Voltage-Dependent Emission Varying from Blue to Orange–Red from a Nondoped Organic Light-Emitting Diode with a Single Emitter
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Preparation of 2,5-Bis(2-fluorophenyl)pyrimidine
2.3. Preparation of 9,9′-(Pyrimidine-2,5-diylbis(2,1-phenylene))bis(3,6-di-tert-butyl-9H-carbazole) (PDPC)
2.4. Characterization
2.5. Device Fabrication and Characterization
2.6. Calculation
3. Results and Discussions
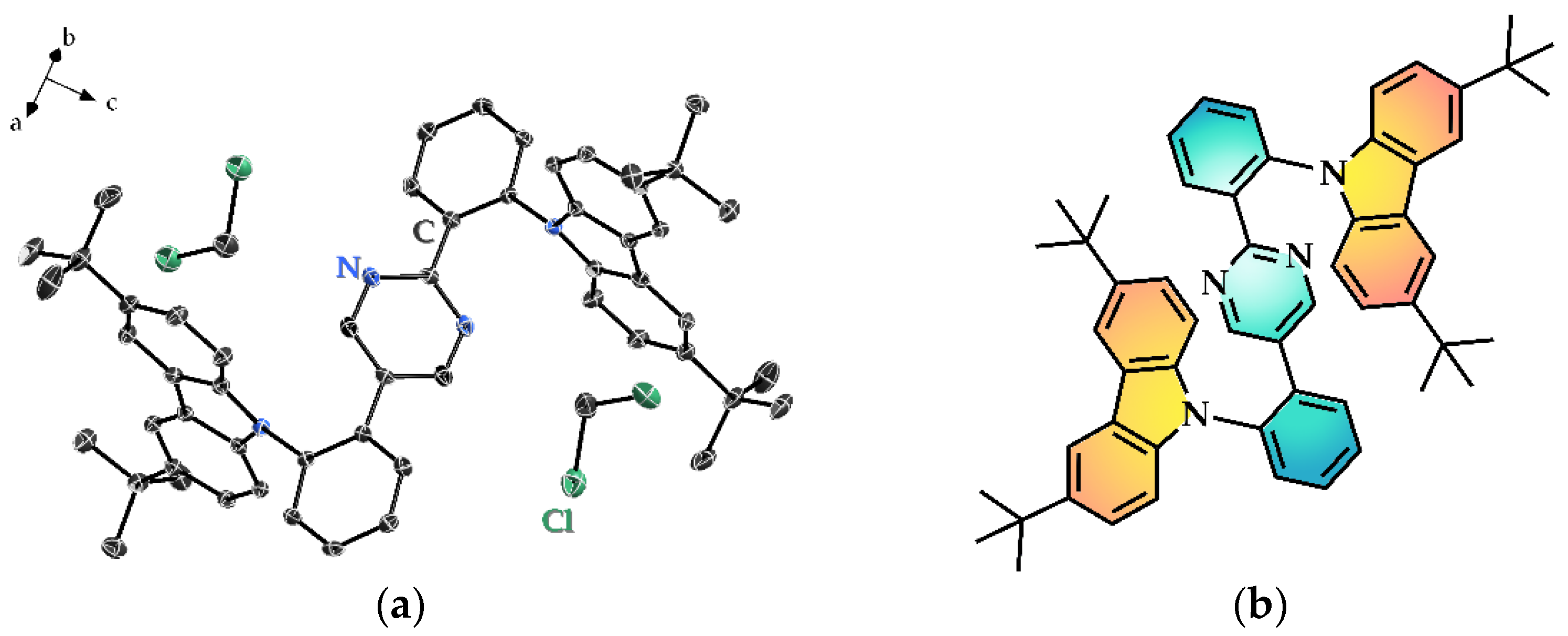
3.1. Single Crystal X-ray Diffraction (SC-XRD)
3.2. UV Diffuse Reflection Analysis
3.3. Thermogravimetric Analysis (TGA)
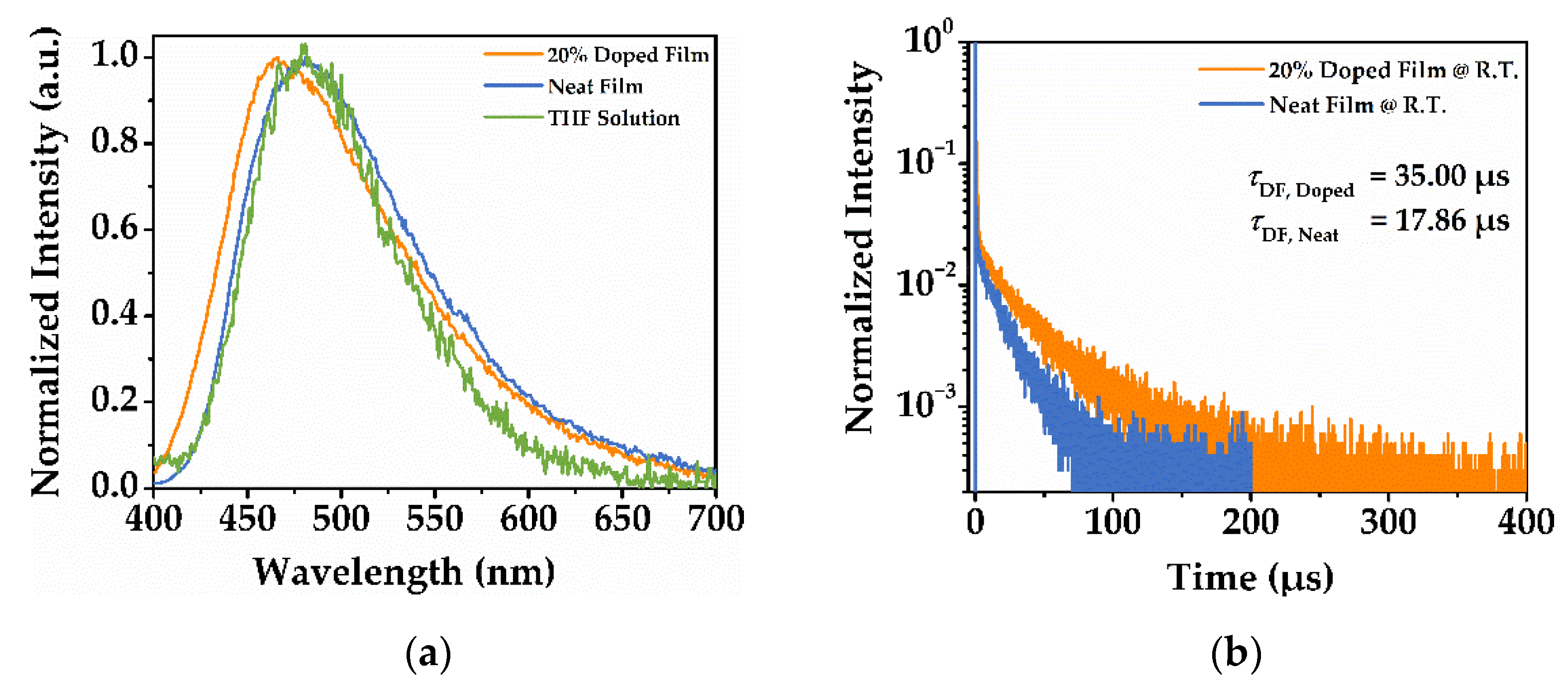
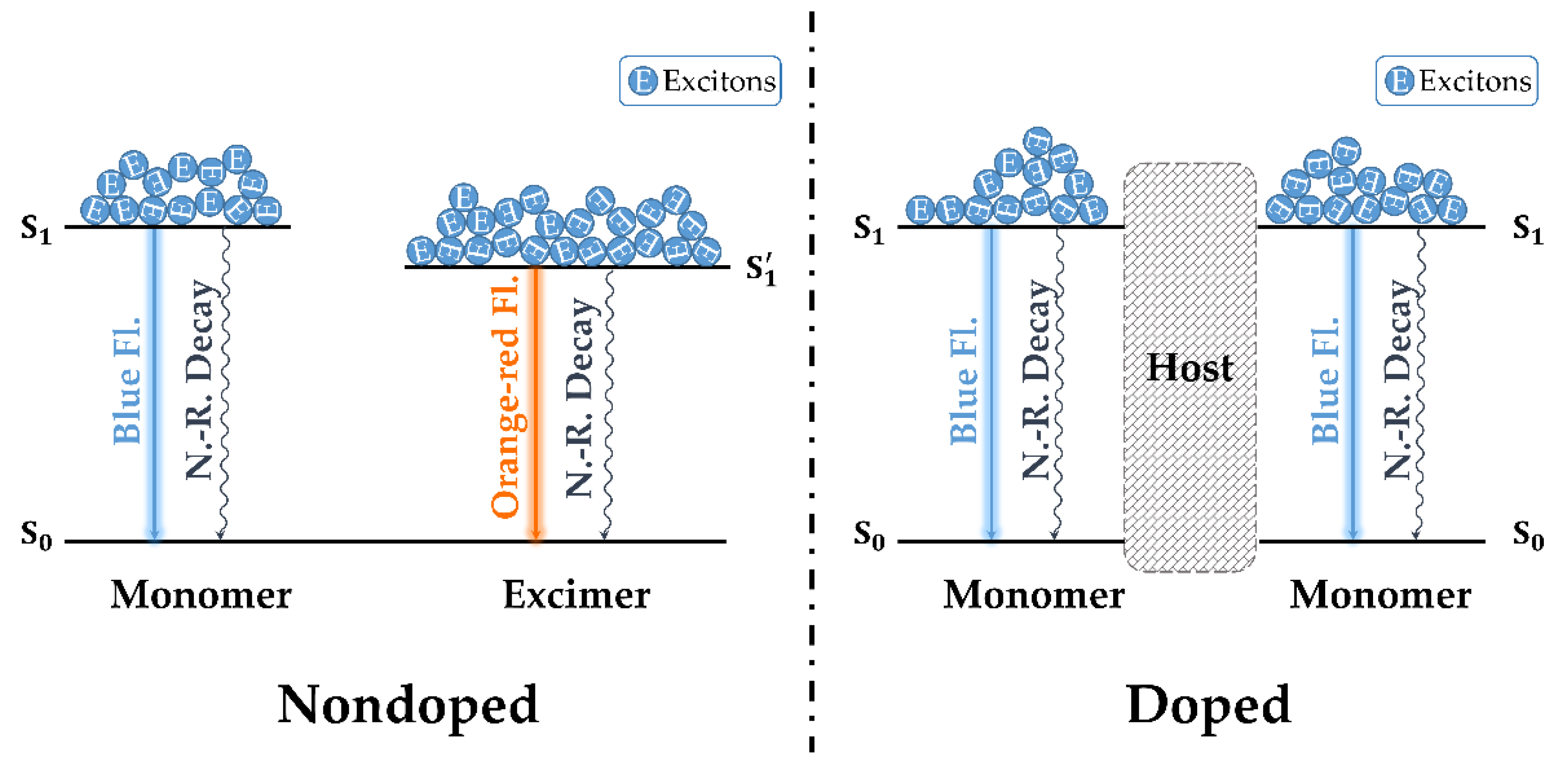
3.4. Photoluminescent Properties
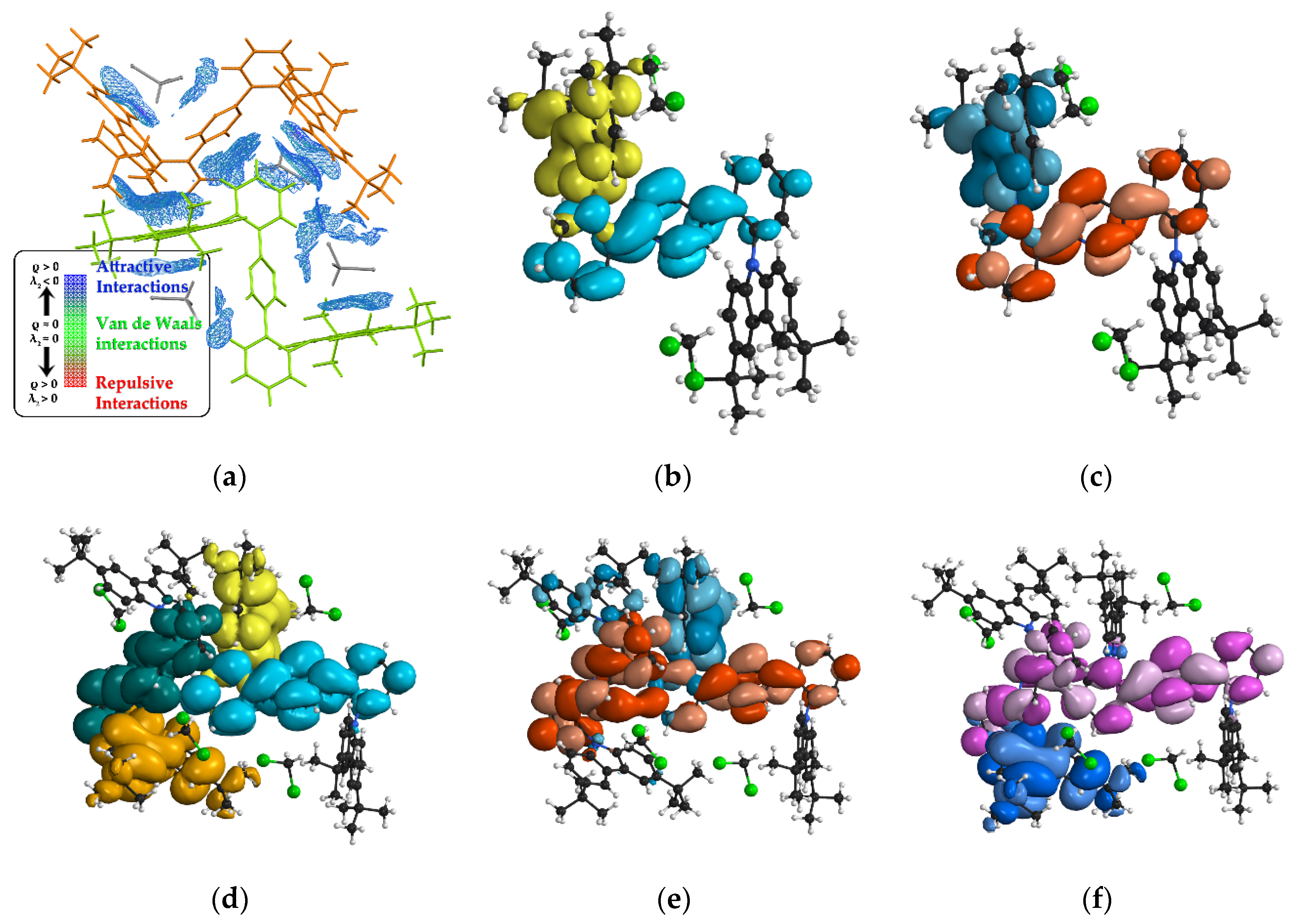
3.5. Theoretical Calculation
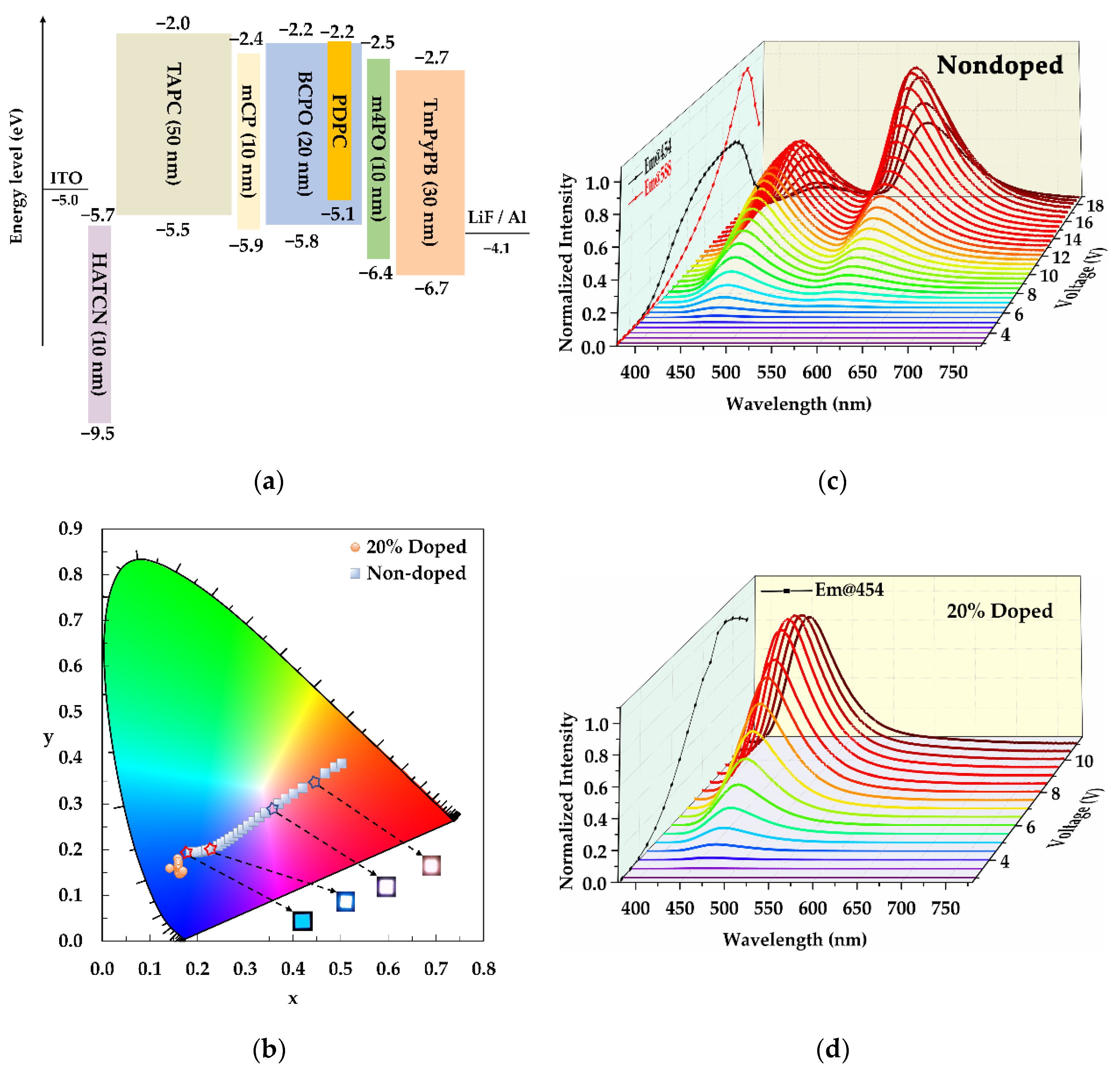
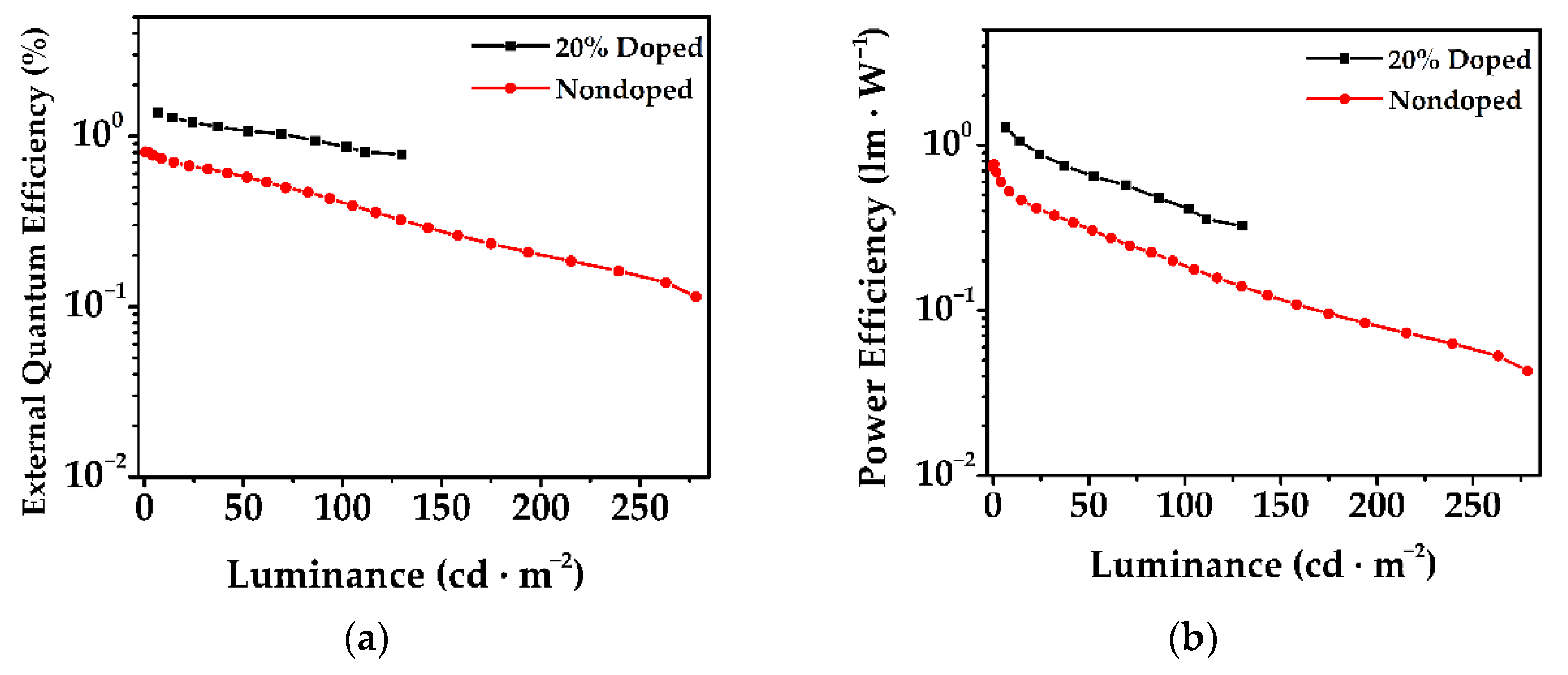
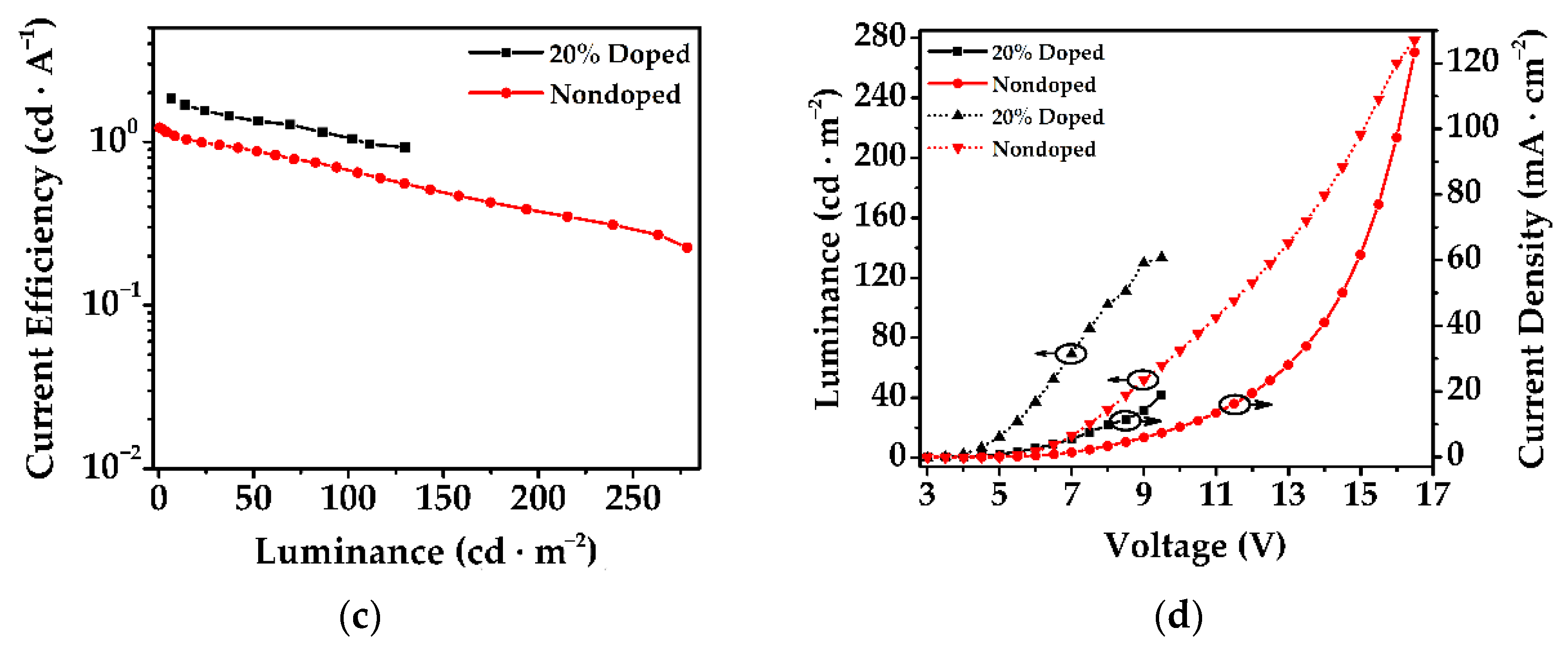
