Optical Dynamics of Copper-Doped Cadmium Sulfide (CdS) and Zinc Sulfide (ZnS) Quantum-Dots Core/Shell Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cadmium Sulfide (CdS) Core
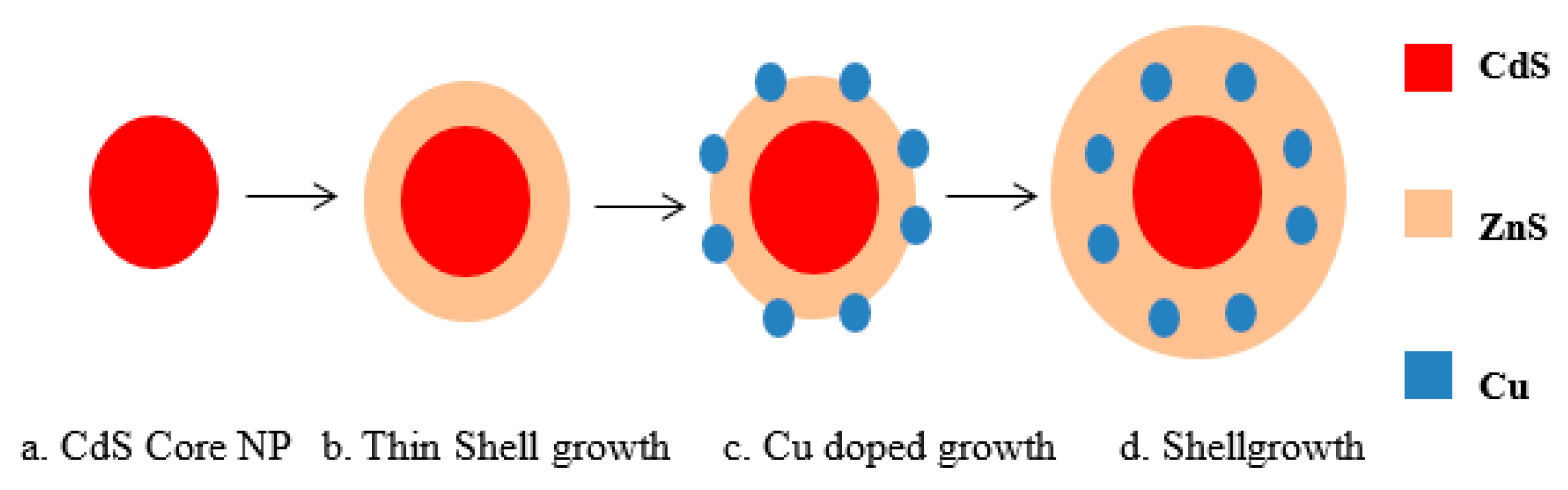
2.2. Copper-Doped Zinc Sulfide (ZnS) Shell
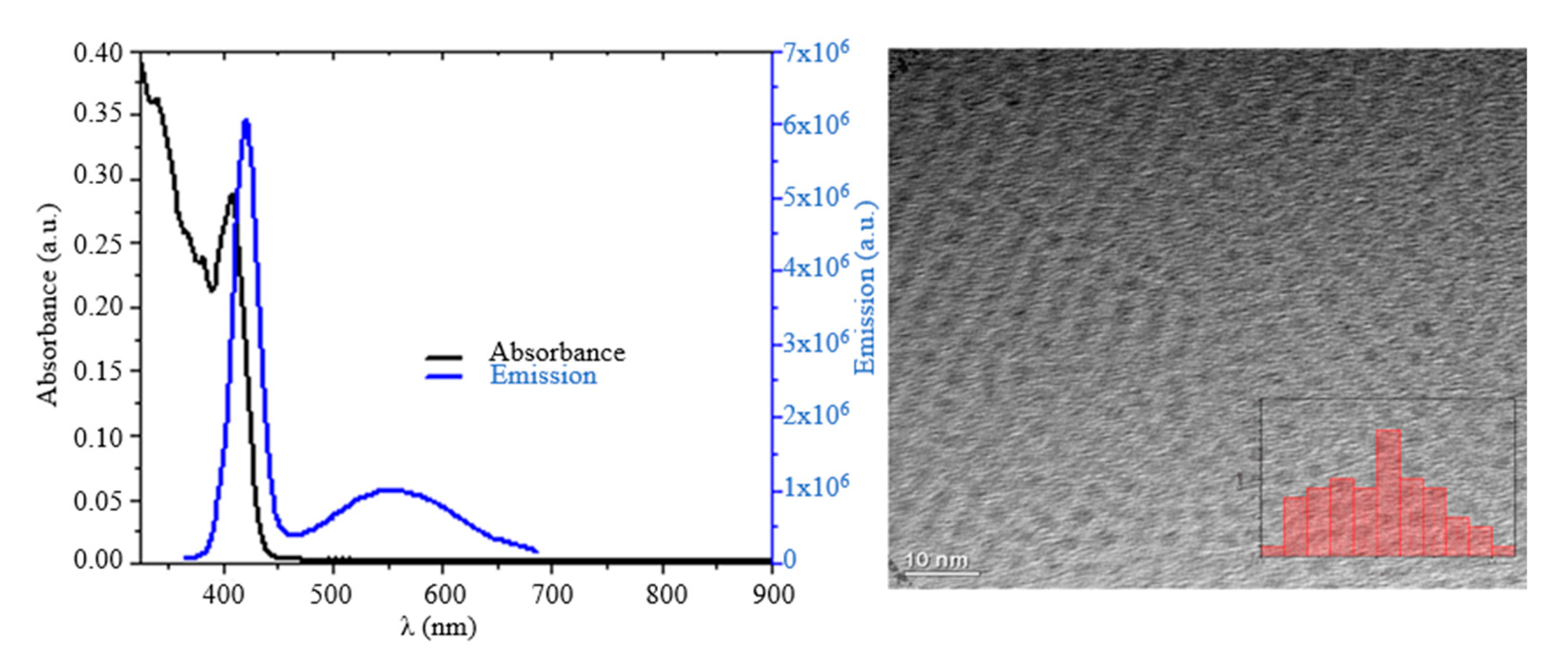
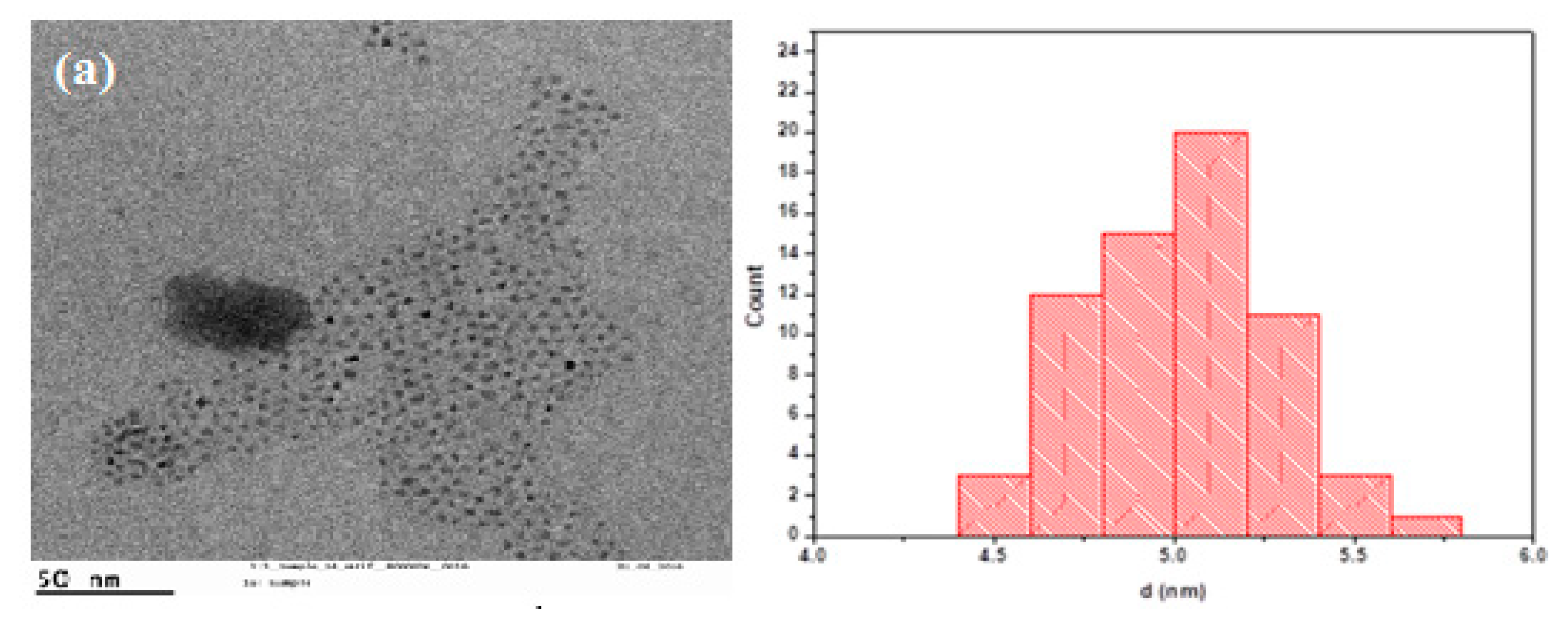
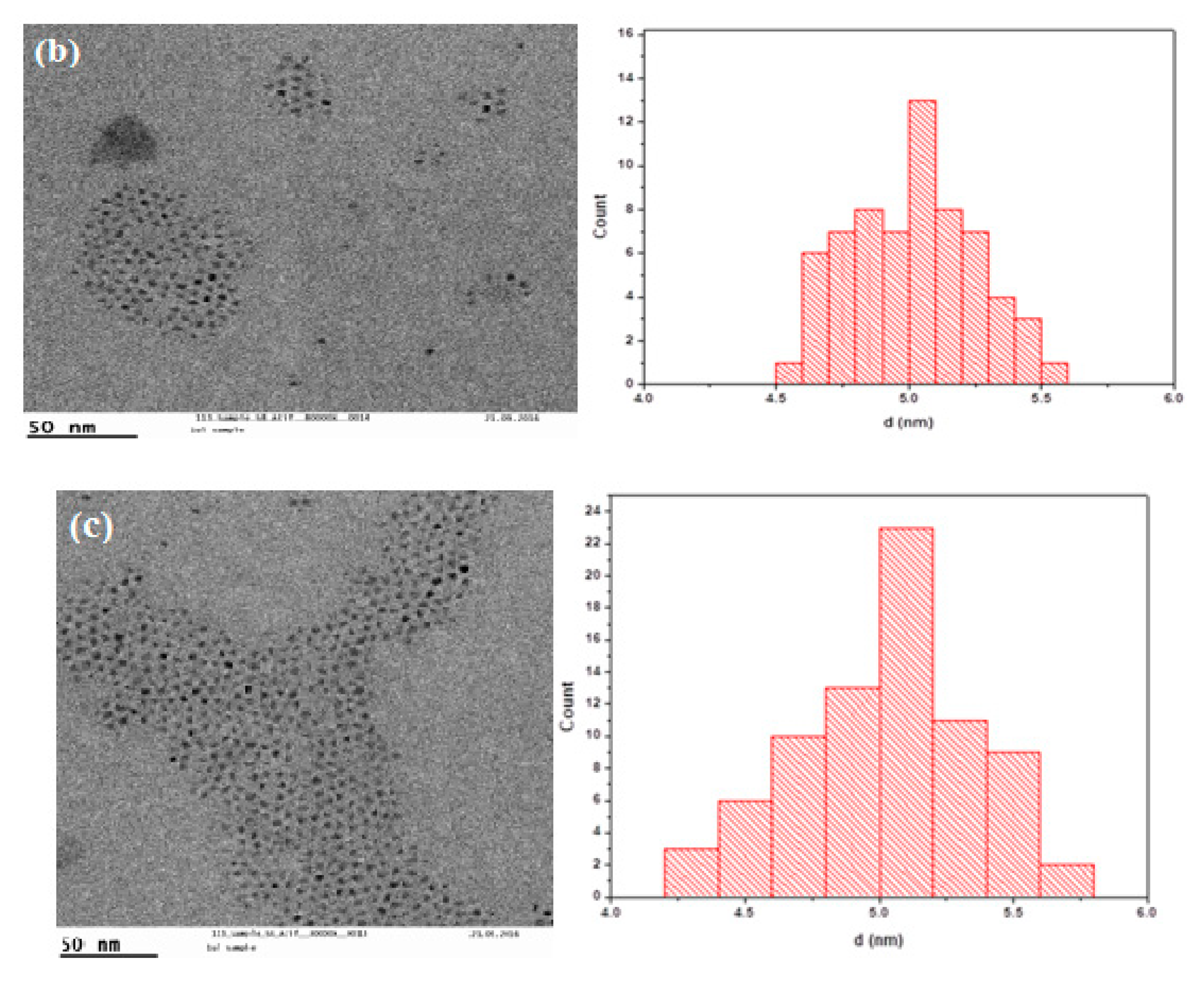
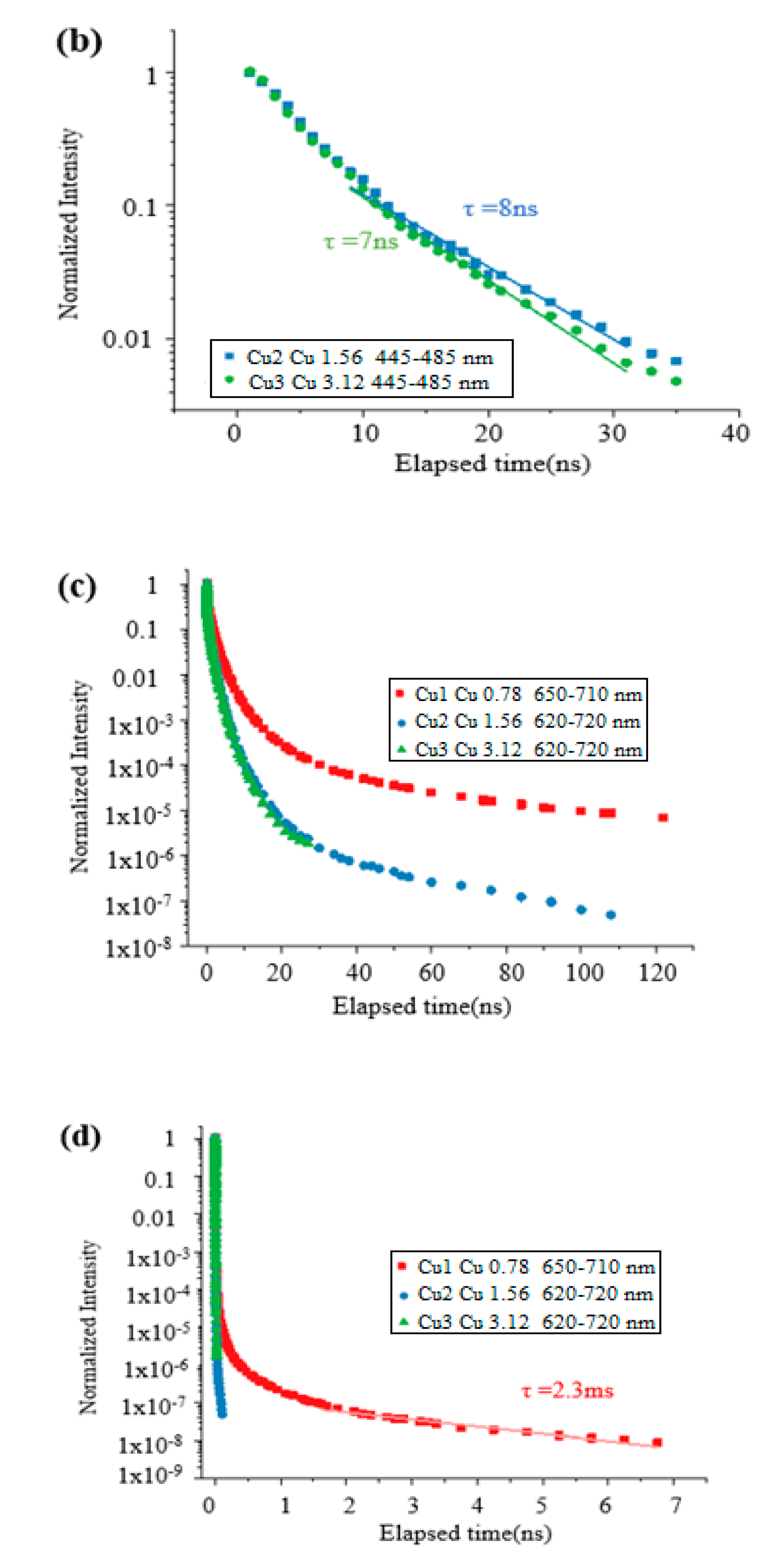
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasudevan, D.; Gaddam, R.R.; Trinchi, A.; Cole, I. Core—Shell quantum dots: Properties and applications. J. Alloys Compds. 2015, 636, 395–404. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X.; Li, W.; Sun, K. Doped quantum dots for white-light-emitting diodes without reabsorption of multiphase phosphors. Adv. Mater. 2012, 24, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Bera, D.; Qian, L.; Tseng, T.K.; Holloway, P.H. Quantum dots and their multimodal applications: A review. J. Mater. 2010, 3, 2260–2345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Yu, J.; Sun, C.; Xu, W.; Chen, J.; Sun, H.; Zong, C.; Liu, Z.; Tang, Y.; Zhao, D. An aqueous route synthesis of transition-metal-ions-doped quantum dots by bimetallic cluster building blocks. J. Am. Chem. Soc. 2020, 142, 16177–16181. [Google Scholar] [CrossRef] [PubMed]
- Aboulaich, A.; Billaud, D.; Abyan, M.; Balan, L.; Gaumet, J.-J.; Medjadhi, G.; Ghanbaja, J.; Schneider, R. One-Pot Noninjection Route to CdS Quantum Dots via Hydrothermal Synthesis. ACS Appl. Mater. Interfaces 2012, 4, 2561–2569. [Google Scholar] [CrossRef]
- Ping, Z.; Zhifeng, L.; Xinqiang, W.; Mu, Z.; Chenghua, H.; Zhou, Z.; Jinghe, W. First-principle study of phase stability, electronic structure and thermodynamic properties of cadmium sulfide under high pressure. J. Phys. Chem. Solids 2014, 75, 662–669. [Google Scholar] [CrossRef]
