A Novel Electrochemical Sensor for Detection of Nicotine in Tobacco Products Based on Graphene Oxide Nanosheets Conjugated with (1,2-Naphthoquinone-4-Sulphonic Acid) Modified Glassy Carbon Electrode
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Apparatus
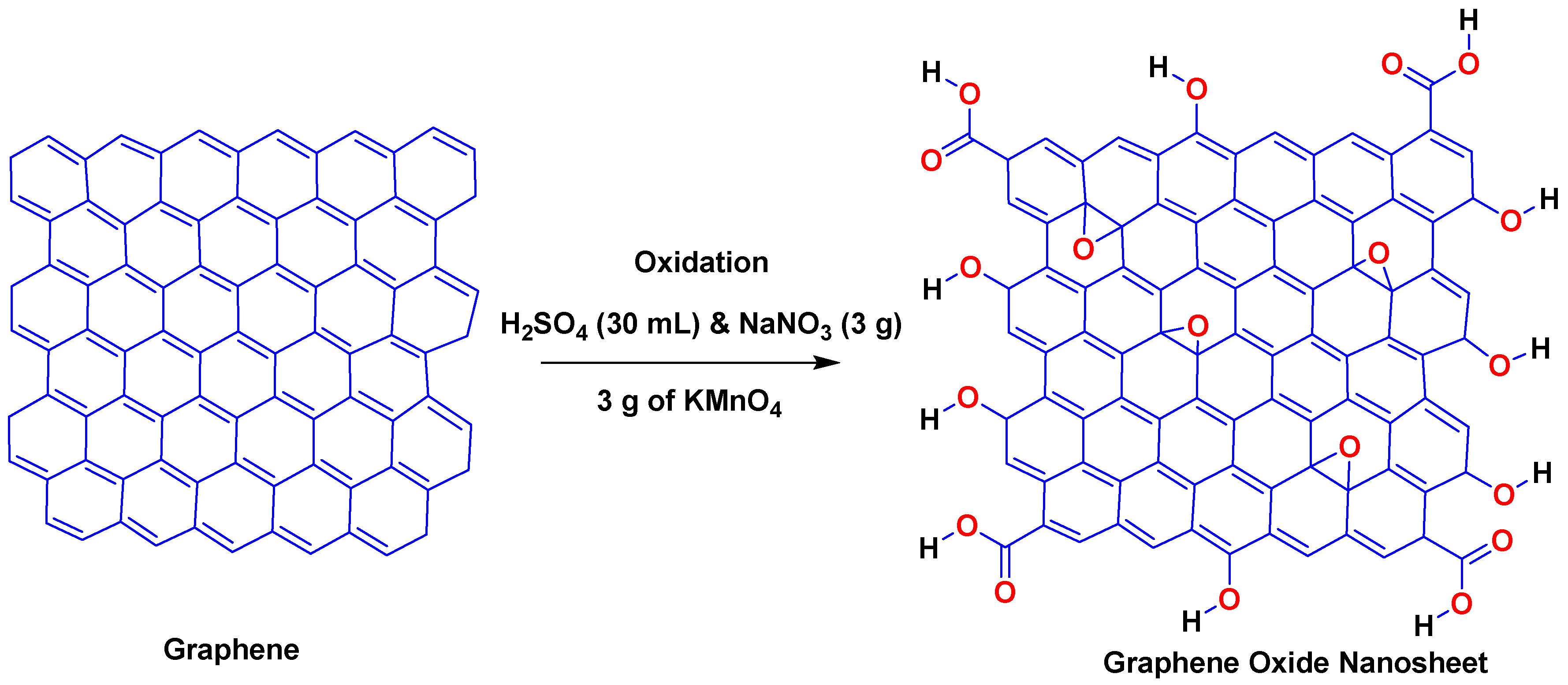
2.3. Synthesis of GO
2.4. GO/Nq/GCE Fabrication
2.5. Sample Preparation
3. Results and Discussion
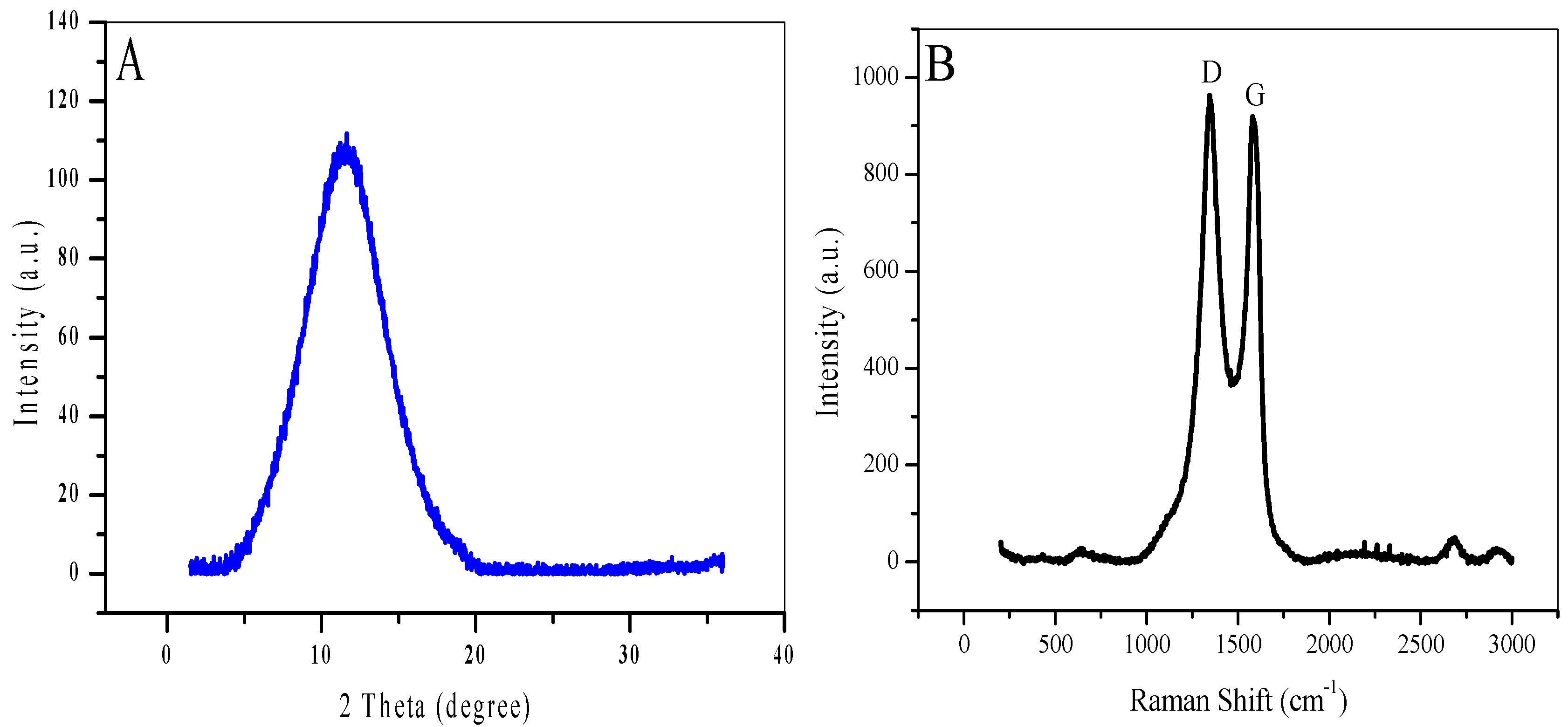
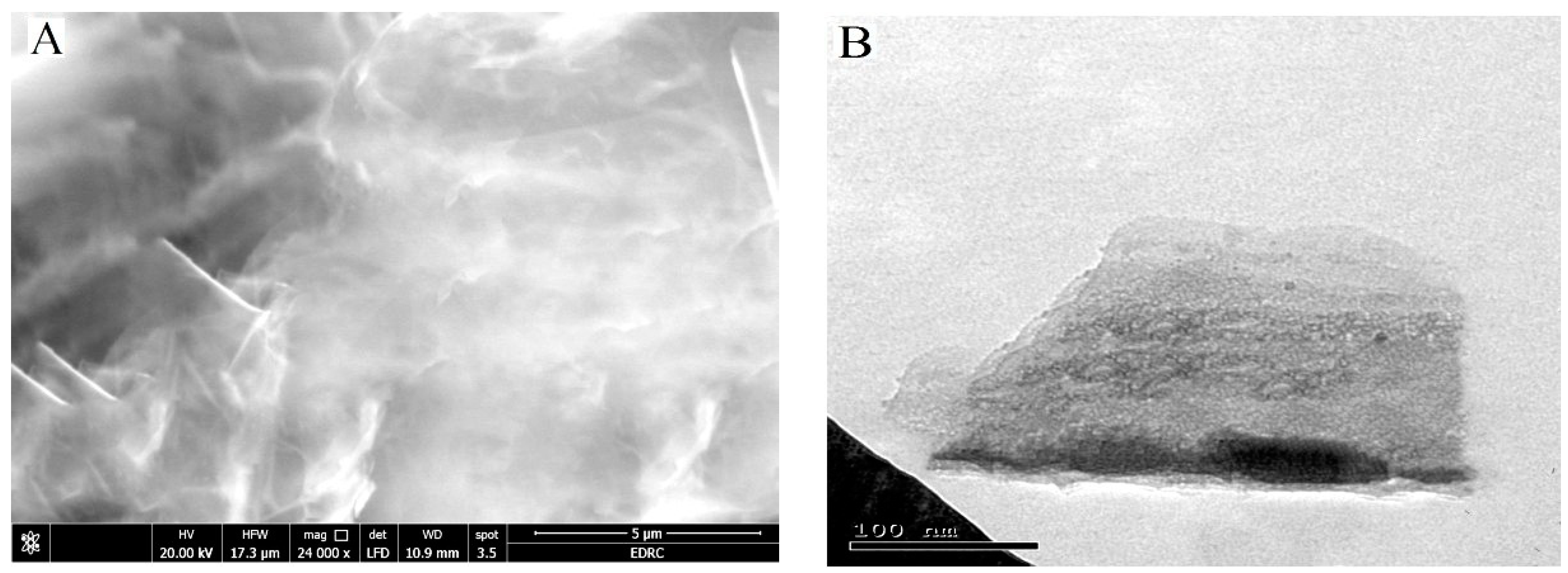
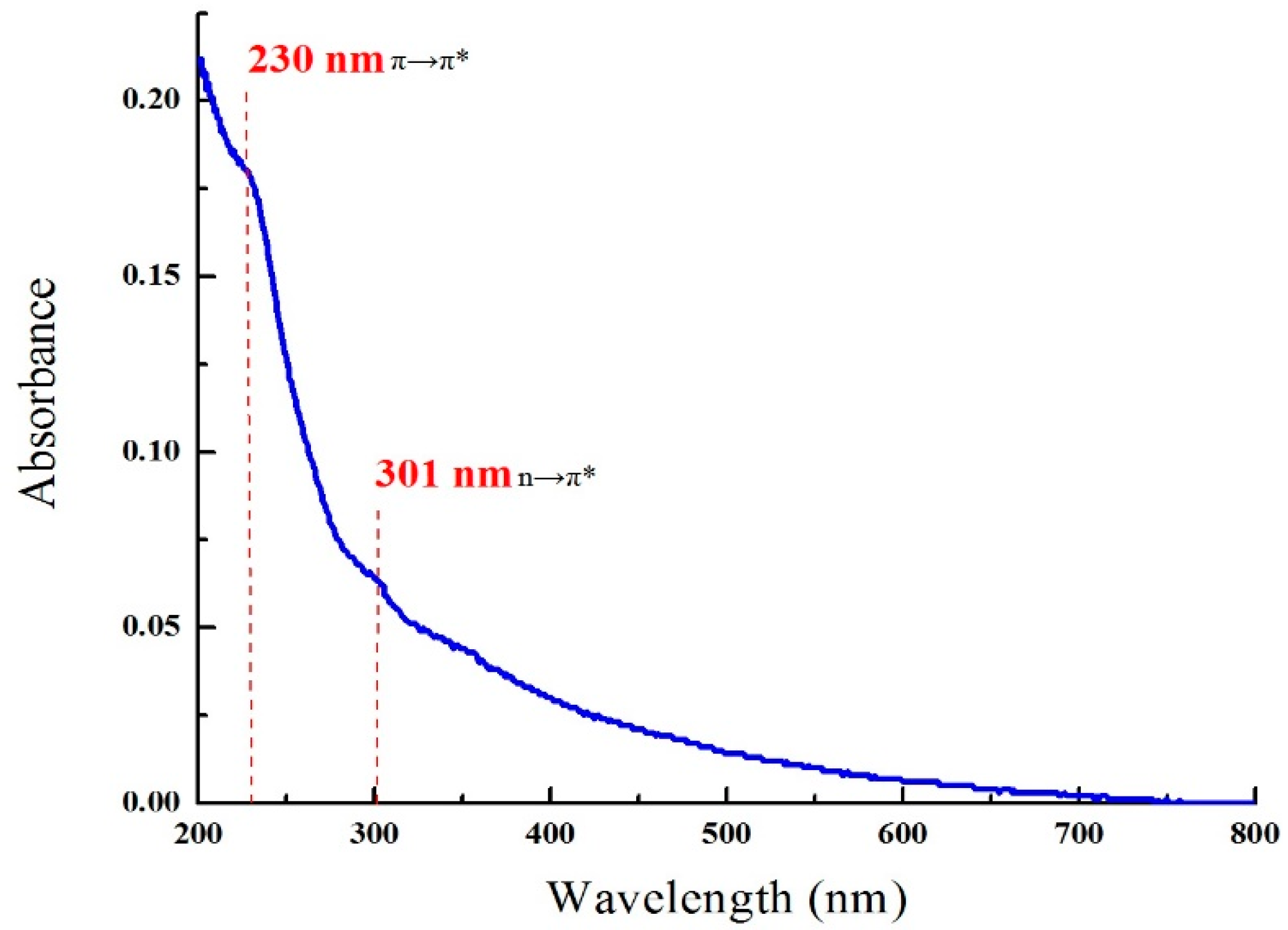
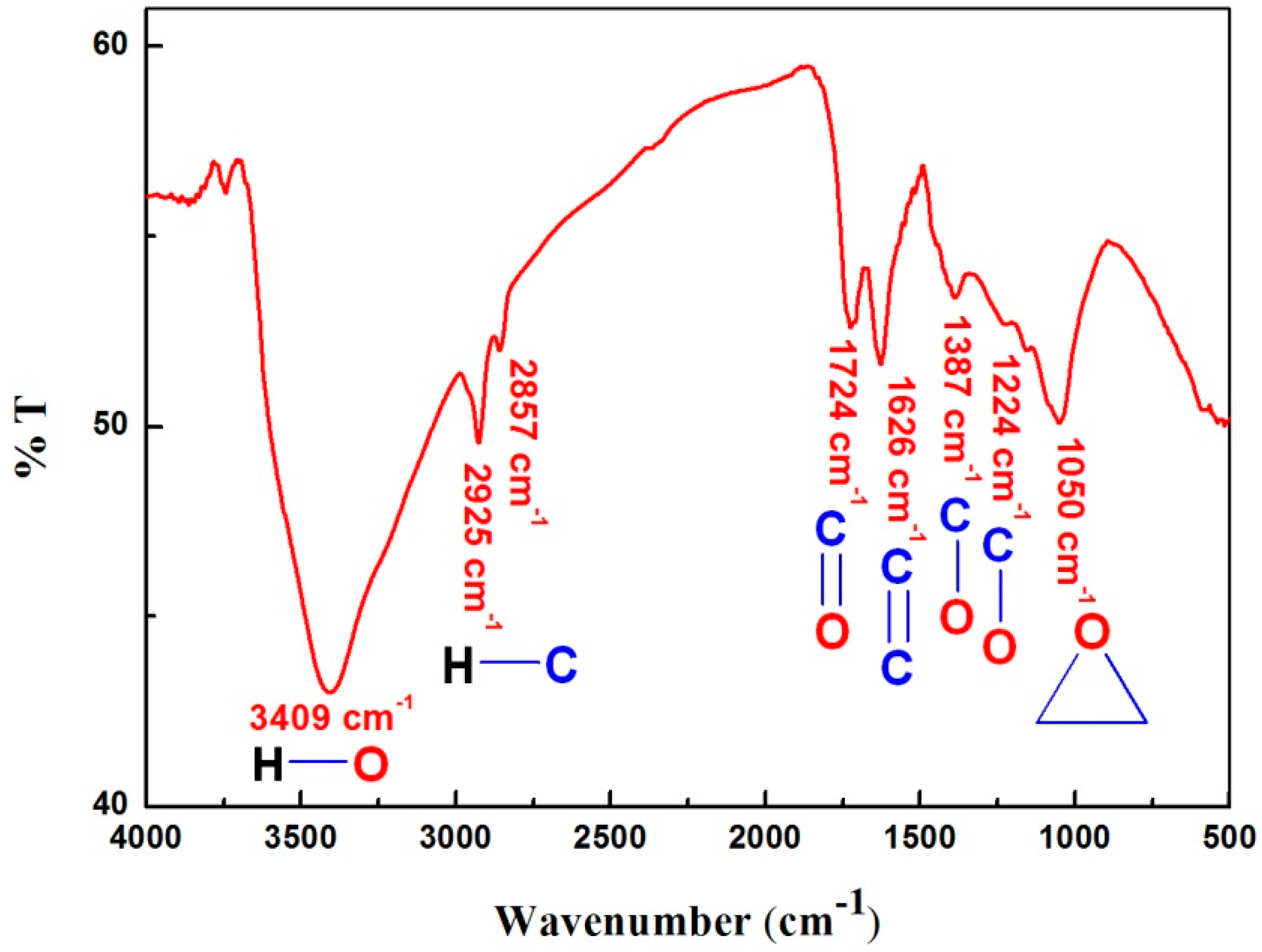