3.6. Electroluminescent Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coe, S.; Woo, W.K.; Bawendi, M.; Bulovic, V. Electroluminescence from Single Monolayers of Nanocrystals in Molecular Organic Devices. Nature 2002, 420, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Han, T.H.; Lee, Y.; Choi, M.R.; Woo, S.H.; Bae, S.H.; Hong, B.H.; Ahn, J.H.; Lee, T.W. Extremely Efficient Flexible Organic Light-Emitting Diodes with Modified Graphene Anode. Nat. Photonics 2012, 6, 105–110. [Google Scholar] [CrossRef]
- Koch, N. Organic Electronic Devices and Their Functional Interfaces. Chemphyschem 2007, 8, 1438–1455. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.L.; Burrows, P.E.; Bulovic, V.; Forrest, S.R.; Thompson, M.E. Three-Color, Tunable, Organic Light-Emitting Devices. Science 1997, 276, 2009–2011. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, D.D.; Bin, Z.Y.; Liu, Z.Y.; Zhang, Y.W.; Lee, H.; Kwon, J.H.; Duan, L. Color-Tunable All-Fluorescent White Organic Light-Emitting Diodes with a High External Quantum Efficiency over 30% and Extended Device Lifetime. Adv. Mater. 2022, 34, e2103102. [Google Scholar] [CrossRef]