- Jamieson, T.; Bakhshi, R.; Petrova, D.; Pocock, R.; Imani, M.; Seifalian, A.M. Biological applications of quantum dots. Biomaterials 2007, 28, 4717–4732. [Google Scholar] [CrossRef]
- Medintz, I.L.; Mattoussi, H.; Clapp, A.R. Potential clinical applications of quantum dots. Int. J. Nanomed. 2008, 3, 151. [Google Scholar]
- Kuang, H.; Zhao, Y.; Ma, W.; Xu, L.; Wang, L.; Xu, C. Recent developments in analytical applications of quantum dots. TrAC Trends Anal. Chem. 2011, 30, 1620–1636. [Google Scholar] [CrossRef]
- Esteve-Turrillas, F.A.; Abad-Fuentes, A. Applications of quantum dots as probes in immunosensing of small-sized analytes. Biosens. Bioelectron. 2013, 15, 12–29. [Google Scholar] [CrossRef] [Green Version]
- Cheki, M.; Moslehi, M.; Assadi, M. Marvelous applications of quantum dots. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1141–1148. [Google Scholar] [PubMed]
- Huang, H.; Zhu, J.J. The electrochemical applications of quantum dots. Analyst 2013, 138, 5855–5865. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, N.; Mehra, N.K.; Jain, K.; Jain, N.K. Pharmaceutical and biomedical applications of quantum dots. Artif. Cells Nanomed. Biotechnol. 2016, 44, 758–768. [Google Scholar] [CrossRef]
- Cheng, Y.; Wan, H.; Liang, T.; Liu, C.; Wu, M.; Hong, H.; Liu, K.; Shen, H. Continuously graded quantum dots: Synthesis, applications in quantum dot light-emitting diodes, and perspectives. J. Phys. Chem. Lett. 2021, 12, 5967–5978. [Google Scholar] [CrossRef] [PubMed]
- Schimpf, C.; Reindl, M.; Basso Basset, F.; Jöns, K.D.; Trotta, R.; Rastelli, A. Quantum dots as potential sources of strongly entangled photons: Perspectives and challenges for applications in quantum networks. Appl. Phys. Lett. 2021, 118, 100502. [Google Scholar] [CrossRef]
