Heteroatom-Doped Porous Carbon-Based Nanostructures for Electrochemical CO2 Reduction
Abstract
:1. Introduction
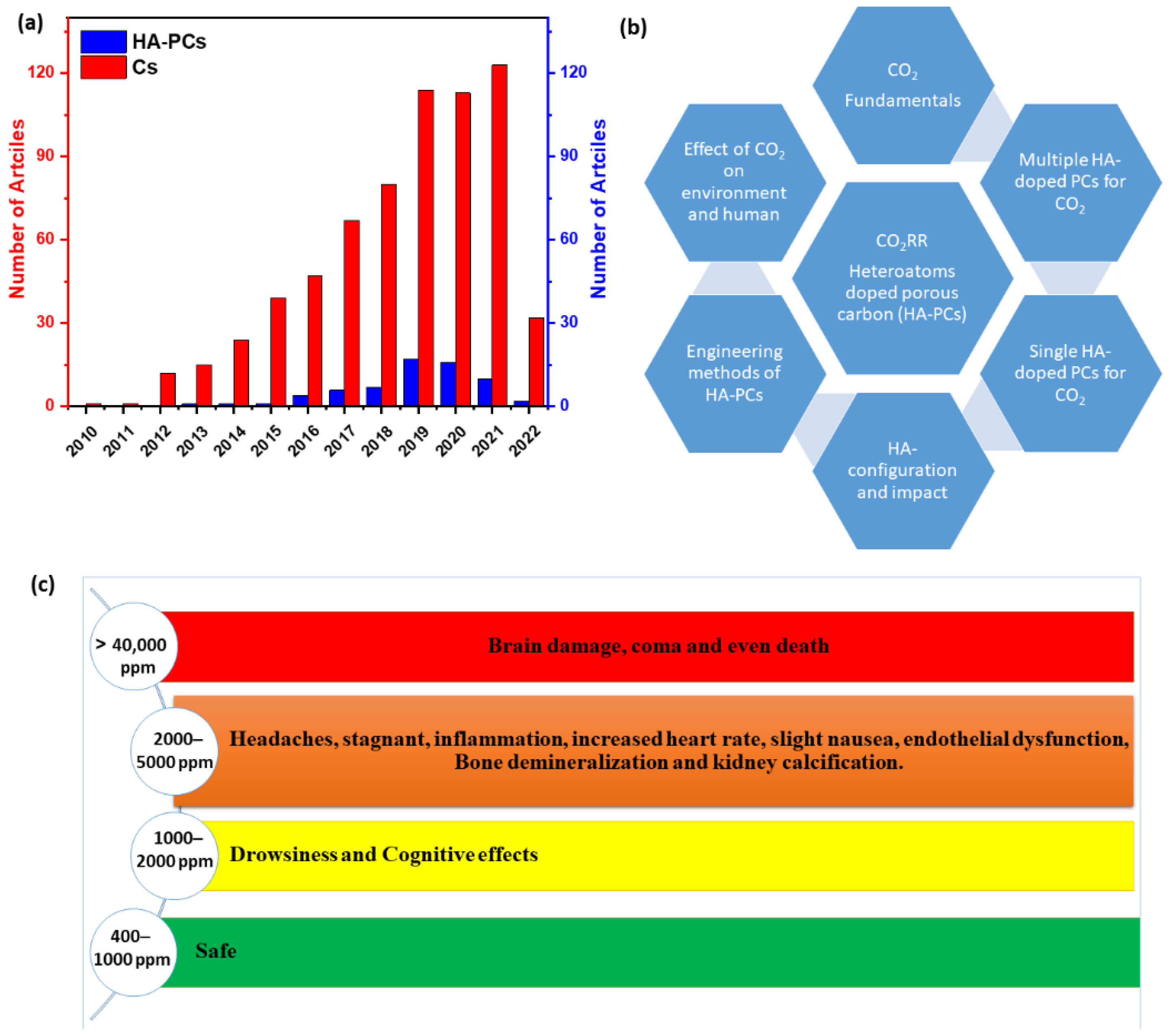
2. Effect of CO2 on the Environment and Human Health
3. Fundamental Parameters for CO2RR Performance
3.1. Faradaic Efficiency (FE)
3.2. Overpotential
3.3. Partial Current Density
3.4. Durability
3.5. Energy Efficiency (Eeff)
3.6. Turnover Frequency (TOF)
4. Engineering Methods of Heteroatom-Doped Porous Carbon
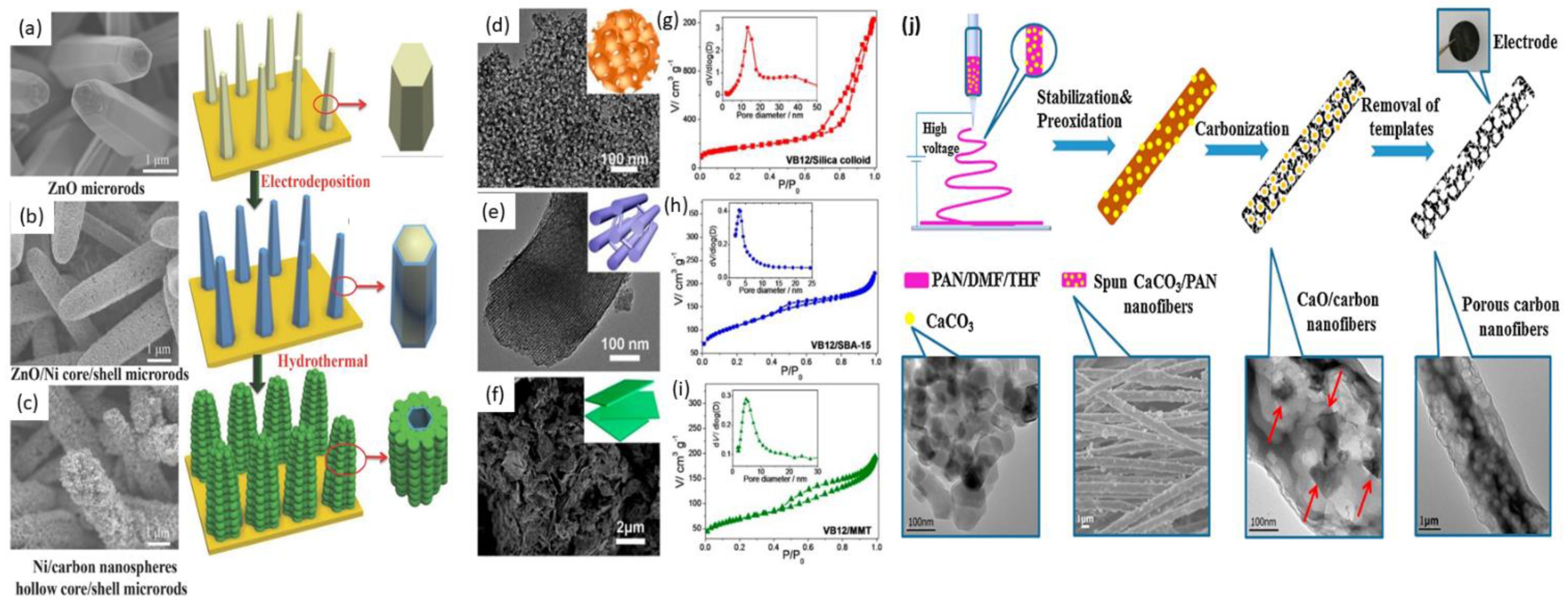
4.1. Template-Based Method
4.1.1. Hard Templates
4.1.2. Ca-Based Templates
4.1.3. New Templates
4.1.4. Organic Soft Templates
4.1.5. Ionic Liquids
4.1.6. MOF Template
4.1.7. Biomass-Derived Carbons
4.2. CO2RR Pathways
4.3. Heteroatom-Doping Configuration and Effects
4.3.1. Advantages of Heteroatoms
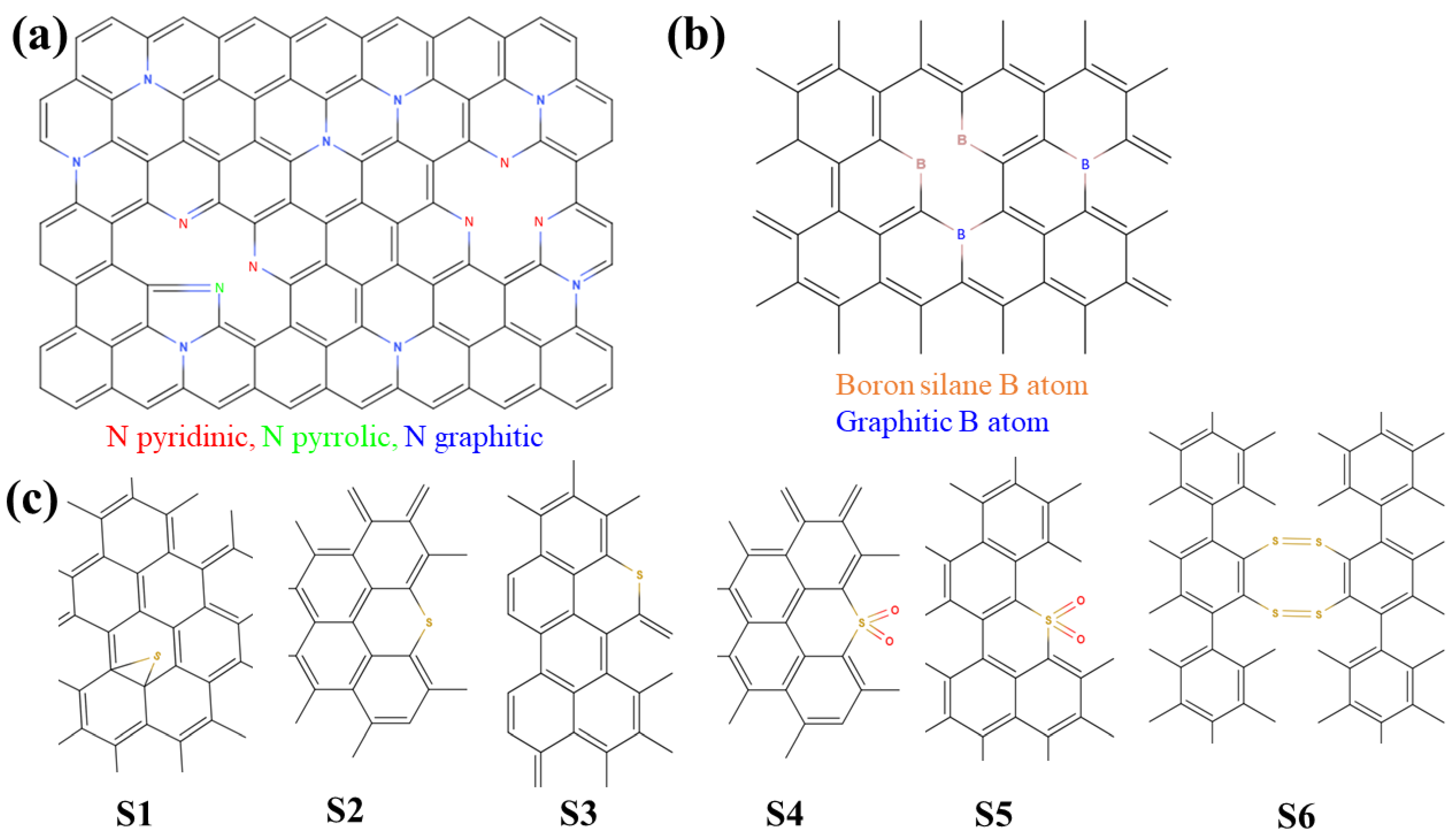
4.3.2. Nitrogen (N) Configuration and Effects
4.3.3. Boron (B) Configuration and Effects
4.3.4. Sulfur (S) Configuration and Effects
4.3.5. Phosphorus (P) Configuration and Effects
4.3.6. Oxygen (O) Configuration and Effects
4.4. Single Heteroatom-Doped Porous Carbon Materials
4.4.1. Nitrogen-Doped Porous Carbon Materials
4.4.2. Other Metal-Free Heteroatom (S, F, or B)-Doped Porous Carbon Materials
| Electrocatalysts | Synthetic Method | Electrolyte | Main Product | Potential of FEmax | sFEmax (%)/jCO (mAcm−2) | Durability | Refs. |
|---|---|---|---|---|---|---|---|
| (vs. RHE) | |||||||
| NC-900 | Hydrothermal synthesis and calcination of Typha in NH3 at 900 °C | 0.5 M KHCO3 | CO | −0.5 | 82%/~1.25 mA·cm−2 | FECO stability 75% after 10 h | [116] |
| N-GRW (GM2) | The first polymerization of melamine and L-cysteine to form C3N4 at 600 °C, followed by carbonization at higher temperatures | 0.5 M KHCO3 | CO | −0.4 | 87.6%/~7.8 mA·cm−2 | FECO stability 80% after 16 h | [110] |
| TTF-1 | Thermal treatment of 2, 6- dicyanopyridine and ZnCl2 at 600 °C for 40 h | 0.5 M KHCO3 | CO | −0.68 | 82%/~−1 mA·cm−2 | FECO stability 75% after 12 h | [115] |
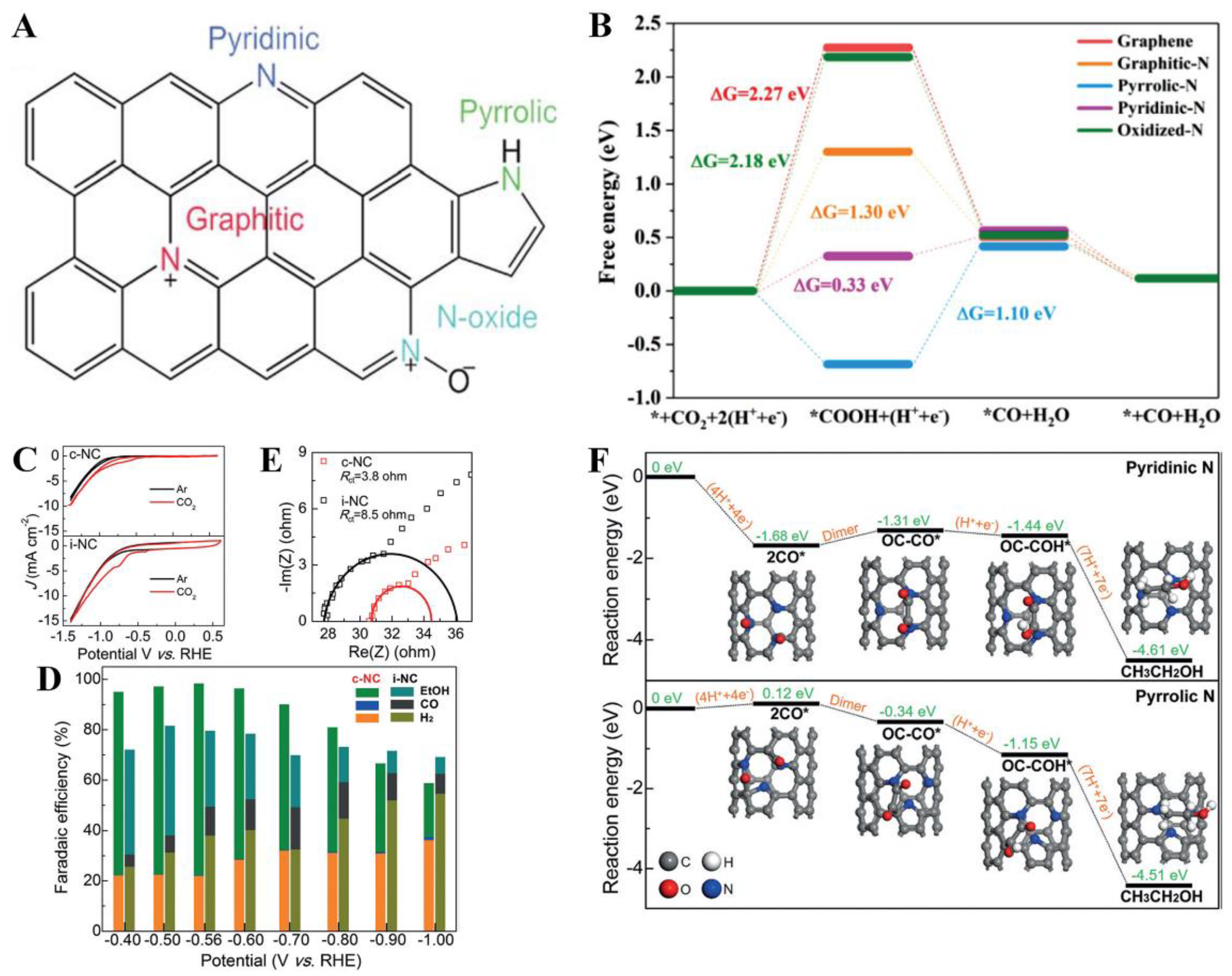
| c-NC | A soft-template method via the self-assembly of resol, F127, and dicyandiamide | 0.1 M KHCO3 | CH3CH2OH | −0.56 | 77%/~−0.35 mA·cm−2 | FECO stability 77% after 6 h | [120] |
| MNC-D | Pyrolysis of ZIF-8 at 900 °C for 3 h and mixed with HCl, followed by treatment in dimethylformamide | 0.1 M KHCO3 | CO | −0.58 | ∼ 92%/∼−6.1 mA·cm−2 | FECO stability ∼86% after 16 h | [108] |
| NPC-1000 | High-temperature annealing of the mixture of oxygen-rich Zn-MOF-74 and melamine at 1000 °C | 0.5 M KHCO3 | CO | −0.55 | 98.4%/−3.01 mA·cm−2 | FECO stability ∼98% after 21 h | [119] |
| NPC-900 | One-step pyrolysis method via the self-assembly of anthracite coal, KOH, and dicyandiamide | 0.5 M KHCO3 | CO | −0.67 | 95%/−4.8 mA cm−2 | FECO stability ∼ 80% after 10 h | [118] |
| BAX-M-950 | Soaking commercial activated carbon BAX-1500 in a melamine suspension in ethanol followed by evaporation and drying, then heating at 950 °C in N2 | 0.1 M KHCO3 | CO, CH4 | −0.66, −0.76 | 40%, 1.2%/−3 mA cm−2 | FECO stability ∼20%, 1.1% after 24 h | [111] |
| CNPC-1100 | Etching coal powder in ammonia atmosphere | 0.1 M KHCO3 | CO | −0.6 | 92%/−4.6mA cm−2 | FECO stability ∼62.5% after 8 h | [112] |
| WNCNs-1000 | An NH3 etching strategy by using NaCl and coal tar pitch as templates and precursor | 0.1 M KHCO3 | CO | −0.49 (overpotential) | 84%/~−1.26 mA cm−2 | FECO stability ∼81% after 8 h | [106] |
| NDC-700 | One-step pyrolysis of wheat flour and KOH | 0.5 M NaHCO3 | CO | −0.82 | 83.7%/~−8 mA cm−2 | FECO stability ∼79.4% after 2 h | [109] |
| PNC | High-temperature calcination by using melamine as the nitrogen source and pentaerythritol as the carbon source | 0.1 M KHCO3 | CO | −0.6 | 74%/~−4 mA cm−2 | FECO stability ∼70% after 10 h | [121] |
| N/C-Cl-1100 | Halogen-assisted calcination of ZIF-8 at 1100 °C | 0.1 M KHCO3 | CO | −0.5 | 99.5%/~−2.6mA cm−2 | FECO stability ∼99% after 20 h | [117] |
| HPC | Hydrothermal treatment of moss at 180 °C for 24 h, followed by pyrolyzing at 900 °C for 2 h and acidic etching | 0.5 M KHCO3 | CH4, C2H5OH, CH3OH | −1.2 (vs. Ag/AgCl) | 56, 26, 10.5%/~−15 mA cm−2 | FECO stability ∼92.6% after 30 h | [123] |
| NDAPC | Pyrolysis of petroleum pitch under nitrogen atmosphere followed by ammonia etching | 0.1 M KHCO3 | CO | −0.9 | 83%/~−3.76 mA cm−2 | FECO stability ∼80% after 8 h | [113] |
| NG-800 | The first formation of 3D graphene foam by chemical vapor deposition and post-doped with graphitic-C3N4, followed by etching Ni with HCl | 0.1 M KHCO3 | CO | −0.58 | 85%/~−1.8 mA cm−2 | FECO stability ∼80% after 5 h | [114] |
| NPC-600 | Hydrothermal treatment of SBA-15 and digested sludge | 0.1 M NaHCO3 | Formate | −1.5 (vs. SCE) | 68%/~−7.5 mA cm−2 | FECO stability ∼68% after 4 h | [122] |
| P-NC | The calcination of sucrose, urea, and NaCl at 800 °C for 4 h | 0.5 M KHCO3 | CO | −0.8 | 81.3%/~−7.2 mA cm−2 | FECO stability ∼81% after 6 h | [104] |
| NC1100 | The calcination of ZIF-8 at 1100°C in Ar | 0.5 M KHCO3 | CO | −0.5 | 95.4%/~−3 mA cm−2 | FECO stability ∼90% after 20 h | [107] |
| F-CPC | An aldol reaction conducted at SiO2 surface, followed by calcination at 900℃, activation with CO2, and removal with HF | 0.5 M KHCO3 | CO | −1.0 | 88.3%/~−37.5 mA cm−2 | FECO stability ∼85% after 12 h | [127] |
| FC | Pyrolyzing the mixture of commercial BP 2000 and polytetrafluoroethylene | 0.1 M NaClO4 | CO | −0.62 | 89.6%/~−0.25 mA cm−2 | - | [128] |
| BG | Heating the uniform mixture of graphene oxide and boric acid at 900 °C in Ar | 0.1 M KHCO3 | HCOOH | −1.4 (vs. SCE) | 66%/~−3 mA cm−2 | FECO stability ∼66% after 4 h | [129] |
4.5. Binary Heteroatom-Doped Porous Carbon Materials
4.5.1. Nitrogen, Sulfur Co-Doped Porous Carbon Materials
4.5.2. Nitrogen, Phosphorus Co-Doped Porous Carbon Materials
4.5.3. Nitrogen, Boron Co-Doped Porous Carbon Materials
| Electrocatalysts | Synthetic Method | Electrolyte | Main Products | Potential of FEmax | FEmax (%)/jCO (mAcm−2) | Durability | Refs. |
|---|---|---|---|---|---|---|---|
| (vs. RHE) | |||||||
| Binary HA-PCs | |||||||
| CPSN | The carbonization of poly(4-styrenesulfonic acid-co-maleic acid) sodium salt at 800 °C, followed by impregnation with urea-saturated solution and holding at 800 °C in N2 for 30 min | 0.1 M KHCO3 | CO | −0.99 | 11.3, 0.18%/ ~−4 mA cm−2 | FECO stability ∼8, 0.126% after 27, 2 h | [130] |
| CH4 | |||||||
| NSHCF900 | The carbonization of polymer nanofiber at 900 °C in Ar | 0.1 M KHCO3 | CO | −0.7 | 94%/ ~−103 mA cm−2 | FECO stability ∼93% after 36 h | [133] |
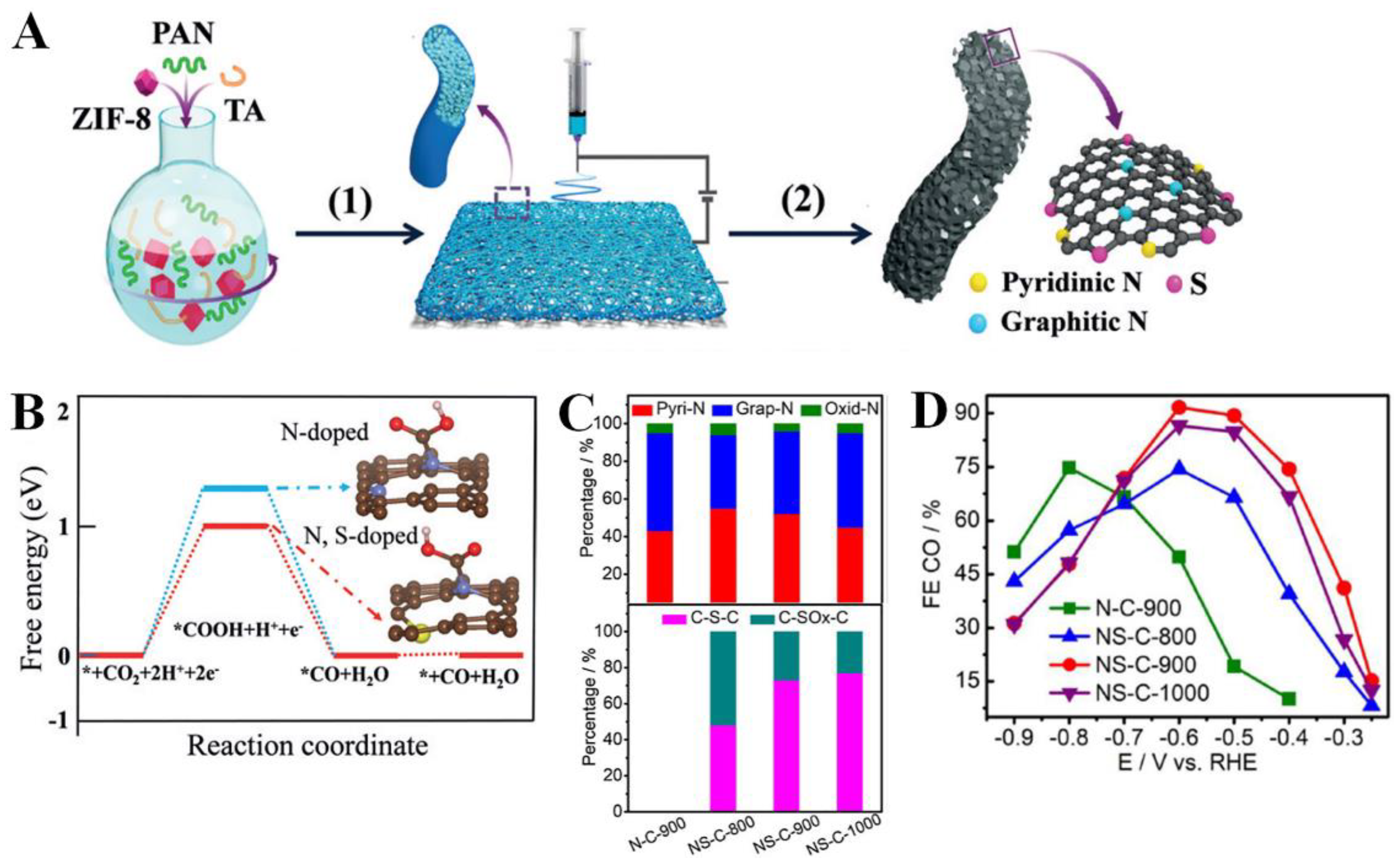
