Boosting the Electrocatalytic CO2 Reduction Reaction by Nanostructured Metal Materials via Defects Engineering
Abstract
:1. Introduction
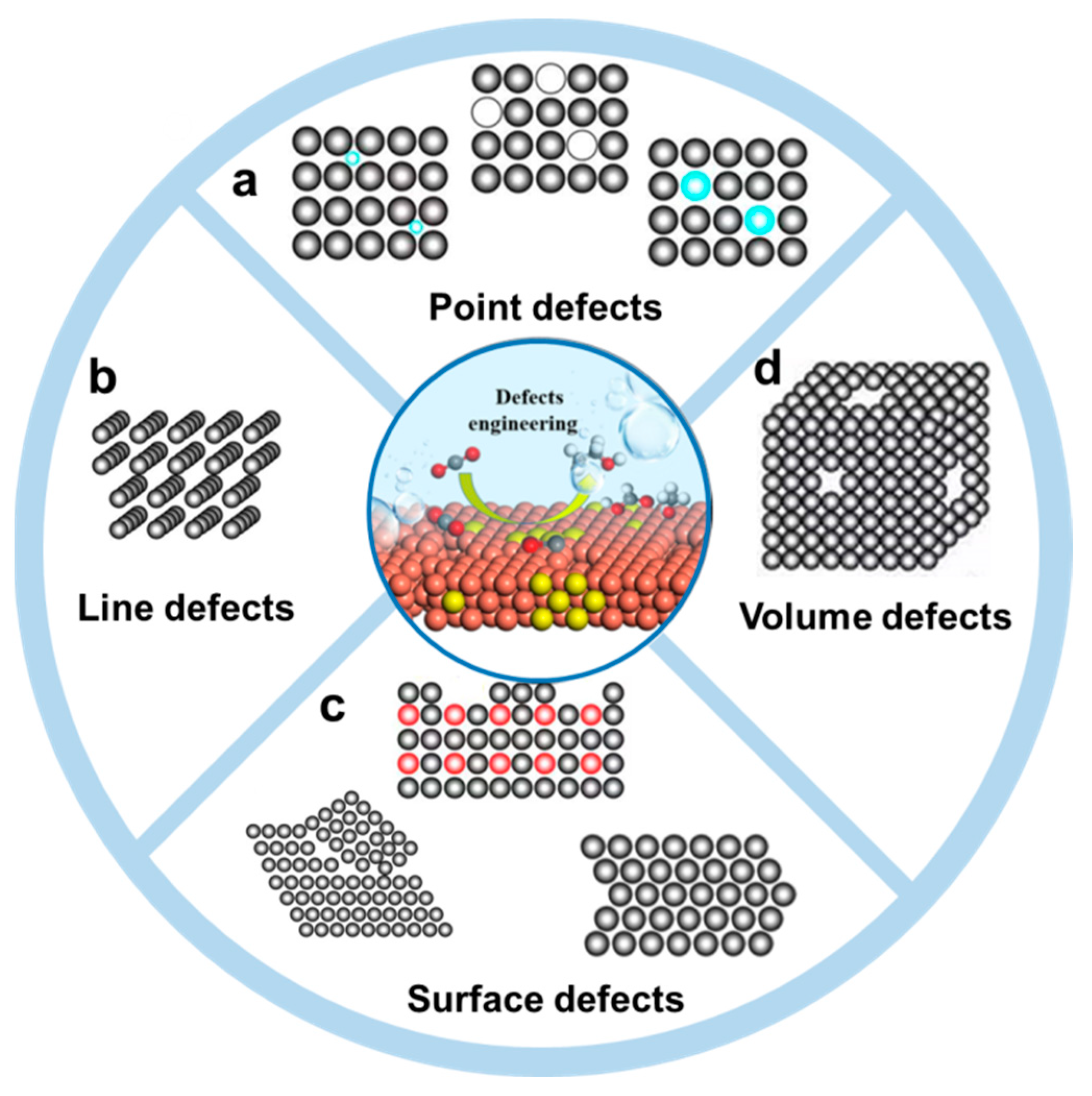
2. Defects in Metal Nanomaterials
2.1. Point Defects
2.2. Line Defects
2.3. Surface Defects
2.4. Volume Defects
3. Relationship between Defects and CO2RR
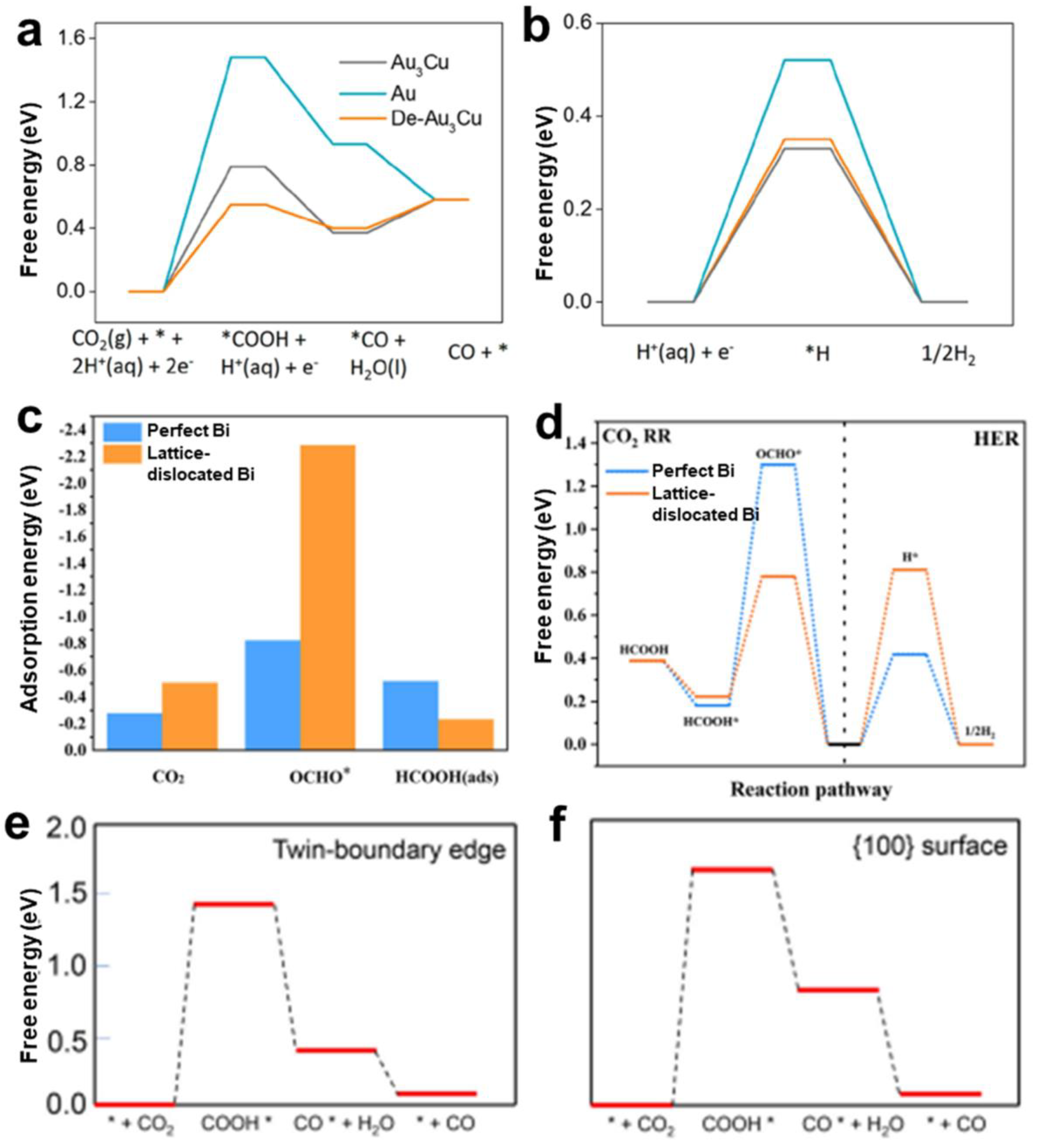
4. Defects Engineering
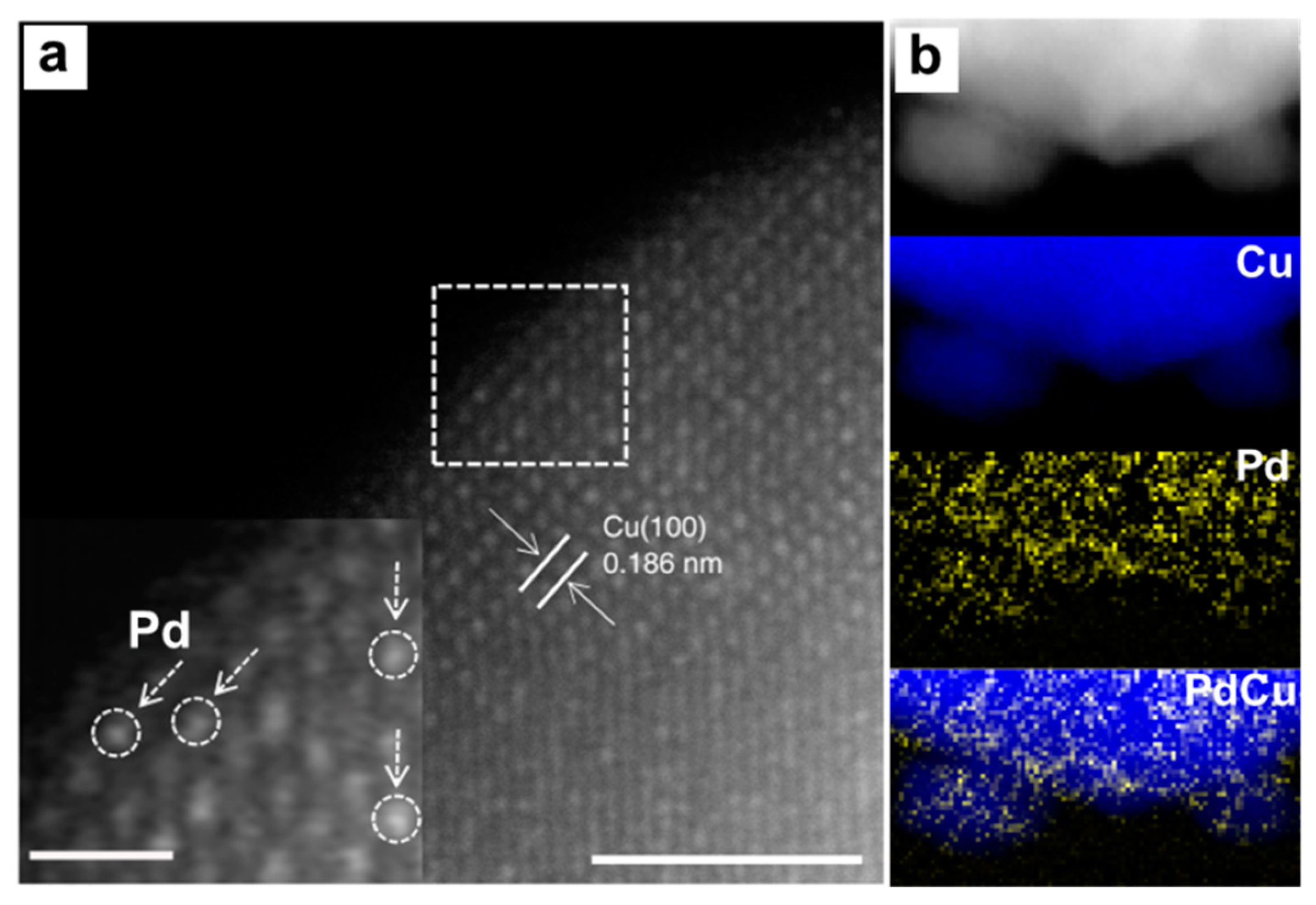
4.1. Point Defects Engineering
4.2. Line Defects Engineering
4.3. Surface Defects Engineering
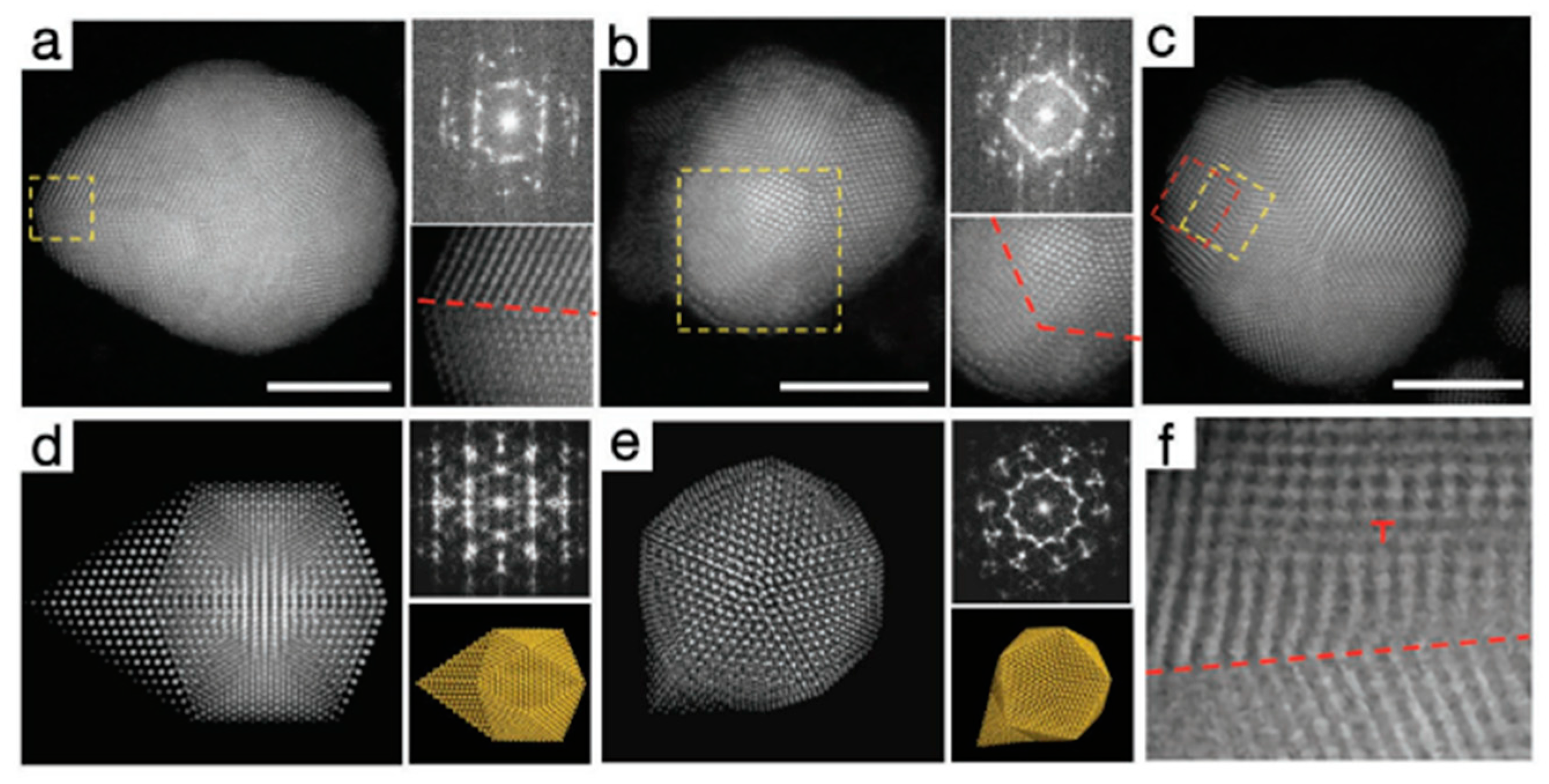
4.4. Volume Defects Engineering
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seneviratne, S.I.; Donat, M.G.; Pitman, A.J.; Knutti, R.; Wilby, R.L. Allowable CO2 emissions based on regional and impact−related climate targets. Nature 2016, 529, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Xia, C.; Yang, F.; Wang, J.; Wang, H.; Lu, Y. Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products. Sci. Adv. 2020, 6, eaay3111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef] [PubMed]
- Al−Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon Capture and Utilization Update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Peng, B.; Peng, T. Recent Advances in Heterogeneous Photocatalytic CO2 Conversion to Solar Fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef]
- Gallo, A.; Snider, J.L.; Sokaras, D.; Nordlund, D.; Kroll, T.; Ogasawara, H.; Kovarik, L.; Duyar, M.S.; Jaramillo, T.F. Ni5Ga3 catalysts for CO2 reduction to methanol: Exploring the role of Ga surface oxidation/reduction on catalytic activity. Appl. Catal. B–Environ. 2020, 267, 118369. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Wu, M.; Du, Y.; Fan, X.; Wang, M.; Zhang, L.; Kong, Q.; Shi, J. Mesostructured CeO2/g−C3N4 nanocomposites: Remarkably enhanced photocatalytic activity for CO2 reduction by mutual component activations. Nano Energy 2016, 19, 145–155. [Google Scholar] [CrossRef]