- Janbandhu, S.Y.; Suhaila, C.T.; Munishwar, S.R.; Jayaramaiah, J.R.; Gedam, R.S. Borosilicate glasses containing CdS/ZnS QDs: A heterostructured composite with enhanced degradation of IC dye under visible-light. Chemosphere 2022, 286, 131672. [Google Scholar] [CrossRef]
- Cho, H.; Jung, S.; Kim, M.; Kwon, H.; Bang, J. Effects of Zn impurity on the photoluminescence properties of InP quantum dots. J. Lumin. 2022, 245, 118647. [Google Scholar] [CrossRef]
- Alipour, A.; Lakouraj, M.M.; Tashakkorian, H. Study of the effect of band gap and photoluminescence on biological properties of polyaniline/CdS QD nanocomposites based on natural polymer. Sci. Rep. 2021, 11, 1913. [Google Scholar] [CrossRef]
- Rismaningsih, N.; Yamauchi, H.; Kameyama, T.; Yamamoto, T.; Morita, S.; Yukawa, H.; Uematsu, T.; Baba, Y.; Kuwabata, S.; Torimoto, T. Photoluminescence properties of quinary Ag–(In, Ga)–(S, Se) quantum dots with a gradient alloy structure for in vivo bioimaging. J. Mater. Chem. C. 2021, 9, 12791–12801. [Google Scholar] [CrossRef]
- Yang, H.; Li, R.; Zhang, Y.; Yu, M.; Wang, Z.; Liu, X.; You, W.; Tu, D.; Sun, Z.; Zhang, R.; et al. Colloidal alloyed quantum dots with enhanced photoluminescence quantum yield in the NIR-II window. J. Am. Chem. Soc. 2021, 143, 2601–2607. [Google Scholar] [CrossRef]
- du Fossé, I.; Boehme, S.C.; Infante, I.; Houtepen, A.J. Dynamic Formation of Metal-Based Traps in Photoexcited Colloidal Quantum Dots and Their Relevance for Photoluminescence. Chem. Mater. 2021, 33, 3349–3358. [Google Scholar] [CrossRef] [PubMed]
