Natural Halloysite-Templated Synthesis of Highly Graphitic Boron-Doped Hollow Carbon Nanocapsule Webs
Abstract
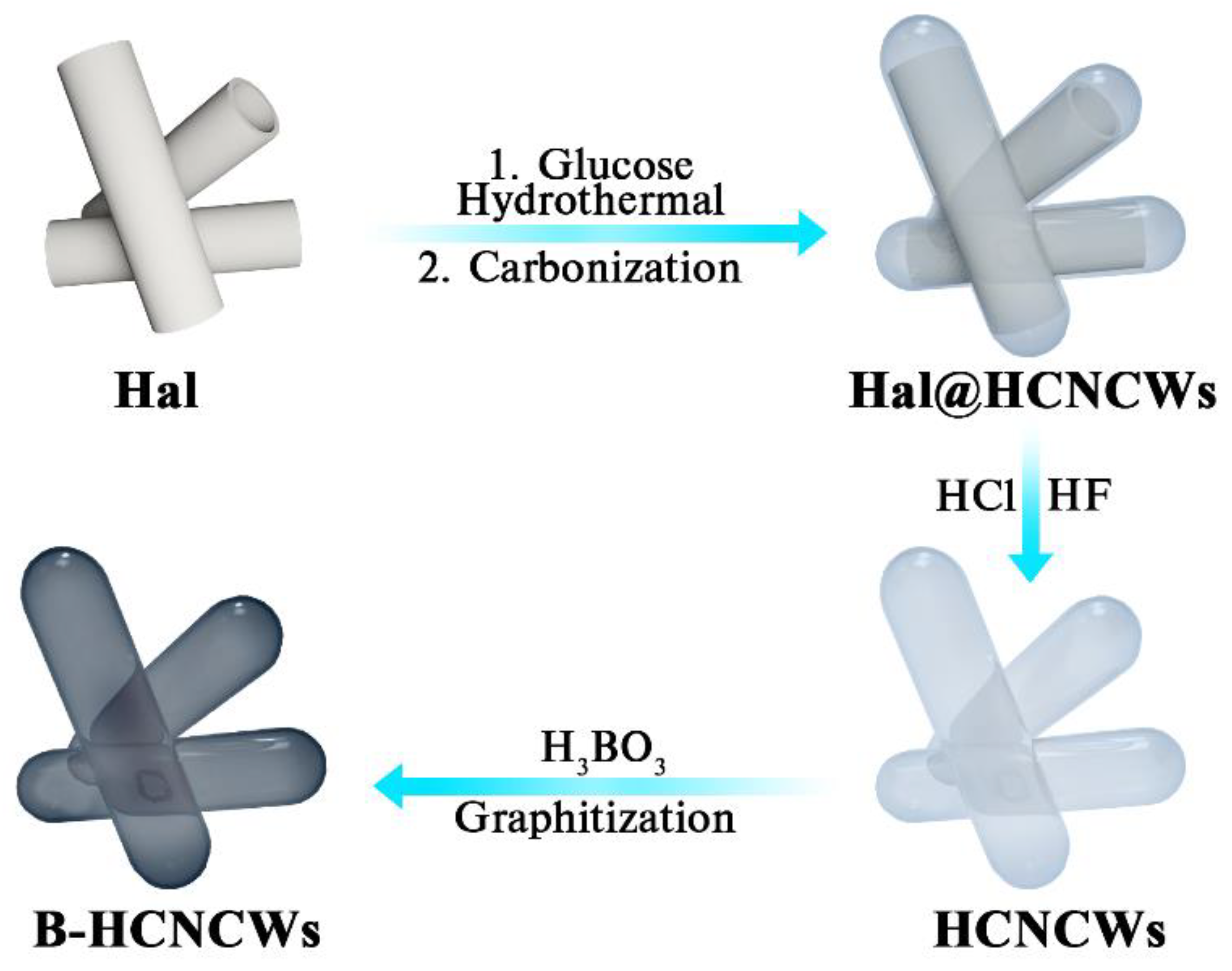
:1. Introduction
2. Materials and Methods
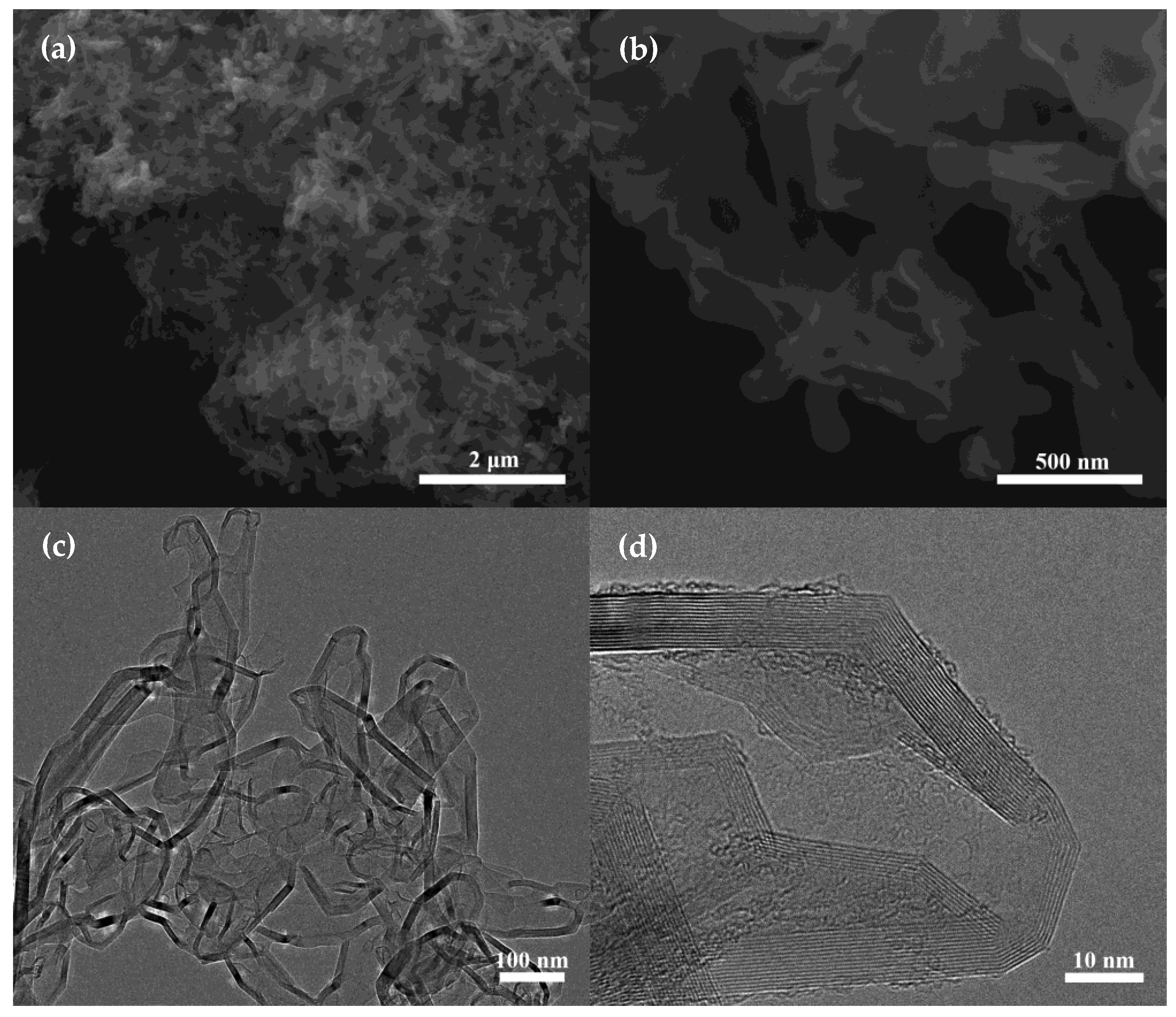
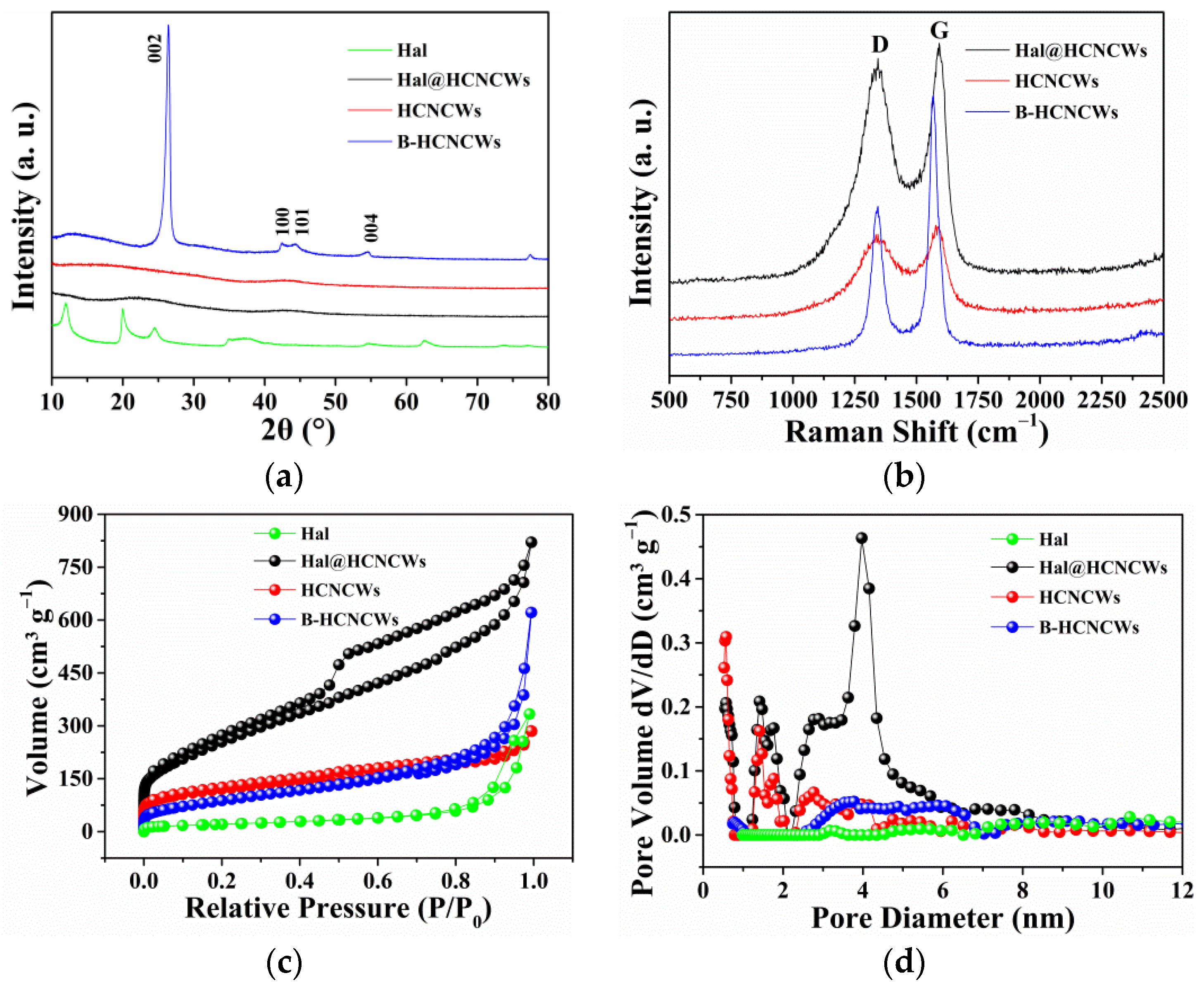
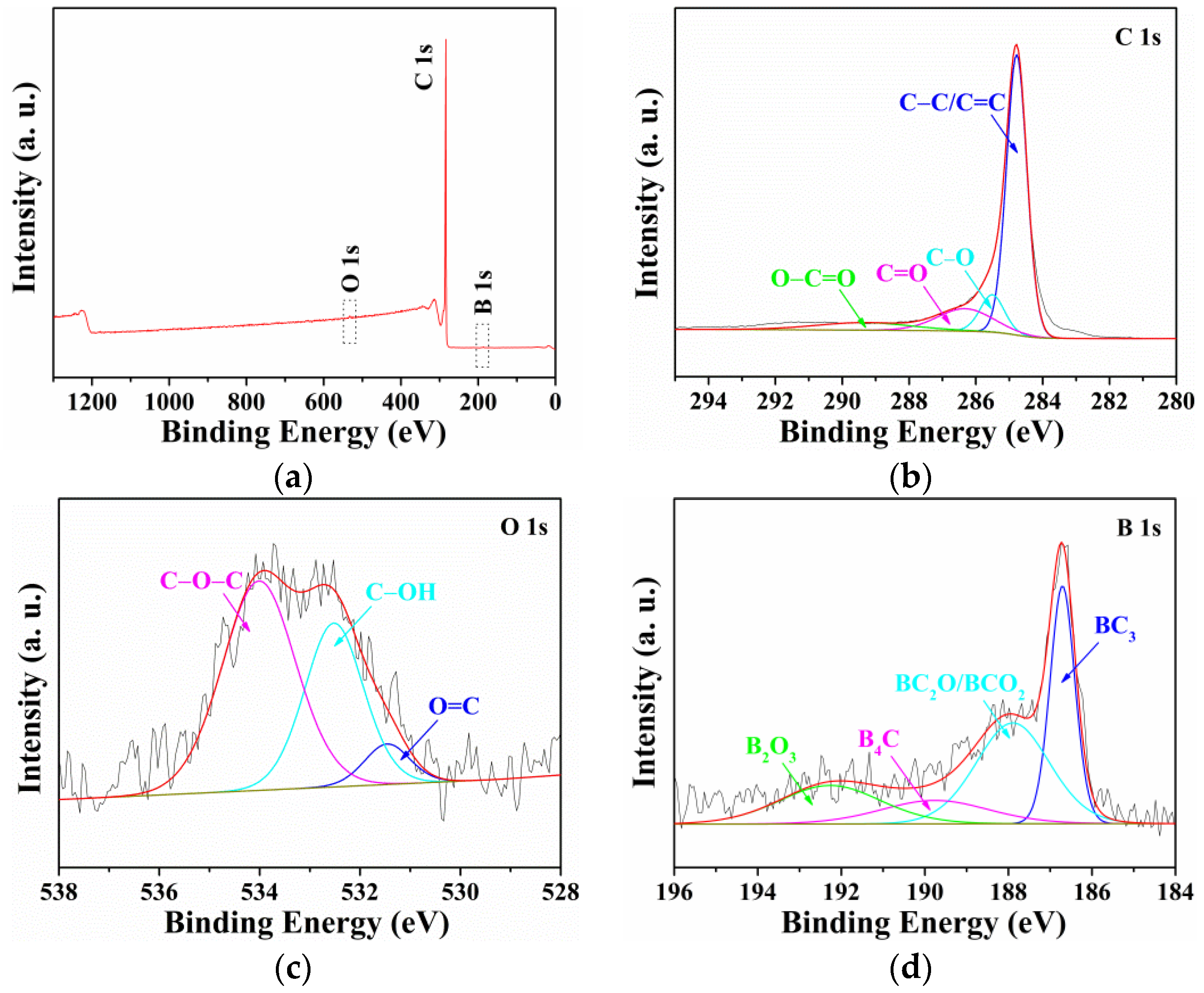
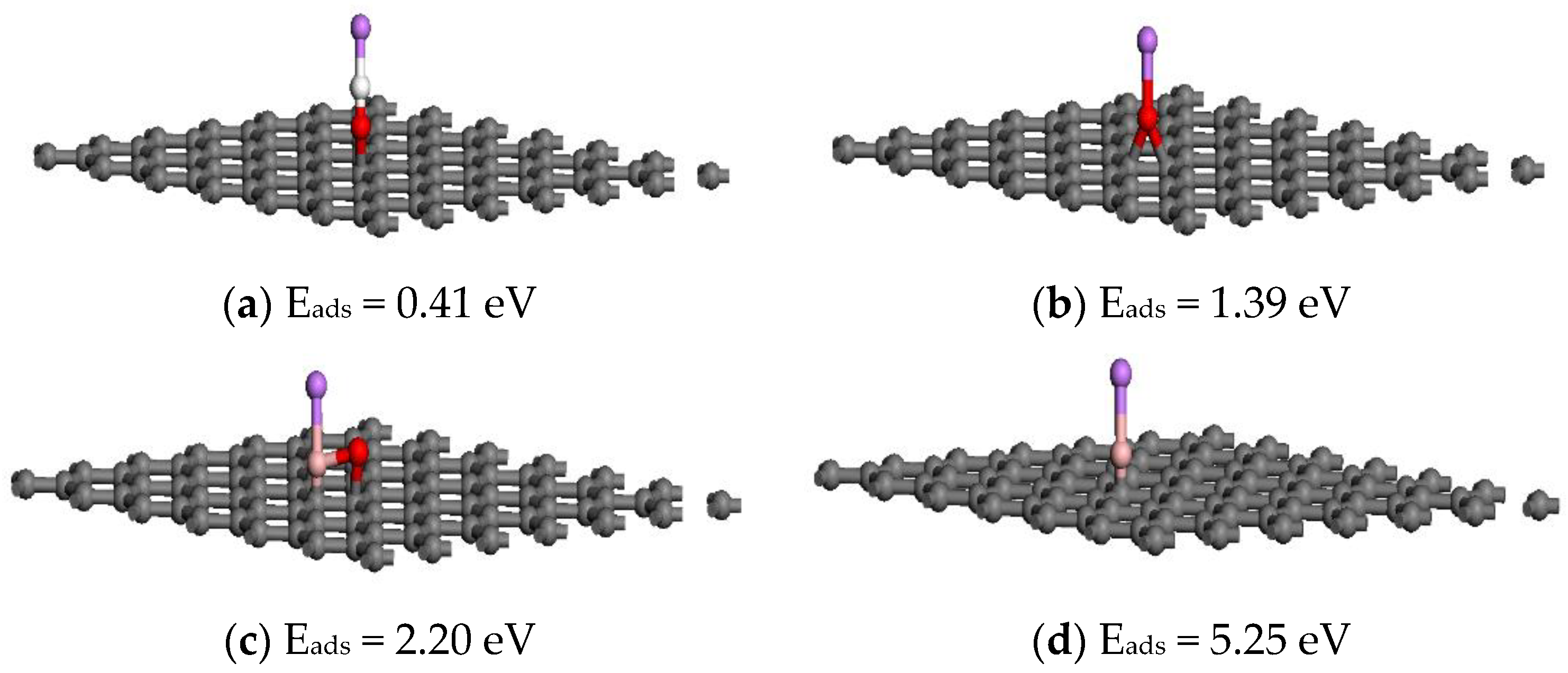
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Q.-L.; Pachfule, P.; Strubel, P.; Li, Z.; Zou, R.; Liu, Z.; Kaskel, S.; Xu, Q. Fabrication of nitrogen and sulfur co-doped hollow cellular carbon nanocapsules as efficient electrode materials for energy storage. Energy Storage Mater. 2018, 13, 72–79. [Google Scholar] [CrossRef]
- Wang, L.; Lu, B.; Wang, S.; Cheng, W.; Zhao, Y.; Zhang, J.; Sun, X. Ultrahigh-performance of Li/Na ion batteries using N/O dual dopants porous hollow carbon nanocapsules as anode. J. Mater. Chem. A 2019, 7, 11117–11126. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Preuß, K.; Qiao, M.; Titirici, M.