Inorganic Nanomaterials versus Polymer-Based Nanoparticles for Overcoming Neurodegeneration
Abstract
1. Overview
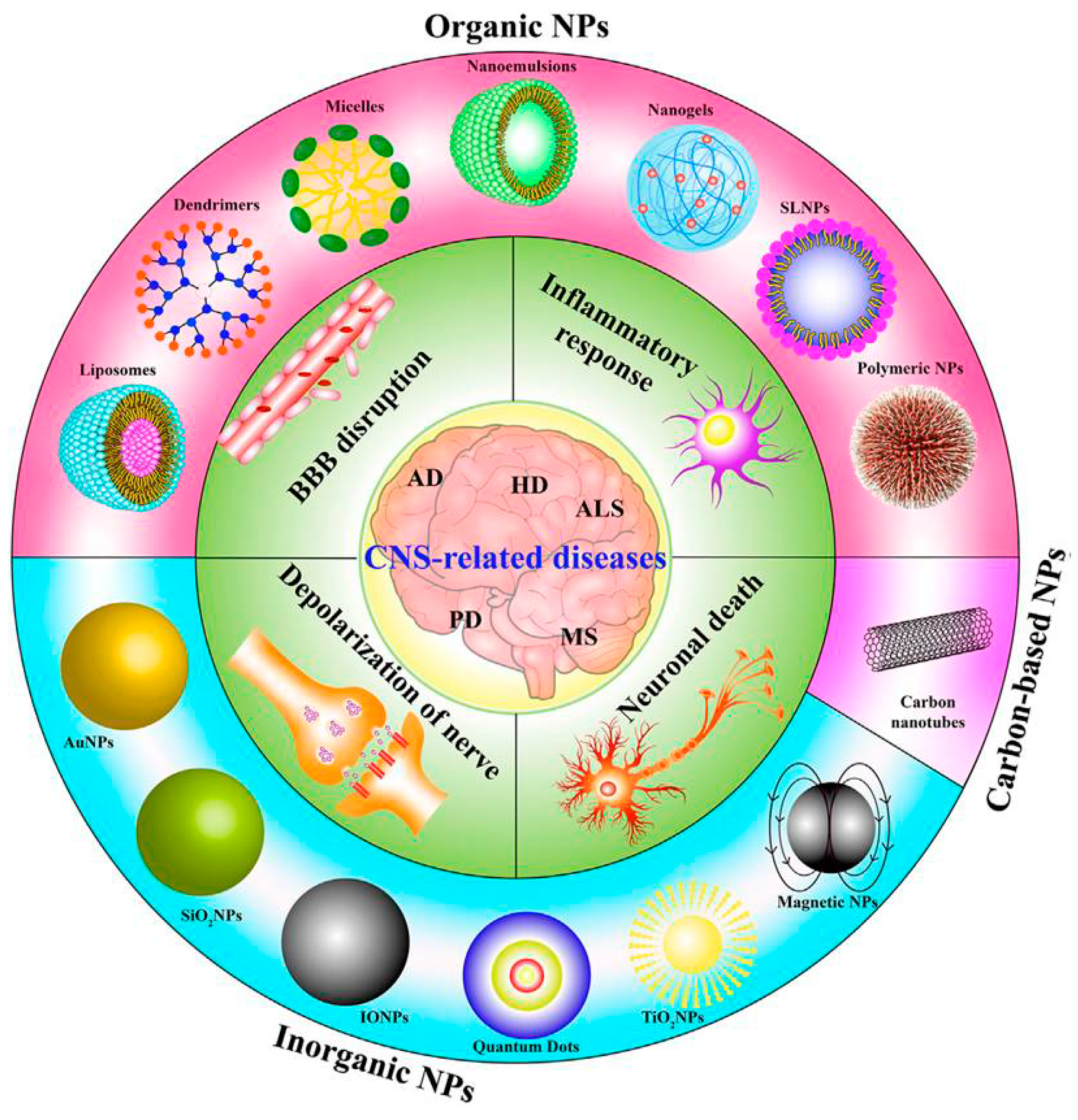
1.1. Neurodegenerative Diseases (NDs): Properties and Conventional Treatments
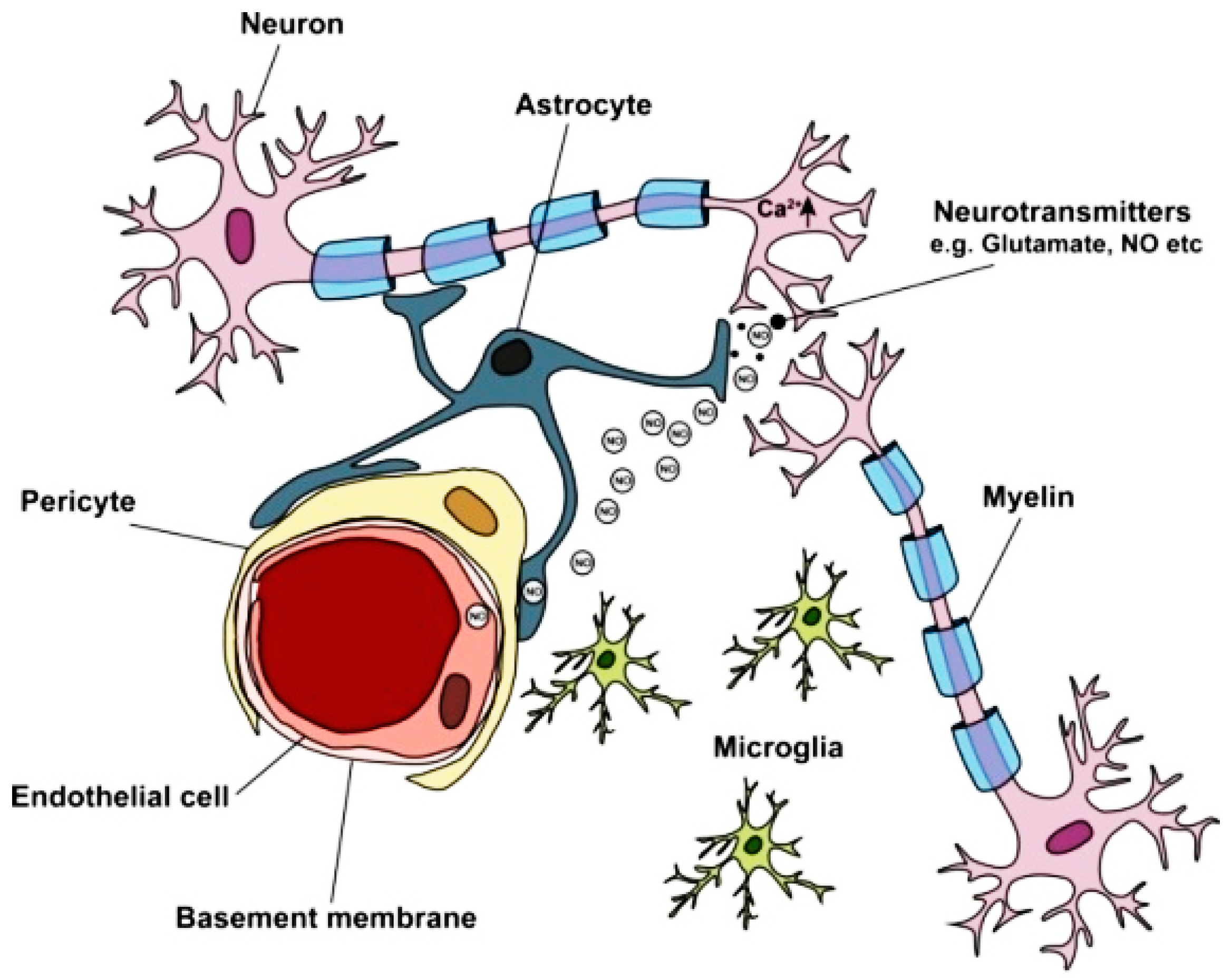
1.2. Blood–Brain Barrier (BBB)
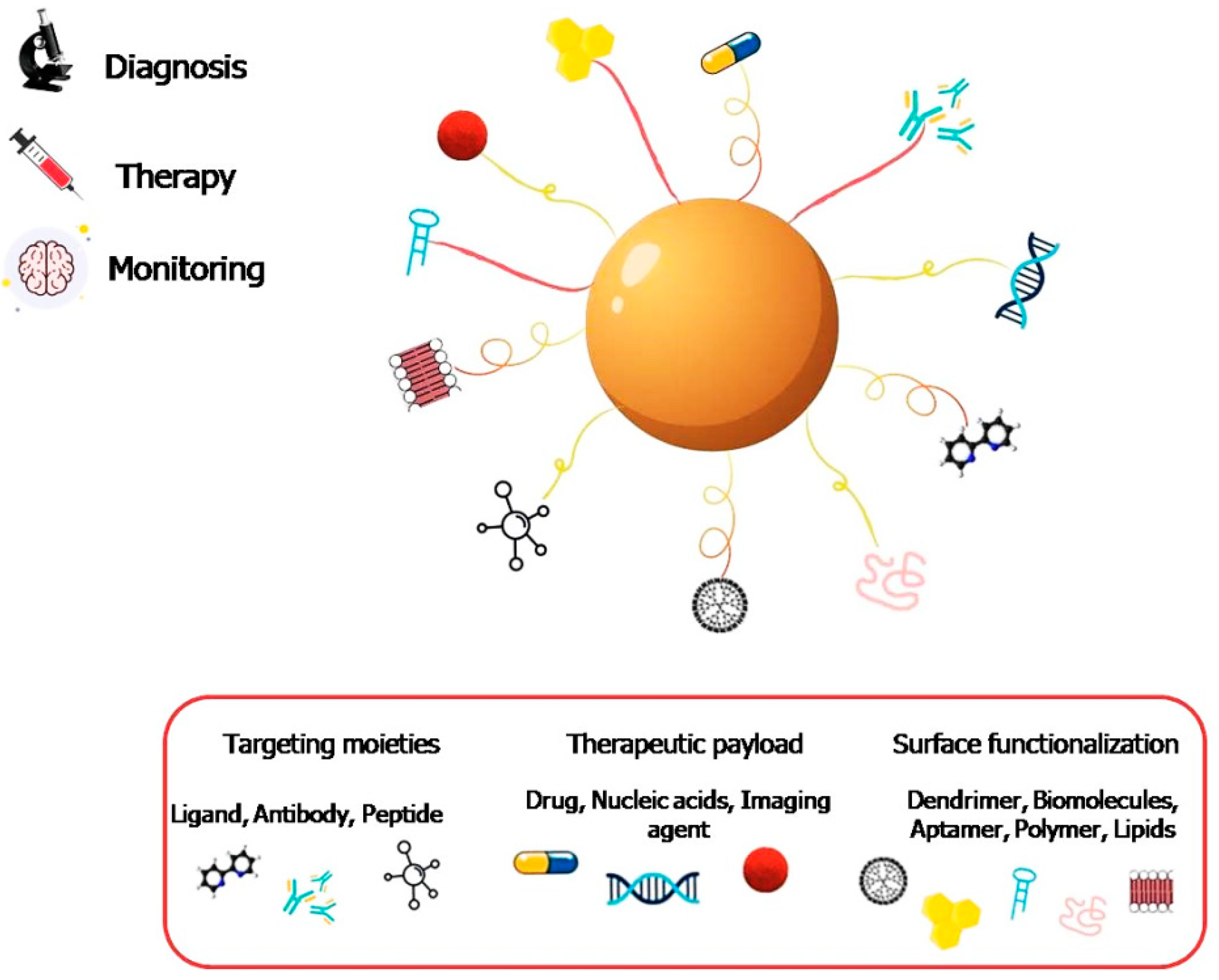
2. Application of Nanotechnology Tools for Neurodegeneration Therapy
2.1. General Aspects
2.2. Nanomaterials As Active Therapeutic Agents
3. NPs Uptake through BBB
4. Antioxidants for Neuroprotection
5. Nutraceutical-Loaded NPs and Green NPs
6. Nanocarriers for Brain Targeting
6.1. Inorganic NPs
6.1.1. Cerium Oxide Nanoparticles (CeO2NPs)
6.1.2. Selenium NPs (SeNPs)
6.1.3. Gold Nanoparticles (AuNPs)
6.1.4. Silver Nanoparticles (AgNPs)
6.1.5. Magnetic Nanoparticles (MNPs)
6.2. Organic Nanoparticles
6.2.1. Poly-Butylcyanoacrylate (PBCA) NPs
6.2.2. Poly (Lactic-Co-Glycolic Acid) (PLGA NPs)
6.2.3. Chitosan Nanoparticles (CS NPs)
6.2.4. Carbon Nanomaterials
6.2.5. Lipid Nanocarriers
6.2.6. Solid Lipid NPs (SLNs)
7. Challenges and Limitations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovacs, G.G. Molecular pathology of neurodegenerative diseases: Principles and practice. J. Clin. Pathol. 2019, 72, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.L.; Gallo, V. The diversity and disparity of the glial scar. Nat. Neurosci. 2018, 21, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Yacoubian, T.A. Neurodegenerative disorders: Why do we need new therapies? In Drug Discovery Approaches for the Treatment of Neurodegenerative Disorders; Academic Press: Cambridge, MA, USA, 2017; pp. 1–16. [Google Scholar]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer’s disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Wei, D.; Zhang, X.; Wang, J.; Wu, X.; Chang, J. Mitochondria-targeted nanoparticles in treatment of neurodegenerative diseases. Exploration 2021, 1, 20210115. [Google Scholar] [CrossRef]
- Abbott, N.J.; Friedman, A. Overview and introduction: The blood-brain barrier in health and disease. Epilepsia 2012, 53, 1–6. [Google Scholar] [CrossRef]
- Binda, A.; Murano, C.; Rivolta, I. Innovative therapies and nanomedicine applications for the treatment of alzheimer’s disease: A state-of-the-art (2017–2020). Int. J. Nanomed. 2020, 15, 6113–6135. [Google Scholar] [CrossRef]
- Tahir, M.S.; Almezgagi, M.; Zhang, Y.; Bashir, A.; Abdullah, H.M.; Gamah, M.; Wang, X.; Zhu, Q.; Shen, X.; Ma, Q.; et al. Mechanistic new insights of flavonols on neurodegenerative diseases. Biomed. Pharmacother. 2021, 137, 111253. [Google Scholar] [CrossRef]
- Di Stefano, A.; Iannitelli, A.; Laserra, S.; Sozio, P. Drug delivery strategies for Alzheimer’s disease treatment. Expert Opin. Drug Deliv. 2011, 8, 581–603. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Current insights on lipid nanocarrier-assisted drug delivery in the treatment of neurodegenerative diseases. Eur. J. Pharm. Biopharm. 2020, 149, 192–217. [Google Scholar] [CrossRef]
- Sachdeva, A.K.; Misra, S.; Pal Kaur, I.; Chopra, K. Neuroprotective potential of sesamol and its loaded solid lipid nanoparticles in ICV-STZ-induced cognitive deficits: Behavioral and biochemical evidence. Eur. J. Pharmacol. 2015, 747, 132–140. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A.N.; et al. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Oertel, W.H. Recent advances in treating Parkinson’s disease. F1000Research 2017, 6, 260. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Lin, Q.; He, S.; Wang, L.; Fu, Y.; Zhang, Z.; Zhang, L. A brain targeting functionalized liposomes of the dopamine derivative N-3,4-bis(pivaloyloxy)-dopamine for treatment of Parkinson’s disease. J. Control. Release 2018, 277, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Miao, W.; Gao, L.; Zhao, X.; Teng, J. Ultrasound-triggered effects of the microbubbles coupled to GDNF plasmid-loaded PEGylated liposomes in a rat model of Parkinson’s disease. Front. Neurosci. 2018, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Yu-Taeger, L.; Bonin, M.; Stricker-Shaver, J.; Riess, O.; Nguyen, H.H.P. Dysregulation of gene expression in the striatum of BACHD rats expressing full-length mutant huntingtin and associated abnormalities on molecular and protein levels. Neuropharmacology 2017, 117, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Fachel, F.N.S.; Schuh, R.S.; Veras, K.S.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Braganhol, E.; Teixeira, H.F. An overview of the neuroprotective potential of rosmarinic acid and its association with nanotechnology-based delivery systems: A novel approach to treating neurodegenerative disorders. Neurochem. Int. 2019, 122, 47–58. [Google Scholar] [CrossRef]
- Couly, S.; Paucard, A.; Bonneaud, N.; Maurice, T.; Benigno, L.; Jourdan, C.; Cohen-Solal, C.; Vignes, M.; Maschat, F. Improvement of BDNF signalling by P42 peptide in Huntington’s disease. Hum. Mol. Genet. 2018, 27, 3012–3028. [Google Scholar] [CrossRef]
- Osorio-Querejeta, I.; Alberro, A.; Muñoz-Culla, M.; Mäger, I.; Otaegui, D. Therapeutic potential of extracellular vesicles for demyelinating diseases; Challenges and opportunities. Front. Mol. Neurosci. 2018, 11, 434. [Google Scholar] [CrossRef]
- Tsang, B.K.T.; Macdonell, R. Multiple sclerosis: Diagnosis, management and prognosis. Aust. Fam. Physician 2011, 40, 948–955. [Google Scholar]
- Dong, X. Current strategies for brain drug delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Cascione, M.; De Matteis, V.; Leporatti, S.; Rinaldi, R. The new frontiers in neurodegenerative diseases treatment: Liposomal-based strategies. Front. Bioeng. Biotechnol. 2020, 8, 566767. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Calkins, D.J. The Neurovascular Unit in Glaucomatous Neurodegeneration. Front. Cell Dev. Biol. 2020, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Madamsetty, V.S.; Bhattacharya, D.; Roy Chowdhury, S.; Paul, M.K.; Mukherjee, A. Recent Advancements of Nanomedicine in Neurodegenerative Disorders Theranostics. Adv. Funct. Mater. 2020, 30, 2003054. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.; Lungu, I.I.; Radu, C.I.; Vlad, O.; Roza, E.; Cost, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Jagaran, K.; Singh, M. Nanomedicine for neurodegenerative disorders: Focus on alzheimer’s and parkinson’s diseases. Int. J. Mol. Sci. 2021, 22, 9082. [Google Scholar] [CrossRef]