3.1. Characterization of the Synthesized GO
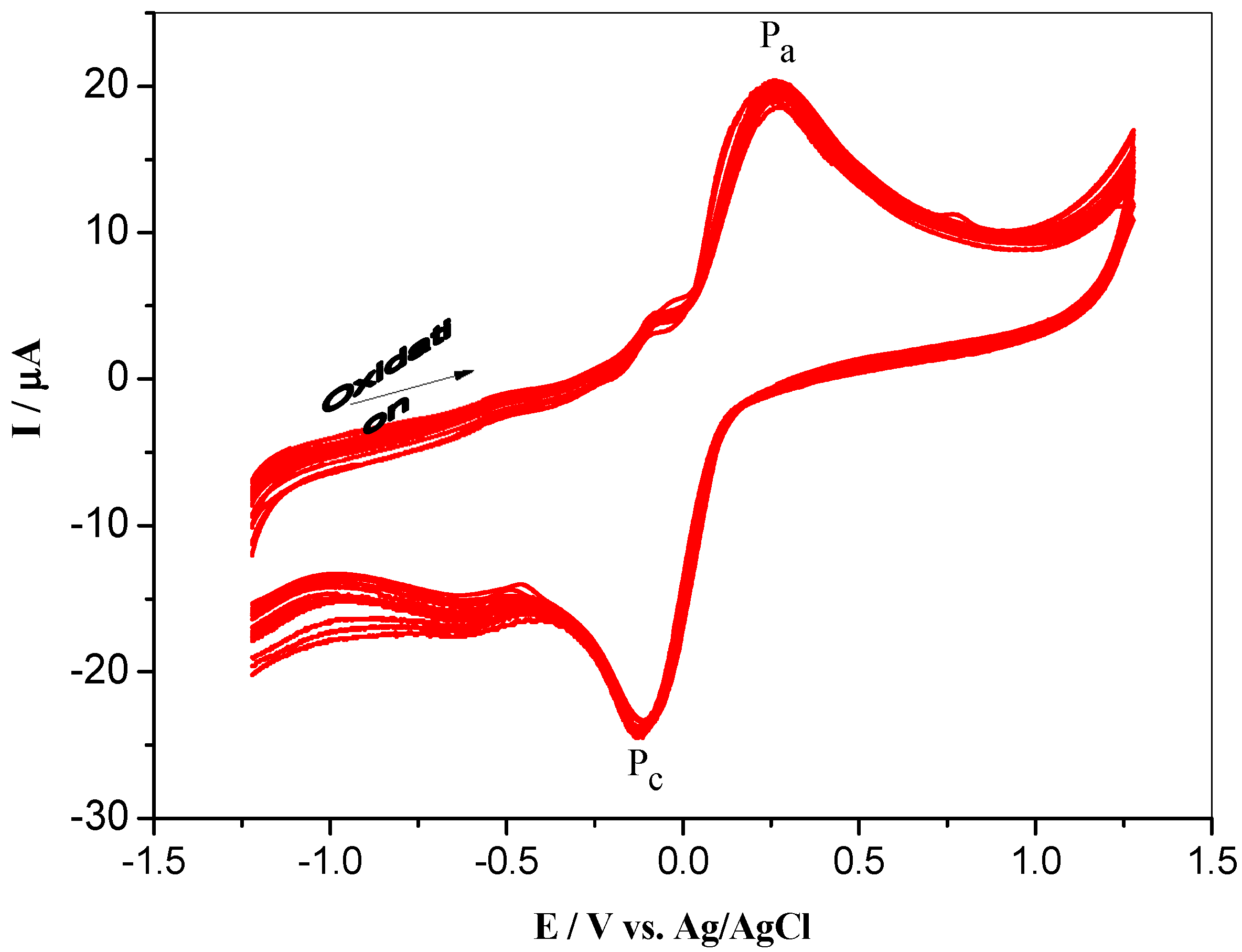
3.2. Electrochemical Behaviorof Nq
3.3. Voltammetric Determination of NIC
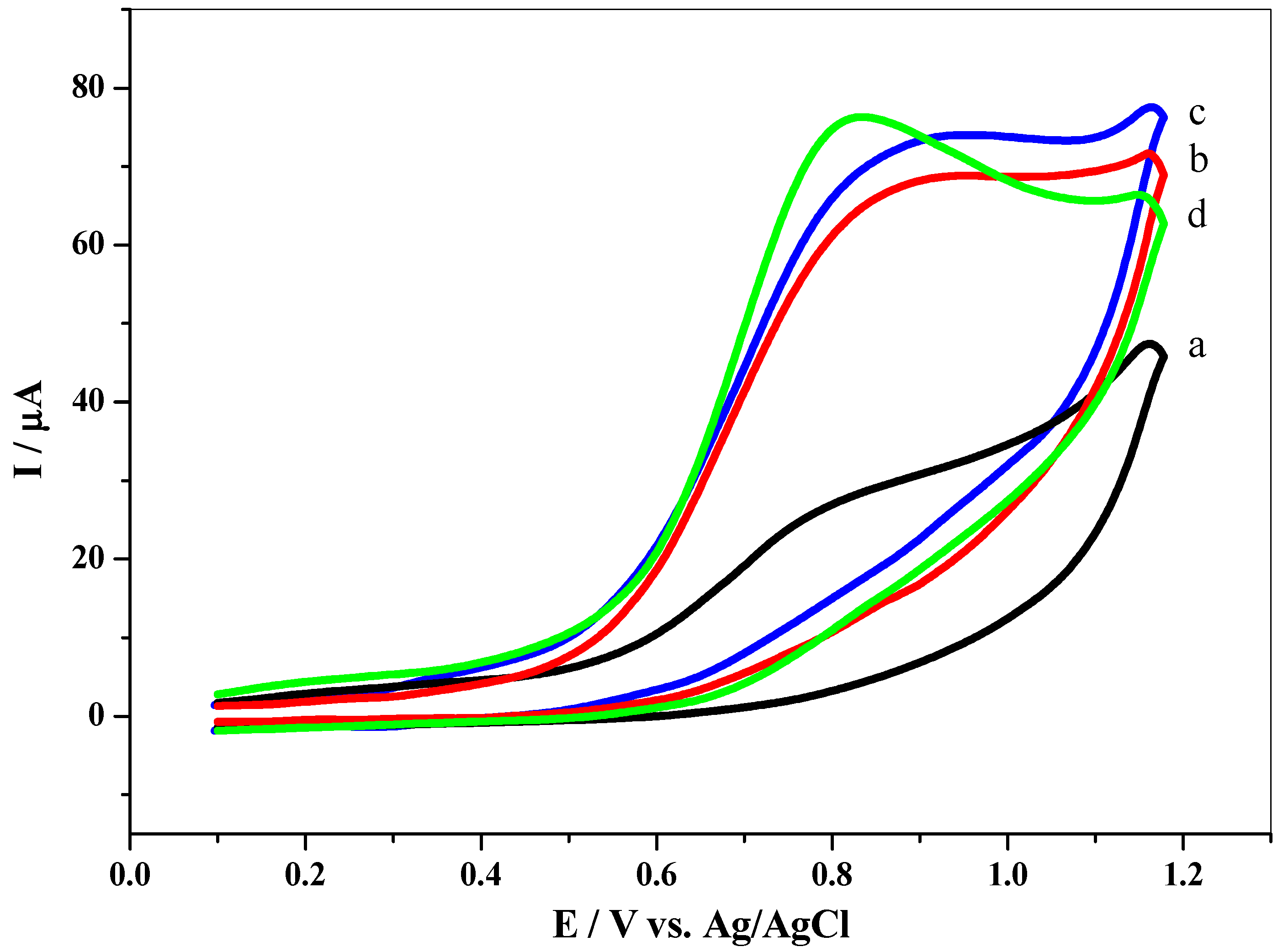
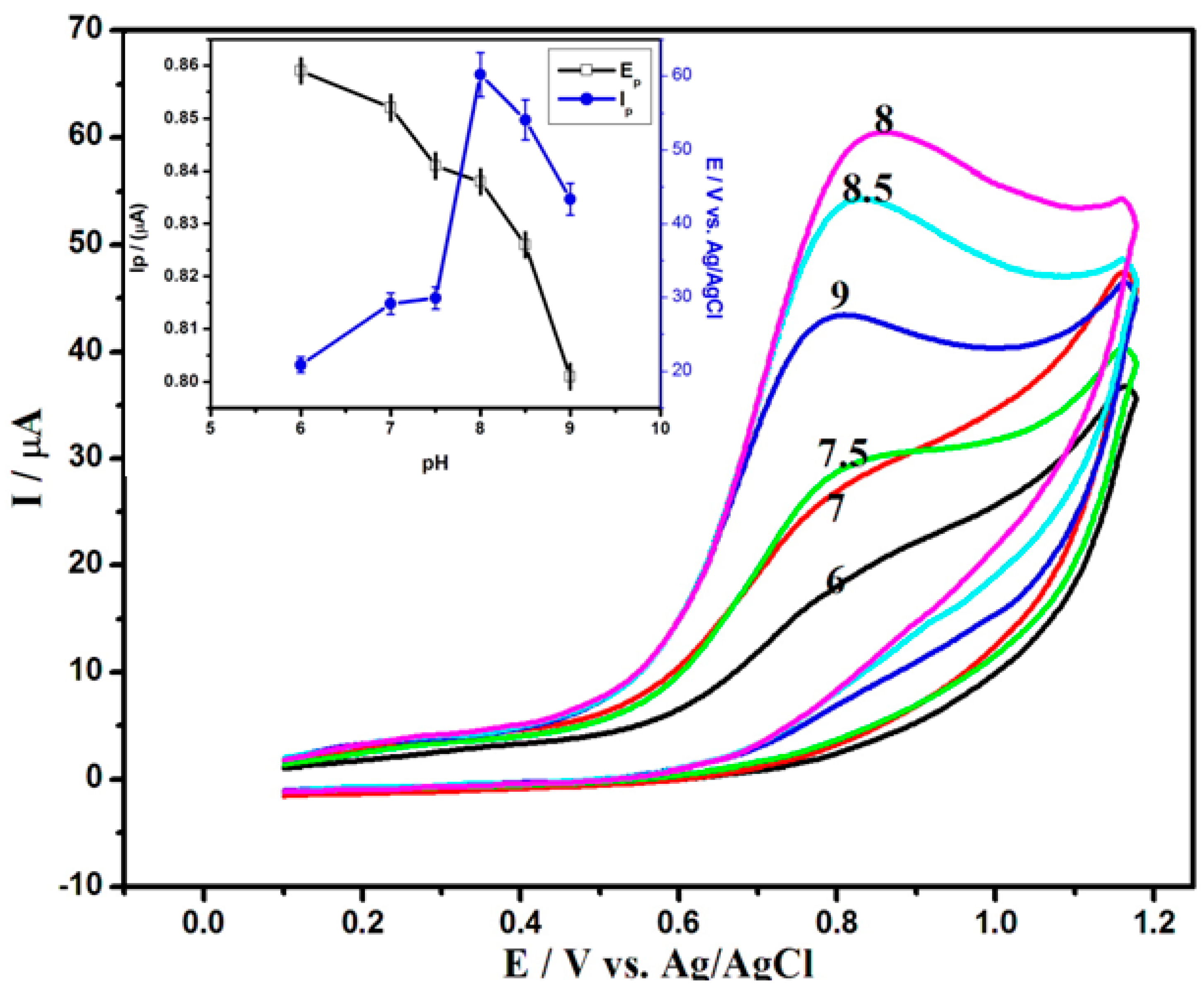
3.4. Effect of pH