- Li, Q.; Wang, S.; Sun, Z.; Tang, Q.; Liu, Y.; Wang, L.; Wang, H.; Wu, Z. Enhanced CH4 selectivity in CO2 photocatalytic reduction over carbon quantum dots decorated and oxygen doping g–C3N4. Nano Res. 2019, 12, 2749–2759. [Google Scholar] [CrossRef]
- Wang, H.; Tzeng, Y.K.; Ji, Y.; Li, Y.; Li, J.; Zheng, X.; Yang, A.; Liu, Y.; Gong, Y.; Cai, L.; et al. Synergistic enhancement of electrocatalytic CO2 reduction to C2 oxygenates at nitrogen−doped nanodiamonds/Cu interface. Nat. Nanotechnol. 2020, 15, 131–137. [Google Scholar] [CrossRef]
- Kan, M.; Wang, Q.; Hao, S.; Guan, A.; Chen, Y.; Zhang, Q.; Han, Q.; Zheng, G. System Engineering Enhances Photoelectrochemical CO2 Reduction. J. Phys. Chem. C 2022, 126, 1689–1700. [Google Scholar] [CrossRef]
- Cai, S.; Chen, J.; Li, Q.; Jia, H. Enhanced Photocatalytic CO2 Reduction with Photothermal Effect by Cooperative Effect of Oxygen Vacancy and Au Cocatalyst. ACS Appl. Mater. Interfaces 2021, 13, 14221–14229. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, L.; Huang, W.; Xu, C.; Zhang, Y. Photothermal Catalysis for Selective CO2 Reduction on the Modified Anatase TiO2 (101) Surface. ACS Appl. Energy Mater. 2021, 4, 7702–7709. [Google Scholar] [CrossRef]
- Ross, M.B.; De Luna, P.; Li, Y.; Dinh, C.-T.; Kim, D.; Yang, P.; Sargent, E.H. Designing materials for electrochemical carbon dioxide recycling. Nat. Catal. 2019, 2, 648–658. [Google Scholar] [CrossRef] [Green Version]
- Shao, Q.; Wang, P.; Zhu, T.; Huang, X. Low Dimensional Platinum–Based Bimetallic Nanostructures for Advanced Catalysis. Acc. Chem. Res. 2019, 52, 3384–3396. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Peter, S.C. An overview on Pd–based electrocatalysts for the hydrogen evolution reaction. Inorg. Chem. Front. 2018, 5, 2060–2080. [Google Scholar] [CrossRef]