- Soni, S.; Ruhela, R.K.; Medhi, B. Nanomedicine in central nervous system (CNS) disorders: A present and future prospective. Adv. Pharm. Bull. 2016, 6, 319–335. [Google Scholar] [CrossRef]
- D’Agata, F.; Ruffinatti, F.A.; Boschi, S.; Stura, I.; Rainero, I.; Abollino, O.; Cavalli, R.; Guiot, C. Magnetic nanoparticles in the central nervous system: Targeting principles, applications and safety issues. Molecules 2018, 23, 9. [Google Scholar] [CrossRef]
- Akhtar, A.; Andleeb, A.; Waris, T.S.; Bazzar, M.; Moradi, A.R.; Awan, N.R.; Yar, M. Neurodegenerative diseases and effective drug delivery: A review of challenges and novel therapeutics. J. Control. Release. 2021, 10, 1152–1167. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Amanda, G.; Chopra, H.; Rahman, M. Applications of Phyto-Nanotechnology for the Treatment of of Neurodegenerative Disorders. Materials 2022, 15, 804. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The future of cancer diagnosis and therapy. CA Cancer J. Clin. 2013, 63, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.D.; Yang, C.; Lake, A.M. Systems biology in the central nervous system: A brief perspective on essential recent advancements. Curr. Opin. Syst. Biol. 2017, 3, 67–76. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Azhar, A.; Ashraf, G.M.; Zia, Q.; Ansari, S.A.; Perveen, A.; Hafeez, A.; Saeed, M.; Kamal, M.A.; Alexiou, A.; Ganash, M.; et al. Frontier View on Nanotechnological Strategies for Neuro-therapy. Curr. Drug Metab. 2018, 19, 596–604. [Google Scholar] [CrossRef]
- Jin, G.Z.; Chakraborty, A.; Lee, J.H.; Knowles, J.C.; Kim, H.W. Targeting with nanoparticles for the therapeutic treatment of brain diseases. J. Tissue Eng. 2020, 11. [Google Scholar] [CrossRef]
- Sheikh, S.; Safia; Haque, E.; Mir, S.S. Neurodegenerative Diseases: Multifactorial Conformational Diseases and Their Therapeutic Interventions. J. Neurodegener. Dis. 2013, 2013, 563481. [Google Scholar] [CrossRef]
- Masoudi Asil, S.; Ahlawat, J.; Guillama Barroso, G.; Narayan, M. Nanomaterial based drug delivery systems for the treatment of neurodegenerative diseases. Biomater. Sci. 2020, 8, 4088–4107. [Google Scholar] [CrossRef]
- Antimisiaris, S.; Mourtas, S.; Papadia, K. Brain targeting with lipidic nanocarriers. In Design of Nanostructures for Versatile Therapeutic Applications; William Andrew: Norwich, NY, USA, 2018; pp. 255–324. [Google Scholar]
- Grabrucker, A.M.; Chhabra, R.; Belletti, D.; Forni, F.; Vandelli, M.A.; Ruozi, B.; Tosi, G. Nanoparticles as blood–brain barrier permeable CNS targeted drug delivery systems. In The Blood Brain Barrier (BBB); Springer: Berlin, Germany, 2013; pp. 71–89. [Google Scholar]
- Gonzalez-Carter, D.A.; Ong, Z.Y.; McGilvery, C.M.; Dunlop, I.E.; Dexter, D.T.; Porter, A.E. L-DOPA functionalized, multi-branched gold nanoparticles as brain-targeted nano-vehicles. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Rautio, J.; Laine, K.; Gynther, M.; Savolainen, J. Prodrug approaches for CNS delivery. AAPS J. 2008, 10, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.; Singh, D.; Prakash, A.; Mishra, N. Development, characterization and nasal delivery of rosmarinic acid-loaded solid lipid nanoparticles for the effective management of Huntingtons disease. Drug Deliv. 2015, 22, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Fan, W.; Lau, J.; Deng, L.; Shen, Z.; Chen, X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 2019, 48, 2967–3014. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, D.M. Antioxidant therapies for neuroprotection-a review. J. Clin. Med. 2019, 8, 1659. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Siddiqi, M.K.; Malik, S.; Chaturvedi, S.K.; Uddin, M.; Khan, R.H. Elucidating the inhibitory potential of Vitamin A against fibrillation and amyloid associated cytotoxicity. Int. J. Biol. Macromol. 2019, 129, 333–338. [Google Scholar] [CrossRef]
- Mohamed, W.M.; Sayeed, S.; Saxena, A.K.; Oothuman, P. Oxidative stress status and neuroprotection of tocotrienols in chronic cerebral hypoperfusion-induced neurodegeneration rat animal model. Int. J. Nutr. Pharmacol. Neurol. Dis. 2018, 8, 47. [Google Scholar]
- Pangeni, R.; Sharma, S.; Mustafa, G.; Ali, J.; Baboota, S. Vitamin e loaded resveratrol nanoemulsion for brain targeting for the treatment of Parkinson’s disease by reducing oxidative stress. Nanotechnology 2014, 25, 485102. [Google Scholar] [CrossRef]
- Man Anh, H.; Linh, D.M.; My Dung, V.; Thi Phuong Thao, D. Evaluating Dose- and Time-Dependent Effects of Vitamin C Treatment on a Parkinson’s Disease Fly Model. Parkinsons. Dis. 2019, 2019, 9720546. [Google Scholar] [CrossRef]
- Salmaso, S.; Pappalardo, J.S.; Sawant, R.R.; Musacchio, T.; Rockwell, K.; Caliceti, P.; Torchilin, V.P. Targeting glioma cells in vitro with ascorbate-conjugated pharmaceutical nanocarriers. Bioconjug. Chem. 2009, 20, 2348–2355. [Google Scholar] [CrossRef]
- Wang, C.; Cai, X.; Hu, W.; Li, Z.; Kong, F.; Chen, X.; Wang, D. Investigation of the neuroprotective effects of crocin via antioxidant activities in HT22 cells and in mice with Alzheimer’s disease. Int. J. Mol. Med. 2019, 43, 956–966. [Google Scholar] [CrossRef]
- Laura, A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–271. [Google Scholar]
- Gan, R.Y.; Chan, C.L.; Yang, Q.Q.; Li, H.B.; Zhang, D.; Ge, Y.Y.; Gunaratne, A.; Ge, J.; Corke, H. Bioactive compounds and beneficial functions of sprouted grains. In Sprouted Grains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 191–246. [Google Scholar]
