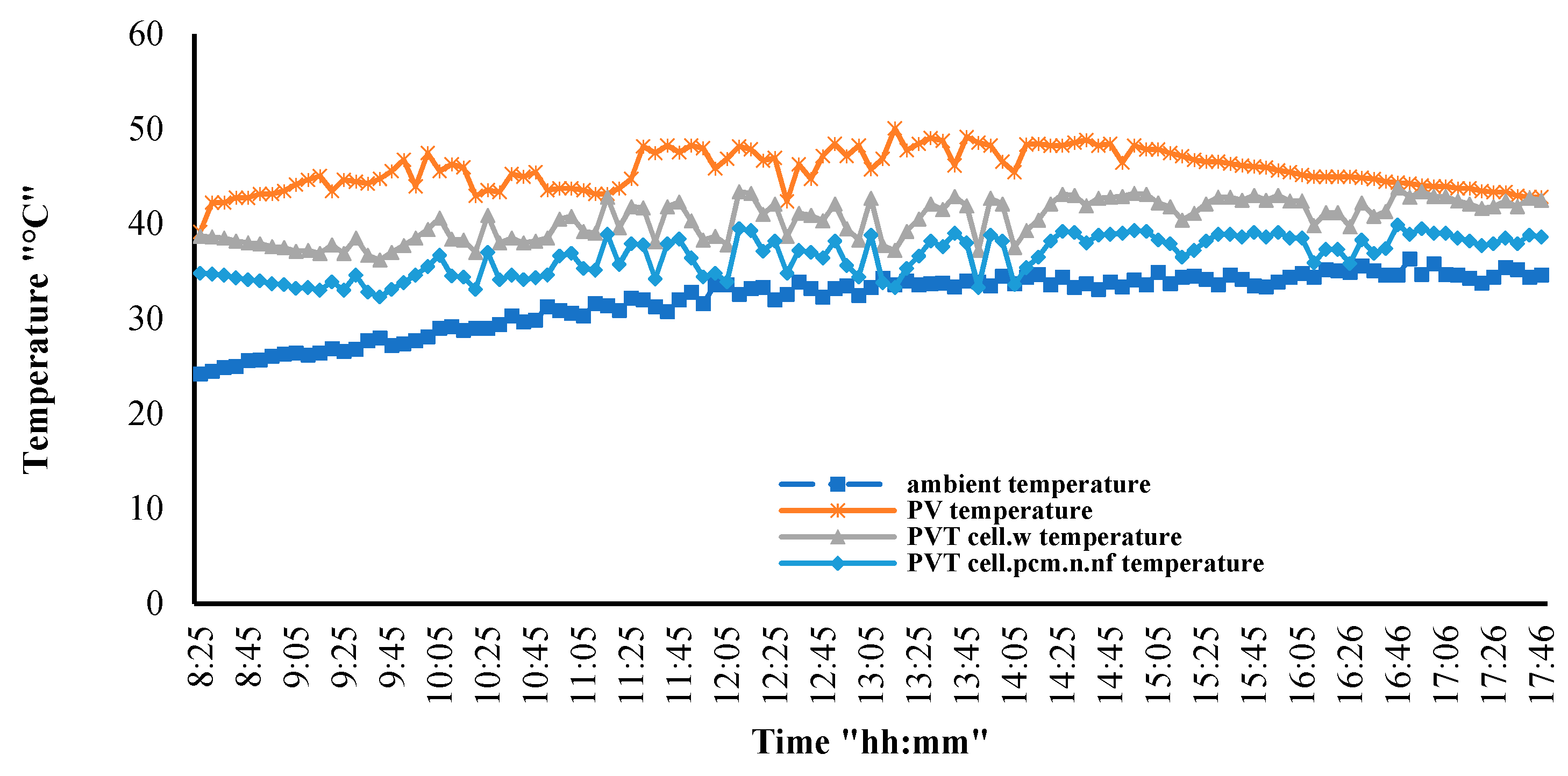
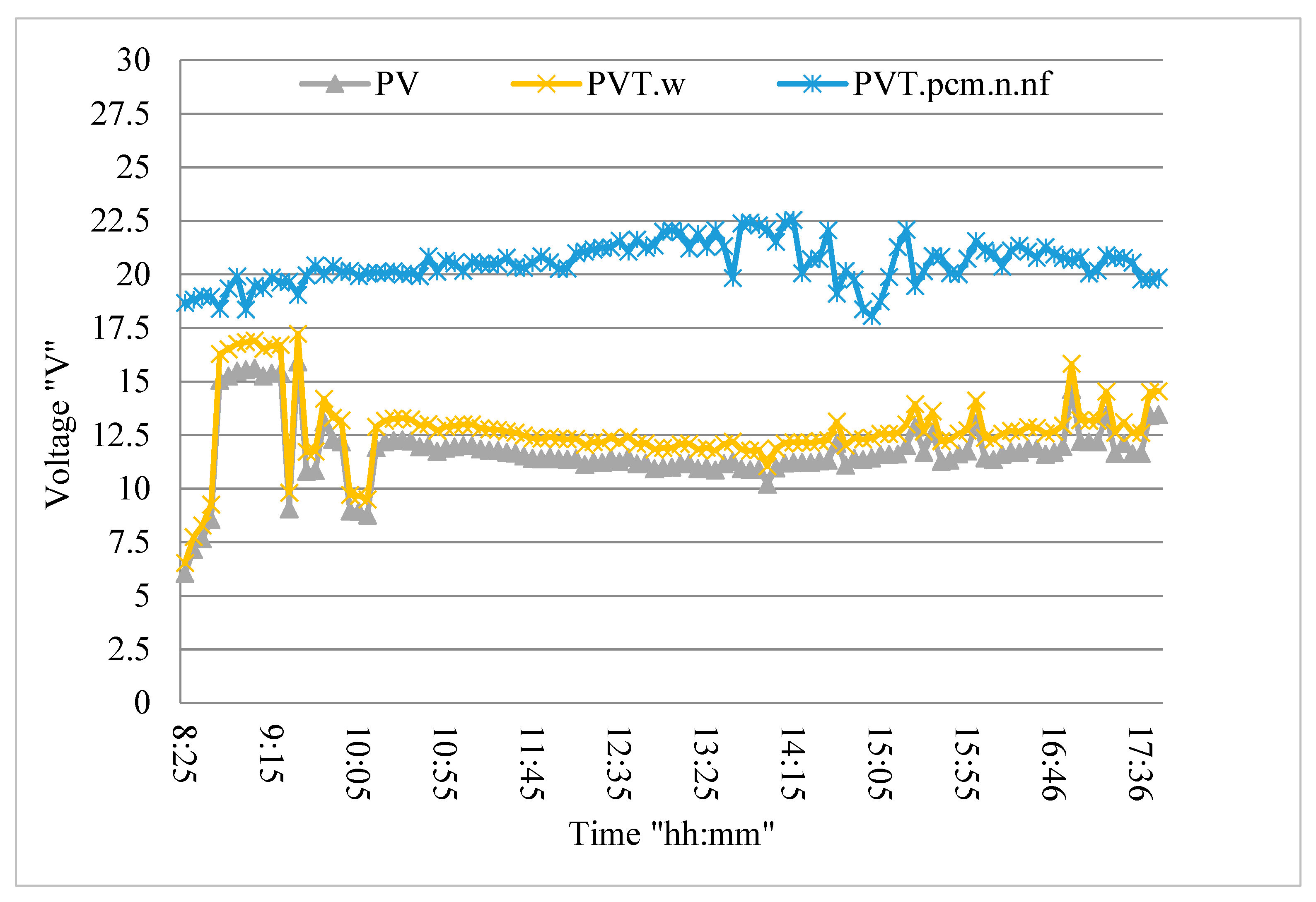
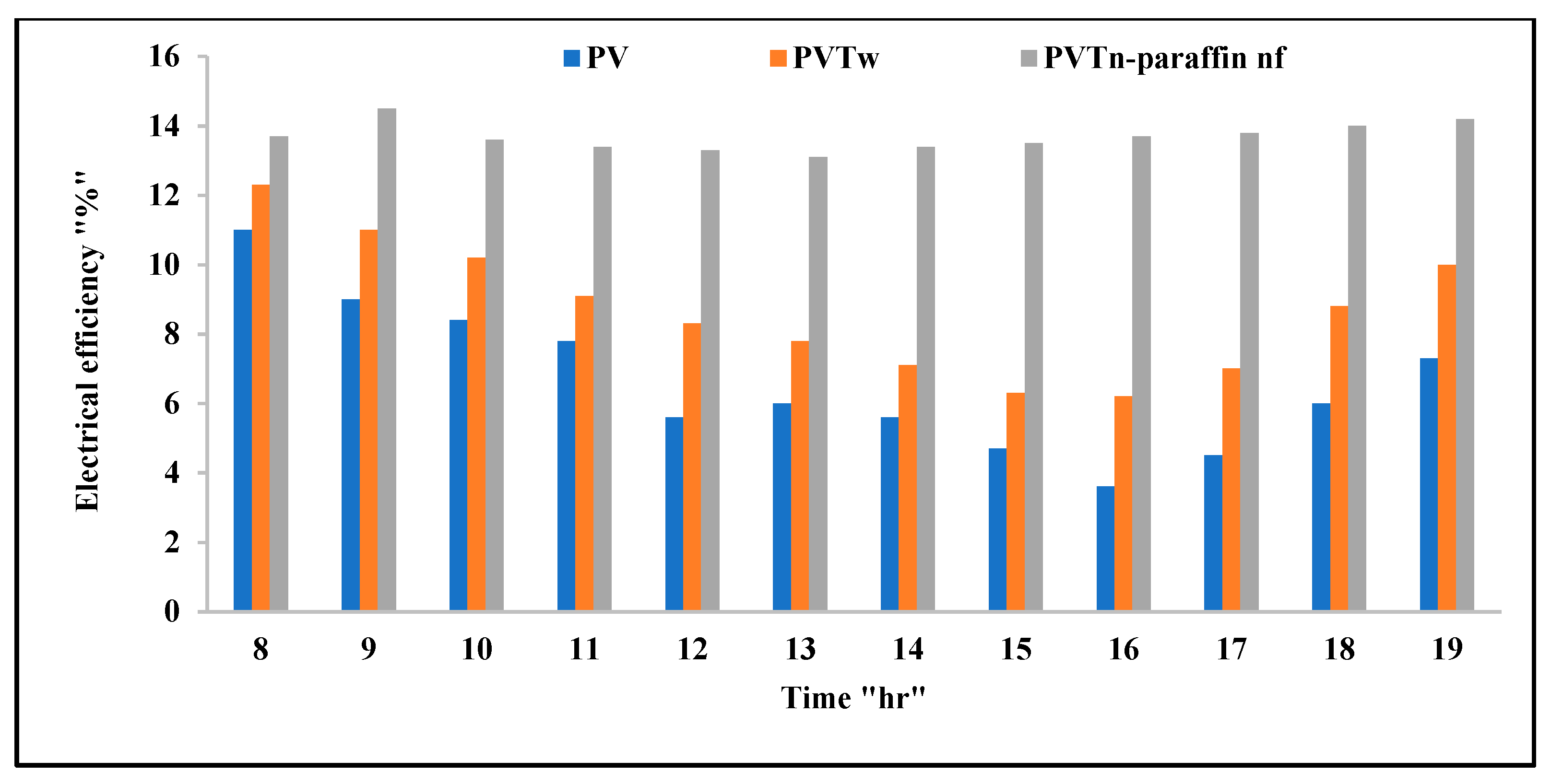
3.6. PV/T System Performance
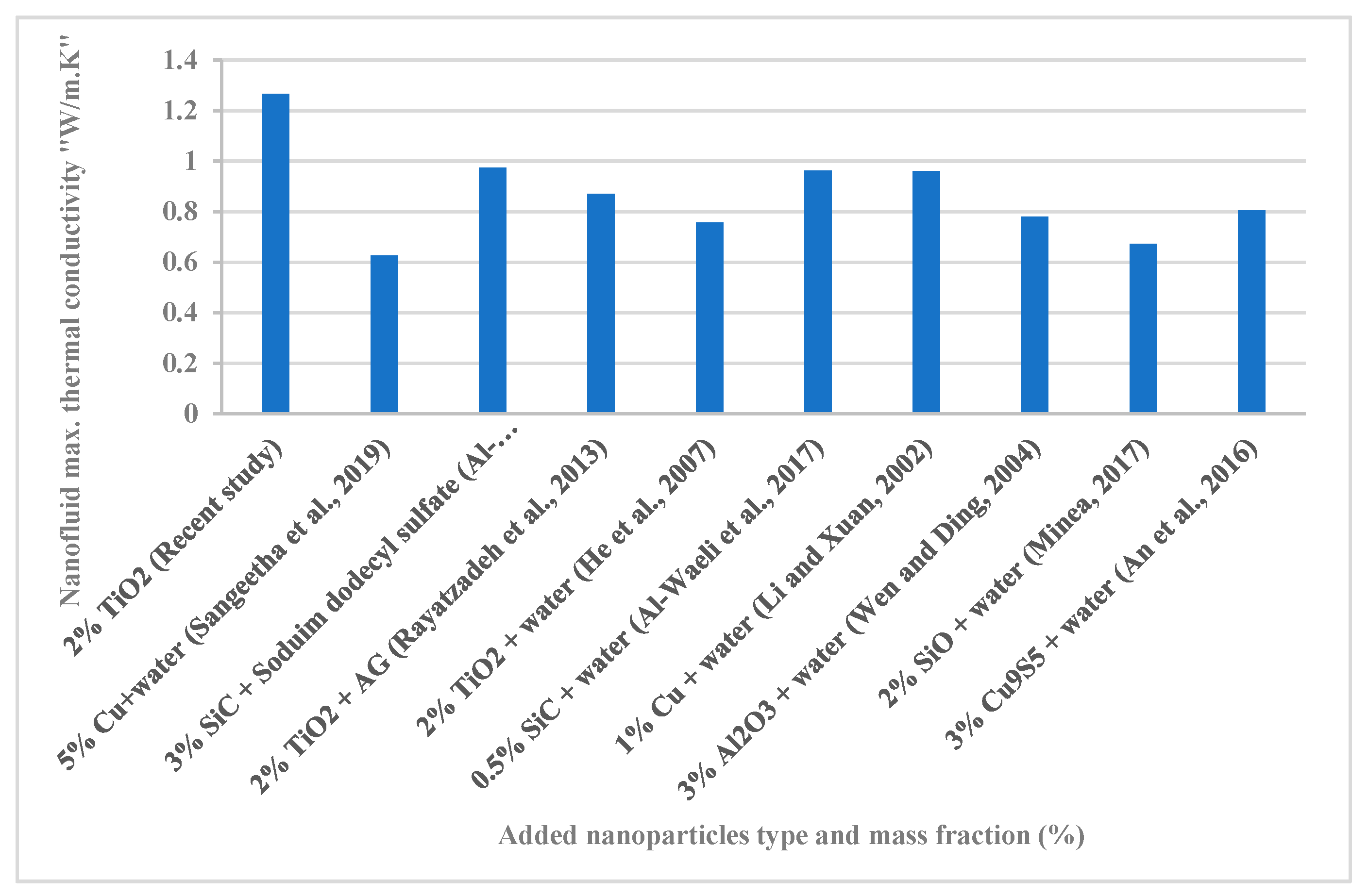
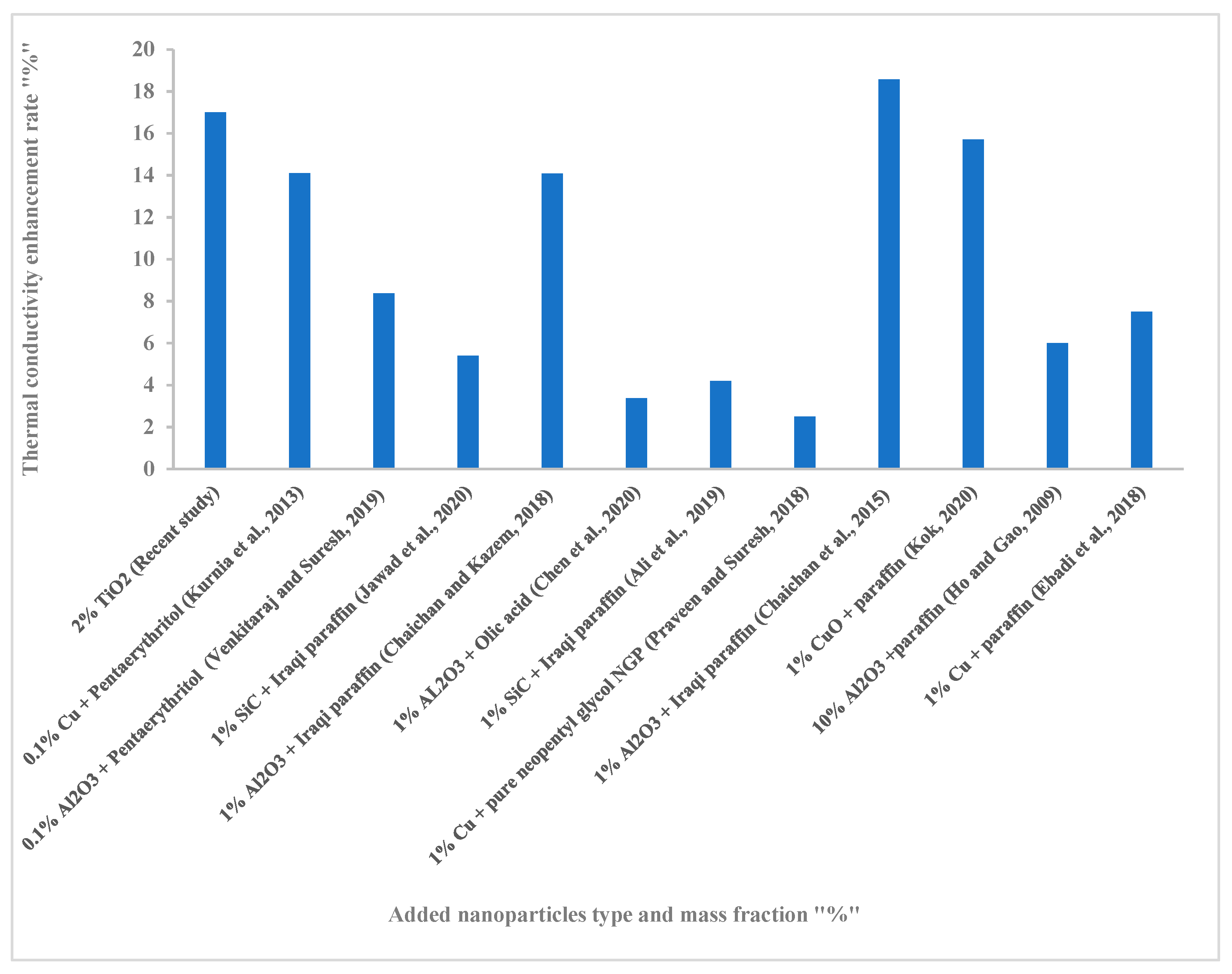
3.7. Comparison with Other Works from Literature
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, W.K.; Abed, T.A.; Salam, A.Q.; Reza, K.S.; Mahdiy, M.T.; Chaichan, M.T. Environmental Impact of Using Generators in the University of Technology in Baghdad, Iraq. J. Therm. Eng. 2020, 6, 272–281. [Google Scholar] [CrossRef]
- Chaichan, M.T.; Kazem, H.A.; Abed, T. Traffic and outdoor air pollution levels near highways in Baghdad, Iraq. Environ. Dev. Sustain. 2018, 20, 589–603. [Google Scholar] [CrossRef]
- Chaichan, M.T.; Kazem, H.A. Generating Electricity Using Photovoltaic Solar Plants in Iraq; Springer International Publishing: Cham, Switzerland, 2018; pp. 47–82. [Google Scholar]
- Kazem, H.A.; Chaichan, M.T.; Al-Waeli, A.H.; Sopian, K. A review of dust accumulation and cleaning methods for solar photovoltaic systems. J. Clean. Prod. 2020, 276, 123187. [Google Scholar] [CrossRef]
- Alnasser, T.M.; Mahdy, A.M.; Abass, K.I.; Chaichan, M.T.; Kazem, H.A. Impact of dust ingredient on photovoltaic performance: An experimental study. Sol. Energy 2020, 195, 651–659. [Google Scholar] [CrossRef]
- Chaichan, M.T.; Kazem, H.A. Experimental evaluation of dust composition impact on photovoltaic performance in Iraq. Energy Sources, Part A Recover. Util. Environ. Eff. 2020, 1–22. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.; Sopian, K.; Kazem, H.A.; Chaichan, M.T. Photovoltaic solar thermal (PV/T) collectors past, present and future: A review. Int. J. Appl. Eng. Res. 2016, 11, 10757–10765. [Google Scholar]
- Al-Damook, M.; Al Qubeissi, M.; Khatir, Z.; Dixon-Hardy, D.; Heggs, P.J. Thermal and Electrical Performance Evaluation and Design Optimization of Hybrid PV/T Systems. In Advances in Heat Transfer and Thermal Engineering; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2021; pp. 805–813. [Google Scholar]
- Gao, Z.; Yu, N.; Cao, X.; Sun, L.; Zhou, J.; Yuan, Y. A Comparison Study on Energy Performance between Different PV/T Systems and PV System in Highland Cold Winter. T Systems and PV System in Highland Cold Winter; SSRN: Rochester, NY, USA, 2021. [Google Scholar]
- Sangeetha, M.; Manigandan, S.; Chaichan, M.T.; Kumar, V. Progress of MWCNT, Al2O3, and CuO with water in enhancing the photovoltaic thermal system. Int. J. Energy Res. 2019, 44, 821–832. [Google Scholar] [CrossRef]