- Xie, H.; Wang, T.; Liang, J.; Li, Q.; Sun, S. Cu–based nanocatalysts for electrochemical reduction of CO2. Nano Today 2018, 21, 41–54. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.W.; Kanan, M.W. Aqueous CO2 reduction at very low overpotential on oxide–derived Au nanoparticles. J. Am. Chem. Soc. 2012, 134, 19969–19972. [Google Scholar] [CrossRef]
- Shi, R.; Guo, J.; Zhang, X.; Waterhouse, G.I.N.; Han, Z.; Zhao, Y.; Shang, L.; Zhou, C.; Jiang, L.; Zhang, T. Efficient wettability–controlled electroreduction of CO2 to CO at Au/C interfaces. Nat. Commun. 2020, 11, 3028. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, X.; Tao, L.; Jiang, P.; Ye, C.; Lin, R.; Huang, Z.; Li, A.; Pang, D.; Yan, H.; et al. Silver Single–Atom Catalyst for Efficient Electrochemical CO2 Reduction Synthesized from Thermal Transformation and Surface Reconstruction. Angew. Chem. Int. Ed. 2021, 60, 6170–6176. [Google Scholar] [CrossRef]
- Gao, D.; Zhou, H.; Wang, J.; Miao, S.; Yang, F.; Wang, G.; Wang, J.; Bao, X. Size–dependent electrocatalytic reduction of CO2 over Pd nanoparticles. J. Am. Chem. Soc. 2015, 137, 4288–4291. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, S.; Guo, T.; Zhang, S.; Wang, J.; Wu, Y.; Chen, Y. Advances in Sn–Based Catalysts for Electrochemical CO2 Reduction. Nanomicro. Lett. 2019, 11, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Zhao, P.; Xue, X.; Chen, R.; Yang, S.; Ma, J.; Liu, J.; et al. Liquid−phase exfoliated ultrathin Bi nanosheets: Uncovering the origins of enhanced electrocatalytic CO2 reduction on two–dimensional metal nanostructure. Nano Energy 2018, 53, 808–816. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Zhang, Y.; Tao, L.; Wang, T.; Zou, Y.; Wang, Y.; Chen, R.; Wang, S. Defect Engineering for Fuel–Cell Electrocatalysts. Adv. Mater. 2020, 32, e1907879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, L.; Tao, L.; Lu, Y.; Wang, S. Defect–Based Single–Atom Electrocatalysts. Small Methods 2018, 3, 1800406. [Google Scholar] [CrossRef]