- González-Sarrías, A.; Núñez-Sánchez, M.Á.; Tomás-Barberán, F.A.; Espín, J.C. Neuroprotective effects of bioavailable polyphenol-derived metabolites against oxidative stress-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Biernasiuk, A.; Wozniak, M.; Bogucka-Kocka, A. Determination of free and bounded phenolic acids in the rhizomes and herb of Sanguisorba officinalis L. Curr. Issues Pharm. Med. Sci. 2015, 28, 254–256. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Rendeiro, C.; Spencer, J.P.E.; del Coso, D.G.; de Llano, M.D.G.; Bartolomé, B.; Moreno-Arribas, M.V. Neuroprotective Effects of Selected Microbial-Derived Phenolic Metabolites and Aroma Compounds from Wine in Human SH-SY5Y Neuroblastoma Cells and Their Putative Mechanisms of Action. Front. Nutr. 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Kanubaddi, K.R.; Yang, S.H.; Wu, L.W.; Lee, C.H.; Weng, C.F. Nanoparticle-conjugated nutraceuticals exert prospectively palliative of amyloid aggregation. Int. J. Nanomed. 2018, 13, 8473–8485. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Sun, C.; Dai, L.; Yang, S.; Wei, Y.; Mao, L.; Yuan, F.; Gao, Y. Effect of molecular weight of hyaluronan on zein-based nanoparticles: Fabrication, structural characterization and delivery of curcumin. Carbohydr. Polym. 2018, 201, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J.; McClements, D.J. Biopolymer-based delivery systems: Challenges and opportunities. Curr. Top. Med. Chem. 2016, 16, 1026–1039. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Huang, J.; Dai, L.; Du, J.; McClements, D.J.; Mao, L.; Liu, J.; Gao, Y. Fabrication and Characterization of Layer-by-Layer Composite Nanoparticles Based on Zein and Hyaluronic Acid for Codelivery of Curcumin and Quercetagetin. ACS Appl. Mater. Interfaces 2019, 11, 16922–16933. [Google Scholar] [CrossRef]
- Okuda, M.; Fujita, Y.; Sugimoto, H. The additive effects of low dose intake of ferulic acid, phosphatidylserine and curcumin, not alone, improve cognitive function in APPswe/PS1dE9 transgenic mice. Biol. Pharm. Bull. 2019, 42, 1694–1706. [Google Scholar] [CrossRef]
- Marcus, M.; Smith, A.; Maswadeh, A.; Shemesh, Z.; Zak, I.; Motiei, M.; Schori, H.; Margel, S.; Sharoni, A.; Shefi, O. Magnetic targeting of growth factors using iron oxide nanoparticles. Nanomaterials 2018, 8, 707. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, Y.; Qin, Y.X. Engineered nanomedicine for neuroregeneration: Light emitting diode-mediated superparamagnetic iron oxide-gold core-shell nanoparticles functionalized by nerve growth factor. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102052. [Google Scholar] [CrossRef]
- Katebi, S.; Esmaeili, A.; Ghaedi, K.; Zarrabi, A. Superparamagnetic iron oxide nanoparticles combined with NGF and quercetin promote neuronal branching morphogenesis of PC12 cells. Int. J. Nanomed. 2019, 14, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, E.; Fu, A. Promotion of SH-SY5Y Cell Growth by Gold Nanoparticles Modified with 6-Mercaptopurine and a Neuron-Penetrating Peptide. Nanoscale Res. Lett. 2017, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Krishnamoorthy, B.; Tamilselvan, N.; Siram, K.; Karthik, S.; Hariprasad, R. Nanomaterials in drug delivery: Existing scenario and potential scope. Nanobiomater. Drug Deliv. 2016, 9, 197–228. [Google Scholar]
- Comoglu, T.; Arisoy, S.; Burcu Akkus, Z. Nanocarriers for effective brain drug delivery. Curr. Top. Med. Chem. 2017, 17, 1490–1506. [Google Scholar] [CrossRef]
- Kevadiya, B.D.; Ottemann, B.M.; Thomas, M.B.; Mukadam, I.; Nigam, S.; Mcmillan, J.; Gorantla, S.; Bronich, T.K.; Gendelman, H.E. Neurotheranostics as personalized medicines. Adv. Drug Deliv. Rev. 2020, 148, 252–289. [Google Scholar] [CrossRef] [PubMed]
- Claudio, P.; Reatul, K.; Brigitte, E.; Geraldine, P. Drug-delivery nanocarriers to cross the blood–brain barrier. In Nanobiomaterials in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 333–370. [Google Scholar]
- Zhu, F.D.; Hu, Y.J.; Yu, L.; Zhou, X.G.; Wu, J.M.; Tang, Y.; Qin, D.L.; Fan, Q.Z.; Wu, A.G. Nanoparticles: A Hope for the Treatment of Inflammation in CNS. Front. Pharmacol. 2021, 12, 683935. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neuronanomedicine: An up-to-date overview. Pharmaceutics 2019, 11, 101. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-brain delivery methods using nanotechnology. Pharmaceutics 2018, 10, 269. [Google Scholar] [CrossRef]
- D’Angelo, B.; Santucci, S.; Benedetti, E.; Di Loreto, S.; Phani, R.; Falone, S.; Amicarelli, F.; Ceru, M.; Cimini, A. Cerium Oxide Nanoparticles Trigger Neuronal Survival in a Human Alzheimer Disease Model By Modulating BDNF Pathway. Curr. Nanosci. 2009, 5, 167–176. [Google Scholar] [CrossRef]
- Das, S.; Dowding, J.M.; Klump, K.E.; Mcginnis, J.F.; Self, W.; Seal, S. Cerium oxide nanoparticles: Applications and prospects in nanomedicine. Nanomedicine 2013, 8, 1483–1508. [Google Scholar] [CrossRef]
- Charbgoo, F.; Ahmad, M.B.; Darroudi, M. Cerium oxide nanoparticles: Green synthesis and biologica. Int. J. Nanomed. 2017, 12, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Thovhogi, N.; Diallo, A.; Gurib-Fakim, A.; Maaza, M. Nanoparticles green synthesis by Hibiscus sabdariffa flower extract: Main physical properties. J. Alloys Compd. 2015, 647, 392–396. [Google Scholar] [CrossRef]
- Kargar, H.; Ghazavi, H.; Darroudi, M. Size-controlled and bio-directed synthesis of ceria nanopowders and their in vitro cytotoxicity effects. Ceram. Int. 2015, 41, 4123–4128. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Turovsky, E.A.; Blinova, E.V. Therapeutic potential and main methods of obtaining selenium nanoparticles. Int. J. Mol. Sci. 2021, 22, 10808. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Mal’tseva, V.N.; Sarimov, R.M.; Simakin, A.V.; Gudkov, S.V.; Plotnikov, E.Y. Features of the cytoprotective effect of selenium nanoparticles on primary cortical neurons and astrocytes during oxygen–glucose deprivation and reoxygenation. Sci. Rep. 2022, 12, 1710. [Google Scholar] [CrossRef]
- Solovyev, N.D. Importance of selenium and selenoprotein for brain function: From antioxidant protection to neuronal signalling. J. Inorg. Biochem. 2015, 153, 1–12. [Google Scholar] [CrossRef]
- Bahamonde, J.; Brenseke, B.; Chan, M.Y.; Kent, R.D.; Vikesland, P.J.; Prater, M.R. Gold Nanoparticle Toxicity in Mice and Rats: Species Differences. Toxicol. Pathol. 2018, 46, 431–443. [Google Scholar] [CrossRef]