-M.; Varcoe, J.; Cai, Q. Halloysite-derived nitrogen doped carbon electrocatalysts for anion exchange membrane fuel cells. J. Power Sources 2017, 372, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yu, Y.; Liu, B.; Liu, L.; Lv, H.; Chen, A. Synthesis of mesoporous tubular carbon using natural tubular Halloysite as template for supercapacitor. J. Mater. Sci.: Mater. Electron. 2018, 29, 12187–12194. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Chen, M.; Yu, B.; Wang, B.; Wang, X.; Zhang, W.; Yang, D. MOF derived multi-metal oxides anchored N, P-doped carbon matrix as efficient and durable electrocatalyst for oxygen evolution reaction. J. Colloid Interf. Sci. 2021, 581, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Liu, L.; Dong, Y.; Li, W.; Li, J.; Liu, Z.; Wang, X. Synthesis of hierarchically porous boron-doped carbon material with enhanced surface hydrophobicity and porosity for improved supercapacitor performance. Electrochim. Acta 2021, 370, 137801. [Google Scholar] [CrossRef]
- Chen, L.; Feng, J.; Zhou, H.; Fu, C.; Wang, G.; Yang, L.; Xu, C.; Chen, Z.; Yang, W.; Kuang, Y. Hydrothermal preparation of nitrogen, boron co-doped curved graphene nanoribbons with high dopant amounts for high-performance lithium sulfur battery cathodes. J. Mater. Chem. A 2017, 5, 7403–7415. [Google Scholar] [CrossRef]
- Srivastava, S.; Jain, S.K.; Gupta, G.; Senguttuvan, T.D.; Gupta, B.K. Boron-doped few-layer graphene nanosheet gas sensor for enhanced ammonia sensing at room temperature. RSC Adv. 2020, 10, 1007–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Sun, N.; Soomro, R.A.; Guan, Z.; Ma, L.; Jiang, M.; Zhu, Q.; Xu, B. Structurally engineered hollow graphitized carbon nanocages as high-performance anodes for potassium ion batteries. ACS Nano 2020, 14, 16161. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, X.; Liao, J.; Yang, S.; Zhou, X.; Gao, Q.; Zhang, S.; Fang, Y.; Zhong, X.; Zhang, S. FeNi intermetallic compound nanoparticles wrapped by N-doped graphitized carbon: A novel cocatalyst for boosting photocatalytic hydrogen evolution. J. Mater. Chem. A 2020, 8, 3481–3490. [Google Scholar] [CrossRef]