- Yonemoto, D.T.; Papa, C.M.; Sheykhi, S.; Castellano, F.N. Controlling thermally activated delayed photoluminescence in CdSe quantum dots through triplet acceptor surface coverage. J. Phys.Chem. 2021, 12, 3718–3723. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xiao, R.; Zhou, S.; Yang, Y.; Zhang, G.; Li, B.; Guo, W.; Han, X.; Wang, D.; Bai, X.; et al. Efficient, Stable, and Photoluminescence Intermittency-Free CdSe-Based Quantum Dots in the Full-Color Range. ACS Photonics 2021, 8, 2538–2547. [Google Scholar] [CrossRef]
- Krishnamoorthy, A.; Sakthivel, P.; Devadoss, I.; Rajathi, V.M. Role of Bi3+ ions on structural, optical, photoluminescence and electrical performance of Cd0.9-xZn0.1BixS QDs. SN Appl. Sci. 2021, 3, 694. [Google Scholar] [CrossRef]
- Stroyuk, O.; Raievska, O.; Kupfer, C.; Solonenko, D.; Osvet, A.; Batentschuk, M.; Brabec, C.J.; Zahn, D.R. High-Throughput Time-Resolved Photoluminescence Study of Composition-and Size-Selected Aqueous Ag–In–S Quantum Dots. J. Phys. J. Phys. Chem. C 2021, 125, 12185–12197. [Google Scholar] [CrossRef]
- Chen, X.; Ren, P.; Li, M.; Lyu, Q.; Zhang, L.; Zhu, J. Dynamic regulation of photoluminescence based on mechanochromic photonic elastomers. Chem. Eng. J. 2021, 426, 131259. [Google Scholar] [CrossRef]
- Al Masud, A.; Arefin, S.M.N.; Fairooz, F.; Fu, X.; Moonschi, F.; Srijanto, B.R.; Neupane, K.R.; Aryal, S.; Calabro, R.; Kim, D.-Y.; et al. Photoluminescence Enhancement, Blinking Suppression, and Improved Biexciton Quantum Yield of Single Quantum Dots in Zero Mode Waveguides. J. Phys. Chem. 2021, 12, 3303–3311. [Google Scholar] [CrossRef]
- Takekuma, H.; Leng, J.; Tateishi, K.; Xu, Y.; Chan, Y.; Ryuzaki, S.; Wang, P.; Okamoto, K.; Tamada, K. Layer Number-Dependent Enhanced Photoluminescence from a Quantum Dot Metamaterial Optical Resonator. ACS Appl. Electron. Mater. 2021, 3, 468–475. [Google Scholar] [CrossRef]