- Yan, D.; Li, H.; Chen, C.; Zou, Y.; Wang, S. Defect Engineering Strategies for Nitrogen Reduction Reactions under Ambient Conditions. Small Methods 2018, 3, 1800331. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Huo, J.; Chen, R.; Dai, L.; Wang, S. Defect Chemistry of Nonprecious−Metal Electrocatalysts for Oxygen Reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, K.; Wang, H.; Yao, X. The Role of Defect Sites in Nanomaterials for Electrocatalytic Energy Conversion. Chem 2019, 5, 1371–1397. [Google Scholar] [CrossRef]
- Xie, C.; Yan, D.; Chen, W.; Zou, Y.; Chen, R.; Zang, S.; Wang, Y.; Yao, X.; Wang, S. Insight into the design of defect electrocatalysts: From electronic structure to adsorption energy. Mater. Today 2019, 31, 47–68. [Google Scholar] [CrossRef]
- Li, G.; Blake, G.R.; Palstra, T.T. Vacancies in functional materials for clean energy storage and harvesting: The perfect imperfection. Chem. Soc. Rev. 2017, 46, 1693–1706. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, L.; Yang, P.; Hu, C.; Dong, H.; Zhao, Z.-T.; Mu, R.; Gong, J. Formation of Enriched Vacancies for Enhanced CO2 Electrocatalytic Reduction over AuCu Alloys. ACS Energy Lett. 2018, 3, 2144–2149. [Google Scholar] [CrossRef]
- Kotakoski, J.; Mangler, C.; Meyer, J.C. Imaging atomic-level random walk of a point defect in graphene. Nat. Commun. 2014, 5, 3991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Chen, Z.; Han, Y.; Deb, P.; Gao, H.; Xie, S.; Purohit, P.; Tate, M.W.; Park, J.; Gruner, S.M.; et al. Electron ptychography of 2D materials to deep sub–angstrom resolution. Nature 2018, 559, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Van Benthem, K.; Lupini, A.R.; Kim, M.; Baik, H.S.; Doh, S.; Lee, J.