- Xu, H.; Wang, N.; Zhang, C.; Qu, Z.; Karimi, F. Energy conversion performance of a PV/T-PCM system under different thermal regulation strategies. Energy Convers. Manag. 2021, 229, 113660. [Google Scholar] [CrossRef]
- Chaichan, M.T.; Abaas, K.I.; Kazem, H.A. Design and assessment of solar concentrator distillating system using phase change materials (PCM) suitable for desertic weathers. Desalination Water Treat. 2015, 57, 1–11. [Google Scholar] [CrossRef]
- Charalambous, P.G.; Maidment, G.G.; Kalogirou, S.A.; Yiakoumetti, K. Photovoltaic thermal (PV/T) collectors: A review. Appl. Therm. Eng. 2007, 27, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Kiran, S.; Devadiga, U. Performance Analysis of Hybrid PV/Thermal Systems. Int. J. Emerg. Technol. Adv. Eng. 2014, 4, 80–86. [Google Scholar]
- Khanjari, Y.; Pourfayaz, F.; Kasaeian, A. Numerical investigation on using of nanofluid in a water-cooled photovoltaic thermal system. Energy Convers. Manag. 2016, 122, 263–278. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, C.; Wei, S.; Zhang, Y.; Sun, C. Flexible management of heat/electricity of novel PV/T systems with spectrum regulation by Ag nanofluids. Energy 2021, 221, 119903. [Google Scholar] [CrossRef]
- Nkurikiyimfura, I.; Wang, Y.; Safari, B.; Nshingabigwi, E. Electrical and thermal performances of photovoltaic/thermal systems with magnetic nanofluids: A review. Particuology 2021, 54, 181–200. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.A.; Kazem, H.A.; Sopian, K.; Chaichan, M.T. Techno-economical assessment of grid connected PV/T using nanoparticles and water as base-fluid systems in Malaysia. Int. J. Sustain. Energy 2018, 37, 558–575. [Google Scholar]
- Gupta, S.K.; Pradhan, S. A review of recent advances and the role of nanofluid in solar photovoltaic thermal (PV/T) system. Mater. Today Proc. 2021, 44, 782–791. [Google Scholar]
- Alwaeli, A.H.A.; Chaichan, M.T.; Sopian, K.; Kazem, H.A. Influence of the base fluid on the thermo-physical properties of PV/T nanofluids with surfactant. Case Stud. Therm. Eng. 2019, 13, 100340. [Google Scholar]
- Purohit, N.; Jakhar, S.; Gullo, P.; Dasgupta, M.S. Heat transfer and entropy generation analysis of alumina/water nanofluid in a flat plate PV/T collector under equal pumping power comparison criterion. Renew. Energy 2018, 120, 14–22. [Google Scholar] [CrossRef]