- Padgaonkar, S.; Eckdahl, C.T.; Sowa, J.K.; López-Arteaga, R.; Westmoreland, D.E.; Woods, E.F.; Irgen-Gioro, S.; Nagasing, B.; Seideman, T.; Hersam, M.C.; et al. Light-Triggered Switching of Quantum Dot Photoluminescence through Excited-State Electron Transfer to Surface-Bound Photochromic Molecules. Nano Lett. 2021, 21, 854–860. [Google Scholar] [CrossRef]
- May, B.M.; Fakayode, O.J.; Bambo, M.F.; Sidwaba, U.; Nxumalo, E.N.; Mishra, A.K. Stable magneto-fluorescent gadolinium-doped AgInS2 core quantum dots (QDs) with enhanced photoluminescence properties. Mater. Lett. 2021, 305, 130776. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, Y.; Liu, F. Preparation and characterization of CdS/ZnS core-shell nanoparticles. J. Dispers Sci. Technol. 2020, 41, 725–732. [Google Scholar] [CrossRef]
- Bukowski, T.J.; Simmons, J.H. Quantum dot research current state and future prospects. Crit. Rev. Solid State Mater. Sci. 2002, 27, 119–142. [Google Scholar] [CrossRef]
- Farkhani, S.M.; Valizadeh, A. Three synthesis methods of CdX (X= Se, S or Te) quantum dots. IET Nanobiotechnol. 2014, 8, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Rodriguez, G.; Pal, U.; Sánchez-Zeferino, R.; Álvarez-Ramos, M.E. Tunable white-light emission of Co2+ and Mn2+ co-doped ZnS nanoparticles by energy transfer between dopant ions. J. Phys. Chem. C 2020, 124, 3857–3866. [Google Scholar] [CrossRef]
- Zhang, K.; Ling, J.; Yuncan, Y.; Kechun, G.; Fang, H. Novel method of constructing CdS/ZnS heterojunction for high performance and stable photocatalytic activity. J. Photochem. Photobiol A Chem. 2019, 380, 111859. [Google Scholar] [CrossRef]
- Reddy, N.L.; Vempuluru, N.R.; Murikinati, N.K.; Marappan, S.; Shankar, M.V. Development of high quantum efficiency CdS/ZnS core/shell structured photocatalyst for the enhanced solar hydrogen evolution. Int. J. Hydrogy Energy 2018, 43, 22315–22328. [Google Scholar] [CrossRef]