-H.; Oxley, M.P.; Findlay, S.D.; Allen, L.J.; Luck, J.T.; et al. Three–dimensional imaging of individual hafnium atoms inside a semiconductor device. Appl. Phys. Lett. 2005, 87, 034104. [Google Scholar] [CrossRef]
- Zhu, Y.; Tao, L.; Chen, X.; Ma, Y.; Ning, S.; Zhou, J.; Zhao, X.; Bosman, M.; Liu, Z.; Du, S.; et al. Anisotropic point defects in rhenium diselenide monolayers. iScience 2021, 24, 103456. [Google Scholar] [CrossRef]
- Li, J.; Xu, A.; Li, F.; Wang, Z.; Zou, C.; Gabardo, C.M.; Wang, Y.; Ozden, A.; Xu, Y.; Nam, D.H.; et al. Enhanced multi–carbon alcohol electroproduction from CO via modulated hydrogen adsorption. Nat. Commun. 2020, 11, 3685. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, G.; Cui, X.; Gong, Y.; Yi, D.; Zhang, Q.; Zhu, C.; Saleem, F.; Chen, B.; Lai, Z.; et al. Evoking ordered vacancies in metallic nanostructures toward a vacated Barlow packing for high–performance hydrogen evolution. Sci. Adv. 2021, 7, eabd6647. [Google Scholar] [CrossRef]
- Shao, W.; Pan, Q.; Chen, Q.; Zhu, C.; Tao, W.; Zhu, H.; Song, H.; Liu, X.; Tan, P.H.; Sheng, G.; et al. Symmetry Breaking in Monometallic Nanocrystals toward Broadband and Direct Electron Transfer Enhanced Plasmonic Photocatalysis. Adv. Funct. Mater. 2020, 31, 2006738. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, M.; Yang, T.-H.; Xia, Y. Decahedral nanocrystals of noble metals: Synthesis, characterization, and applications. Mater. Today 2019, 22, 108–131. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, S.; Gilroy, K.D.; Cai, Z.; Xia, Y. Icosahedral nanocrystals of noble metals: Synthesis and applications. Nano Today 2017, 15, 121–144. [Google Scholar] [CrossRef]