- Chaichan, M.T.; Kazem, H.A.; Al-Waeli, A.H.; Sopian, K. Controlling the melting and solidification points temperature of PCMs on the performance and economic return of the water-cooled photovoltaic thermal system. Sol. Energy 2021, 224, 1344–1357. [Google Scholar] [CrossRef]
- Vakhshouri, A.R. Paraffin as Phase Change Material; IntechOpen: London, UK, 2018; pp. 1–23. [Google Scholar]
- Chaichan, M.T.; Kazem, H.A. Using Aluminum Powder with PCM (paraffin wax) to Enhance Single Slope Solar Water Distillation Productivity in Baghdad-Iraq Winter Weathers. Int. J. Renew. Energy Res. 2015, 5, 251–257. [Google Scholar]
- Mahmoud, B.K.; Ibrahim, S.I.; Abass, K.I.; Ali, A.J.; Chaichan, M.T. Flat solar air heater collector with phase change materials for domestic purposes in Iraqi climate. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 022099. [Google Scholar] [CrossRef]
- Habib, N.A.; Ali, A.J.; Chaichan, M.T.; Kareem, M. Carbon nanotubes/paraffin wax nanocomposite for improving the performance of a solar air heating system. Therm. Sci. Eng. Prog. 2021, 23, 100877. [Google Scholar] [CrossRef]
- Shojaeefard, M.H.; Sakran, N.B.; Sharfabadi, M.M.; Davoudi, N. A Novel Review on Nano-fluid and Phase Change Material based Photovoltaic Thermal (PV/T) Systems. IOP Conf. Series: Mater. Sci. Eng. 2021, 1067, 012099. [Google Scholar] [CrossRef]
- Khodadadi, M.; Sheikholeslami, M. Numerical simulation on the efficiency of PVT system integrated with PCM under the influence of using fins. Sol. Energy Mater. Sol. Cells 2021, 233, 111402. [Google Scholar] [CrossRef]
- Rossi, R.M.; Bolli, W.P. Phase Change Materials for the Improvement of Heat Protection. Adv. Eng. Mater. 2005, 7, 368–373. [Google Scholar] [CrossRef]
- Fallahi, A.; Guldentops, G.; Tao, M.; Granados-Focil, S.; Van Dessel, S. Review on solid-solid phase change materials for thermal energy storage: Molecular structure and thermal properties. Appl. Therm. Eng. 2017, 127, 1427–1441. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Rayatzadeh, H.R.; Saffar-Avval, M.; Mansourkiaei, M.; Abbassi, A. Effects of continuous sonication on laminar convective heat transfer inside a tube using water–TiO2 nanofluid. Exp. Therm. Fluid Sci. 2013, 48, 8–14. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.; Kazem, H.A.; Chaichan, M.T.; Sopian, K. Photovoltaic/Thermal (PV/T) Systems: Principles, Design, and Applications; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Ho, C.J.; Gao, J. An experimental study on melting heat transfer of paraffin dispersed with Al2O3 nanoparticles in a vertical enclosure. Int. J. Heat Mass Transf. 2013, 62, 2–8. [Google Scholar] [CrossRef]