- Xiao, L.; Wei, F.; Zhou, Y.; Anderson, G.J.; Frazer, D.M.; Lim, Y.C.; Liu, T.; Xiao, Y. Dihydrolipoic acid-gold nanoclusters regulate microglial polarization and have the potential to alter neurogenesis. Nano Lett. 2020, 20, 478–495. [Google Scholar] [CrossRef]
- Chahardoli, A.; Karimi, N.; Sadeghi, F.; Fattahi, A. Green approach for synthesis of gold nanoparticles from Nigella arvensis leaf extract and evaluation of their antibacterial, antioxidant, cytotoxicity and catalytic activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 579–588. [Google Scholar] [CrossRef]
- Subakanmani, S.; Murugan, S.; Uma Devi, P. Green synthesis of gold nanoparticles using Hypericum hookerianum and its antiparkinson like effect in haloperidol induced swiss albino mice. Int. J. Biol. Chem. 2015, 9, 220–234. [Google Scholar] [CrossRef][Green Version]
- Xue, J.; Liu, T.; Liu, Y.; Jiang, Y.; Seshadri, V.D.D.; Mohan, S.K.; Ling, L. Neuroprotective effect of biosynthesised gold nanoparticles synthesised from root extract of Paeonia moutan against Parkinson disease—In vitro & In vivo model. J. Photochem. Photobiol. B Biol. 2019, 200, 111635. [Google Scholar]
- Henn, A.; Lund, S.; Hedtjärn, M.; Schrattenholz, A.; Pörzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX Altern. Anim. Exp. 2009, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Badeggi, U.M.; Ismail, E.; Adeloye, A.O.; Botha, S.; Badmus, J.A.; Marnewick, J.L.; Cupido, C.N.; Hussein, A.A. Green synthesis of gold nanoparticles capped with procyanidins from Leucosidea sericea as potential antidiabetic and antioxidant agents. Biomolecules 2020, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Jiang, Y.; Liu, Y.; Li, H.; Bari, A.; Ullah, R.; Xue, J. Role of gold nanoparticle from Cinnamomum verum against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) induced mice model. J. Photochem. Photobiol. B Biol. 2019, 201, 111657. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Yi, E.H.; Kim, Y.; Park, G. Anti-neuroinflammatory effects of Ephedra sinica stapf extract-capped gold nanoparticles in microglia. Int. J. Nanomed. 2019, 14, 2861–2877. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Kim, M.J.; Rehman, S.U.; Ahmad, A.; Kim, M.O. Anthocyanin-Loaded PEG-Gold Nanoparticles Enhanced the Neuroprotection of Anthocyanins in an Aβ1–42 Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 54, 6490–6506. [Google Scholar] [CrossRef]
- Talebpour, F.; Ghahghaei, A. Effect of Green Synthesis of Gold Nanoparticles (AuNPs) from Hibiscus sabdariffa on the Aggregation of α-Lactalbumin. Int. J. Pept. Res. Ther. 2020, 26, 2297–2306. [Google Scholar] [CrossRef]
- Prades, R.; Guerrero, S.; Araya, E.; Molina, C.; Salas, E.; Zurita, E.; Selva, J.; Egea, G.; López-Iglesias, C.; Teixidó, M.; et al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials 2012, 33, 7194–7205. [Google Scholar] [CrossRef]
- Sanati, M.; Khodagholi, F.; Aminyavari, S.; Ghasemi, F.; Gholami, M.; Kebriaeezadeh, A.; Sabzevari, O.; Hajipour, M.J.; Imani, M.; Mahmoudi, M.; et al. Impact of Gold Nanoparticles on Amyloid β-Induced Alzheimer’s Disease in a Rat Animal Model: Involvement of STIM Proteins. ACS Chem. Neurosci. 2019, 10, 2299–2309. [Google Scholar] [CrossRef]
- Yoo, J.; Lee, E.; Kim, H.Y.; Youn, D.H.; Jung, J.; Kim, H.; Chang, Y.; Lee, W.; Shin, J.; Baek, S.; et al. Electromagnetized gold nanoparticles mediate direct lineage reprogramming into induced dopamine neurons in vivo for Parkinson’s disease therapy. Nat. Nanotechnol. 2017, 12, 1006–1014. [Google Scholar] [CrossRef]
- Olmedo, I.; Araya, E.; Sanz, F.; Medina, E.; Arbiol, J.; Toledo, P.; Álvarez-Lueje, A.; Giralt, E.; Kogan, M.J. How changes in the sequence of the peptide CLPFFD-NH2 can modify the conjugation and stability of gold nanoparticles and their affinity for β-amyloid fibrils. Bioconjug. Chem. 2008, 19, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Gendelman, H.E. Nanomedicine in the diagnosis and therapy of neurodegenerative disorders. Prog. Polym. Sci. 2007, 32, 1054–1082. [Google Scholar] [CrossRef]
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. J. Nanomater. 2021, 2021, 6687290. [Google Scholar] [CrossRef]
- Darroudi, M.; Ahmad, M.B.; Abdullah, A.H.; Ibrahim, N.A. Green synthesis and characterization of gelatin-based and sugar-reduced silver nanoparticles. Int. J. Nanomed. 2011, 6, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Patakfalvi, R.; Diaz, D.; Velasco-Arias, D.; Rodriguez-Gattorno, G.; Santiago-Jacinto, P. Synthesis and direct interactions of silver colloidal nanoparticles with pollutant gases. Colloid Polym. Sci. 2008, 286, 67–77. [Google Scholar] [CrossRef]
- Prozorova, G.F.; Pozdnyakov, A.S.; Kuznetsova, N.P.; Korzhova, S.A.; Emel’yanov, A.I.; Ermakova, T.G.; Fadeeva, T.V.; Sosedova, L.M. Green synthesis of water-soluble nontoxic polymeric nanocomposites containing silver nanoparticles. Int. J. Nanomed. 2014, 9, 1883–1889. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, Y. Green synthetic nanoarchitectonics of gold and silver nanoparticles prepared using quercetin and their cytotoxicity and catalytic applications. J. Nanosci. Nanotechnol. 2020, 20, 2781–2790. [Google Scholar] [CrossRef]
- Youssif, K.A.; Haggag, E.G.; Elshamy, A.M.; Rabeh, M.A.; Gabr, N.M.; Seleem, A.; Alaraby Salem, M.; Hussein, A.S.; Krischke, M.; Mueller, M.J.; et al. Anti-Alzheimer potential, metabolomic profiling and molecular docking of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea aqueous extracts. PLoS ONE 2019, 14, e0223781. [Google Scholar] [CrossRef]
- Chinnasamy, G.; Chandrasekharan, S.; Bhatnagar, S. Biosynthesis of silver nanoparticles from Melia azedarach: Enhancement of antibacterial, wound healing, antidiabetic and antioxidant activities. Int. J. Nanomed. 2019, 14, 9823–9836. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Panda, S.K.; Jayabalan, R.; Sharma, N.; Bastia, A.K.; Mohanta, T.K. Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (Roxb.). Front. Mol. Biosci. 2017, 4, 14. [Google Scholar] [CrossRef]
- Dehvari, M.; Ghahghaei, A. The effect of green synthesis silver nanoparticles (AgNPs) from Pulicaria undulata on the amyloid formation in α-lactalbumin and the chaperon action of α-casein. Int. J. Biol. Macromol. 2018, 108, 1128–1139. [Google Scholar] [CrossRef]