- Lee, S.R.; Vara, M.; Hood, Z.D.; Zhao, M.; Gilroy, K.D.; Chi, M.; Xia, Y. Rhodium Decahedral Nanocrystals: Facile Synthesis, Mechanistic Insights, and Experimental Controls. ChemNanoMat 2018, 4, 66–70. [Google Scholar] [CrossRef]
- Tang, Y.; Edelmann, R.E.; Zou, S. Length tunable penta–twinned palladium nanorods: Seedless synthesis and electrooxidation of formic acid. Nanoscale 2014, 6, 5630–5633. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, P.; Song, L.; Wang, J.X.; Liu, L.F.; Liu, D.F.; Xiang, Y.J.; Zhang, Z.X.; Zhao, X.W.; Dou, X.Y.; et al. Studies on silver nanodecahedrons synthesized by PVP–assisted N,N–dimethylformamide (DMF) reduction. J. Crys. Growth 2006, 289, 376–380. [Google Scholar] [CrossRef]
- Song, M.; Wu, Z.; Lu, N.; Li, D. Strain Relaxation-Induced Twin Interface Migration and Morphology Evolution of Silver Nanoparticles. Chem. Mater. 2019, 31, 842–850. [Google Scholar] [CrossRef]
- Choi, C.; Cheng, T.; Flores Espinosa, M.; Fei, H.; Duan, X.; Goddard, W.A., 3rd; Huang, Y. A Highly Active Star Decahedron Cu Nanocatalyst for Hydrocarbon Production at Low Overpotentials. Adv. Mater. 2019, 31, e1805405. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.L.; Snoeck, E.; Ezcurdia, M.; Rodriguez-Gonzalez, B.; Pastoriza-Santos, I.; Liz-Marzan, L.M.; Hytch, M.J. Effects of elastic anisotropy on strain distributions in decahedral gold nanoparticles. Nat. Mater. 2008, 7, 120–124. [Google Scholar] [CrossRef]
- Chatterjee, D.; Shetty, S.; Muller-Caspary, K.; Grieb, T.; Krause, F.F.; Schowalter, M.; Rosenauer, A.; Ravishankar, N. Ultrathin Au–Alloy Nanowires at the Liquid–Liquid Interface. Nano Lett. 2018, 18, 1903–1907. [Google Scholar] [CrossRef]
- Yu, Y.; Cui, F.; Sun, J.; Yang, P. Atomic Structure of Ultrathin Gold Nanowires. Nano Lett. 2016, 16, 3078–3084. [Google Scholar] [CrossRef]