- Mishra, A.K.; Lahiri, B.; Philip, J. Thermal conductivity enhancement in organic phase change material (phenol-water system) upon addition of Al2O3, SiO2 and TiO2 nano-inclusions. J. Mol. Liq. 2018, 269, 47–63. [Google Scholar] [CrossRef]
- Colangelo, G.; Favale, E.; Miglietta, P.; Milanese, M.; de Risi, A. Thermal conductivity, viscosity and stability of Al2O3 -diathermic oil nanofluids for solar energy systems. Energy 2016, 95, 124–136. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.; Nabil, M.; Mamat, R. Experimental investigation of nanoparticle mixture ratios on TiO2–SiO2 nanofluids heat transfer performance under turbulent flow. Int. J. Heat Mass Transf. 2018, 118, 617–627. [Google Scholar] [CrossRef]
- Azmi, W.; Hamid, K.A.; Usri, N.; Mamat, R.; Sharma, K. Heat transfer augmentation of ethylene glycol: Water nanofluids and applications—A review. Int. Commun. Heat Mass Transf. 2016, 75, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Sharar, D.J.; Donovan, B.F.; Warzoha, R.J.; Wilson, A.A.; Leff, A.C.; Hanrahan, B.M. Solid-state thermal energy storage using reversible martensitic transformations. Appl. Phys. Lett. 2019, 114, 143902. [Google Scholar] [CrossRef]
- Iacobazzi, F.; Milanese, M.; Colangelo, G.; De Risi, A. A critical analysis of clustering phenomenon in Al2O3 nanofluids. J. Therm. Anal. 2019, 135, 371–377. [Google Scholar] [CrossRef]
- Zawawi, N.N.M.; Azmi, W.; Redhwan, A.; Sharif, M.; Sharma, K.V. Thermo-physical properties of Al2O3-SiO2/PAG composite nanolubricant for refrigeration system. Int. J. Refrig. 2017, 80, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ghadimi, A.; Saidur, R.; Metselaar, H.S.C. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 4051–4068. [Google Scholar] [CrossRef]
- Afzal, A.; Nawfal, I.; Mahbubul, I.M.; Kumbar, S.S. An overview on the effect of ultrasonication duration on different properties of nanofluids. J. Therm. Anal. 2018, 135, 393–418. [Google Scholar] [CrossRef]
- Chen, Z.; Shahsavar, A.; Al-Rashed, A.A.; Afrand, M. The impact of sonication and stirring durations on the thermal conductivity of alumina-liquid paraffin nanofluid: An experimental assessment. Powder Technol. 2020, 360, 1134–1142. [Google Scholar] [CrossRef]