- Tang, J.; Xiong, L.; Zhou, G.; Wang, S.; Wang, J.; Liu, L.; Li, J.; Yuan, F.; Lu, S.; Wan, Z.; et al. Silver nanoparticles crossing through and distribution in the blood-brain barrier in vitro. J. Nanosci. Nanotechnol. 2010, 10, 6313–6317. [Google Scholar] [CrossRef]
- Zhan, L.; Li, P.; Huang, C.-Z. Stable silver nanoparticles–aptamer bioconjugates for cellular prion protein imaging. Chinese Sci. Bull. 2014, 59, 964–970. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Omar Zaki, S.S.; Ibrahim, M.N.; Katas, H. Particle size affects concentration-dependent cytotoxicity of chitosan nanoparticles towards mouse hematopoietic stem cells. J. Nanotechnol. 2015, 2015, 919658. [Google Scholar] [CrossRef]
- Chertok, B.; Moffat, B.A.; David, A.E.; Yu, F.; Bergemann, C.; Ross, B.D.; Yang, V.C. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials 2008, 29, 487–496. [Google Scholar] [CrossRef]
- Singh, D.; Mcmillan, J.E.M.; Kabanov, A.V.; Sokolsky-Papkov, M.; Gendelman, H.E. Bench-to-bedside translation of magnetic nanoparticles. Nanomedicine 2014, 9, 501–516. [Google Scholar] [CrossRef]
- Zhao, M.; Liang, C.; Li, A.; Chang, J.; Wang, H.; Yan, R.; Zhang, J.; Tai, J. Magnetic paclitaxel nanoparticles inhibit glioma growth and improve the survival of rats bearing glioma xenografts. Anticancer Res. 2010, 30, 2217–2223. [Google Scholar]
- Qiao, R.; Jia, Q.; Hüwel, S.; Xia, R.; Liu, T.; Gao, F.; Galla, H.J.; Gao, M. Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier. ACS Nano 2012, 6, 3304–3310. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Busquets, M.A.; Espargaro, A.; Sabaté, R.; Estelrich, J. Magnetic nanoparticles cross the blood-brain barrier: When physics rises to a challenge. Nanomaterials 2015, 5, 2231–2248. [Google Scholar] [CrossRef]
- Enteshari Najafabadi, R.; Kazemipour, N.; Esmaeili, A.; Beheshti, S.; Nazifi, S. Using superparamagnetic iron oxide nanoparticles to enhance bioavailability of quercetin in the intact rat brain. BMC Pharmacol. Toxicol. 2018, 19, 59. [Google Scholar] [CrossRef]
- Liu, X.G.; Zhang, L.; Lu, S.; Liu, D.Q.; Huang, Y.R.; Zhu, J.; Zhou, W.W.; Yu, X.L.; Liu, R.T. Superparamagnetic iron oxide nanoparticles conjugated with Aβ oligomer-specific scFv antibody and class A scavenger receptor activator show therapeutic potentials for Alzheimer’s Disease. J. Nanobiotechnol. 2020, 18, 160. [Google Scholar] [CrossRef]
- Han, D.; Zhang, B.; Dong, J.; Yang, B.; Peng, Y.; Wang, J.; Wang, L. 1,2-Dimyristoyl-: Sn-glycero -3-phosphocholine promotes the adhesion of nanoparticles to bio-membranes and transport in rat brain. RSC Adv. 2021, 11, 35455–35462. [Google Scholar] [CrossRef]
- Sengel-Turk, C.T.; Gumustas, M.; Uslu, B.; Ozkan, S.A. Nanosized drug carriers for oral delivery of anticancer compounds and the importance of the chromatographic techniques. In Nano- and Microscale Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 165–195. [Google Scholar]
- Singh, N.; Joshi, A.; Toor, A.P.; Verma, G. Drug delivery: Advancements and challenges. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 865–886. [Google Scholar]
- Modi, G.; Pillay, V.; Choonara, Y.E.; Ndesendo, V.M.K.; du Toit, L.C.; Naidoo, D. Nanotechnological applications for the treatment of neurodegenerative disorders. Prog. Neurobiol. 2009, 88, 272–285. [Google Scholar] [CrossRef]
- Behan, N.; Birkinshaw, C.; Clarke, N. Poly n-butyl cyanoacrylate nanoparticles: A mechanistic study of polymerisation and particle formation. Biomaterials 2001, 22, 1335–1344. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Ji, W.H.; Xiao, Z.B.; Liu, G.Y.; Zhang, X. Development and application of nano-flavor-drug carriers in neurodegenerative diseases. Chin. Chem. Lett. 2017, 28, 1829–1834. [Google Scholar] [CrossRef]
- Kurakhmaeva, K.B.; Djindjikhashvili, I.A.; Petrov, V.E.; Balabanyan, V.U.; Voronina, T.A.; Trofimov, S.S.; Kreuter, J.; Gelperina, S.; Begley, D.; Alyautdin, R.N. Brain targeting of nerve growth factor using poly(butyl cyanoacrylate) nanoparticles. J. Drug Target. 2009, 17, 564–574. [Google Scholar] [CrossRef]
- McLachlan, D.C.; Kruck, T.P.A.; Kalow, W.; Andrews, D.F.; Dalton, A.J.; Bell, M.Y.; Smith, W.L. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 1991, 337, 1304–1308. [Google Scholar] [CrossRef]
- Liu, G.; Men, P.; Kudo, W.; Perry, G.; Smith, M.A. Nanoparticle-chelator conjugates as inhibitors of amyloid-β aggregation and neurotoxicity: A novel therapeutic approach for Alzheimer disease. Neurosci. Lett. 2009, 455, 187–190. [Google Scholar] [CrossRef]
- Wilson, B.; Geetha, K.M. Neurotherapeutic applications of nanomedicine for treating Alzheimer’s disease. J. Control. Release 2020, 325, 25–37. [Google Scholar] [CrossRef]
- Li, J.; Sabliov, C. PLA/PLGA nanoparticles for delivery of drugs across the blood-brain barrier. Nanotechnol. Rev. 2013, 2, 241–257. [Google Scholar] [CrossRef]
- Bi, C.C.; Wang, A.P.; Chu, Y.C.; Liu, S.; Mu, H.J.; Liu, W.H.; Wu, Z.M.; Sun, K.X.; Li, Y.X. Intranasal delivery of rotigotine to the brain with lactoferrin-modified PEG-PLGA nanoparticles for Parkinson’s disease treatment. Int. J. Nanomed. 2016, 11, 6547–6559. [Google Scholar] [CrossRef]
- Hu, K.; Shi, Y.; Jiang, W.; Han, J.; Huang, S.; Jiang, X. Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: Preparation, characterization and efficacy in Parkinsons disease. Int. J. Pharm. 2011, 415, 273–283. [Google Scholar] [CrossRef]
- Noshita, T.; Murayama, N.; Oka, T.; Ogino, R.; Nakamura, S.; Inoue, T. Effect of bFGF on neuronal damage induced by sequential treatment of amyloid β and excitatory amino acid in vitro and in vivo. Eur. J. Pharmacol. 2012, 695, 76–82. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, C.; Liu, Q.; Shao, X.; Feng, C.; Shen, Y.; Zhang, Q.; Jiang, X. Solanum tuberosum lectin-conjugated PLGA nanoparticles for nose-to-brain delivery: In vivo and in vitro evaluations. J. Drug Target. 2012, 20, 174–184. [Google Scholar] [CrossRef]
- Wang, Z.H.; Wang, Z.Y.; Sun, C.S.; Wang, C.Y.; Jiang, T.Y.; Wang, S.L. Trimethylated chitosan-conjugated PLGA nanoparticles for the delivery of drugs to the brain. Biomaterials 2010, 31, 908–915. [Google Scholar] [CrossRef]