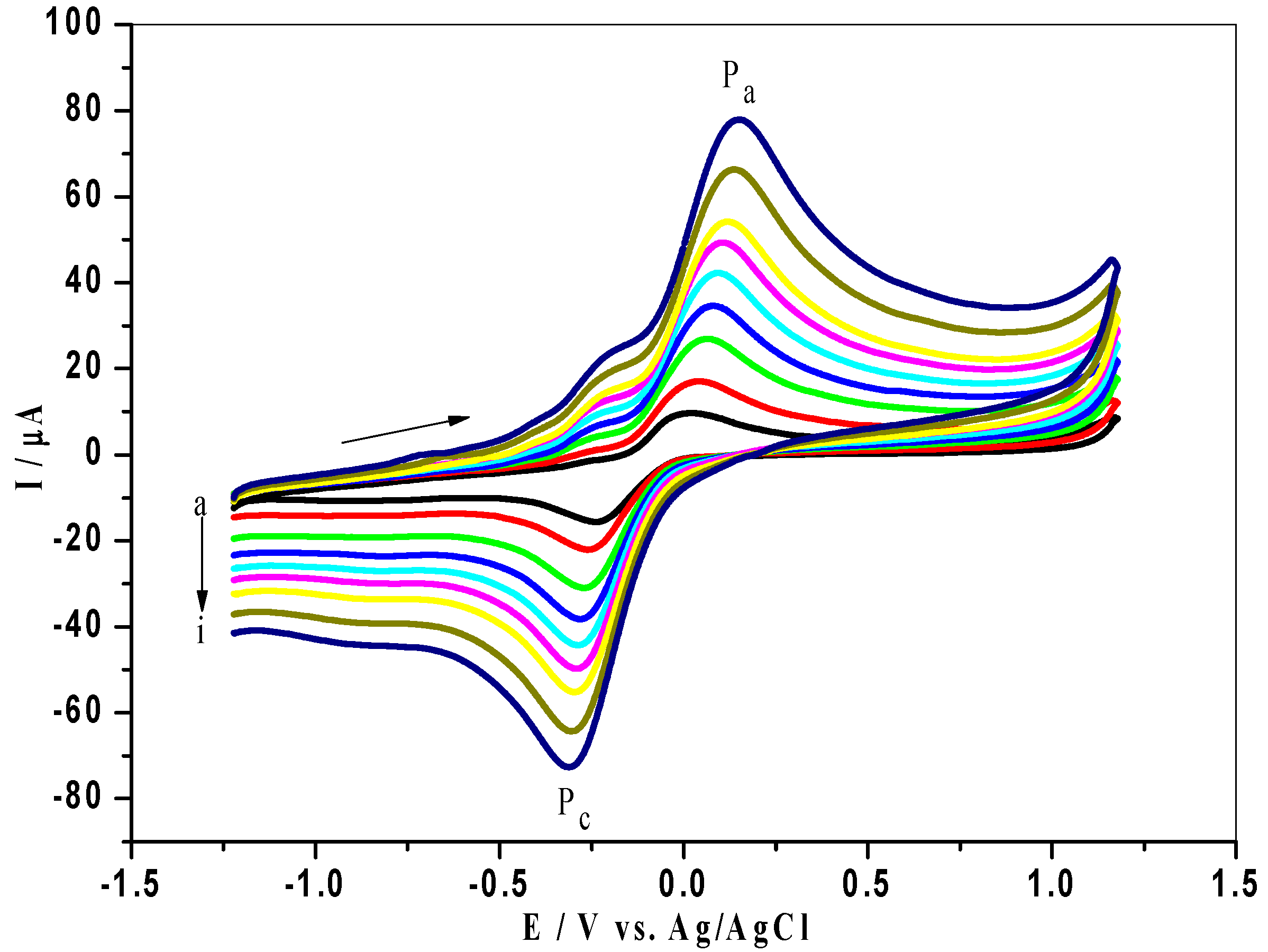
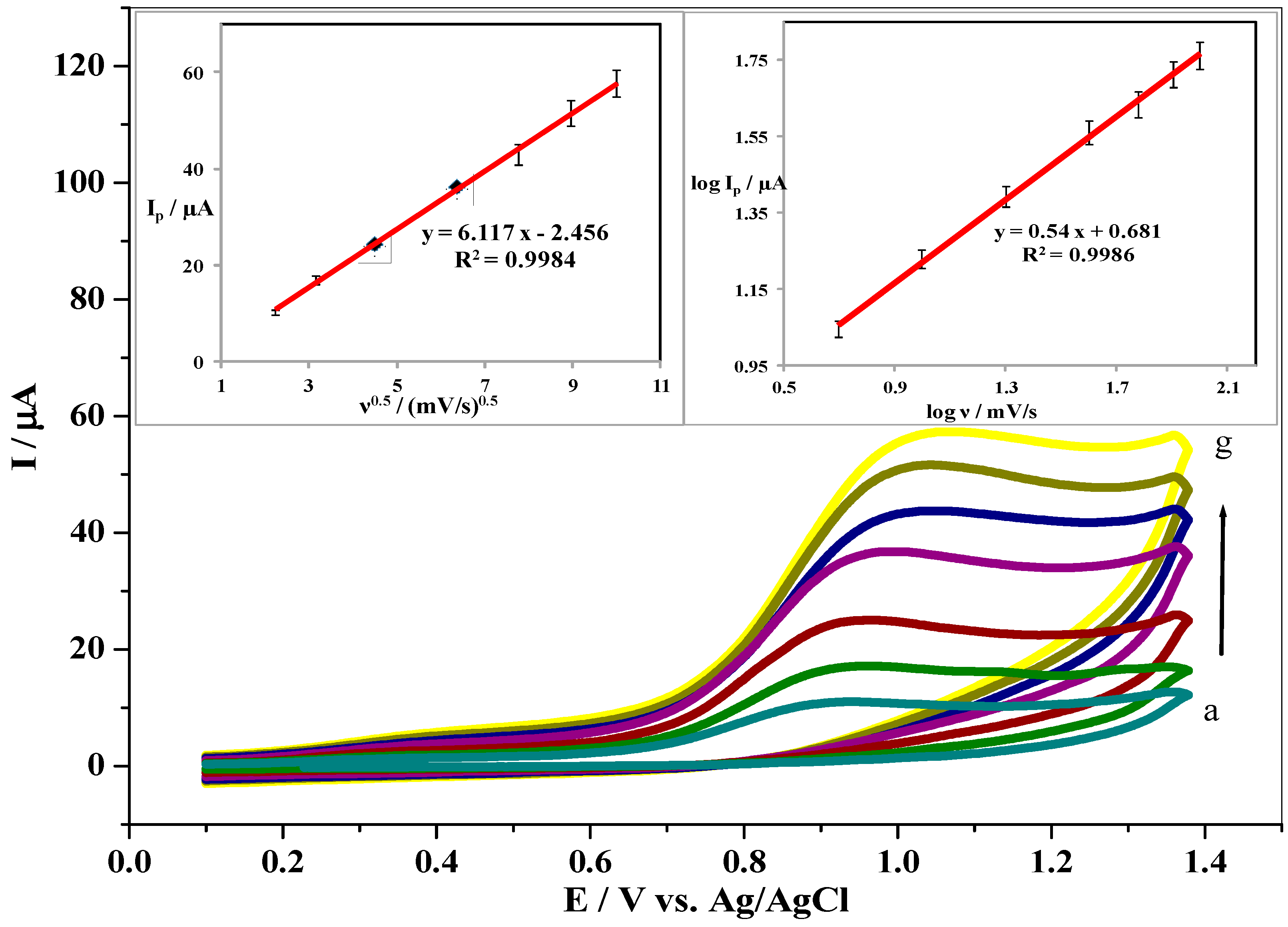
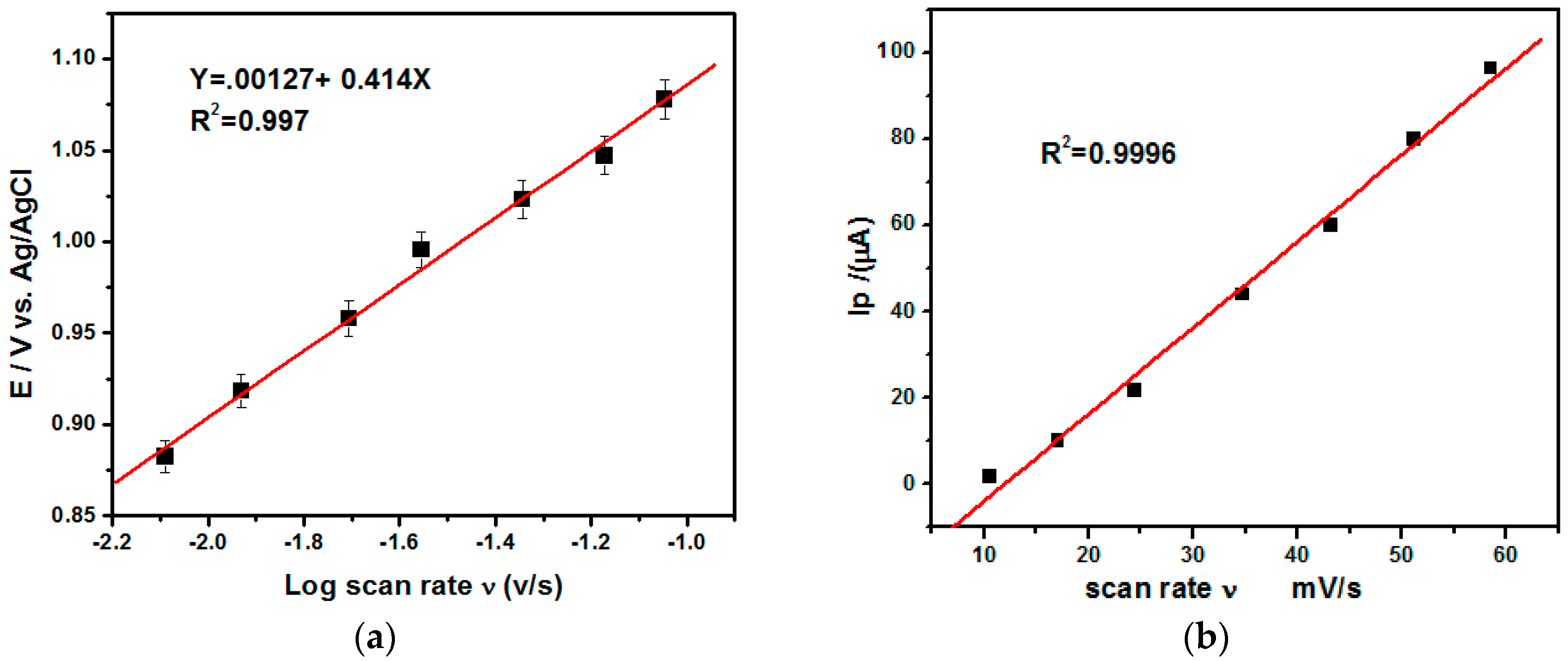
3.5. Effect of Scan Rate
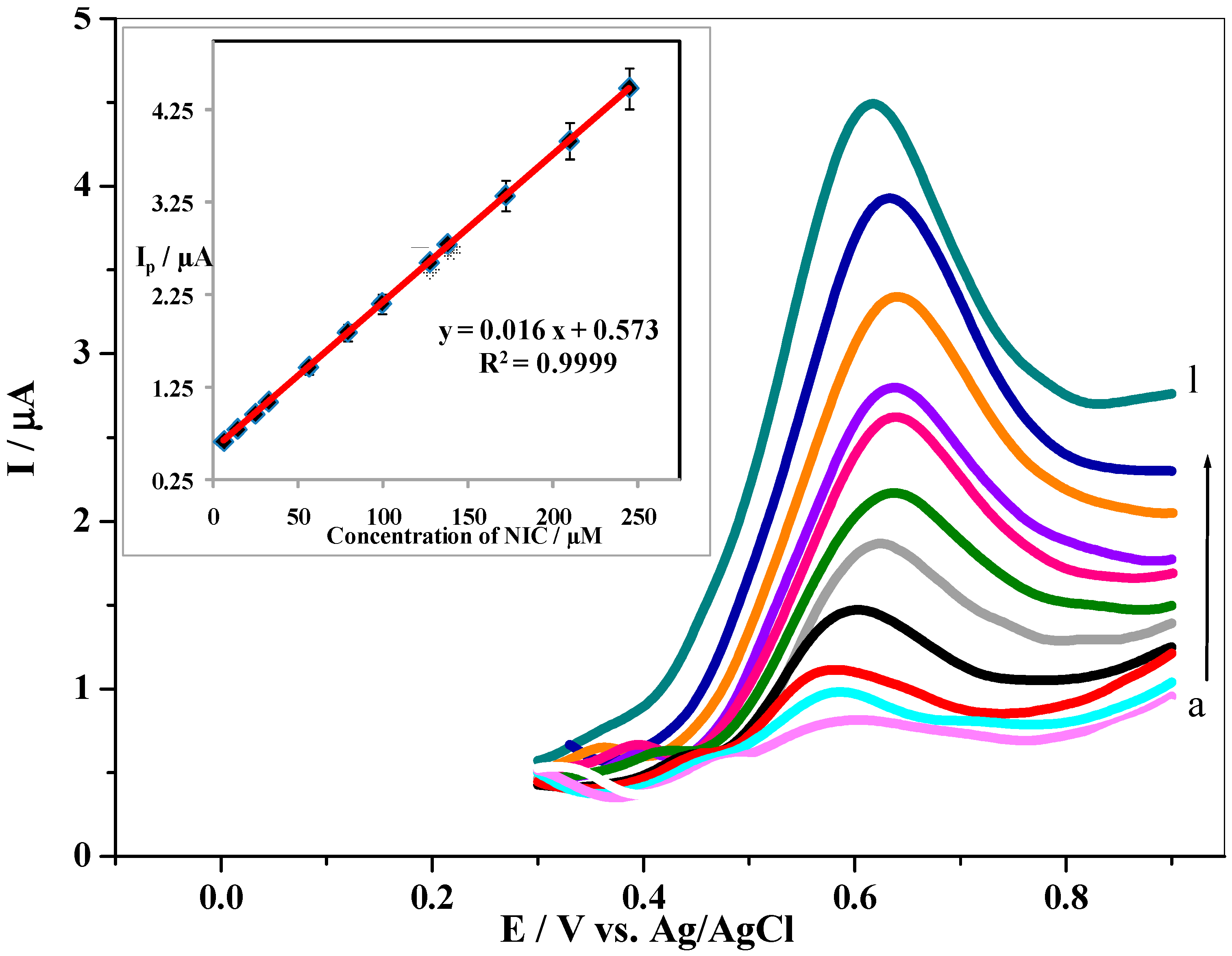
3.6. Effect of Concentration and Calibration Curve
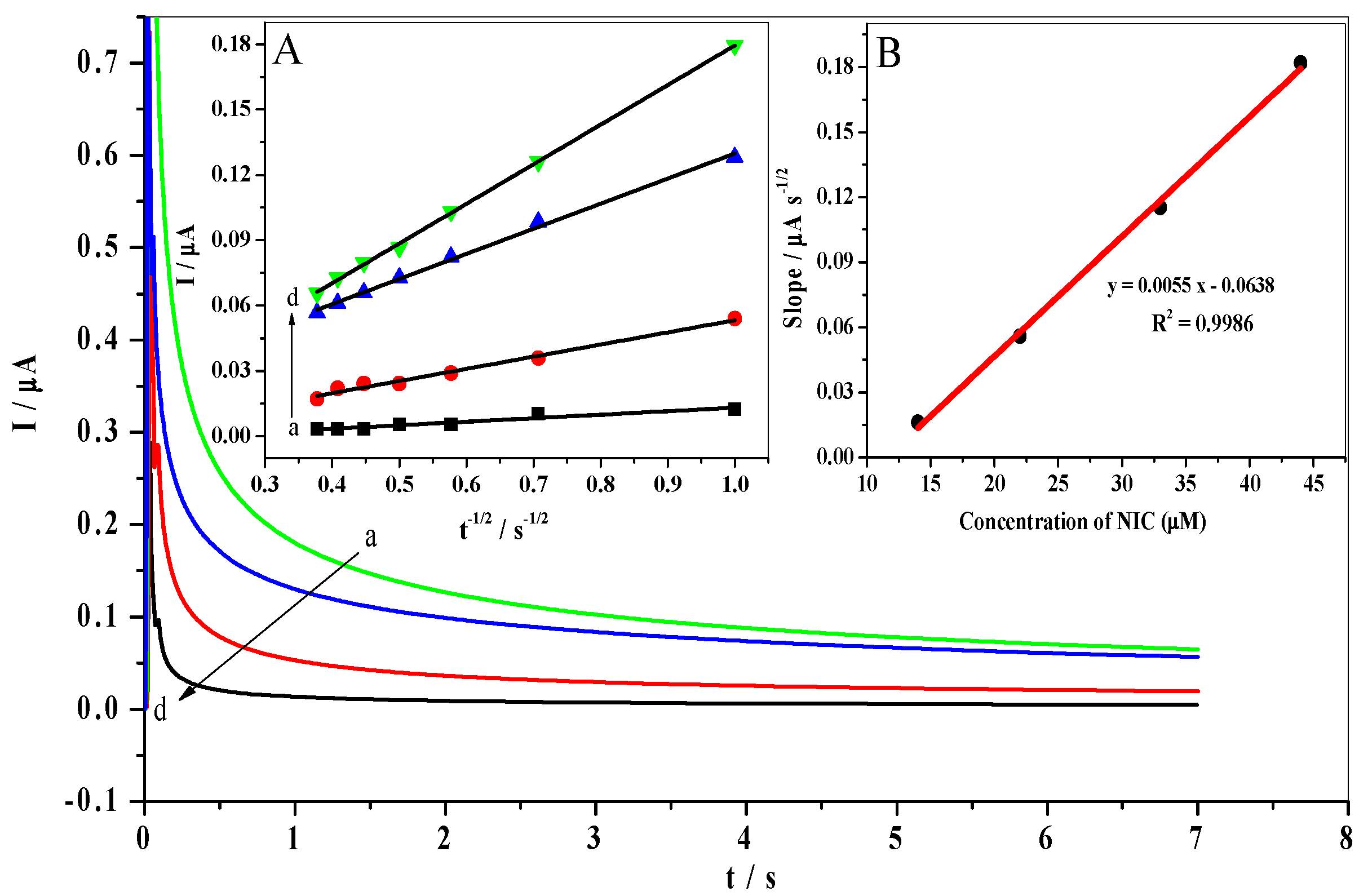