- Alwaeli, A.H.A.; Chaichan, M.T.; Kazem, H.A.; Sopian, K.; Safaei, J. Numerical study on the effect of operating nanofluids of photovoltaic thermal system (PV/T) on the convective heat transfer. Case Stud. Therm. Eng. 2018, 12, 405–413. [Google Scholar] [CrossRef]
- Babu, J.R.; Kumar, K.K.; Rao, S.S. State-of-art review on hybrid nanofluids. Renew. Sustain. Energy Rev. 2017, 77, 551–565. [Google Scholar] [CrossRef]
- Li, D.; Fang, W.; Feng, Y.; Geng, Q.; Song, M. Stability properties of water-based gold and silver nanofluids stabilized by cationic gemini surfactants. J. Taiwan Inst. Chem. Eng. 2019, 97, 458–465. [Google Scholar] [CrossRef]
- Moldoveanu, G.M.; Huminic, G.; Minea, A.A.; Huminic, A. Experimental study on thermal conductivity of stabilized Al2O3 and SiO2 nanofluids and their hybrid. Int. J. Heat Mass Transf. 2018, 127, 450–457. [Google Scholar] [CrossRef]
- He, Y.; Jin, Y.; Chen, H.; Ding, Y.; Cang, D.; Lu, H. Heat transfer and flow behaviour of aqueous suspensions of TiO2 nanoparticles (nanofluids) flowing upward through a vertical pipe. Int. J. Heat Mass Transf. 2007, 50, 2272–2281. [Google Scholar] [CrossRef]
- Khairul, M.; Shah, K.; Doroodchi, E.; Azizian, R.; Moghtaderi, B. Effects of surfactant on stability and thermo-physical properties of metal oxide nanofluids. Int. J. Heat Mass Transf. 2016, 98, 778–787. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.; Chaichan, M.T.; Kazem, H.A.; Sopian, K.; Ibrahim, A.; Mat, S.; Ruslan, M.H. Comparison study of indoor/outdoor experiments of a photovoltaic thermal PV/T system containing SiC nanofluid as a coolant. Energy 2018, 151, 33–44. [Google Scholar] [CrossRef]
- Lewis, R.J., Sr. Hazardous Chemical Desk Reference; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Lai, Y.-K.; Sun, L.; Chen, Y.; Zhuang, H.; Lin, C.; Chin, J.W. Effects of the Structure of TiO2 Nanotube Array on Ti Substrate on Its Photocatalytic Activity. J. Electrochem. Soc. 2006, 153, D123–D127. [Google Scholar] [CrossRef]
- Goia, F.; Zinzi, M.; Carnielo, E.; Serra, V. Characterization of the optical properties of a PCM glazing system. Energy Procedia 2012, 30, 428–437. [Google Scholar] [CrossRef]
- Atal, A.; Wang, Y.; Harsha, M.; Sengupta, S. Effect of porosity of conducting matrix on a phase change energy storage device. Int. J. Heat Mass Transf. 2016, 93, 9–16. [Google Scholar] [CrossRef]