- Li, M.C.; Zhang, X.J.; Zhang, H.R.; Chen, W.B.; Ma, L.; Wang, X.J.; Liu, Y.L.; Lei, B.F. Highly Efficient and Dual Broad Emitting Light Convertor: An Option for Next-Generation Plant Growth LEDs. J. Mater. Chem. C 2019, 7, 3617–3622. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Gong, Y.Y.; Chen, S.M.; Shen, X.Y.; Lam, J.W.Y.; Lu, P.; Lu, Y.W.; Wan, Z.M.; Hu, R.R.; Xie, N.; et al. Efficient Solid Emitters with Aggregation-Induced Emission and Intramolecular Charge Transfer Characteristics: Molecular Design, Synthesis, Photophysical Behaviors, and OLED Application. Chem. Mater. 2012, 24, 1518–1528. [Google Scholar] [CrossRef]
- Wang, C.; Hwang, D.; Yu, Z.B.; Takei, K.; Park, J.; Chen, T.; Ma, B.W.; Javey, A. User-Interactive Electronic Skin for Instantaneous Pressure Visualization. Nat. Mater. 2013, 12, 899–904. [Google Scholar] [CrossRef]
- Mao, M.; Lam, T.L.; To, W.P.; Lao, X.; Liu, W.; Xu, S.; Cheng, G.; Che, C.M. Stable, High-Efficiency Voltage-Dependent Color-Tunable Organic Light-Emitting Diodes with a Single Tetradentate Platinum(II) Emitter Having Long Operational Lifetime. Adv. Mater. 2021, 33, e2004873. [Google Scholar] [CrossRef]