| NS-C | The calcination of citric acid and thiourea at 550 °C for 2 h under Ar | 0.1 M KHCO3 | CO | 0.49 (overpotential) | 92%/ ~−2.63 mA cm−2 | FECO stability ∼91% after 20 h | [40] |
| NS-CNSs-1000 | Two-step pyrolysis of the mixture of iron-oleate, Na2SO4 and urea and acid etching | 0.5 M KHCO3 | CO | −0.55 | 85.4%/ ~−2.5 mA cm−2 | FECO stability over 80% after 20 h | [43] |
| NSHPC | The pyrolysis of glucosamine hydrochloride and thiocyanuric acid precursor using SiO2 as hard templates | 0.1 M KHCO3 | CO | −0.6 | 87.8%/ ~−2.2 mA cm−2 | FECO stability ∼80% after 10 h | [134] |
| SZ-HCN | One-step pyrolysis of N-containing polymer and S powder | 0.1 M KHCO3 | CO | −0.6 | 93%/ ~−5.2 mA cm−2 | FECO stability ∼90% after 20 h | [136] |
| BAX-TU-20 | High-temperature treatment of commercial wood-based carbon impregnated with thiourea | 0.1 M KHCO3 | CO | 0.67 | 29, 0.27%/ ~−1.5 mA cm−2 | FECO stability ∼22.5, 0.25% after 40, 50 h | [132] |
| CH4 | |||||||
| NPC-900-2 | Pyrolysis-controlled sacrificial templating approach using citric acid, melamine and NH3, and phytic acid as carbon, nitrogen, and phosphorous source, respectively | 0.5 M KHCO3 | CO | −0.41 | 88%/ ~−1.71 mA cm−2 | FECO stability ∼80% after 27 h | [140] |
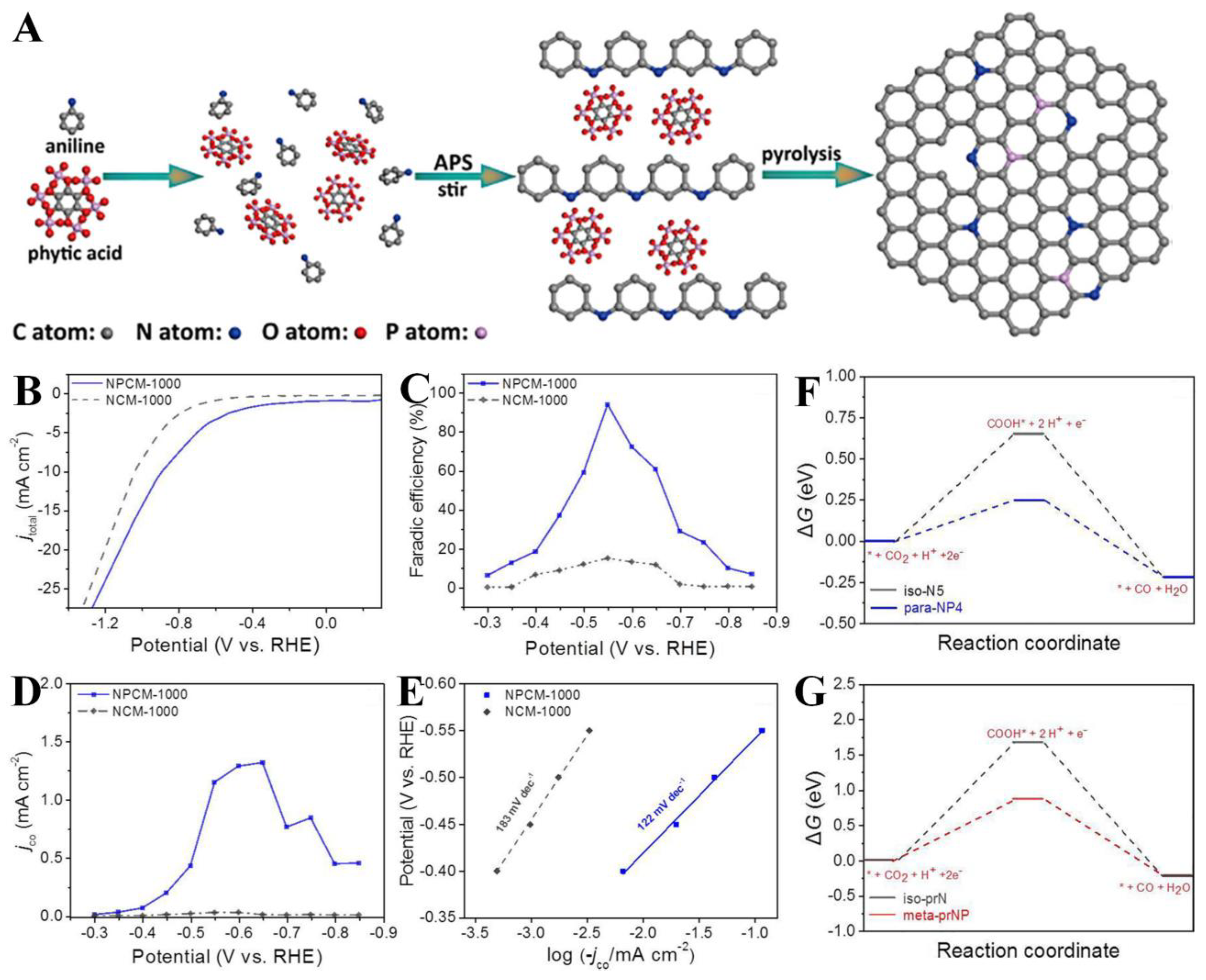
| NPCM-1000 | One-pot synthesis by using aniline monomer and phytic acid as nitrogen, carbon, and phosphorus source | 0.5 M NaHCO3 | CO | −0.55 | 92%/ ~−1.25 mA cm−2 | FECO stability ∼75% after 24 h | [139] |
| MPC-1000 | Pyrolysis of vitamin B12 in NaCl assembly-enclosed nanoreactors | 0.1 M KHCO3 | CO | −0.7 | 62%/ ~−3.1 mA cm−2 | FECO stability ∼60% after 20 h | [137] |
| N, P-FC | One-step soft-template pyrolysis method by using phytic acid as P source, dicyandiamide as N source, and polyethylene glycol as soft template | 0.5 M NaHCO3 | CO | −0.52 | 83.3%/ ~−8.52 mA cm−2 | FECO stability ∼80% after 12.5 h | [138] |
| NBPC | Liquid nitrogen-assisted freeze-drying of the NaCl-glucose solution containing carbon, nitrogen, and boron precursors and two-stage solid pyrolysis | 0.5 M KHCO3 | CO | −0.4 | 83%/ ~−0.5 mA cm−2 | FECO stability ∼80% after 20 h | [144] |
| BND3 | The deposition of BND film on Si substrate using hot filament chemical vapor deposition method with a gas mixture of CH4/B2H6/N2/H2 | 0.1 M NaHCO3 | CH3CH2OH | −1.0 | 93.2%/ ~−0.5 mA cm−2 | FECO stability ∼93.2%) after 48 h | [141] |
| CH3OH | |||||||
| HCOO− | |||||||
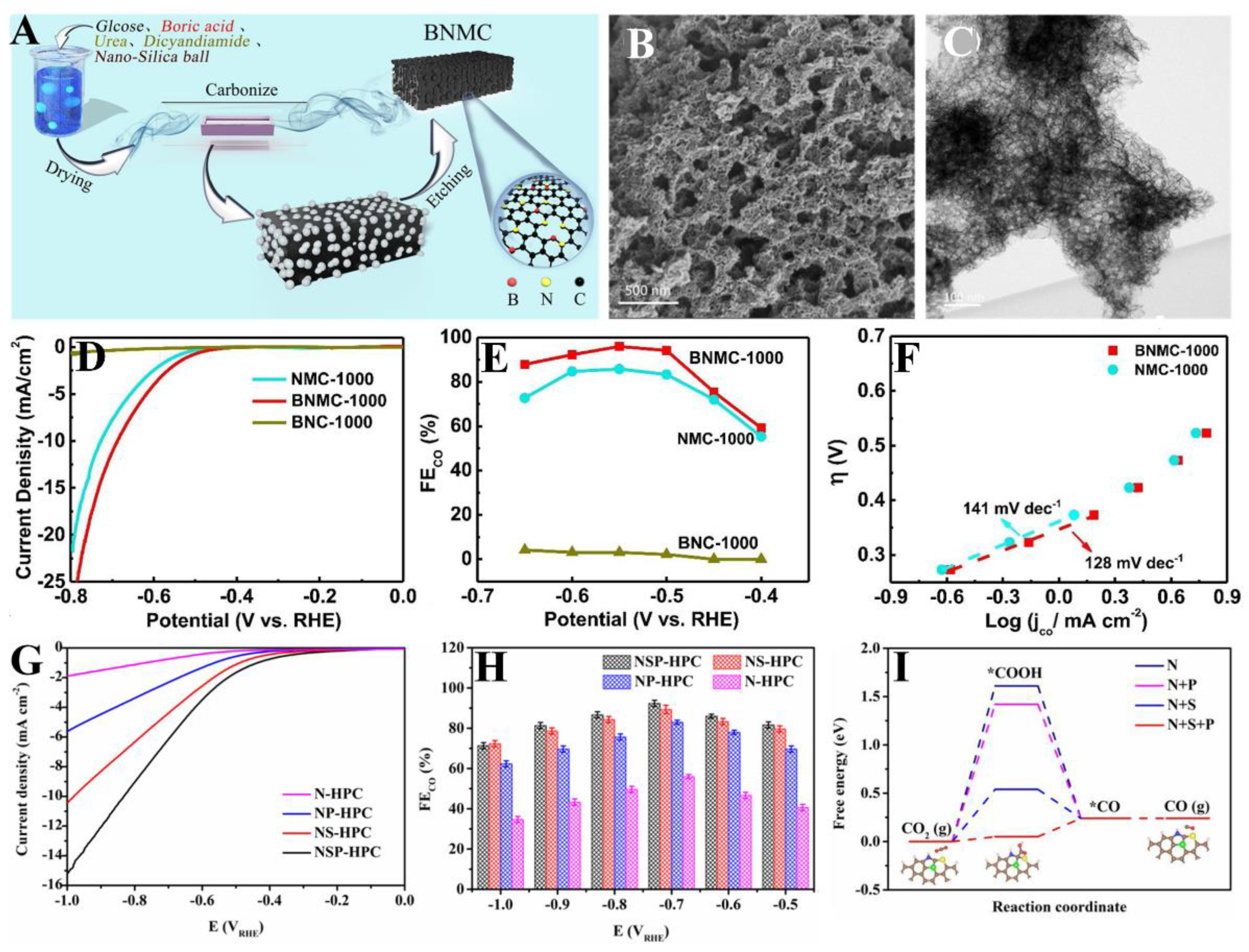
| BNMC-1000 | The carbonization of a precursor containing urea, dicyandiamide, glucose, and boric acid along with silica as templates | 0.1 M KHCO3 | CO | −0.55 | 95%/~−2.7 mA cm−2 | FECO stability ∼90% after 10 h | [142] |
| Ternary HA-PCs | |||||||
| NSP-HPC | A H2SO4-H3PO4 binary-acids activation method | 0.5 M KHCO3 | CO | −0.7, −1 | 92, 98.5%/~−5.2, −186 mA cm−2 | FECO stability ∼91, 94% after 50 h | [145] |
| LC-3 | The carbonization of the mixture of lignin, urea, melamine, NaCl, and ZnCl2 at 1000 °C for 2 h in Ar, followed by impregnating in HCl for 24 h | 0.1 M KHCO3 | CO | −0.6 | 95.9%/~−1.98 mA cm−2 | FECO stability ∼95.9% after 18 h | [135] |
4.6. Ternary Heteroatom-Doped Porous Carbon Materials
5. Conclusions and Outlook
- The current preparation approaches of heteroatom-doped porous carbon-based nanocatalysts involve multiple reaction steps, energy consumption, and hazardous reagents, making them impractical. Thus, they should be prepared using green materials under ambient conditions to meet sustainability requirements.
- Using biomass wastes is a promising approach to synthesizing HA-PCs with tunable porosity and surface area under ambient conditions; however, they are rarely reported for CO2RR.
- The CO2RR performance of HA-PCs is mainly measured in CO3-based electrolytes, so other organic, ionic liquid, and hybrid electrolytes should be studied to produce liquid products other than CO. Moreover, the effect of electrolytes and cell design on the CO2RR of HA-PCs has not yet been reported.
- Integration of HA-PCs with other materials such as carbon nitride [1,146,147,148], MXenes [23,149,150,151], carboxylated carbon/graphene [152,153], and graphdiyne [41] can enhance their CO2RR owing to their rich electron density, unique physicochemical properties, and catalytic/photocatalytic merits. Using HA-PCs with 3D porous multi-metallic nanocrystals (i.e., cages, branched, dendrites, and yolk-shell) can improve the CO2RR selectivity.
- Computational studies could be conducted with experimental studies to allow the synthesis of novel HA-PCs and to examine their CO2RR activity, mechanism, and pathways.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Q.; Eid, K.; Li, W.; Abdullah, A.M.; Xu, G.; Varma, R.S. Engineering graphitic carbon nitride (gC3N4) for catalytic reduction of CO2 to fuels and chemicals: Strategy and mechanism. Green Chem. 2021, 23, 5394–5428. [Google Scholar] [CrossRef]
- Eid, K.; Sliem, M.H.; Eldesoky, A.S.; Al-Kandari, H.; Abdullah, A.M. Rational synthesis of one-dimensional carbon nitride-based nanofibers atomically doped with Au/Pd for efficient carbon monoxide oxidation. Int. J. Hydrog. Energy 2019, 44, 17943–17953. [Google Scholar] [CrossRef]
- Eid, K.; Sliem, M.H.; Abdullah, A.M. Unraveling template-free fabrication of carbon nitride nanorods codoped with Pt and Pd for efficient electrochemical and photoelectrochemical carbon monoxide oxidation at room temperature. Nanoscale 2019, 11, 11755–11764. [Google Scholar] [CrossRef]
- Eid, K.; Sliem, M.H.; Al-Kandari, H.; Sharaf, M.A.; Abdullah, A.M. Rational synthesis of porous graphitic-like carbon nitride nanotubes codoped with Au and Pd as an efficient catalyst for carbon monoxide oxidation. Langmuir 2019, 35, 3421–3431. [Google Scholar] [CrossRef]
- Lu, Q.; Li, J.; Eid, K.; Gu, X.; Wan, Z.; Li, W.; Al-Hajri, R.S.; Abdullah, A.M. Facile One-step Aqueous-phase Synthesis of Porous PtBi Nanosponges for Efficient Electrochemical Methanol Oxidation with a High CO Tolerance. J. Electroanal. Chem. 2022, 916, 116361. [Google Scholar] [CrossRef]
- Eid, K.; Abdullah, A.M. Porous Ternary Pt-based Branched Nanostructures for Electrocatalytic Oxygen Reduction. Electrochem. Commun. 2022, 136, 107237. [Google Scholar] [CrossRef]
- Ahsan, M.A.; He, T.; Eid, K.; Abdullah, A.M.; Sanad, M.F.; Aldalbahi, A.; Alvarado-Tenorio, B.; Du, A.; Puente Santiago, A.R.; Noveron, J.C. Controlling the Interfacial Charge Polarization of MOF-Derived 0D–2D vdW Architectures as a Unique Strategy for Bifunctional Oxygen Electrocatalysis. ACS Appl. Mater. Interfaces 2022, 14, 3919–3929. [Google Scholar] [CrossRef]