- Alwaeli, A.H.A.; Chaichan, M.T.; Sopian, K.; Kazem, H.A.; Mahood, H.B.; Khadom, A.A. Modeling and experimental validation of a PVT system using nanofluid coolant and nano-PCM. Sol. Energy 2019, 177, 178–191. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.; Chaichan, M.T.; Kazem, H.A.; Sopian, K. Evaluation and analysis of nanofluid and surfactant impact on photovoltaic-thermal systems. Case Stud. Therm. Eng. 2019, 13, 100392. [Google Scholar] [CrossRef]
- Holman, J.P. Experimental Methods for Engineers, 8th ed.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Alwaeli, A.H.A.; Sopian, K.; Chaichan, M.T.; Kazem, H.A.; Ibrahim, A.; Mat, S.; Ruslan, M.H. Evaluation of the nanofluid and nano-PCM based photovoltaic thermal (PVT) system: An experimental study. Energy Convers. Manag. 2017, 151, 693–708. [Google Scholar] [CrossRef]
- Li, Q.; Xuan, Y. Convective heat transfer and flow characteristics of Cu–water nanofluid. Sci. China Ser. E Technol. Sci. 2002, 45, 408–416. [Google Scholar]
- Wen, D.; Ding, Y. Experimental investigation into convective heat transfer of nanofluids at the entrance region under laminar flow conditions. Int. J. Heat Mass Transf. 2004, 47, 5181–5188. [Google Scholar] [CrossRef]
- Minea, A.A. Hybrid nanofluids based on Al2O3, TiO2 and SiO2: Numerical evaluation of different approaches. Int. J. Heat Mass Transf. 2017, 104, 852–860. [Google Scholar] [CrossRef]
- An, W.; Wu, J.; Zhu, T.; Zhu, Q. Experimental investigation of a concentrating PV/T collector with Cu9S5 nanofluid spectral splitting filter. Appl. Energy 2016, 184, 197–206. [Google Scholar] [CrossRef]
- Chaichan, M.T.; Kamel, S.H.; Al-Ajeely, A.N.M. Thermal conductivity enhancement by using nano-material in phase change material for latent heat thermal energy storage Systems. Saussurea 2015, 5, 48–55. [Google Scholar]
- Kurnia, J.C.; Sasmito, A.P.; Jangam, S.V.; Mujumdar, A.S. Improved design for heat transfer performance of a novel phase change material (PCM) thermal energy storage (TES). Appl. Therm. Eng. 2013, 50, 896–907. [Google Scholar] [CrossRef]
- Venkitaraj, K.P.; Suresh, S. Effects of Al2O3, CuO and TiO2 nanoparticles son thermal, phase transition and crystallization properties of solid-solid phase change material. Mech. Mater. 2019, 128, 64–88. [Google Scholar] [CrossRef]
- Jawad, Q.A.; Mahdy, A.M.; Khuder, A.H.; Chaichan, M.T. Improve the performance of a solar air heater by adding aluminum chip, paraffin wax, and nano-SiC. Case Stud. Therm. Eng. 2020, 19, 100622. [Google Scholar] [CrossRef]