- Frobel, M.; Schwab, T.; Kliem, M.; Hofmann, S.; Leo, K.; Gather Malte, C. Get It White: Color-Tunable AC/DC OLEDs. Light-Sci. Appl. 2015, 4, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.B.; Lian, J.R.; Chen, S.M.; Kwok, H.S. Fabrication of Color Tunable Organic Light-Emitting Diodes by an Alignment Free Mask Patterning Method. Org. Electron. 2013, 14, 2001–2006. [Google Scholar] [CrossRef]
- Wong, K.M.C.; Zhu, X.L.; Hung, L.L.; Zhu, N.Y.; Yam, V.W.W.; Kwok, H.S. A Novel Class of Phosphorescent Gold(III) Alkynyl-Based Organic Light-Emitting Devices with Tunable Colour. Chem. Commun. 2005, 2906–2908. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Chan, K.T.; To, W.P.; Che, C.M. Color Tunable Organic Light-Emitting Devices with External Quantum Efficiency Over 20% Based on Strongly Luminescent Gold(III) Complexes Having Long-Lived Emissive Excited States. Adv. Mater. 2014, 26, 2540–2546. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Wu, R.F.; Huang, J.; Yu, J.S. Color-Tunable and High-Efficiency Organic Light-Emitting Diode by Adjusting Exciton Bilateral Migration Zone. Appl. Phys. Lett. 2013, 103, 133307. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, J.Q.; Ma, D.G.; Cheng, Y.X.; Wang, L.X.; Jing, X.B.; Wang, F.S. Harvesting Excitons via Two Parallel Channels for Efficient White Organic LEDs with Nearly 100% Internal Quantum Efficiency: Fabrication and Emission-Mechanism Analysis. Adv. Funct. Mater. 2009, 19, 84–95. [Google Scholar] [CrossRef]
- Mazzeo, M.; Vitale, V.; Della Sala, F.; Anni, M.; Barbarella, G.; Favaretto, L.; Sotgiu, G.; Cingolani, R.; Gigli, G. Bright White Organic Light-Emitting Devices from a Single Active Molecular Material. Adv. Mater. 2005, 17, 34–39. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph Model. 1995, 14, 33–38. [Google Scholar] [CrossRef]
- Ji, S.C.; Jiang, S.S.; Zhao, T.X.; Meng, L.Y.; Chen, X.L.; Lu, C.Z. Efficient yellow and red thermally activated delayed fluorescence materials based on a quinoxaline-derived electron-acceptor. New J. Chem. 2022, 46, 8991–8998. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Independent Gradient Model Based on Hirshfeld Partition: A New Method for Visual Study of Interactions in Chemical Systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef]
- Tao, X.-D.; Wei, Z.; Meng, L.; Chen, X.-L.; Yang, M.; Jing, Y.-Y.; Zhang, D.-H.; Lu, C.-Z. A Meta-Linkage Strategy Towards High-Performance Hosts for Efficient Blue Thermally Activated Delayed Fluorescence OLEDs. Mater. Chem. Front. 2022, 6, 748–756. [Google Scholar] [CrossRef]
- Jing, Y.-Y.; Tao, X.-D.; Yang, M.-X.; Chen, X.-L.; Lu, C.-Z. Triptycene-Imbedded Thermally Activated Delayed Fluorescence Emitters with Excellent Film Morphologies for Applications in Efficient Nondoped and Doped Organic Light-Emitting Devices. Chem. Eng. J. 2021, 413, 127418. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Liang, Q.; Wei, Y.; Duan, C.; Han, C.; Xu, H. Charge-Transfer Exciton Manipulation Based on Hydrogen Bond for Efficient White Thermally Activated Delayed Fluorescence. Adv. Funct. Mater. 2019, 30, 1908568. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Y.; Yu, L.; Zhao, C.; Yang, D.; Qiao, X.; Chen, J.; Yang, C.; Kleemann, H.; Leo, K.; et al. Strategic-tuning of radiative excitons for efficient and stable fluorescent white organic light-emitting diodes. Nat. Commun. 2019, 10, 2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Su, S.J. Molecular and Device Design Strategies for Ideal Performance White Organic Light-Emitting Diodes. Chem. Rec. 2019, 19, 1518–1530. [Google Scholar] [CrossRef]
- OriginPro; Learning Edition; OriginLab Corporation: Northampton, MA, USA; Available online: https://www.originlab.com/originprolearning.aspx (accessed on 6 June 2022).

| Film | λPL (a) (nm) | ΦPL(b) (%) | τPF/τDF(c) (ns/μs) | kPF (d) (108 s−1) | kDF (e) (104 s−1) | krS/ knrS (f) (108 s−1) | kRISC(g) (104 s−1) |
|---|---|---|---|---|---|---|---|
| Doped | 466 | 52 | 1.12 (h)/35.00 | 8.93 | 2.86 | 4.64/4.29 | 2.86 |
| Neat | 484 | 46 | 2.23 (h)/17.86 | 4.48 | 5.60 | 2.06/2.42 | 5.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Zhao, T.-X.; Ji, S.-C.; Tao, X.-D.; Chen, X.-L.; Meng, L.; Liang, D.; Lu, C.-Z. Voltage-Dependent Emission Varying from Blue to Orange–Red from a Nondoped Organic Light-Emitting Diode with a Single Emitter. Nanomaterials 2022, 12, 2333. https://doi.org/10.3390/nano12142333
Yang M, Zhao T-X, Ji S-C, Tao X-D, Chen X-L, Meng L, Liang D, Lu C-Z. Voltage-Dependent Emission Varying from Blue to Orange–Red from a Nondoped Organic Light-Emitting Diode with a Single Emitter. Nanomaterials. 2022; 12(14):2333. https://doi.org/10.3390/nano12142333
Chicago/Turabian StyleYang, Mingxue, Tian-Xiang Zhao, Si-Chao Ji, Xiao-Dong Tao, Xu-Lin Chen, Lingyi Meng, Dong Liang, and Can-Zhong Lu. 2022. "Voltage-Dependent Emission Varying from Blue to Orange–Red from a Nondoped Organic Light-Emitting Diode with a Single Emitter" Nanomaterials 12, no. 14: 2333. https://doi.org/10.3390/nano12142333
APA StyleYang, M., Zhao, T.-X., Ji, S.-C., Tao, X.-D., Chen, X.-L., Meng, L., Liang, D., & Lu, C.-Z. (2022). Voltage-Dependent Emission Varying from Blue to Orange–Red from a Nondoped Organic Light-Emitting Diode with a Single Emitter. Nanomaterials, 12(14), 2333. https://doi.org/10.3390/nano12142333