- Wang, H.; Yin, S.; Eid, K.; Li, Y.; Xu, Y.; Li, X.; Xue, H.; Wang, L. Fabrication of mesoporous cage-bell Pt nanoarchitectonics as efficient catalyst for oxygen reduction reaction. ACS Sustain. Chem. Eng. 2018, 6, 11768–11774. [Google Scholar] [CrossRef]
- Wu, F.; Eid, K.; Abdullah, A.M.; Niu, W.; Wang, C.; Lan, Y.; Elzatahry, A.A.; Xu, G. Unveiling one-pot template-free fabrication of exquisite multidimensional PtNi multicube nanoarchitectonics for the efficient electrochemical oxidation of ethanol and methanol with a great tolerance for CO. ACS Appl. Mater. Interfaces 2020, 12, 31309–31318. [Google Scholar] [CrossRef]
- Logeshwaran, N.; Panneerselvam, I.R.; Ramakrishnan, S.; Kumar, R.S.; Kim, A.R.; Wang, Y.; Yoo, D.J. Quasihexagonal Platinum Nanodendrites Decorated over CoS2-N-Doped Reduced Graphene Oxide for Electro-Oxidation of C1-, C2-, and C3-Type Alcohols. Adv. Sci. 2022, 9, 2105344. [Google Scholar] [CrossRef]
- Ghanem, A.; Mandor, M.A.; El-Nagar, R.; Eid, K. Atomic and Molecular Functionalization of Graphitic Carbon Nitride for Solar Cell Applications. In Carbon Nitride Nanostructures for Sustainable Energy Production and Environmental Remediation; RSC: London, UK, 2021; pp. 221–261. [Google Scholar]
- Chen, L.; Xu, C.; Qin, Y.; He, X.; Bian, H.; Xu, G.; Niu, L.; Song, Q. An Inverted Perovskite Solar Cell with Good Comprehensive Performance Realized by Reducing the Concentration of Precursors. Nanomaterials 2022, 12, 1736. [Google Scholar] [CrossRef] [PubMed]
- Eid, K.; Sliem, M.H.; Abdullah, A.M. Tailoring the defects of sub-100 nm multipodal titanium nitride/oxynitride nanotubes for efficient water splitting performance. Nanoscale Adv. 2021, 3, 5016–5026. [Google Scholar] [CrossRef]
- Eid, K.; Soliman, K.A.; Abdulmalik, D.; Mitoraj, D.; Sleim, M.H.; Liedke, M.O.; El-Sayed, H.A.; AlJaber, A.S.; Al-Qaradawi, I.Y.; Reyes, O.M. Tailored fabrication of iridium nanoparticle-sensitized titanium oxynitride nanotubes for solar-driven water splitting: Experimental insights on the photocatalytic–activity–defects relationship. Catal. Sci. Technol. 2020, 10, 801–809. [Google Scholar] [CrossRef]
- Ahmad, Y.H.; Eid, K.A.; AlQaradawi, S.Y.; Allam, N.K. Highly active, durable and pH-universal hybrid oxide nanocrystals for efficient oxygen evolution. Sustain. Energy Fuels 2017, 1, 1123–1129. [Google Scholar] [CrossRef]
- Ipadeola, A.K.; Lebechi, A.K.; Gaolatlhe, L.; Haruna, A.B.; Chitt, M.; Eid, K.; Abdullah, A.M.; Ozoemena, K.I. Porous High-Entropy Alloys as Efficient Electrocatalysts for Water-Splitting Reactions. Electrochem. Commun. 2022, 136, 107207. [Google Scholar] [CrossRef]
- Ipadeola, A.K.; Haruna, A.B.; Gaolatlhe, L.; Lebechi, A.K.; Meng, J.; Pang, Q.; Eid, K.; Abdullah, A.M.; Ozoemena, K.I. Efforts at Enhancing Bifunctional Electrocatalysis and Related Events for Rechargeable Zinc-Air Batteries. ChemElectroChem 2021, 8, 3998–4018. [Google Scholar] [CrossRef]
- Logeshwaran, N.; Ramakrishnan, S.; Chandrasekaran, S.S.; Vinothkannan, M.; Kim, A.R.; Sengodan, S.; Velusamy, D.B.; Varadhan, P.; He, J.-H.; Yoo, D.J. An efficient and durable trifunctional electrocatalyst for zinc–air batteries driven overall water splitting. Appl. Catal. B 2021, 297, 120405. [Google Scholar] [CrossRef]
- Soliman, A.; AlAmoodi, N.; Karanikolos, G.N.; Doumanidis, C.C.; Polychronopoulou, K. A review on new 3-D printed materials’ geometries for catalysis and adsorption: Paradigms from reforming reactions and CO2 capture. Nanomaterials 2020, 10, 2198. [Google Scholar] [CrossRef]
- Wan, Y.; Miao, Y.; Qiu, T.; Kong, D.; Wu, Y.; Zhang, Q.; Shi, J.; Zhong, R.; Zou, R. Tailoring Amine-Functionalized Ti-MOFs via a Mixed Ligands Strategy for High-Efficiency CO2 Capture. Nanomaterials 2021, 11, 3348. [Google Scholar] [CrossRef]
- Modak, A.; Bhanja, P.; Dutta, S.; Chowdhury, B.; Bhaumik, A. Catalytic reduction of CO2 into fuels and fine chemicals. Green Chem. 2020, 22, 4002–4033. [Google Scholar] [CrossRef]
- Guzmán, H.; Russo, N.; Hernández, S. CO2 valorisation towards alcohols by Cu-based electrocatalysts: Challenges and perspectives. Green Chem. 2021, 23, 1896–1920. [Google Scholar] [CrossRef]
- Eid, K.; Lu, Q.; Abdel-Azeim, S.; Soliman, A.; Abdullah, A.M.; Abdelgwad, A.M.; Forbes, R.P.; Ozoemena, K.I.; Varma, R.S.; Shibl, M.F. Highly exfoliated Ti3C2Tx MXene nanosheets atomically doped with Cu for efficient electrochemical CO2 reduction: An experimental and theoretical study. J. Mater. Chem. A 2022, 10, 1965–1975. [Google Scholar] [CrossRef]
- Bian, J.; Zhang, Z.; Feng, J.; Thangamuthu, M.; Yang, F.; Sun, L.; Li, Z.; Qu, Y.; Tang, D.; Lin, Z. Energy Platform for Directed Charge Transfer in the Cascade Z-Scheme Heterojunction: CO2 Photoreduction without a Cocatalyst. Angew. Chem. Int. Ed. 2021, 60, 20906–20914. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liu, S.; Li, H.; Ding, J.; Liu, L.; Kuang, Z.; Li, L.; Yang, H.; Bai, F.; Huang, Y. Electron-withdrawing functional ligand promotes CO2 reduction catalysis in single atom catalyst. Sci. China Chem. 2020, 63, 1727–1733. [Google Scholar] [CrossRef]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.-W.; Zhou, Z.-H.; He, L.-N. Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem. 2017, 19, 3707–3728. [Google Scholar] [CrossRef]
- Franco, F.; Rettenmaier, C.; Jeon, H.S.; Roldan Cuenya, B. Transition metal-based catalysts for the electrochemical CO2 reduction: From atoms and molecules to nanostructured materials. Chem. Soc. Rev. 2020, 49, 6884–6946. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, S.; Hou, J.; Li, Z.; Zhang, B.; Zhai, P.; Zhang, Y.; Sun, L. Rational Design of Nanocatalysts with Nonmetal Species Modification for Electrochemical CO2 Reduction. Adv. Energy Mater. 2020, 10, 2000588. [Google Scholar] [CrossRef]
- Eid, K.; Abdullah, A.M. Data on the catalytic CO oxidation and CO2 reduction durability on gC3N4 nanotubes Co-doped atomically with Pd and Cu. Data Br. 2019, 26, 104495. [Google Scholar] [CrossRef]
- Eid, K.; Sliem, M.H.; Jlassi, K.; Eldesoky, A.S.; Abdo, G.G.; Al-Qaradawi, S.Y.; Sharaf, M.A.; Abdullah, A.M.; Elzatahry, A.A. Precise fabrication of porous one-dimensional gC3N4 nanotubes doped with Pd and Cu atoms for efficient CO oxidation and CO2 reduction. Inorg. Chem. Commun. 2019, 107, 107460. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.-J.; Zhang, J.-X.; Bai, F.-Q.; Zhang, H.-X. First-principles investigation on the interfacial interaction and electronic structure of BiVO4/WO3 heterostructure semiconductor material. Appl. Surf. Sci. 2021, 549, 149309. [Google Scholar] [CrossRef]