- Chaichan, M.T.; Kazem, H.A. Single slope solar distillator productivity improvement using phase change material and Al2O3 nanoparticle. Sol. Energy 2018, 164, 370–381. [Google Scholar] [CrossRef]
- Ali, A.H.; Ibrahim, S.I.; Jawad, Q.A.; Jawad, R.S.; Chaichan, M.T. Effect of nanomaterial addition on the thermophysical properties of Iraqi paraffin wax. Case Stud. Therm. Eng. 2019, 15, 100537. [Google Scholar] [CrossRef]
- Praveen, B.; Suresh, S. Experimental study on heat transfer performance of neopentyl glycol/CuO composite solid-solid PCM in TES based heat sink. Eng. Sci. Technol. Int. J. 2018, 21, 1086–1094. [Google Scholar] [CrossRef]
- Kok, B. Examining effects of special heat transfer fins designed for the melting process of PCM and Nano-PCM. Appl. Therm. Eng. 2020, 170, 114989. [Google Scholar] [CrossRef]
- Ho, C.J.; Gao, J. Preparation and thermophysical properties of nanoparticle-in-paraffin emulsion as phase change material. Int. Commun. Heat Mass Transf. 2009, 36, 467–470. [Google Scholar] [CrossRef]
- Ebadi, S.; Tasnim, S.H.; Aliabadi, A.A.; Mahmud, S. Geometry and nanoparticle loading effects on the bio-based nano-PCM filled cylindrical thermal energy storage system. Appl. Therm. Eng. 2018, 141, 724–740. [Google Scholar] [CrossRef]




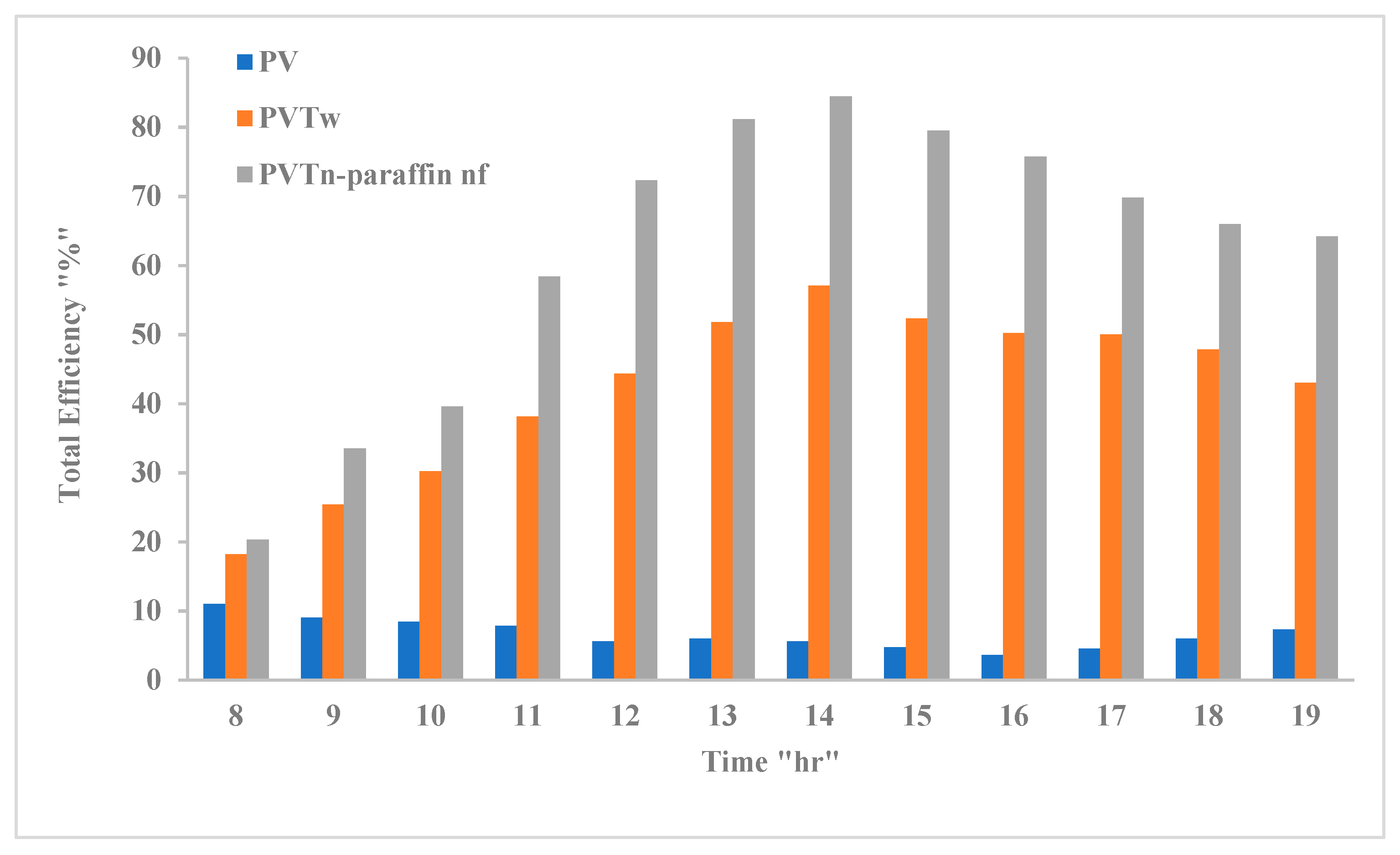
| Governorate | Station Location | Designed Power (MW) | Voltage Level (KV) |
|---|---|---|---|
| Al-Muthana | Sawa-1 | 30 | 33 |
| Al-Muthana | Sawa-2 | 50 | 132 |
| Al-Muthana | Khidhir | 50 | 132 |
| Karbala | Karbala | 300 | 132 |
| Babilon | Al-Iskandaria | 225 | 132 |
| Diwania | Ramla | 50 | 132 |
| Wassit | Jassan | 50 | 132 |
| Electrical Properties (Standard Test Conditions) | Symbol | Specification |
|---|---|---|
| Model | - | STF–120P6 |
| Max. power | Pmax | 120 ± 3% |
| Voltage (Open-circuit) | Voc | 22 V |
| Current (Short-circuit) | Isc | 7.63 A |
| Voltage at maximum power | VMP | 17.40 V |
| Current at maximum power | IMP | 6.89 A |
| Max. electrical efficiency | 14% |
| Item | Nano-TiO2 |
|---|---|
| Manufacturer | Hongwu Nanometer |
| Appearance | White colored powder |
| Purity | 99.7% |
| pH | 7.4 |
| Grain size (nm) | 20–50 nm |
| Density (g/cm3) | 3.92 |
| Loss when dried %≤ | 0.23 |
| Zeta potential (mV) | 38.5 |
| Molar mass (g/mole) | 79.87 |
| Melting point (°C) | 1843 |
| TC (W/m K) | 280–750 |
| Property | Range | Units |
|---|---|---|
| Chemical composition | C20H42-C27H56 | - |
| The temperature of melting point | 43.5 | °C |
| Liquid state density | 836 | kg/m3 |