- Sun, D.; Li, N.; Zhang, W.; Zhao, Z.; Mou, Z.; Huang, D.; Liu, J.; Wang, W. Design of PLGA-functionalized quercetin nanoparticles for potential use in Alzheimer’s disease. Colloids Surf. B Biointerfaces 2016, 148, 116–129. [Google Scholar] [CrossRef]
- Xie, C.L.; Li, J.H.; Wang, W.W.; Zheng, G.Q.; Wang, L.X. Neuroprotective effect of ginsenoside-Rg1 on cerebral ischemia/reperfusion injury in rats by downregulating protease-activated receptor-1 expression. Life Sci. 2015, 121, 145–151. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.Q.; Lu, L.; Fu, D.L.; Liu, A.J.; Li, J.H.; Zheng, G.Q. Ginsenoside Rg1 provides neuroprotection against blood brain barrier disruption and neurological injury in a rat model of cerebral ischemia/reperfusion through downregulation of aquaporin 4 expression. Phytomedicine 2014, 21, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Aalinkeel, R.; Kutscher, H.L.; Singh, A.; Cwiklinski, K.; Khechen, N.; Schwartz, S.A.; .Prasad, P.N.; Mahajan, S.D. Neuroprotective effects of a biodegradable poly (lactic-co-glycolic acid)-ginsenoside Rg3 nanoformulation: A potential nanotherapy for Alzheimer’s disease? J. Drug Target. 2018, 26, 182–193. [Google Scholar] [CrossRef]
- Limpeanchob, N.; Jaipan, S.; Rattanakaruna, S.; Phrompittayarat, W.; Ingkaninan, K. Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J. Ethnopharmacol. 2008, 120, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Sowmya, S.; Cinu, T.A.; Aleykutty, N.A.; Thomas, S.; Souto, E.B. Surface modified PLGA nanoparticles for brain targeting of Bacoside-A. Eur. J. Pharm. Sci. 2014, 63, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.K.; Wu, L.; Kou, W.; Ghorpade, A.; Labhasetwar, V. Superoxide dismutase-loaded PLGA nanoparticles protect cultured human neurons under oxidative stress. Appl. Biochem. Biotechnol. 2008, 151, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Bhavna, B.; Shadab, M.; Ali, M.; Baboota, S.; Sahni, J.K.; Bhatnagar, A.; Ali, J. Preparation, characterization, in vivo biodistribution and pharmacokinetic studies of donepezil-loaded PLGA nanoparticles for brain targeting. Drug Dev. Ind. Pharm. 2014, 40, 278–287. [Google Scholar]
- Baysal, I.; Ucar, G.; Gultekinoglu, M.; Ulubayram, K.; Yabanoglu-Ciftci, S. Donepezil loaded PLGA-b-PEG nanoparticles: Their ability to induce destabilization of amyloid fibrils and to cross blood brain barrier in vitro. J. Neural Transm. 2017, 124, 33–45. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Tsai, H.C. Rosmarinic acid- and curcumin-loaded polyacrylamide-cardiolipin-poly(lactide-co-glycolide) nanoparticles with conjugated 83-14 monoclonal antibody to protect β-amyloid-insulted neurons. Mater. Sci. Eng. C 2018, 91, 445–457. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Hanafy, A.S.; Farid, R.M.; ElGamal, S.S. Complexation as an approach to entrap cationic drugs into cationic nanoparticles administered intranasally for Alzheimer’s disease management: Preparation and detection in rat brain. Drug Dev. Ind. Pharm. 2015, 41, 2055–2068. [Google Scholar] [CrossRef]
- Wang, X.; Chi, N.; Tang, X. Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur. J. Pharm. Biopharm. 2008, 70, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Kölsch, H.; Rao, M.L. Neuroprotective effects of estradiol-17β: Implications for psychiatric disorders. Arch. Womens Ment. Health 2002, 5, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Wairkar, S.; Sridhar, V.; Gaud, R. Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: Development, characterization and in vivo anti-Parkinson activity. Int. J. Biol. Macromol. 2018, 109, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Intranasal Piperine-Loaded Chitosan Nanoparticles as Brain-Targeted Therapy in Alzheimer’s Disease: Optimization, Biological Efficacy, and Potential Toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef]
- Yusuf, M. Formulation and cognitive evaluation of self-assembled phosphatidylserine-chitosan nanoparticles of lycopene, an innovative technique to lessen STZ-induced oxidative stress: A vital persuader of major neurological diseases. J. Drug Deliv. Sci. Technol. 2021, 63, 102534. [Google Scholar] [CrossRef]
- Ahlawat, J.; Neupane, R.; Deemer, E.; Sreenivasan, S.T.; Narayan, M. Chitosan-Ellagic Acid Nanohybrid for Mitigating Rotenone-induced Oxidative Stress. ACS Appl. Mater. Interfaces 2020, 12, 18964–18977. [Google Scholar] [CrossRef]
- Song, G.; Liu, J.; Wang, Q.; Wang, D.; Chu, B.; Li, L.; Xiao, G.; Gong, J.; Zheng, F. Layer-by-layer self-assembly of hollow dextran sulfate/chitosan-coated zein nanoparticles loaded with crocin: Fabrication, structural characterization and potential biological fate. Food Hydrocoll. 2022, 125, 107420. [Google Scholar] [CrossRef]
- Malmo, J.; Sandvig, A.; Vårum, K.M.; Strand, S.P. Nanoparticle Mediated P-Glycoprotein Silencing for Improved Drug Delivery across the Blood–Brain Barrier: A siRNA-Chitosan Approach. PLoS ONE 2013, 8, e54182. [Google Scholar] [CrossRef]
- Engineering, B.; Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R. Carbon-Based Nanomaterials: Multifunctional Materials for Biomedical Engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar]
- Zhou, K.; Motamed, S.; Thouas, G.A.; Bernard, C.C.; Li, D.; Parkington, H.C.; Coleman, H.A.; Finkelstein, D.I.; Forsythe, J.S. Graphene functionalized scaffolds reduce the inflammatory response and supports endogenous neuroblast migration when implanted in the Adult Brain. PLoS ONE 2016, 11, e0151589. [Google Scholar] [CrossRef]
- Goswami, A.D.; Trivedi, D.H.; Jadhav, N.L.; Pinjari, D.V. Sustainable and green synthesis of carbon nanomaterials: A review. J. Environ. Chem. Eng. 2021, 9, 106118. [Google Scholar] [CrossRef]
- Gust, D.; Moore, T.A.; Moore, A.L. Photochemistry of supramolecular systems containing C60. J. Photochem. Photobiol. B Biol. 2000, 58, 63–71. [Google Scholar] [CrossRef]
- Ajitha, A.; Akhina, H.; Aswathi, M.; LovelyMathew, P.; Sabu, T. Carbon nanotubes: An ideal candidate for biomedical applications. JSM Nanotechnol. Nanomed. 2018, 6, 1065. [Google Scholar]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neurotoxicity of nanomaterials: An up-to-date overview. Nanomaterials 2019, 9, 96. [Google Scholar] [CrossRef]