- Cui, H.; Guo, Y.; Guo, L.; Wang, L.; Zhou, Z.; Peng, Z. Heteroatom-doped carbon materials and their composites as electrocatalysts for CO2 reduction. J. Mater. Chem. A 2018, 6, 18782–18793. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, X.; Peng, C.; Priest, C.; Mei, Y.; Wu, G. Carbon-Supported Single Metal Site Catalysts for Electrochemical CO2 Reduction to CO and Beyond. Small 2021, 17, 2005148. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Liu, Y.; Zhang, J.; Wang, F.; Miao, Z.; Diao, L.; Mu, J.; Zhou, J.; Zhuo, S. Understanding the role of metal and N species in M@NC catalysts for electrochemical CO2 reduction reaction. Appl. Catal. B 2022, 306, 121115. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, B.; Liu, X.; Ma, T. Surface and interface chemistry in metal-free electrocatalysts for electrochemical CO2 reduction. SmartMat 2022, 3, 5–34. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, C.; Di, Q.; Liu, W.; Sun, X.; Tuo, Y.; Zhou, Y.; Pan, Y.; Feng, X.; Li, L. Dual Role of Pyridinic-N Doping in Carbon-Coated Ni Nanoparticles for Highly Efficient Electrochemical CO2 Reduction to CO over a Wide Potential Range. ACS Catal. 2022, 12, 1364–1374. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, Y.; Wang, D.; Li, Y. Defect engineering in earth-abundant electrocatalysts for CO2 and N2 reduction. Energy Environ. Sci. 2019, 12, 1730–1750. [Google Scholar] [CrossRef]
- Liu, A.; Gao, M.; Ren, X.; Meng, F.; Yang, Y.; Gao, L.; Yang, Q.; Ma, T. Current progress in electrocatalytic carbon dioxide reduction to fuels on heterogeneous catalysts. J. Mater. Chem. A 2020, 8, 3541–3562. [Google Scholar] [CrossRef]
- Pan, F.; Li, B.; Deng, W.; Du, Z.; Gang, Y.; Wang, G.; Li, Y. Promoting electrocatalytic CO2 reduction on nitrogen-doped carbon with sulfur addition. Appl. Catal. B 2019, 252, 240–249. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Z.; Zhao, J. Metal-free graphdiyne doped with sp-hybridized boron and nitrogen atoms at acetylenic sites for high-efficiency electroreduction of CO2 to CH4 and C2H4. J. Mater. Chem. A 2019, 7, 4026–4035. [Google Scholar] [CrossRef]
- Li, G.; Pei, L.; Wu, Y.; Zhu, B.; Hu, Q.; Yang, H.; Zhang, Q.; Liu, J.; He, C. Facile synthesis of polyacrylonitrile-based N/S-codoped porous carbon as an efficient oxygen reduction electrocatalyst for zinc–air batteries. J. Mater. Chem. A 2019, 7, 11223–11233. [Google Scholar] [CrossRef]
- Wang, G.; Liu, M.; Jia, J.; Xu, H.; Zhao, B.; Lai, K.; Tu, C.; Wen, Z. Nitrogen and Sulfur Co-doped Carbon Nanosheets for Electrochemical Reduction of CO2. ChemCatChem 2020, 12, 2203–2208. [Google Scholar] [CrossRef]
- Wang, H.; Shao, Y.; Mei, S.; Lu, Y.; Zhang, M.; Sun, J.-k.; Matyjaszewski, K.; Antonietti, M.; Yuan, J. Polymer-derived heteroatom-doped porous carbon materials. Chem Rev. 2020, 120, 9363–9419. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Feng, X.; Li, J.; Li, M.; Xia, Y.; Yuan, Y.; Yang, C.; Dai, B.; Lin, Z.; Wang, J. Heteroatom-doped porous carbon materials with unprecedented high volumetric capacitive performance. Angew. Chem. Int. Ed. 2019, 131, 2419–2423. [Google Scholar] [CrossRef]
- Rangraz, Y.; Heravi, M.M.; Elhampour, A. Recent Advances on Heteroatom-Doped Porous Carbon/Metal Materials: Fascinating Heterogeneous Catalysts for Organic Transformations. Chem Rec. 2021, 21, 1985–2073. [Google Scholar] [CrossRef]
- Rehman, A.; Nazir, G.; Rhee, K.Y.; Park, S.-J. A rational design of cellulose-based heteroatom-doped porous carbons: Promising contenders for CO2 adsorption and separation. Chem. Eng. J. 2021, 420, 130421. [Google Scholar] [CrossRef]
- Ashourirad, B.; Arab, P.; Islamoglu, T.; Cychosz, K.A.; Thommes, M.; El-Kaderi, H.M. A cost-effective synthesis of heteroatom-doped porous carbons as efficient CO2 sorbents. J. Mater. Chem. A 2016, 4, 14693–14702. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Luo, W.; Sherrell, P.C.; Chen, J.; Yang, J. Heterogeneous single-atom catalysts for electrochemical CO2 reduction reaction. Adv. Mater. 2020, 32, 2001848. [Google Scholar] [CrossRef]
- Duan, X.; Xu, J.; Wei, Z.; Ma, J.; Guo, S.; Wang, S.; Liu, H.; Dou, S. Metal-free carbon materials for CO2 electrochemical reduction. Adv. Mater. 2017, 29, 1701784. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, D.; Jiang, S.; Xia, H.; Yang, Y.; Yan, W.; Hu, J.; Zhang, J. Rational confinement engineering of MOF-derived carbon-based electrocatalysts toward CO2 reduction and O2 reduction reactions. InfoMat 2022, 4, e12257. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Q.; Fu, Y.; Lei, C.; Yang, B.; Li, Z.; Lei, L.; Wu, G.; Hou, Y. Carbon-rich nonprecious metal single atom electrocatalysts for CO2 reduction and hydrogen evolution. Small Methods 2019, 3, 1900210. [Google Scholar] [CrossRef]
- Han, S.G.; Ma, D.D.; Zhu, Q.L. Atomically Structural Regulations of Carbon-Based Single-Atom Catalysts for Electrochemical CO2 Reduction. Small Methods 2021, 5, 2100102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shao, Y.; Wang, H.; Yuan, J. Advanced heteroatom-doped porous carbon membranes assisted by poly(ionic liquid) design and engineering. Acc. Mater. Res. 2020, 1, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Ali, K.; Ahmad, M.I.; Yusup, Y. Issues, impacts, and mitigations of carbon dioxide emissions in the building sector. Sustainability 2020, 12, 7427. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Kler, J.S.; Hernke, M.T.; Braun, R.K.; Meyer, K.C.; Funk, W.E. Direct human health risks of increased atmospheric carbon dioxide. Nature Sustain. 2019, 2, 691–701. [Google Scholar] [CrossRef]
- Gall, E.T.; Cheung, T.; Luhung, I.; Schiavon, S.; Nazaroff, W.W. Real-time monitoring of personal exposures to carbon dioxide. Build. Environ. 2016, 104, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Zheutlin, A.R.; Adar, S.D.; Park, S.K. Carbon dioxide emissions and change in prevalence of obesity and diabetes in the United States: An ecological study. Environ. Int. 2014, 73, 111–116. [Google Scholar] [CrossRef]
- Zappulla, D. Environmental stress, erythrocyte dysfunctions, inflammation, and the metabolic syndrome: Adaptations to CO2 increases? CMSJ 2008, 3, 30–34. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Chen, L.-H.; Li, Y.; Rooke, J.C.; Sanchez, C.; Su, B.-L. Hierarchically porous materials: Synthesis strategies and structure design. Chem. Soc. Rev. 2017, 46, 481–558. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Chen, C.; Chen, L.; Su, B. Hierarchically porous materials: Synthesis strategies and emerging applications. Front. Chem. Sci. Eng. 2016, 10, 301–347. [Google Scholar] [CrossRef]
- Zhao, T.; Elzatahry, A.; Li, X.; Zhao, D. Single-micelle-directed synthesis of mesoporous materials. Nat. Rev. Mater. 2019, 4, 775–791. [Google Scholar] [CrossRef]