| Solid state density | 929 | kg/m3 |
| Latent heat | 197 | kJ/kg |
| Solid state TC | 0.21 | W/m·K |
| Liquid state TC | 0.19 | W/m·K |
| Liquid state specific heat | 2.15 | kJ/kg K |
| Solid state specific heat | 2.23 | kJ/kg K |
| Measured Specification | Device | Uncertainty |
|---|---|---|
| Density | Density tester type (DII-300 L) | ±0.16 |
| Viscosity | Brookfield programmable viscometer (model: LVDV-III) | ±0.12 |
| TC | KD2 Pro-Analyzer | ±0.8 |
| Heat Capacity | KD2 Pro-Analyzer | ±0.94 |
| Temperatures | Thermocouples type K | ±0.93 |
| Weight | Delicate balance type (EJ6I0-E) | ±0.34 |
| Coolants flow rate | HC (US Hunter) | ±0.55 |
| Stability | Zeta-Sizer Nano Analyzer (ZSN) | ±0.88 |
| Nanofluid Mass Fraction (%) | TC (W/m K) | Density (g/cm3) | Viscosity (mPa·s) | Stability Zeta Potential (mV) |
|---|---|---|---|---|
| Water | 0.6 | 1.00 | 1.00 | - |
| Nano-TiO2 (%) 0.5 | 0.82 | 1.0125 | 1.013 | 68 |
| Nano-TiO2 (%) 1.0 | 1.13 | 1.019 | 1.023 | 64 |
| Nano-TiO2 (%) 1.5 | 1.25 | 1.022 | 1.028 | 59 |
| Nano-TiO2 (%) 2.0 | 1.36 | 1.025 | 1.033 | 48 |
| Nanofluid Type Mass Fraction (%) | TC (W/m K) | Density (g/cm3) | Viscosity (mPa·s) | Stability TC Degradation (days) |
|---|---|---|---|---|
| Paraffin | 0.2 | 950 | 0.088–0.077 | - |
| Nano-TiO2 (%) 0.5 | 0.31 | 961.4 | 0.09–0.082 | 98 |
| Nano-TiO2 (%) 1.0 | 0.48 | 967.86 | 0.92–0.085 | 94 |
| Nano-TiO2 (%) 1.5 | 0.54 | 970.14 | 0.094–0.088 | 89 |
| Nano-TiO2 (%) 2.0 | 0.66 | 972.61 | 0.0955–0.09 | 88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaichan, M.T.; Kazem, H.A.; Alamiery, A.A.; Isahak, W.N.R.W.; Kadhum, A.A.H.; Takriff, M.S. Adding Nano-TiO2 to Water and Paraffin to Enhance Total Efficiency of a Photovoltaic Thermal PV/T System Subjected to Harsh Weathers. Nanomaterials 2022, 12, 2266. https://doi.org/10.3390/nano12132266
Chaichan MT, Kazem HA, Alamiery AA, Isahak WNRW, Kadhum AAH, Takriff MS. Adding Nano-TiO2 to Water and Paraffin to Enhance Total Efficiency of a Photovoltaic Thermal PV/T System Subjected to Harsh Weathers. Nanomaterials. 2022; 12(13):2266. https://doi.org/10.3390/nano12132266
Chicago/Turabian StyleChaichan, Miqdam T., Hussein A. Kazem, Ahmed A. Alamiery, Wan Nor Roslam Wan Isahak, Abdul Amir H. Kadhum, and Mohd S. Takriff. 2022. "Adding Nano-TiO2 to Water and Paraffin to Enhance Total Efficiency of a Photovoltaic Thermal PV/T System Subjected to Harsh Weathers" Nanomaterials 12, no. 13: 2266. https://doi.org/10.3390/nano12132266