- Reddy, C.; Shim, J.; Cho, M. Synthesis, structural, optical and photocatalytic properties of CdS/ZnS core/shell nanoparticles. J. Phys. Chem. Solids 2017, 103, 209–217. [Google Scholar] [CrossRef]
- Murugadoss, G.; Kumar, M.R. Optical and structural characterization of CdS/ZnS and CdS: Cu2+/ZnS core–shell nanoparticles. Luminescence 2014, 29, 663–668. [Google Scholar] [CrossRef]
- Soltani, N.; Saion, E.; Yunus, W.M.M.; Erfani, M.; Navasery, M.; Bahmanrokh, G.; Rezaee, K. Enhancement of visible light photocatalytic activity of ZnS and CdS nanoparticles based on organic and inorganic coating. Appl. Surf. Sci. 2014, 290, 663–668. [Google Scholar] [CrossRef]
- Kanagasubbulakshmi, S.; Gowtham, I.; Kadirvelu, K.; Archana, K. Biocompatible methionine-capped CdS/ZnS quantum dots for live cell nucleus imaging. MRS Commun. 2019, 9, 344–351. [Google Scholar] [CrossRef]
- Hofman, E.; Robinson, R.J.; Li, Z.; Dzikovski, B.; Zheng, W. Controlled dopant migration in CdS/ZnS core/shell quantum dots. J. Am. Chem. Soc. 2017, 139, 8878–8885. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.L.; Lo, Y.S. Highly efficient quantum-dot-sensitized solar cell based on co-sensitization of CdS/CdSe. Adv. Func. Mater. 2009, 19, 604–609. [Google Scholar] [CrossRef]
- Tian, X.; Cao, L.; Liu, W.; Su, G.; Dong, B. Synthesis and characterisation of ZnS:Cu and ZnS:Cu/CdS core/shell nanocrystals via a water-soluble route. Micro Nano Lett. 2012, 7, 604–607. [Google Scholar] [CrossRef]
- Thambidurai, M.; Muthukumarasamy, N.; Velauthapillai, D.; Agilan, S.; Balasundaraprabhu, R. Structural, Optical, and Electrical Properties of Cobalt-Doped CdS Quantum Dots. J. Electron. Mater. 2012, 41, 665–672. [Google Scholar] [CrossRef]