3.7. Chronoamperometric Measurements
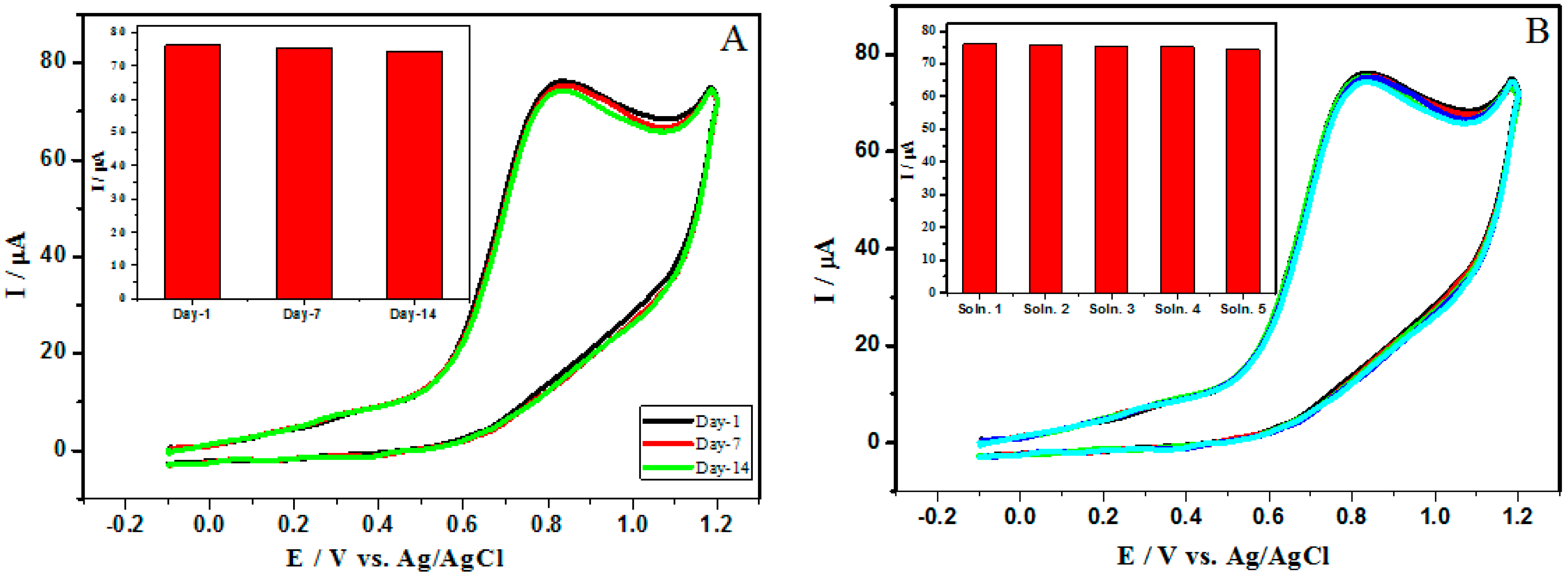
3.8. Stability and Reproducibility
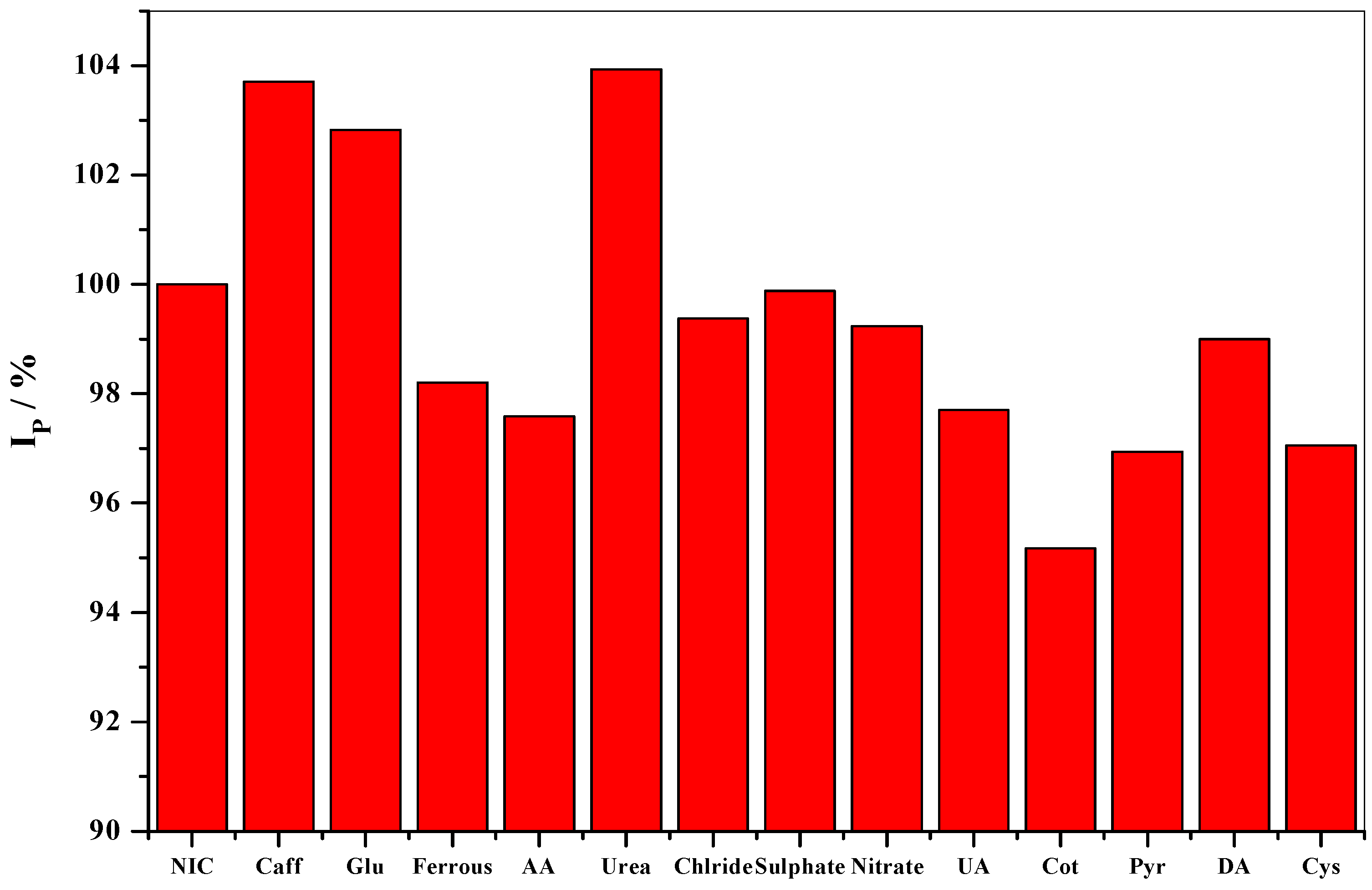
3.9. Interference Study
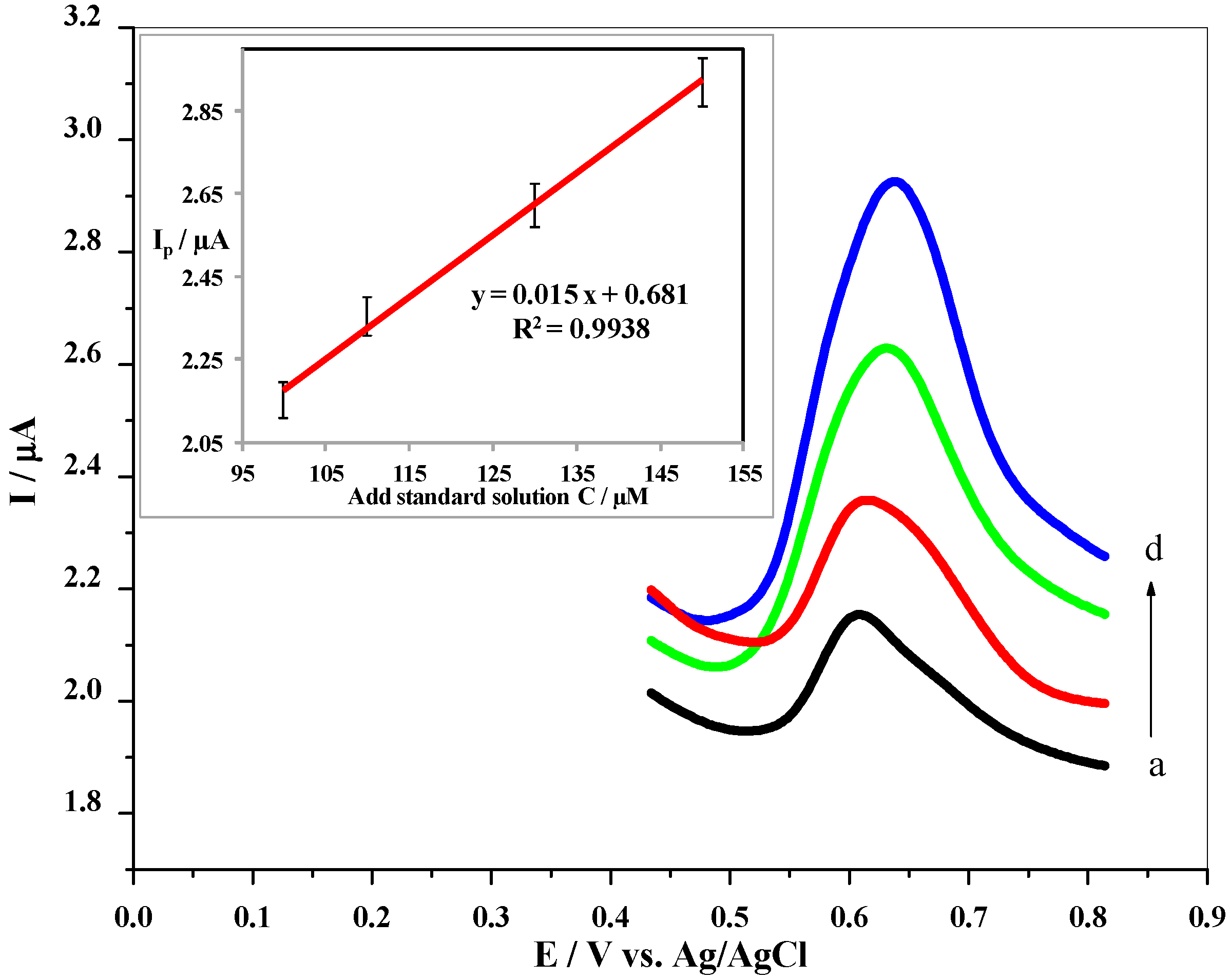
3.10. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Ramady, H.; Abdalla, N.; Alshaal, T.; Elhenawy, A.; Amer, M.; Shehata, A.; Paulsen, J.; Kleinwächter, M.; Selmar, D.; Taha, H. Contamination of Plant Foods with Nicotine: An Overview. DBG Reports. 2015. Available online: https://eprints.dbges.de/1088/ (accessed on 10 April 2022).