- Liu, D.; Hu, Y.-Y.; Zeng, C.; Qu, D.-Y. Soft-templated ordered mesoporous carbon materials: Synthesis, structural modification and functionalization. Acta Phys. Sin. 2016, 32, 2826–2840. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, Y.; Fan, Z.; Chao, D.; Xiong, Q.; Tu, J.; Zhang, H.; Fan, H.J. Novel Metal@Carbon Spheres Core–Shell Arrays by Controlled Self-Assembly of Carbon Nanospheres: A Stable and Flexible Supercapacitor Electrode. Adv. Energy Mater. 2015, 5, 1401709. [Google Scholar] [CrossRef]
- Liang, H.-W.; Wei, W.; Wu, Z.-S.; Feng, X.; Müllen, K. Mesoporous Metal–Nitrogen-Doped Carbon Electrocatalysts for Highly Efficient Oxygen Reduction Reaction. J. Am. Chem. Soc. 2013, 135, 16002–16005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Jiang, Y.; Wang, L.; Zhang, C.; Liu, S. Hierarchical porous carbon nanofibers as binder-free electrode for high-performance supercapacitor. Electrochim. Acta 2016, 196, 189–196. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Wang, Y.; Wei, T.; Zhang, M.; Jing, X.; Fan, Z. Three-dimensional flower-like and hierarchical porous carbon materials as high-rate performance electrodes for supercapacitors. Carbon 2014, 67, 119–127. [Google Scholar] [CrossRef]
- He, X.; Zhang, N.; Shao, X.; Wu, M.; Yu, M.; Qiu, J. A layered-template-nanospace-confinement strategy for production of corrugated graphene nanosheets from petroleum pitch for supercapacitors. Chem. Eng. J. 2016, 297, 121–127. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, Y.; Yan, J.; Ning, G.; Wang, Q.; Wei, T.; Zhi, L.; Wei, F. Template-directed synthesis of pillared-porous carbon nanosheet architectures: High-performance electrode materials for supercapacitors. Adv. Energy Mater. 2012, 2, 419–424. [Google Scholar] [CrossRef]
- Xie, K.; Qin, X.; Wang, X.; Wang, Y.; Tao, H.; Wu, Q.; Yang, L.; Hu, Z. Carbon nanocages as supercapacitor electrode materials. Adv. Mater. 2012, 24, 347–352. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, C.; Aoki, Y.; Habazaki, H. Starch-derived hierarchical porous carbon with controlled porosity for high performance supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 7292–7303. [Google Scholar] [CrossRef]
- Qian, M.; Wang, Y.; Xu, F.; Zhao, W.; Lin, T.; Huang, F. Extraordinary porous few-layer carbons of high capacitance from pechini combustion of magnesium nitrate gel. ACS Appl. Mater. Interfaces 2018, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Song, M.; Wu, G.; Zhou, Y.; Wan, J.; Ren, X.; Ma, F. 3D carbon nanocage networks with multiscale pores for high-rate supercapacitors by flower-like template and in-situ coating. Energy Storage Mater. 2018, 13, 57–65. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, H.-f.; He, X.-j.; Ma, H.; Dong, S.-a.; Xie, X.-y. Synthesis of porous carbons from coal tar pitch for high-performance supercapacitors. New Carbon Mater. 2019, 34, 132–139. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Mao, X.; Xu, X.; Zhang, B.; Yang, J.; Wang, Y.; Zhu, J.; Hou, S. High rate performance carbon nano-cages with oxygen-containing functional groups as supercapacitor electrode materials. Carbon 2017, 111, 207–214. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Xiao, Y.; Wei, T.; Fan, Z.; Zhang, M.; Jing, X. Interconnected porous and nitrogen-doped carbon network for supercapacitors with high rate capability and energy density. Electrochim. Acta 2013, 114, 165–172. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Wei, T.; Feng, J.; Ren, Y.; Fan, Z.; Zhang, M.; Jing, X. Two-dimensional mesoporous carbon sheet-like framework material for high-rate supercapacitors. Carbon 2013, 60, 481–487. [Google Scholar] [CrossRef]
- Zhao, Z.; Hao, S.; Hao, P.; Sang, Y.; Manivannan, A.; Wu, N.; Liu, H. Lignosulphonate-cellulose derived porous activated carbon for supercapacitor electrode. J. Mater. Chem. A 2015, 3, 15049–15056. [Google Scholar] [CrossRef]
- Ma, X.; Gan, L.; Liu, M.; Tripathi, P.K.; Zhao, Y.; Xu, Z.; Zhu, D.; Chen, L. Mesoporous size controllable carbon microspheres and their electrochemical performances for supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 8407–8415. [Google Scholar] [CrossRef]
- Zeng, R.; Tang, X.; Huang, B.; Yuan, K.; Chen, Y. Nitrogen-Doped Hierarchically Porous Carbon Materials with Enhanced Performance for Supercapacitor. ChemElectroChem 2018, 5, 515–522. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Q.; Wei, L.; Zhong, L.; Wang, X. Simple and scalable synthesis of hierarchical porous carbon derived from cornstalk without pith for high capacitance and energy density. J. Mater. Chem. A 2020, 8, 1469–1479. [Google Scholar] [CrossRef]
- Wei, F.; He, X.; Zhang, H.; Liu, Z.; Xiao, N.; Qiu, J. Crumpled carbon nanonets derived from anthracene oil for high energy density supercapacitor. J. Power Sources 2019, 428, 8–12. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Ding, B.; Malgras, V.; Chang, Z.; Hao, X.; Wang, Y.; Dou, H.; Zhang, X.; Yamauchi, Y. Hierarchical porous carbons with layer-by-layer motif architectures from confined soft-template self-assembly in layered materials. Nature Commun. 2017, 8, 15717. [Google Scholar] [CrossRef]
- Li, W.; Li, B.; Shen, M.; Gao, Q.; Hou, J. Use of Gemini surfactant as emulsion interface microreactor for the synthesis of nitrogen-doped hollow carbon spheres for high-performance supercapacitors. Chem. Eng. J. 2020, 384, 123309. [Google Scholar] [CrossRef]
- Estevez, L.; Prabhakaran, V.; Garcia, A.L.; Shin, Y.; Tao, J.; Schwarz, A.M.; Darsell, J.; Bhattacharya, P.; Shutthanandan, V.; Zhang, J.-G. Hierarchically porous graphitic carbon with simultaneously high surface area and colossal pore volume engineered via ice templating. ACS Nano 2017, 11, 11047–11055. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yu, H.; Fan, L.; Yu, M.; Zheng, M. Honeycomb-like porous carbons synthesized by a soft template strategy for supercapacitors. Mater. Lett. 2017, 195, 31–33. [Google Scholar] [CrossRef]
- Peng, H.; Yao, B.; Wei, X.; Liu, T.; Kou, T.; Xiao, P.; Zhang, Y.; Li, Y. Pore and Heteroatom Engineered Carbon Foams for Supercapacitors. Adv. Energy Mater. 2019, 9, 1803665. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, H.; Li, L.; Yao, Y.; Qu, H.; Zhang, C.; Liu, S.; Zhou, Y. Double Soft-Template Synthesis of Nitrogen/Sulfur-Codoped Hierarchically Porous Carbon Materials Derived from Protic Ionic Liquid for Supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 26088–26095. [Google Scholar] [CrossRef]
- Chen, L.-F.; Lu, Y.; Yu, L.; Lou, X.W. Designed formation of hollow particle-based nitrogen-doped carbon nanofibers for high-performance supercapacitors. Energy Environ. Sci. 2017, 10, 1777–1783. [Google Scholar] [CrossRef]