- Saeed, S.E.; Abdel-Mottaleb, M.M.S.; Abdel-Mottaleb, M.S.A. One-Step thermolysis synthesis of divalent transition metal ions monodoped and tridopedCdS and ZnS luminescent nanomaterials. J. Nanomater. 2014, 2014, 873036. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Kumar, K.; Jeon, H.C.; Kang, T.W.; Lee, D.; Kumar, S. Effect of Cu-doping on the photoluminescence and photoconductivity of template synthesized CdS nanowires. J. Phys. Chem. Solids 2019, 124, 1–6. [Google Scholar] [CrossRef]
- Wojnicki, M.; Hessel, V. Quantum Materials Made in Microfluidics—Critical Review and Perspective. Chem. Eng. J. 2022, 438, 135616. [Google Scholar] [CrossRef]
- Yu, W.W.; Peng, X. Formation of High-Quality CdS and Other II-VI Semiconductor Nanocrystals in Noncoordinating SolventsTunable Reactivity of Monomers. Angew. Chem. Int. Ed. 2002, 41, 2368–2371. [Google Scholar]
- Mullamuri, B.; Mosali, V.S.S.; Maseed, H.; Majety, S.S.; Chandu, B. Photocatalytic Activity of Heavy Metal Doped CdS Nanoparticles Synthesized by Using Ocimum sanctum Leaf Extract. Biointerfaces Res. Appl. Chem. 2021, 11, 12547–12559. [Google Scholar]
- Chen, O.; Shelby, D.E.; Yang, Y.; Zhuang, J.; Wang, T.; Niu, C.; Omenetto, N.; Cao, Y.C. Excitation-intensity-dependent color-tunable dual emissions from manganese-doped CdS/ZnS core/shell nanocrystals. Angew. Chem. Int. Ed. 2010, 49, 10132–10135. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Lan, X.; Zhang, Y.; Li, S.; Li, J.; Han, T.; Wang, B.; Zhong, H. Highly luminescent blue emitting CdS/ZnS core/shell quantum dots via a single-molecular precursor for shell growth. Mater. Chem. Phys. 2011, 130, 909–914. [Google Scholar] [CrossRef]