- Rahim, S.; Rauf, A.; Rauf, S.; Shah, M.R.; Malik, M.I. Enhanced electrochemical response of a modified glassy carbon electrode by poly (2-vinlypyridine-b-methyl methacrylate) conjugated gold nanoparticles for detection of nicotine. RSC Adv. 2018, 8, 35776–35786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharenberg, F.; Stegemann, T.; Çiçek, S.S.; Zidorn, C. Sequestration of pyridine alkaloids anabasine and nicotine from Nicotiana (Solanaceae) by Orobanche ramosa (Orobanchaceae). Biochem. Syst. Ecol. 2019, 86, 103908. [Google Scholar] [CrossRef]
- Marsh, A.; Clark, B.J.; Altria, K.D. Orthogonal separations of nicotine and nicotine-related alkaloids by various capillary electrophoretic modes. Electrophoresis 2004, 25, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Kowalcze, M.; Jakubowska, M. Voltammetric determination of nicotine in electronic cigarette liquids using a boron-doped diamond electrode (BDDE). Diam. Relat. Mater. 2020, 103, 107710. [Google Scholar] [CrossRef]
- Hannisdal, A.; Mikkelsen, Ø.; Schrøder, K.H. Analysis of nicotine in antismoking pharmaceutical products by differential pulse polarography and voltammetry. Collect. Czechoslov. Chem. Commun. 2007, 72, 1207–1213. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xiong, H.; Zhang, X.; Wang, S. Electrochemical behaviors of nicotine and its interaction with DNA. Electrochem. Commun. 2009, 11, 2129–2132. [Google Scholar] [CrossRef]
- Wu, C.-T.; Chen, P.-Y.; Chen, J.-G.; Suryanarayanan, V.; Ho, K.-C. Detection of nicotine based on molecularly imprinted TiO2-modified electrodes. Anal. Chim. Acta 2009, 633, 119–126. [Google Scholar] [CrossRef]
- Dushna, O.; Dubenska, L.; Plotycya, S.; Rydchuk, M.; Blazheyevskiy, M. The Alternative Voltammetric Method for the Determination of Nicotine and Its Metabolite Nicotine N-Oxide. J. Electrochem. Soc. 2022, 169, 16513. [Google Scholar] [CrossRef]
- Banerjee, J.; Al-Wadei, H.A.N.; Schuller, H.M. Chronic nicotine inhibits the therapeutic effects of gemcitabine on pancreatic cancer in vitro and in mouse xenografts. Eur. J. Cancer 2013, 49, 1152–1158. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.-C.; Chang, Y.-C. Enhancement of cancer stem-like and epithelial—Mesenchymal transdifferentiation property in oral epithelial cells with long-term nicotine exposure: Reversal by targeting SNAIL. Toxicol. Appl. Pharmacol. 2013, 266, 459–469. [Google Scholar] [CrossRef]
- Suto, M.J.; Zacharias, N. Neuronal nicotinic acetylcholine receptors as drug targets. Expert Opin. Ther. Targets 2004, 8, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, P.A.; Potter, A.; Kelton, M.; Corwin, J. Nicotinic treatment of Alzheimer’s disease. Biol. Psychiatry 2001, 49, 268–278. [Google Scholar] [CrossRef]
- Page-Sharp, M.; Hale, T.W.; Hackett, L.P.; Kristensen, J.H.; Ilett, K.F. Measurement of nicotine and cotinine in human milk by high-performance liquid chromatography with ultraviolet absorbance detection. J. Chromatogr. B 2003, 796, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Man, C.N.; Ismail, S.; Harn, G.L.; Lajis, R.; Awang, R. Determination of hair nicotine by gas chromatography–mass spectrometry. J. Chromatogr. B 2009, 877, 339–342. [Google Scholar] [CrossRef]
- Figueiredo, E.C.; de Oliveira, D.M.; de Siqueira, M.E.P.B.; Arruda, M.A.Z. On-line molecularly imprinted solid-phase extraction for the selective spectrophotometric determination of nicotine in the urine of smokers. Anal. Chim. Acta 2009, 635, 102–107. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, H.; Zhang, L.; Xu, H.; Wu, L.; Sun, J.; Wang, L. A new spectrofluorometric method for the determination of nicotine base on the inclusion interaction of methylene blue and cucurbit [7] uril. Microchim. Acta 2009, 164, 63–68. [Google Scholar] [CrossRef]
- Byrd, G.D.; Davis, R.A.; Ogden, M.W. A rapid LC-MS-MS method for the determination of nicotine and cotinine in serum and saliva samples from smokers: Validation and comparison with a radioimmunoassay method. J. Chromatogr. Sci. 2005, 43, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Mitsubayashi, K.; Nakayama, K.; Taniguchi, M.; Saito, H.; Otsuka, K.; Kudo, H. Bioelectronic sniffer for nicotine using enzyme inhibition. Anal. Chim. Acta 2006, 573, 69–74. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.-T.; Liu, Y.; Qi, X.-M.; Jin, H.-G.; Yang, C.; Liu, J.; Li, G.-L.; He, Q.-G. Recent advances in black phosphorus-based electrochemical sensors: A review. Anal. Chim. Acta 2021, 1170, 338480. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Zhang, G.; Wang, S.; Li, K.; Wu, J.; Wan, X.; Liu, Y.; Li, Q. Low-cost voltammetric sensors for robust determination of toxic Cd(II) and Pb(II) in environment and food based on shuttle-like α-Fe2O3 nanoparticles decorated β-Bi2O3 microspheres. Microchem. J. 2022, 179, 107515. [Google Scholar] [CrossRef]
- Giuliano, C.; Parikh, V.; Ward, J.R.; Chiamulera, C.; Sarter, M. Increases in cholinergic neurotransmission measured by using choline-sensitive microelectrodes: Enhanced detection by hydrolysis of acetylcholine on recording sites? Neurochem. Int. 2008, 52, 1343–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, N.; Li, J.; Chen, H.; Liao, C.; Chen, L. A functional graphene oxide-ionic liquid composites–gold nanoparticle sensing platform for ultrasensitive electrochemical detection of Hg2+. Analyst 2013, 138, 1091–1097. [Google Scholar] [CrossRef]
- Mehmeti, E.; Kilic, T.; Laur, C.; Carrara, S. Electrochemical determination of nicotine in smokers’ sweat. Microchem. J. 2020, 158, 105155. [Google Scholar] [CrossRef]
- Lakshmi, D.; Whitcombe, M.J.; Davis, F.; Sharma, P.S.; Prasad, B.B. Electrochemical detection of uric acid in mixed and clinical samples: A review. Electroanalysis 2011, 23, 305–320. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Lai, G.; Mahon, P.J.; Wang, J.; Zhu, D.; Jia, B.; Malherbe, F.; Yu, A. Carbon nanotube and graphene oxide directed electrochemical synthesis of silver dendrites. Rsc Adv. 2014, 4, 39645–39650. [Google Scholar] [CrossRef]
- Yang, Y.; Kang, M.; Fang, S.; Wang, M.; He, L.; Zhao, J.; Zhang, H.; Zhang, Z. Electrochemical biosensor based on three-dimensional reduced graphene oxide and polyaniline nanocomposite for selective detection of mercury ions. Sens. Actuators B Chem. 2015, 214, 63–69. [Google Scholar] [CrossRef]
- Wang, H.-S.; Li, T.-H.; Jia, W.-L.; Xu, H.-Y. Highly selective and sensitive determination of dopamine using a Nafion/carbon nanotubes coated poly (3-methylthiophene) modified electrode. Biosens. Bioelectron. 2006, 22, 664–669. [Google Scholar] [CrossRef]
- Sun, W.; Jiang, Q.; Yang, M.; Jiao, K. Electrochemical behaviors of hydroquinone on a carbon paste electrode with ionic liquid as binder. Bull. Korean Chem. Soc. 2008, 29, 915–920. [Google Scholar]
- Xue, Z.; Yin, B.; Wang, H.; Li, M.; Rao, H.; Liu, X.; Zhou, X.; Lu, X. An organic indicator functionalized graphene oxide nanocomposite-based colorimetric assay for the detection of sarcosine. Nanoscale 2016, 8, 5488–5496. [Google Scholar] [CrossRef]
- Abo-bakr, A.M.; Abd-Elsabour, M.; Abou-Krisha, M.M. An Efficient Novel Electrochemical Sensor for Simultaneous Determination of Vitamin C and Aspirin Based on a PMR/Zn-Al LDH/GCE. Electroanalysis 2021, 33, 2476–2489. [Google Scholar] [CrossRef]
- Rageh, H.M.; Abou-Krisha, M.M.; Abo-Bakr, A.M.; Abd-Elsabour, M. Electrochemical behavior and the detection limit of ascorbic acid on a Pt modified electrode. Int. J. Electrochem. Sci. 2015, 10, 4105–4115. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Muzyka, R.; Kwoka, M.; Smędowski, Ł.; Díez, N.; Gryglewicz, G. Oxidation of graphene by different modified Hummers methods. New Carbon Mater. 2017, 32, 15–20. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Zhang, D. Aqueous colloids of graphene oxide nanosheets by exfoliation of graphene oxide without ultrasonication. Bull. Mater. Sci. 2011, 34, 25–28. [Google Scholar] [CrossRef]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Kumar, R.; Singh, D.P. Graphene oxide: Strategies for synthesis, reduction and frontier applications. RSC Adv. 2016, 6, 64993–65011. [Google Scholar] [CrossRef]
- Neelakandan, S.; Noel Jacob, K.; Kanagaraj, P.; Sabarathinam, R.M.; Muthumeenal, A.; Nagendran, A. Effect of sulfonated graphene oxide on the performance enhancement of acid-base composite membranes for direct methanol fuel cells. RSC Adv. 2016, 6, 51599–51608. [Google Scholar] [CrossRef]
- Toghan, A.; Abd-Elsabour, M.; Abo-Bakr, A.M. A novel electrochemical sensor based on EDTA-NQS/GC for simultaneous determination of heavy metals. Sensors Actuators A Phys. 2021, 322, 112603. [Google Scholar] [CrossRef]
- Chaiyakun, S.; Witit-Anun, N.; Nuntawong, N.; Chindaudom, P.; Oaew, S.; Kedkeaw, C.; Limsuwan, P. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012, 32, 759–764. [Google Scholar]