- Shi, D.; Mi, G.; Webster, T.J. The synthesis, application, and related neurotoxicity of carbon nanotubes. In Neurotoxicity of Nanomaterials and Nanomedicine; Academic Press: Cambridge, MA, USA, 2017; pp. 259–284. [Google Scholar]
- Yang, J.; Wang, L.; Huang, L.; Che, X.; Zhang, Z.; Wang, C.; Bai, L.; Liu, P.; Zhao, Y.; Hu, X.; et al. Receptor-targeting nanomaterials alleviate binge drinking-induced neurodegeneration as artificial neurotrophins. Exploration 2021, 1, 61–74. [Google Scholar] [CrossRef]
- Mohajeri, M.; Behnam, B.; Barreto, G.E.; Sahebkar, A. Carbon nanomaterials and amyloid-beta interactions: Potentials for the detection and treatment of Alzheimer’s disease? Pharmacol. Res. 2019, 143, 186–203. [Google Scholar] [CrossRef]
- Hsieh, F.Y.; Zhilenkov, A.V.; Voronov, I.I.; Khakina, E.A.; Mischenko, D.V.; Troshin, P.A.; Hsu, S.H. Water-Soluble Fullerene Derivatives as Brain Medicine: Surface Chemistry Determines if They Are Neuroprotective and Antitumor. ACS Appl. Mater. Interfaces 2017, 9, 11482–11492. [Google Scholar] [CrossRef]
- Ali, S.S.; Hardt, J.I.; Dugan, L.L. SOD Activity of carboxyfullerenes predicts their neuroprotective efficacy: A structure-activity study. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 283–294. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Fu, S.; Zhang, A. Facile Synthesis of Water-Soluble Fullerene (C60) Nanoparticles via Mussel-Inspired Chemistry as Efficient Antioxidants. Nanomaterials 2019, 9, 1647. [Google Scholar] [CrossRef]
- Cacciatore, I.; Ciulla, M.; Fornasari, E.; Marinelli, L.; Di Stefano, A. Solid lipid nanoparticles as a drug delivery system for the treatment of neurodegenerative diseases. Expert Opin. Drug Deliv. 2016, 13, 1121–1131. [Google Scholar] [CrossRef]
- Chifiriuc, M.C.; Kamerzan, C.; Lazar, V. Essential oils and nanoparticles: New strategy to prevent microbial biofilms. In Nanostructures for Antimicrobial Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 279–291. [Google Scholar]
- Mandal, A.; Bisht, R.; Pal, D.; Mitra, A.K. Diagnosis and Drug Delivery to the Brain: Novel Strategies; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780323429979. [Google Scholar]
- Kammari, R.; Das, N.G.; Das, S.K. Nanoparticulate systems for therapeutic and diagnostic applications. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 105–144. [Google Scholar]
- Neves, A.R.; Queiroz, J.F.; Weksler, B.; Romero, I.A.; Couraud, P.O.; Reis, S. Solid lipid nanoparticles as a vehicle for brain-targeted drug delivery: Two new strategies of functionalization with apolipoprotein E. Nanotechnology 2015, 26, 495103. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of Nanoparticle-Based Carriers for Targeted Drug Delivery. J. Nanomater. 2016, 2016, 1087250. [Google Scholar] [CrossRef] [PubMed]
- Bondì, M.L.; Craparo, E.F.; Giammona, G.; Drago, F. Brain-targeted solid lipid nanoparticles containing riluzole: Preparation, characterization and biodistribution. Nanomedicine 2010, 5, 25–32. [Google Scholar] [CrossRef]
- Misra, S.; Chopra, K.; Sinha, V.R.; Medhi, B. Galantamine-loaded solid–lipid nanoparticles for enhanced brain delivery: Preparation, characterization, in vitro and in vivo evaluations. Drug Deliv. 2016, 23, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Obregon, D.F.; Rezai-Zadeh, K.; Bai, Y.; Sun, N.; Hou, H.; Ehrhart, J.; Zeng, J.; Mori, T.; Arendash, G.W.; Shytle, D.; et al. ADAM10 activation is required for green tea (-)-epigallocatechin-3-gallate- induced α-secretase cleavage of amyloid precursor protein. J. Biol. Chem. 2006, 281, 16419–16427. [Google Scholar] [CrossRef]
- Smith, A.; Giunta, B.; Bickford, P.C.; Fountain, M.; Tan, J.; Shytle, R.D. Nanolipidic particles improve the bioavailability and α-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer’s disease. Int. J. Pharm. 2010, 389, 207–212. [Google Scholar] [CrossRef]
- Pinheiro, R.G.R.; Granja, A.; Loureiro, J.A.; Pereira, M.C.; Pinheiro, M.; Neves, A.R.; Reis, S. Quercetin lipid nanoparticles functionalized with transferrin for Alzheimer’s disease. Eur. J. Pharm. Sci. 2020, 148, 105314. [Google Scholar]
- Chandra Bhatt, P.; Srivastava, P.; Pandey, P.; Khan, W.; Panda, B.P. Nose to brain delivery of astaxanthin-loaded solid lipid nanoparticles: Fabrication, radio labeling, optimization and biological studies. RSC Adv. 2016, 6, 10001–10010. [Google Scholar] [CrossRef]
- Patel, S.; Nanda, R. Nanotechnology in Healthcare: Applications and Challenges. Med. Chem. 2015, 5, 528–533. [Google Scholar] [CrossRef]
- Zhang, W. Nanoparticle aggregation: Principles and modeling. Nanomaterial 2014, 811, 19–43. [Google Scholar]
- Wu, T.; Tang, M. Review of the effects of manufactured nanoparticles on mammalian target organs. J. Appl. Toxicol. 2018, 38, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Shannahan, J. The biocorona: A challenge for the biomedical application of nanoparticles. Nanotechnol. Rev. 2017, 6, 345–353. [Google Scholar] [CrossRef] [PubMed]



| NPs and NMscomposition | Green Extract | Functionalization and Encapsulation | Beneficial Effect | In Vitro/In Vivo Model | References |
|---|---|---|---|---|---|
| Cerium oxide (CeO2) NPs | - | - | Antioxidant properties | SH-SY5Y | [75] |
| Selenium (Se) NPs | - | Resveratrol (Res) | Antioxidant and antiaggregatory properties | PC12 cells | [80] |
| - | Chondroitin sulfate (ChS) | Protection from Aβ (1–42)-induced cytotoxicity; reduced level of ROS, malondialdehyde (MDA), and hyperphosphorylation of tau | SH-SY5Y | [80] | |
| - | Glycine | Neuroprotection, antioxidant role decreasing MDA levels, and regulating SOD, GSH-PX enzymes | PD-rats | [80] | |
| Gold (Au) NPs | Hypericum hookerianum | - | Antiparkinson-like effect | Swiss albino mice | [86] |
| Paeonia moutan | - | Alleviated neuroinflammation and improved motor coordination | Murine microglial BV2 cells and PD-induced C57BL/6 mice | [87] | |
| Cinnamomum verum | - | Depletion of induced oxidative stress and motor abnormalities | PD-rats | [90] | |