- Dey, C.; Molla, A.R.; Goswami, M.; Kothiyal, G.P.; Karmakar, B. Synthesisandoptical properties of multifunctional CdS nanostructured dielectric nano-composites. JOSA B 2014, 31, 1761–1770. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Q.; Zhang, Z.; Wang, Z.; Gong, Z. Nitrogen and copper (II) co-doped carbon dots as multi-functional fluorescent probes for Fe3+ ions and tetracycline. Microchem. J. 2022, 181, 107628. [Google Scholar] [CrossRef]
- Kaiser, U.; Sabir, N.; Carrillo-Carrion, C.; Pino, P.; Bossi, M.; Heimbrodt, W.; Parak, W. Forster resonance energy transfer mediated enhancement of the flouresence lifetime of organic flourophores to the millisecond range by coupling to Mn-doped Cds/ZnS quantum dotd. Nanotechnology 2016, 5, 055101. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.H.; Koel, A.; Rang, T.; Nasir, N.; Sabir, N.; Ameen, F.; Rasheed, A. Optical Dynamics of Copper-Doped Cadmium Sulfide (CdS) and Zinc Sulfide (ZnS) Quantum-Dots Core/Shell Nanocrystals. Nanomaterials 2022, 12, 2277. https://doi.org/10.3390/nano12132277
Rashid MH, Koel A, Rang T, Nasir N, Sabir N, Ameen F, Rasheed A. Optical Dynamics of Copper-Doped Cadmium Sulfide (CdS) and Zinc Sulfide (ZnS) Quantum-Dots Core/Shell Nanocrystals. Nanomaterials. 2022; 12(13):2277. https://doi.org/10.3390/nano12132277
Chicago/Turabian StyleRashid, Muhammad Haroon, Ants Koel, Toomas Rang, Nadeem Nasir, Nadeem Sabir, Faheem Ameen, and Abher Rasheed. 2022. "Optical Dynamics of Copper-Doped Cadmium Sulfide (CdS) and Zinc Sulfide (ZnS) Quantum-Dots Core/Shell Nanocrystals" Nanomaterials 12, no. 13: 2277. https://doi.org/10.3390/nano12132277
APA StyleRashid, M. H., Koel, A., Rang, T., Nasir, N., Sabir, N., Ameen, F., & Rasheed, A. (2022). Optical Dynamics of Copper-Doped Cadmium Sulfide (CdS) and Zinc Sulfide (ZnS) Quantum-Dots Core/Shell Nanocrystals. Nanomaterials, 12(13), 2277. https://doi.org/10.3390/nano12132277