- Roy, A.; Kundu, S.; Muller, K.; Rosenauer, A.; Singh, S.; Pant, P.; Gururajan, M.P.; Kumar, P.; Weissmuller, J.; Singh, A.K.; et al. Wrinkling of atomic planes in ultrathin Au nanowires. Nano Lett. 2014, 14, 4859–4866. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.; Yang, G.; Chen, Q.; Yin, Y.; Jin, M. Creation of Controllable High–Density Defects in Silver Nanowires for Enhanced Catalytic Property. Nano Lett. 2016, 16, 5669–5674. [Google Scholar] [CrossRef]
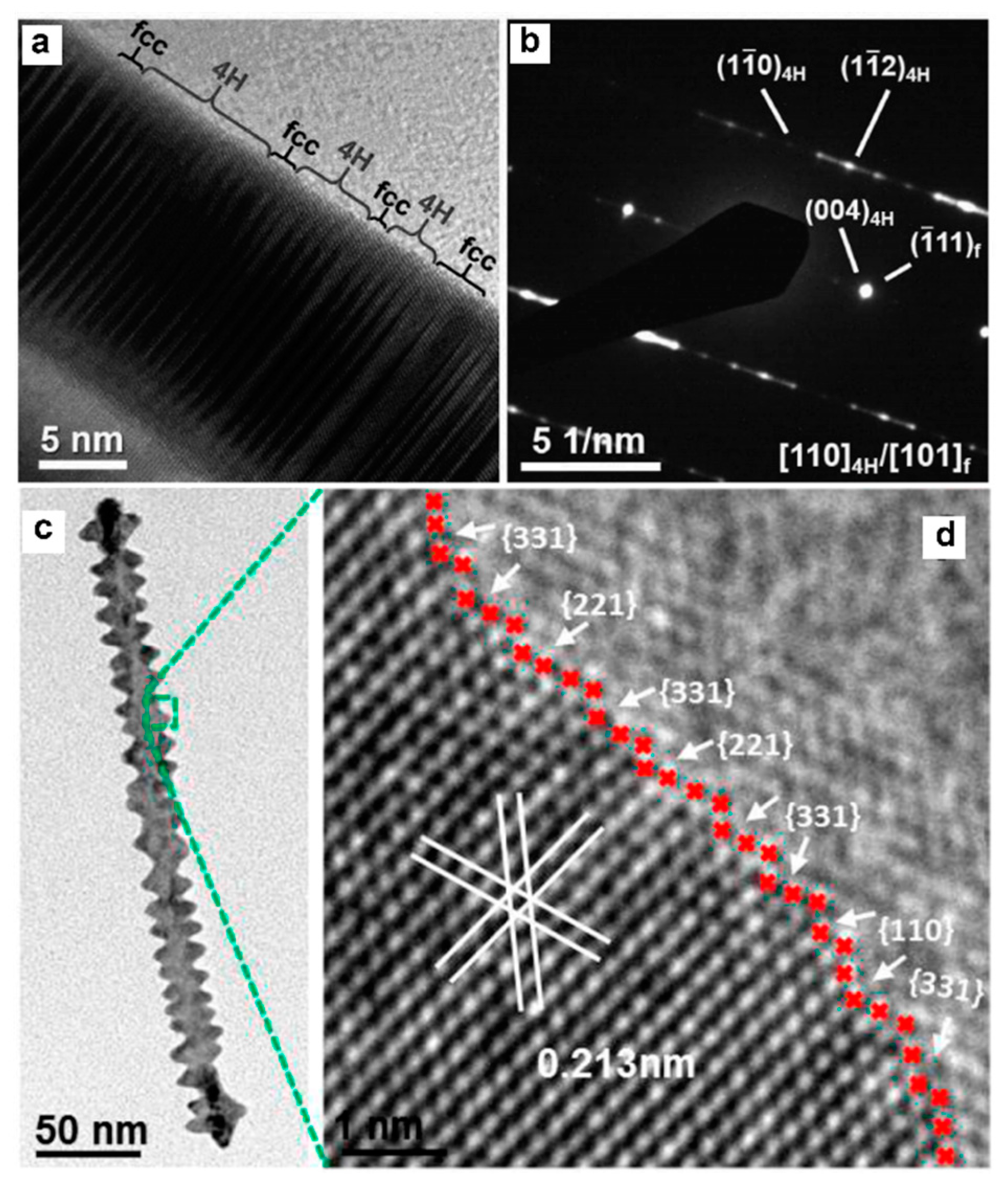
- Fan, Z.; Bosman, M.; Huang, X.; Huang, D.; Yu, Y.; Ong, K.P.; Akimov, Y.A.; Wu, L.; Li, B.; Wu, J.; et al. Stabilization of 4H hexagonal phase in gold nanoribbons. Nat. Commun. 2015, 6, 7684. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, Z.; Luo, Z.; Liu, X.; Lai, Z.; Li, B.; Zong, Y.; Gu, L.; Zhang, H. High–Yield Synthesis of Crystal-Phase–Heterostructured 4H/fcc Au@Pd Core–Shell Nanorods for Electrocatalytic Ethanol Oxidation. Adv. Mater. 2017, 29, 1701331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Bu, L.; Guo, S.; Guo, J.; Huang, X. Screw Thread–Like Platinum–Copper Nanowires Bounded with High–Index Facets for Efficient Electrocatalysis. Nano Lett. 2016, 16, 5037–5043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.Y.; Sheng, T.; Tian, N.; Liu, L.; Xiao, C.; Lu, B.A.; Xu, B.B.; Zhou, Z.Y.; Sun, S.G. Cu overlayers on tetrahexahedral Pd nanocrystals with high–index facets for CO2 electroreduction to alcohols. Chem. Commun. 2017, 53, 8085–8088. [Google Scholar] [CrossRef]
- Koh, J.H.; Won, D.H.; Eom, T.; Kim, N.-K.; Jung, K.D.; Kim, H.; Hwang, Y.J.; Min, B.K. Facile CO2 Electro-Reduction to Formate via Oxygen Bidentate Intermediate Stabilized by High–Index Planes of Bi Dendrite Catalyst. ACS Catal. 2017, 7, 5071–5077. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Lu, Q.; Rivera, S.; Zhou, Y.; Vlachos, D.G.; Jiao, F. Mechanistic Insights into the Electrochemical Reduction of CO2 to CO on Nanostructured Ag Surfaces. ACS Catal. 2015, 5, 4293–4299. [Google Scholar] [CrossRef]