- Zhang, W.; Mu, B.; Wang, A. Halloysite nanotubes template-induced fabrication of carbon/manganese dioxide hybrid nanotubes for supercapacitors. Ionics 2015, 21, 2329–2336. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, F.; Bai, T.; Long, B.; Liao, Q.; Ren, Y.; Yang, J. Interconnected highly graphitic carbon nanosheets derived from wheat stalk as high performance anode materials for lithium ion batteries. Green Chem. 2016, 18, 2078–2088. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Tomiczek, B.; Pawlyta, M.; Nuckowski, P. TEM and XRD study of nanostructured composite materials reinforced with the halloysite particles. Mater. Sci. Forum 2014, 783–786, 1591–1596. [Google Scholar] [CrossRef]
- Yu, K.; Wang, Y.; Wang, X.; Liu, W.; Liang, J.; Liang, C. Preparation of porous carbon anode materials for lithium-ion battery from rice husk. Mater. Lett. 2019, 253, 405–408. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; Wang, A.; Li, W.; Liu, X.; Fu, L.; Huang, W. Novel self-assembled natural graphite based composite anodes with improved kinetic properties in lithium-ion batteries. J. Mater. Chem. A 2016, 4, 9865–9872. [Google Scholar] [CrossRef]
- Greene, M.L.; Schwartz, R.W.; Treleaven, J.W. Short residence time graphitization of mesophase pitch-based carbon fibers. Carbon 2002, 40, 1217–1226. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, F.; Pan, K.; Zhao, Y.; Ma, L.; Wei, S. Studies on kinetics, isotherms, thermodynamics and adsorption mechanism of methylene blue by N and S co-doped porous carbon spheres. Nanomaterials 2021, 11, 1819. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Yin, Y.X.; Ye, H.; Jiang, K.C.; Zhang, J.; Guo, Y.G. Insight into the effect of boron doping on sulfur/carbon cathode in lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2014, 6, 8789–8795. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Meng, Z.; Cai, T.; Han, W.Q. Effect of boron-doping on the graphene aerogel used as cathode for the lithium-sulfur battery. ACS Appl. Mater. Interfaces 2015, 7, 25202–25210. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Gao, Y.; Shi, P.; Cheng, X.; Aramata, A. The effect of boron doping on lithium intercalation performance of boron-doped carbon materials. Mater. Chem. Phys. 2003, 80, 94–101. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Ma, L.; Li, B.; Jiang, P.; Song, Z.; Huang, L. Natural Halloysite-Templated Synthesis of Highly Graphitic Boron-Doped Hollow Carbon Nanocapsule Webs. Nanomaterials 2022, 12, 2352. https://doi.org/10.3390/nano12142352
Chen F, Ma L, Li B, Jiang P, Song Z, Huang L. Natural Halloysite-Templated Synthesis of Highly Graphitic Boron-Doped Hollow Carbon Nanocapsule Webs. Nanomaterials. 2022; 12(14):2352. https://doi.org/10.3390/nano12142352
Chicago/Turabian StyleChen, Feng, Lulu Ma, Bing Li, Peiwen Jiang, Zhimin Song, and Lei Huang. 2022. "Natural Halloysite-Templated Synthesis of Highly Graphitic Boron-Doped Hollow Carbon Nanocapsule Webs" Nanomaterials 12, no. 14: 2352. https://doi.org/10.3390/nano12142352
APA StyleChen, F., Ma, L., Li, B., Jiang, P., Song, Z., & Huang, L. (2022). Natural Halloysite-Templated Synthesis of Highly Graphitic Boron-Doped Hollow Carbon Nanocapsule Webs. Nanomaterials, 12(14), 2352. https://doi.org/10.3390/nano12142352