- Fechler, N.; Fellinger, T.P.; Antonietti, M. “Salt templating”: A simple and sustainable pathway toward highly porous functional carbons from ionic liquids. Adv. Mater. 2013, 25, 75–79. [Google Scholar] [CrossRef]
- Xie, X.; He, X.; Zhang, H.; Wei, F.; Xiao, N.; Qiu, J. Interconnected sheet-like porous carbons from coal tar by a confined soft-template strategy for supercapacitors. Chem. Eng. J. 2018, 350, 49–56. [Google Scholar] [CrossRef]
- Fechler, N.; Zussblatt, N.P.; Rothe, R.; Schlögl, R.; Willinger, M.G.; Chmelka, B.F.; Antonietti, M. Eutectic syntheses of graphitic carbon with high pyrazinic nitrogen content. Adv. Mater. 2016, 28, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Zhu, D.; Duan, H.; Wang, Z.; Lv, Y.; Xiong, W.; Li, L.; Liu, M.; Gan, L. Deep-eutectic-solvent synthesis of N/O self-doped hollow carbon nanorods for efficient energy storage. Chem. Commun. 2019, 55, 11219–11222. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, G.; Wei, T.; Fan, Z.; Yan, P. Nitrogen and sulfur co-doped porous carbon nanosheets derived from willow catkin for supercapacitors. Nano Energy 2016, 19, 165–175. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, Z.; Zhang, M.; Yu, C.; Wang, G.; Dong, Y.; Liu, S.; Wang, Y.; Qiu, J. Sustainable Synthesis and Assembly of Biomass-Derived B/N Co-Doped Carbon Nanosheets with Ultrahigh Aspect Ratio for High-Performance Supercapacitors. Adv. Funct. Mater. 2016, 26, 111–119. [Google Scholar] [CrossRef]
- Gao, F.; Qu, J.; Geng, C.; Shao, G.; Wu, M. Self-templating synthesis of nitrogen-decorated hierarchical porous carbon from shrimp shell for supercapacitors. J. Mater. Chem. A 2016, 4, 7445–7452. [Google Scholar] [CrossRef]
- Ghosh, U.; Majumdar, A.; Pal, A. Photocatalytic CO2 reduction over g-C3N4 based heterostructures: Recent progress and prospects. J. Environ. Chem. Eng. 2020, 9, 104631. [Google Scholar] [CrossRef]
- Ong, W.-J.; Putri, L.K.; Mohamed, A.R. Rational Design of Carbon-Based 2D Nanostructures for Enhanced Photocatalytic CO2 Reduction: A Dimensionality Perspective. Eur. J. Chem. 2020, 26, 9710–9748. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Li, X.; Fan, L.; Li, Z.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Zhu, H. Boron doping of graphene for graphene–silicon p–n junction solar cells. Adv. Energy Mater. 2012, 2, 425–429. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, J.; Li, M.; Xia, Z. Catalytic mechanisms of sulfur-doped graphene as efficient oxygen reduction reaction catalysts for fuel cells. J. Phys. Chem. C 2014, 118, 3545–3553. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Li, X.; Gao, T.; Wang, X.-B. Metal-free carbon-based nanomaterials for electrochemical nitrogen and carbon dioxide reductions. Mater. Res. Bull. 2021, 140, 111294. [Google Scholar] [CrossRef]
- Bing, X.; Wei, Y.; Wang, M.; Xu, S.; Long, D.; Wang, J.; Qiao, W.; Ling, L. Template-free synthesis of nitrogen-doped hierarchical porous carbons for CO2 adsorption and supercapacitor electrodes. J. Colloid Interface Sci. 2017, 488, 207–217. [Google Scholar] [CrossRef]
- Sun, Z.; Li, K.; Wee Koh, S.; Jiao, L. Low-Cost and Scalable Fabrication of Hierarchically Porous N-Doped Carbon for Energy Storage and Conversion Application. ChemistrySelect 2020, 5, 533–537. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Mi, R.; Mei, J.; Liu, L.-M.; Cao, L.; Lau, W.-M.; Liu, H. Porous structure design of carbon xerogels for advanced supercapacitor. Appl. Energy 2015, 153, 32–40. [Google Scholar] [CrossRef]
- Li, H.; Xiao, N.; Hao, M.; Song, X.; Wang, Y.; Ji, Y.; Liu, C.; Li, C.; Guo, Z.; Zhang, F. Efficient CO2 electroreduction over pyridinic-N active sites highly exposed on wrinkled porous carbon nanosheets. Chem. Eng. J. 2018, 351, 613–621. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, P.; Xu, J.; Han, J.; Wang, D.; Hao, C.; Alanagh, H.R.; Long, C.; Shi, X.; Tang, Z. MOF-derived nitrogen-doped nanoporous carbon for electroreduction of CO2 to CO: The calcining temperature effect and the mechanism. Nanoscale 2019, 11, 4911–4917. [Google Scholar] [CrossRef] [PubMed]
- Kuang, M.; Guan, A.; Gu, Z.; Han, P.; Qian, L.; Zheng, G. Enhanced N-doping in mesoporous carbon for efficient electrocatalytic CO2 conversion. Nano Res. 2019, 12, 2324–2329. [Google Scholar] [CrossRef]
- Li, F.; Xue, M.; Knowles, G.P.; Chen, L.; MacFarlane, D.R.; Zhang, J. Porous nitrogen–doped carbon derived from biomass for electrocatalytic reduction of CO2 to CO. Electrochim. Acta 2017, 245, 561–568. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H.; Huang, X.; Liu, L.; Cai, W.; Gao, J.; Li, X.; Zhang, T.; Huang, Y.; Liu, B. Identifying active sites of nitrogen-doped carbon materials for the CO2 reduction reaction. Adv. Funct. Mater. 2018, 28, 1800499. [Google Scholar] [CrossRef]
- Li, W.; Herkt, B.; Seredych, M.; Bandosz, T.J. Pyridinic-N groups and ultramicropore nanoreactors enhance CO2 electrochemical reduction on porous carbon catalysts. Appl. Catal. B 2017, 207, 195–206. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Xiao, N.; Li, H.; Ji, Y.; Guo, Z.; Liu, C.; Qiu, J. Nitrogen-doped porous carbon from coal for high efficiency CO2 electrocatalytic reduction. Carbon 2019, 151, 46–52. [Google Scholar] [CrossRef]
- Ning, H.; Guo, D.; Wang, X.; Tan, Z.; Wang, W.; Yang, Z.; Li, L.; Zhao, Q.; Hao, J.; Wu, M. Efficient CO2 electroreduction over N-doped hieratically porous carbon derived from petroleum pitch. J. Energy Chem. 2021, 56, 113–120. [Google Scholar] [CrossRef]
- Wu, J.; Liu, M.; Sharma, P.P.; Yadav, R.M.; Ma, L.; Yang, Y.; Zou, X.; Zhou, X.-D.; Vajtai, R.; Yakobson, B.I. Incorporation of nitrogen defects for efficient reduction of CO2 via two-electron pathway on three-dimensional graphene foam. Nano Lett. 2016, 16, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tian, C.; Wu, H.; He, Y.; He, L.; Wang, H.; Zhuang, X.; Liu, H.; Xia, C.; Dai, S. Pyrolyzed triazine-based nanoporous frameworks enable electrochemical CO2 reduction in water. ACS Appl. Mater. Interfaces 2018, 10, 43588–43594. [Google Scholar] [CrossRef]
- Yao, P.; Qiu, Y.; Zhang, T.; Su, P.; Li, X.; Zhang, H. N-doped nanoporous carbon from biomass as a highly efficient electrocatalyst for the CO2 reduction reaction. ACS Sustain. Chem. Eng. 2019, 7, 5249–5255. [Google Scholar] [CrossRef]
- Shu, Z.; Ye, G.; Wang, J.; Liu, S.; He, Z.; Zhu, W.; Liu, B.; Liu, M. Nitrogen-doped carbon with high graphitic-N exposure for electroreduction of CO2 to CO. Ionics 2021, 27, 3089–3098. [Google Scholar] [CrossRef]