- Manavalan, S.; Govindasamy, M.; Chen, S.-M.; Rajaji, U.; Chen, T.-W.; Ali, M.A.; Al-Hemaid, F.M.A.; Elshikh, M.S.; Farah, M.A. Reduced graphene oxide supported raspberry-like SrWO4 for sensitive detection of catechol in green tea and drinking water samples. J. Taiwan Inst. Chem. Eng. 2018, 89, 215–223. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Kang, X.; Wang, C.; Wang, D.; Liu, J.; Aksay, I.A.; Lin, Y. Glucose biosensor based on immobilization of glucose oxidase in platinum nanoparticles/graphene/chitosan nanocomposite film. Talanta 2009, 80, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Lin, E.; Su, X.; Liu, Y.; Li, H.; Yuan, X.; Ping, L.; Fan, Y. Electrodeposition of Au nanoparticles on poly(diallyldimethylammonium chloride) functionalized reduced graphene oxide sheets for voltammetric determination of nicotine in tobacco products and anti-smoking pharmaceuticals. RSC Adv. 2016, 6, 26247–26253. [Google Scholar] [CrossRef]
- Cheng, M.M.; Huang, L.J.; Wang, Y.X.; Tang, J.G.; Wang, Y.; Zhao, Y.C.; Liu, G.F.; Zhang, Y.; Kipper, M.J.; Wickramasinghe, S.R. Reduced graphene oxide–gold nanoparticle membrane for water purification. Sep. Sci. Technol. 2019, 54, 1079–1085. [Google Scholar] [CrossRef]
- You, Z.; Fu, Y.; Xiao, A.; Liu, L.; Huang, S. Magnetic molecularly imprinting polymers and reduced graphene oxide modified electrochemical sensor for the selective and sensitive determination of luteolin in natural extract. Arab. J. Chem. 2021, 14, 102990. [Google Scholar] [CrossRef]
- Mohammadi, S.Z.; Beitollahi, H.; Dehghan, Z.; Hosseinzadeh, R. Electrochemical determination of ascorbic acid, uric acid and folic acid using carbon paste electrode modified with novel synthesized ferrocene derivative and core–shell magnetic nanoparticles in aqueous media. Appl. Organomet. Chem. 2018, 32, e4551. [Google Scholar] [CrossRef]
- Rageh, H.; Abdel-sabour, M. Pharmaceutical electrochemistry: The electrochemical behaviour of paracetamol at ZnO nanoparticales/1, 2-napthaquinone-4-sulphonic acid glassy carbon modified electrode. Anal Bioanal Electrochem. 2017, 9, 351–364. [Google Scholar]
- Suffredini, H.B.; de Santos, M.C.; De Souza, D.; Codognoto, L.; Homem-de-Mello, P.; Honório, K.M.; Da Silva, A.B.F.; Machado, S.A.S.; Avaca, L.A. Electrochemical behavior of nicotine studied by voltammetric techniques at boron-doped diamond electrodes. Anal. Lett. 2005, 38, 1587–1599. [Google Scholar] [CrossRef]
- Levent, A.; Yardim, Y.; Senturk, Z. Voltammetric behavior of nicotine at pencil graphene electrode and its enhancement determination in the presence of anionic surfactant. Electrochim. Acta 2009, 55, 190–195. [Google Scholar] [CrossRef]
- Khodari, M.; Rabie, E.M.; Assaf, H.F. A new electrochemical sensor based on TiO2 nanoparticles modified carbon paste electrode for voltammetric determination of phenol. Int. J. Sci. Res. 2015, 5, 1501–1505. [Google Scholar]
- Švorc, Ľ.; Stanković, D.M.; Kalcher, K. Boron-doped diamond electrochemical sensor for sensitive determination of nicotine in tobacco products and anti-smoking pharmaceuticals. Diam. Relat. Mater. 2014, 42, 1–7. [Google Scholar] [CrossRef]
- Assaf, H.F.; Salah, H.; Hashem, N.; Khodari, M.; Toghan, A. Fabrication of an electrochemical sensor based on copper waste wire recycling and its application. Sens. Actuators A Phys. 2021, 331, 112962. [Google Scholar] [CrossRef]
- Yang, S.S.; Smetena, I. Evaluation of capillary electrophoresis for the analysis of nicotine and selected minor alkaloids from tobacco. Chromatographia 1995, 40, 375–378. [Google Scholar] [CrossRef]
- Swain, G.M. Solid electrode materials: Pretreatment and activation. In Handbook of Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2007; pp. 111–153. [Google Scholar]
- Nicholson, R.S.; Shain, I. Theory of Stationary Electrode Polarography. Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems. Anal. Chem. 1964, 36, 706–723. [Google Scholar] [CrossRef]
- Rabie, E.M.; Assaf, H.F.; Shamroukh, A.A.; Khodari, M. Fabrication of a New Electrochemical Sensor Based on Carbon Paste Electrode Modified by Silica gel/MWCNTs for the Voltammetric Determination of Salicylic Acid in Tomato. Egypt. J. Chem. 2019, 62, 165–175. [Google Scholar] [CrossRef]
- Highton, L.; Kadara, R.O.; Jenkinson, N.; Logan Riehl, B.; Banks, C.E. Metallic free carbon nanotube cluster modified screen printed electrodes for the sensing of nicotine in artificial saliva. Electroanalysis 2009, 21, 2387–2389. [Google Scholar] [CrossRef]
- Miller, J.C.; Miller, J.N. Basic statistical methods for analytical chemistry. Part, I. Statistics of repeated measurements. A review. Analyst 1988, 113, 1351–1356. [Google Scholar] [CrossRef]
- Karthika, A.; Karuppasamy, P.; Selvarajan, S.; Suganthi, A.; Rajarajan, M. Electrochemical sensing of nicotine using CuWO4 decorated reduced graphene oxide immobilized glassy carbon electrode. Ultrason. Sonochem. 2019, 55, 196–206. [Google Scholar] [CrossRef]
- Lo, T.W.B.; Aldous, L.; Compton, R.G. The use of nano-carbon as an alternative to multi-walled carbon nanotubes in modified electrodes for adsorptive stripping voltammetry. Sens. Actuators B Chem. 2012, 162, 361–368. [Google Scholar] [CrossRef]
- Bai, H.; Li, C.; Wang, X.; Shi, G. A pH-sensitive graphene oxide composite hydrogel. Chem. Commun. 2010, 46, 2376–2378. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Yu, B.; Li, P.; Xiong, B.; Cheng, Y.; Li, Y.; Li, C.; Xiao, X.; Chen, M.; Chen, L. Synthesis of graphene/DPA composite for determination of nicotine in tobacco products. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, M.J.; Rees, N.V.; Dickinson, E.J.F.; Compton, R.G. Effects of thin-layer diffusion in the electrochemical detection of nicotine on basal plane pyrolytic graphene (BPPG) electrodes modified with layers of multi-walled carbon nanotubes (MWCNT-BPPG). Sens. Actuators B Chem. 2010, 144, 153–158. [Google Scholar] [CrossRef]
- Geto, A.; Amare, M.; Tessema, M.; Admassie, S. Voltammetric Determination of Nicotine at Poly (4-Amino-3-Hydroxynaphthalene Sulfonic Acid)-Modified Glassy Carbon Electrode. Electroanalysis 2012, 24, 659–665. [Google Scholar] [CrossRef]
- Kassa, H.; Geto, A.; Admassie, S. Voltammetric determination of nicotine in cigarette tobacco at electrochemically activated glassy carbon electrode. Bull. Chem. Soc. Ethiop. 2013, 27, 321–328. [Google Scholar] [CrossRef]
- Jing, Y.; Yuan, X.; Yuan, Q.; He, K.; Liu, Y.; Lu, P.; Li, H.; Li, B.; Zhan, H.; Li, G. Determination of nicotine in tobacco products based on mussel-inspired reduced graphene oxide-supported gold nanoparticles. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bard, A.J.; Faulkner, L.R. Faulkner, Electrochemical Methods: Fundamentals and Applications, 2nd ed; Wiley: New York, NY, USA, 2001. [Google Scholar] [CrossRef]

| Technique | Working Electrode | LOD (M) | Linear Range (µM) | Ref. |
|---|---|---|---|---|
| DPV | SPCE | 0.6 × 10−6 | 1–375 | [24] |
| Amp | CuWO4/rGO/Nf/GCE | 3.5 × 10−8 | 0.1–0.9 | [61] |
| CV | MWCNTs/CuNPs | 6.0 × 10−9 | 1–1000 | [62] |
| DPV | Poly(ARS)-GR/SPCE | 4.6 × 10−6 | 30–1000 | [63] |
| DPV | RGO/DPA/PGE | 7.60 × 10−6 | 31–1900 | [64] |
| CV | MWCNT-BPPG | 1.50 × 10−6 | 10–1000 | [65] |
| SWV | p-AHNSA/GCE | 0.90 × 10−6 | 1–200 | [66] |
| SWV | EA/GCE | 0.70 × 10−6 | 1–200 | [67] |
| SWV | CNC/SPCE | 2.00 × 10−6 | 10–1000 | [59] |
| Amp | PDA-RGO/Au/GCE | 1.50 × 10−8 | 0.05–500 | [68] |
| DPV | GO/Nq/GCE | 1.27 × 10−8 | 6.5–245 | This work |
| Sample | Added (µM) | Founded (µM) | Recovery (%) |
|---|---|---|---|
| Cigarette | 0.0 | 21.13 | – |
| 50 | 70.73 | 99.44 | |
| 100 | 121.53 | 100.33 | |
| Urine | 50 | 50.07 | 100.14 |
| 100 | 99.63 | 99.63 | |
| 200 | 199.4 | 99.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Elsabour, M.; Alsoghier, H.M.; Alhamzani, A.G.; Abou-Krisha, M.M.; Yousef, T.A.; Assaf, H.F. A Novel Electrochemical Sensor for Detection of Nicotine in Tobacco Products Based on Graphene Oxide Nanosheets Conjugated with (1,2-Naphthoquinone-4-Sulphonic Acid) Modified Glassy Carbon Electrode. Nanomaterials 2022, 12, 2354. https://doi.org/10.3390/nano12142354
Abd-Elsabour M, Alsoghier HM, Alhamzani AG, Abou-Krisha MM, Yousef TA, Assaf HF. A Novel Electrochemical Sensor for Detection of Nicotine in Tobacco Products Based on Graphene Oxide Nanosheets Conjugated with (1,2-Naphthoquinone-4-Sulphonic Acid) Modified Glassy Carbon Electrode. Nanomaterials. 2022; 12(14):2354. https://doi.org/10.3390/nano12142354
Chicago/Turabian StyleAbd-Elsabour, M., Hesham M. Alsoghier, Abdulrahman G. Alhamzani, Mortaga M. Abou-Krisha, Tarek A. Yousef, and Hytham F. Assaf. 2022. "A Novel Electrochemical Sensor for Detection of Nicotine in Tobacco Products Based on Graphene Oxide Nanosheets Conjugated with (1,2-Naphthoquinone-4-Sulphonic Acid) Modified Glassy Carbon Electrode" Nanomaterials 12, no. 14: 2354. https://doi.org/10.3390/nano12142354