| Ephedra Sinica | - | Depletion intopro-neuroinflammatory cytokines and mediators; reduced ROS levels | Mouse primary microglia and immortal BV-2 mouse microglial cells | [91] | |
| - | Anthocyanin | Ameliorated memory impairments; protective role in pre- and post-synapticproteins | Aβ (1–42) mouse | [92] | |
| - | Engineered β-sheet breaker peptide (CLPFFD) | Increased permeability in the brain; Disrupted Aβ toxic aggregates | Co-cultured bovine microvessel brain endothelial cells and newbornratastrocytes; Male Sprague–Dawley rats | [94] | |
| Silver (Ag) NPs | Regulation of gene and protein expressions of Aβ depositions | Rat brain microvessel vascular endothelial cells (BMVECs) | |||
| Lampranthus coccineus and Malephora lutea | - | Anti-Alzheimer and antioxidant activity | AD-inducedrats | [104] | |
| Melia azedarach | - | increased antioxidant activity | [105] | ||
| Erythrinasuberosa | - | ROS scavenger | A-431 osteosarcoma cell line | [106] | |
| Pulicaria undulata L. | - | Prevented amyloid aggregation | α-lactalbumin (amyloid model) | [107] | |
| PEG-coated Fe3O4 NPs | - | Lactoferrin | Enhanced permeability across the BBB | Primary porcine and bovine brain capillary endothelial cells (PBCECs); Sprague–Dawley rats | [115] |
| Dextran-coated Fe3O4 NPs | - | Quercetin | Enhanced bioavailability | Wistar male rats | [118] |
| Fe3O4 NPs | - | W20 antibody and XD4 peptide | Microglialphagocytosis of AβO ligomers, restoration of cognitive deficits, and alleviated neuropathology of AD | SH-SY5Y cells; AD mice | [119] |
| PVP-SPIONs | - | 1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC) | Fast brain delivery, activation of neuron membrane channels | Rat adrenal pheochromocytoma (PC-12) cells; Sprague–Dawley rats | [120] |
| - | Great brain uptake, decrease in brain Aβ deposition | APP2576 transgenic mice | |||
| PEG-PLGA NPs | - | Lactoferrin | Optimal drug delivery to the brain | 16HBE and SH-SY5Y cells | [132] |
| - | Lactoferrin and Coumarin-6 | Brain parenchyma-targeting ability, high cellular uptake, | Mouse brain endothelial cell line (b.End3); BALB/c mice | [133] | |
| PS80-PBCA NPs | - | Nerve growth factor (NGF) | Reversed scopolamine-induced amnesia, improved memory and recognition, reduction of the basic symptoms of Parkinsonism | C57Bl/6 mice | [127] |
| PEG-PLGA NPs | - | Solanum Tuberosum Lectin (STL) | High brain-targeting efficiency; noninvasive brain drug-delivery system | Calu-3 cells (human lung adenocarcinoma); Sprague–Dawley rats | [135] |
| - | STL and basic fibroblast growth factor (bFGF) | Neuroprotective effect, improved spatial learning and memory | Sprague–Dawley rats | [130] | |
| PLGA NPs | |||||
| - | Curcumin | Neural stem cell proliferation and neuronal cell differentiation; reversed learning and memory defects | AD-induced rats | [130] | |
| - | Quercetin | Inhibited and disassembled Aβ 42 fibrils; ameliorated cognition and memory deficits | SH-SY5Y cells; APP/PS1 mice | [137] | |
| - | Angiopep-2, Thioflavin T, ginsenoside Rg3 | Reduction of Aβ plaques, decreased ROS generation, inhibiting Aβ-mediated neuronal mitochondrial stress | C6 ratglial cells and THP-1 human monocytic cells | [140] | |
| PS80-PLGA NPs | - | Bacoside-A | Brain targeting nanodelivery, sustained release pattern | Wistar albino rats | [142] |
| PAAM-CL-PLGA NPs | - | 83–14 MAb, rosmarinic acid, curcumin | Enhanced viability in the presence of β-amyloid (Aβ) deposits | SK-N-MC cells (human neuroblastoma) | [146] |
| Chitosan (CS) NPs | - | Estradiol | Improved nasal absorption and brain targeting | Wistar rats | [149] |
| - | Pramipexole dihydrochloride | Antioxidant role; enhancement of dopamine level in the brain, increased locomotor activity | Sprague–Dawley rats | [151] | |
| - | Piperine | Improvement in cognitive function | Wistar rats | [152] | |
| PS80-CS NPs | - | Lycopene, Phosphatidylserine | Improved antioxidant enzymatic activity of CAT, SOD, GPx; Ameliorated behavioral and cognitive impairments | Albino mice | [153] |
| PEG-CS NPs | - | Ellagic acid | Prevent oxidative stress in vitro | SH-SY5Y cells | [154] |
| Fullerenols and fullerene | - | - | Neuroprotective effect, limited excitotoxicity and apoptosis; delayed onset of motor degeneration | Cortical neurons; familial ALS mouse model | [37] |
| Single-walled carbon nanotubes (SWNTs) | - | - | Learning and memory restoring | Sprague–Dawley rats | [164] |
| Fullerene derivatives | - | - | Induced proliferation of NSC; preserved CNS functions | Neural stem cell (NSC); Zebra fish | [166] |
| Carboxy fullerene | - | SOD mimetics | Neuroprotection | Cortical neurons | [167] |
| C60 NPs | Polydopamine (PD) and Reduced Glutathione (GSH) | Free radicals scavenging | HEK-a, HUVEC, HM, L02 cell lines | [168] | |
| Nanolipid particles | - | Epigallocatechin-3-gallate (EGCG) | Improved neuronal α- secretase | SweAPP N2a cells | [178] |
| PS-80 SLN | - | Rosmarinic acid | Attenuated behavioral, locomotor, and body weight deficits | HD rat model | [45] |
| SLN | - | Quercetin and transferrin | BBB permeation | hCMEC/D3 cell line | [179] |
| - | Astaxanthin | Neuroprotection | PC12 cell line | [180] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martano, S.; De Matteis, V.; Cascione, M.; Rinaldi, R. Inorganic Nanomaterials versus Polymer-Based Nanoparticles for Overcoming Neurodegeneration. Nanomaterials 2022, 12, 2337. https://doi.org/10.3390/nano12142337
Martano S, De Matteis V, Cascione M, Rinaldi R. Inorganic Nanomaterials versus Polymer-Based Nanoparticles for Overcoming Neurodegeneration. Nanomaterials. 2022; 12(14):2337. https://doi.org/10.3390/nano12142337
Chicago/Turabian StyleMartano, Simona, Valeria De Matteis, Mariafrancesca Cascione, and Rosaria Rinaldi. 2022. "Inorganic Nanomaterials versus Polymer-Based Nanoparticles for Overcoming Neurodegeneration" Nanomaterials 12, no. 14: 2337. https://doi.org/10.3390/nano12142337
APA StyleMartano, S., De Matteis, V., Cascione, M., & Rinaldi, R. (2022). Inorganic Nanomaterials versus Polymer-Based Nanoparticles for Overcoming Neurodegeneration. Nanomaterials, 12(14), 2337. https://doi.org/10.3390/nano12142337