- Liu, W.; Qi, J.; Bai, P.; Zhang, W.; Xu, L. Utilizing spatial confinement effect of N atoms in micropores of coal-based metal-free material for efficiently electrochemical reduction of carbon dioxide. Appl. Catal. B 2020, 272, 118974. [Google Scholar] [CrossRef]
- Ye, L.; Ying, Y.; Sun, D.; Zhang, Z.; Fei, L.; Wen, Z.; Qiao, J.; Huang, H. Highly Efficient Porous Carbon Electrocatalyst with Controllable N-Species Content for Selective CO2 Reduction. Angew. Chem. Int. Ed. 2020, 59, 3244–3251. [Google Scholar] [CrossRef]
- Song, Y.; Chen, W.; Zhao, C.; Li, S.; Wei, W.; Sun, Y. Metal-free nitrogen-doped mesoporous carbon for electroreduction of CO2 to ethanol. Angew. Chem. 2017, 129, 10980–10984. [Google Scholar] [CrossRef]
- Chen, K.; Deng, J.; Zhao, J.; Liu, X.; Imhanria, S.; Wang, W. Electrocatalytic Production of Tunable Syngas from CO2 via a Metal-Free Porous Nitrogen-Doped Carbon. Ind. Eng. Chem. Res. 2021, 60, 7739–7745. [Google Scholar] [CrossRef]
- Qin, Z.; Jiang, X.; Cao, Y.; Dong, S.; Wang, F.; Feng, L.; Chen, Y.; Guo, Y. Nitrogen-doped porous carbon derived from digested sludge for electrochemical reduction of carbon dioxide to formate. Sci. Total Environ. 2021, 759, 143575. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Qian, X.; Luo, B.; Wang, L.; Deng, L.; Chen, Y. Carbon dioxide reduction to multicarbon hydrocarbons and oxygenates on plant moss-derived, metal-free, in situ nitrogen-doped biochar. Sci. Total Environ. 2020, 739, 140340. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fechler, N.; Bandosz, T.J. Chemically heterogeneous nitrogen sites of various reactivity in porous carbons provide high stability of CO2 electroreduction catalysts. Appl. Catal. B 2018, 234, 1–9. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Ren, T.-Z. Porous carbon modified with sulfur in energy related applications. Carbon 2017, 118, 561–577. [Google Scholar] [CrossRef]
- Wu, J.; Yadav, R.M.; Liu, M.; Sharma, P.P.; Tiwary, C.S.; Ma, L.; Zou, X.; Zhou, X.-D.; Yakobson, B.I.; Lou, J. Achieving highly efficient, selective, and stable CO2 reduction on nitrogen-doped carbon nanotubes. ACS Nano 2015, 9, 5364–5371. [Google Scholar] [CrossRef]
- Ni, W.; Xue, Y.; Zang, X.; Li, C.; Wang, H.; Yang, Z.; Yan, Y.-M. Fluorine doped cagelike carbon electrocatalyst: An insight into the structure-enhanced CO selectivity for CO2 reduction at high overpotential. ACS Nano 2020, 14, 2014–2023. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, X.; Wu, M.; Li, Q.; Wang, Y.; Yao, J. Metal-free fluorine-doped carbon electrocatalyst for CO2 reduction outcompeting hydrogen evolution. Angew. Chem. 2018, 130, 9788–9792. [Google Scholar] [CrossRef]
- Sreekanth, N.; Nazrulla, M.A.; Vineesh, T.V.; Sailaja, K.; Phani, K.L. Metal-free boron-doped graphene for selective electroreduction of carbon dioxide to formic acid/formate. Chem. Commun. 2015, 51, 16061–16064. [Google Scholar] [CrossRef]
- Li, W.; Seredych, M.; Rodríguez-Castellón, E.; Bandosz, T.J. Metal-free nanoporous carbon as a catalyst for electrochemical reduction of CO2 to CO and CH4. ChemSusChem 2016, 9, 606–616. [Google Scholar] [CrossRef]
- Nakata, K.; Ozaki, T.; Terashima, C.; Fujishima, A.; Einaga, Y. High-yield electrochemical production of formaldehyde from CO2 and seawater. Angew. Chem. Int. Ed. 2014, 53, 871–874. [Google Scholar] [CrossRef]
- Li, W.; Bandosz, T.J. Analyzing the effect of nitrogen/sulfur groups’ density ratio in porous carbons on the efficiency of CO2 electrochemical reduction. Appl. Surf. Sci. 2021, 569, 151066. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Lin, Q.; Fan, L.; Chai, X.; Zhang, Q.; Liu, J.; He, C.; Lin, Z. Composition Tailoring via N and S Co-doping and structure tuning by constructing hierarchical pores: Metal-free catalysts for high-performance electrochemical reduction of CO2. Angew. Chem. 2018, 130, 15702–15706. [Google Scholar] [CrossRef]
- Li, R.; Liu, F.; Zhang, Y.; Guo, M.; Liu, D. Nitrogen, sulfur co-doped hierarchically porous carbon as a metal-free electrocatalyst for oxygen reduction and carbon dioxide reduction reaction. ACS Appl. Mater. Interfaces 2020, 12, 44578–44587. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Qin, B.; Li, Y.; Zhang, Q.; Yang, G.; Wang, H.; Cao, Y.; Yu, H.; Peng, F. Chlorine-Promoted Nitrogen and Sulfur Co-Doped Biocarbon Catalyst for Electrochemical Carbon Dioxide Reduction. ChemElectroChem 2020, 7, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Qin, Y.; Wu, Y.; Pei, L.; Hu, Q.; Yang, H.; Zhang, Q.; Liu, J.; He, C. Nitrogen and sulfur dual-doped high-surface-area hollow carbon nanospheres for efficient CO2 reduction. Chin. J. Catal. 2020, 41, 830–838. [Google Scholar] [CrossRef]
- Pan, F.; Liang, A.; Duan, Y.; Liu, Q.; Zhang, J.; Li, Y. Self-growth-templating synthesis of 3D N, P, Co-doped mesoporous carbon frameworks for efficient bifunctional oxygen and carbon dioxide electroreduction. J. Mater. Chem. A 2017, 5, 13104–13111. [Google Scholar] [CrossRef]
- Xue, X.; Yang, H.; Yang, T.; Yuan, P.; Li, Q.; Mu, S.; Zheng, X.; Chi, L.; Zhu, J.; Li, Y. N, P-coordinated fullerene-like carbon nanostructures with dual active centers toward highly-efficient multi-functional electrocatalysis for CO2RR, ORR and Zn-air battery. J. Mater. Chem. A 2019, 7, 15271–15277. [Google Scholar] [CrossRef]
- Chen, S.; Liu, T.; Olanrele, S.O.; Lian, Z.; Si, C.; Chen, Z.; Li, B. Boosting electrocatalytic activity for CO2 reduction on nitrogen-doped carbon catalysts by co-doping with phosphorus. J. Energy Chem. 2021, 54, 143–150. [Google Scholar] [CrossRef]
- Liang, X.; Tian, N.; Zhou, Z.; Sun, S. N, P Dual-Doped Porous Carbon Nanosheets for High-Efficiency CO2 Electroreduction. ACS Sustain. Chem. Eng. 2022, 10, 1880–1887. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Cheng, K.; Quan, X.; Fan, X.; Su, Y.; Chen, S.; Zhao, H.; Zhang, Y.; Yu, H. Selective electrochemical reduction of carbon dioxide to ethanol on a boron-and nitrogen-Co-doped nanodiamond. Angew. Chem. 2017, 129, 15813–15817. [Google Scholar] [CrossRef]
- Ma, X.; Du, J.; Sun, H.; Ye, F.; Wang, X.; Xu, P.; Hu, C.; Zhang, L.; Liu, D. Boron, nitrogen co-doped carbon with abundant mesopores for efficient CO2 electroreduction. Appl. Catal. B 2021, 298, 120543. [Google Scholar] [CrossRef]
- Ghosh, S.; Ramaprabhu, S. Boron and nitrogen co-doped carbon nanosheets encapsulating nano iron as an efficient catalyst for electrochemical CO2 reduction utilizing a proton exchange membrane CO2 conversion cell. J. Colloid Interface Sci. 2020, 559, 169–177. [Google Scholar] [CrossRef]
- Jia, C.; Ren, W.; Chen, X.; Yang, W.; Zhao, C. (N, B) Dual heteroatom-doped hierarchical porous carbon framework for efficient electroreduction of carbon dioxide. ACS Sustain. Chem. Eng. 2020, 8, 6003–6010. [Google Scholar] [CrossRef]
- Yang, F.; Yu, H.; Mao, X.; Meng, Q.; Chen, S.; Deng, Q.; Zeng, Z.; Wang, J.; Deng, S. Boosting electrochemical CO2 reduction on ternary heteroatoms-doped porous carbon. Chem. Eng. J. 2021, 425, 131661. [Google Scholar] [CrossRef]
- Eid, K.; Ahmad, Y.H.; Mohamed, A.T.; Elsafy, A.G.; Al-Qaradawi, S.Y. Versatile synthesis of Pd and Cu Co-doped porous carbon nitride nanowires for catalytic CO oxidation reaction. Catalysts 2018, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Eid, K.A.M.; Abdullah, A.M. Carbon Nitride Nanostructures for Sustainable Energy Production and Environmental Remediation; RSC: London, UK, 2021; Volume 51. [Google Scholar]
- Eid, K.; Hailan, S.M.; Ibrahim, Y.S.; Salah, B.; Abdullah, A.M. Recent Advances in the Controlled Design of One-dimensional Carbon Nitrides for Thermal CO Oxidation Reaction. In Carbon Nitride Nanostructures for Sustainable Energy Production and Environmental Remediation; RSC: London, UK, 2021; pp. 1–37. [Google Scholar]
- Ibrahim, Y.; Meslam, M.; Eid, K.; Salah, B.; Abdullah, A.M.; Ozoemena, K.I.; Elzatahry, A.; Sharaf, M.A.; Sillanpää, M. A review of MXenes as emergent materials for dye removal from wastewater. Sep. Purif. Technol. 2022, 282, 120083. [Google Scholar] [CrossRef]
- Salah, B.; Eid, K.; Abdelgwad, A.M.; Ibrahim, Y.; Abdullah, A.M.; Hassan, M.K.; Ozoemena, K.I. Titanium Carbide (Ti3C2Tx) MXene Ornamented with Palladium Nanoparticles for Electrochemical CO Oxidation. Electroanalysis 2022, 34, 677–683. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Mohamed, A.; Abdelgawad, A.M.; Eid, K.; Abdullah, A.M.; Elzatahry, A. The recent advances in the mechanical properties of self-standing two-dimensional MXene-based nanostructures: Deep insights into the supercapacitor. Nanomaterials 2020, 10, 1916. [Google Scholar] [CrossRef]
- Abdu, H.I.; Eid, K.; Abdullah, A.M.; Han, Z.; Ibrahim, M.H.; Shan, D.; Chen, J.; Elzatahry, A.A.; Lu, X. Unveiling one-pot scalable fabrication of reusable carboxylated heterogeneous carbon-based catalysts from eucalyptus plant with the assistance of dry ice for selective hydrolysis of eucalyptus biomass. Renew. Energy 2020, 153, 998–1004. [Google Scholar] [CrossRef]
- Abdu, H.I.; Eid, K.; Abdullah, A.M.; Sliem, M.H.; Elzatahry, A.; Lu, X. Dry ice-mediated rational synthesis of edge-carboxylated crumpled graphene nanosheets for selective and prompt hydrolysis of cellulose and eucalyptus lignocellulose under ambient reaction conditions. Green Chem. 2020, 22, 5437–5446. [Google Scholar] [CrossRef]




| Title | Focus | Ref. |
|---|---|---|
| Heteroatom-Doped Porous Carbon-based Nanostructures for Electrochemical CO2 reduction | Engineering of heteroatom (i.e., N, S, P, and B)-doped porous carbon materials for the electrochemical CO2 reduction reaction (CO2RR). The effect of mono, binary, and ternary dopants on CO2RR, and their fundamentals and mechanisms, are discussed, in addition to the effect of CO2 on the environment and human health. | This work |
| Heterogeneous Single-Atom Catalysts for Electrochemical CO2 Reduction Reaction | Synthesis of metal single-atom catalysts (SACs) supported carbon, graphene, and metal–organic framework for CO2RR to CO and its fundamental mechanism. | [49] |
| Advanced Heteroatom-Doped Porous Carbon Membranes Assisted by Poly(ionic liquid) Design and Engineering | Controlling structures and properties of heteroatom-doped porous carbon membranes (HPCMMs) using porous polymer membranes as sacrificial templates built up from heteroatom-rich poly(ionic liquid) for fuel cells and water electrolysis applications. | [54] |
| Metal-Free Carbon Materials for CO2 Electrochemical Reduction | Fabrication of carbon-based catalysts (i.e., carbon fibers, carbon nanotubes, graphene, diamond, nanoporous carbon, and graphene dots) doped with heteroatoms (e.g., N, S, and B) for the CO2RR as well as the identification of active sites and pathways. | [50] |
| Carbon-Supported Single Metal Site Catalysts for Electrochemical CO2 Reduction to CO and Beyond | Fabrication of metal single-atoms embedded in carbon-based SACs (denoted as MNxCy, where M = Ni, Fe, and Co) for electrocatalytic CO2RR to CO, C1, and C2 products. This is in addition to the effect of precursors and synthetic conditions on the structure of SACs. | [34] |
| Rational confinement engineering of MOF-derived carbon-based electrocatalysts toward CO2 reduction and O2 reduction reactions | The fabrication methods of MOF-derived carbon-based electrocatalysts supported (atoms, atomic clusters, and nanoparticles) for oxygen reduction reaction and CO2RR. | [51] |
| Carbon-rich nonprecious metal single atom electrocatalysts for CO2 reduction and hydrogen evolution | The fabrication process of carbon (diamond, MOF, graphene, nanotubes, etc.)-supported single-atom (Ni, Co, Fe, Zn, and Sn) catalysts for CO2RR and supported single-atom (Ni, Co, Fe, Mo, and W) catalysts for hydrogen evolution reaction. | [52] |
| Atomically Structural Regulations of Carbon-Based Single-Atom Catalysts for Electrochemical CO2 Reduction | The fabrication and characterization of carbon-supported metal single-atom catalysts for CO2RR in addition to the recent progress in controlling coordination structures of heteroatom coordination, coordination numbers, diatomic metal centers, and the microenvironments for CO2RR catalytic performance. | [53] |
| Half Reactions of CO2R | E0/V vs. SHE |
|---|---|
| CO2 + e− → CO2− | −1.90 |
| CO2 + 2 H+ + 2 e− → HCOOH | −0.61 |
| CO2 + 2 H+ + 2 e− → CO + H2O | −0.53 |
| CO2 + 4 H+ + 4 e− → HCHO + H2O | −0.48 |
| CO2 + 6 H+ + 6 e− → CH3OH + H2O | −0.38 |
| CO2 + 8 H+ + 8 e− → CH4+ 2H2O | −0.24 |
| 2 CO2 (g) + 10 H2O (l) + 14e− → C2H6 (g) + 14 OH− | −0.270 |
| 3 CO2 (g) + 13 H2O (l) + 18e− → C3H7OH (l) + 18 OH− | −0.320 |
| 2 CO2 (g) + 9 H2O (l) + 12e− → C2H5OH (l) + 12 OH− | −0.33 |
| 2 CO2 (g) + 8 H2O (l) + 12e− → C2H4 (g) + 12 OH− | −0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Eid, K.; Li, W. Heteroatom-Doped Porous Carbon-Based Nanostructures for Electrochemical CO2 Reduction. Nanomaterials 2022, 12, 2379. https://doi.org/10.3390/nano12142379
Lu Q, Eid K, Li W. Heteroatom-Doped Porous Carbon-Based Nanostructures for Electrochemical CO2 Reduction. Nanomaterials. 2022; 12(14):2379. https://doi.org/10.3390/nano12142379
Chicago/Turabian StyleLu, Qingqing, Kamel Eid, and Wenpeng Li. 2022. "Heteroatom-Doped Porous Carbon-Based Nanostructures for Electrochemical CO2 Reduction" Nanomaterials 12, no. 14: 2379. https://doi.org/10.3390/nano12142379