- Huo, H.; Wang, J.; Fan, Q.; Hu, Y.; Yang, J. Cu–MOFs Derived Porous Cu Nanoribbons with Strengthened Electric Field for Selective CO2 Electroreduction to C2+ Fuels. Adv. Energy Mater. 2021, 11, 2102447. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Liu, J.; Dong, C.; Xiao, C.; Cheng, L.; Jiang, H.; Jiang, H.; Li, C. BiPO4–Derived 2D Nanosheets for Efficient Electrocatalytic Reduction of CO2 to Liquid Fuel. Angew. Chem. Int. Ed. 2021, 60, 7681–7685. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Kim, Y.; Hwang, Y.J.; Won, D.H. Progress in development of electrocatalyst for CO2 conversion to selective CO production. Carbon Energy 2019, 2, 72–98. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, X.X.; Lang, Z.L.; Liu, Y.; Liu, J.; Yuan, L.; Lu, W.Y.; Xia, Y.S.; Dong, L.Z.; Yuan, D.Q.; et al. Enhanced Cuprophilic Interactions in Crystalline Catalysts Facilitate the Highly Selective Electroreduction of CO2 to CH4. J. Am. Chem. Soc. 2021, 143, 3808–3816. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Hua, M.; Wan, Q.; Su, Z.; Tan, X.; Liu, L.; Zhang, F.; Chen, G.; Tan, D.; et al. Highly Electrocatalytic Ethylene Production from CO2 on Nanodefective Cu Nanosheets. J. Am. Chem. Soc. 2020, 142, 13606–13613. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Fu, X.; Zhang, L.; Zeng, Y.-J.; Huang, H. Point–defect–optimized electron distribution for enhanced electrocatalysis: Towards the perfection of the imperfections. Nano Today 2020, 31, 100833. [Google Scholar] [CrossRef]
- Wu, D.; Feng, R.; Xu, C.; Sui, P.F.; Zhang, J.; Fu, X.Z.; Luo, J.L. Regulating the Electron Localization of Metallic Bismuth for Boosting CO2 Electroreduction. Nanomicro Lett. 2021, 14, 38. [Google Scholar] [CrossRef] [PubMed]
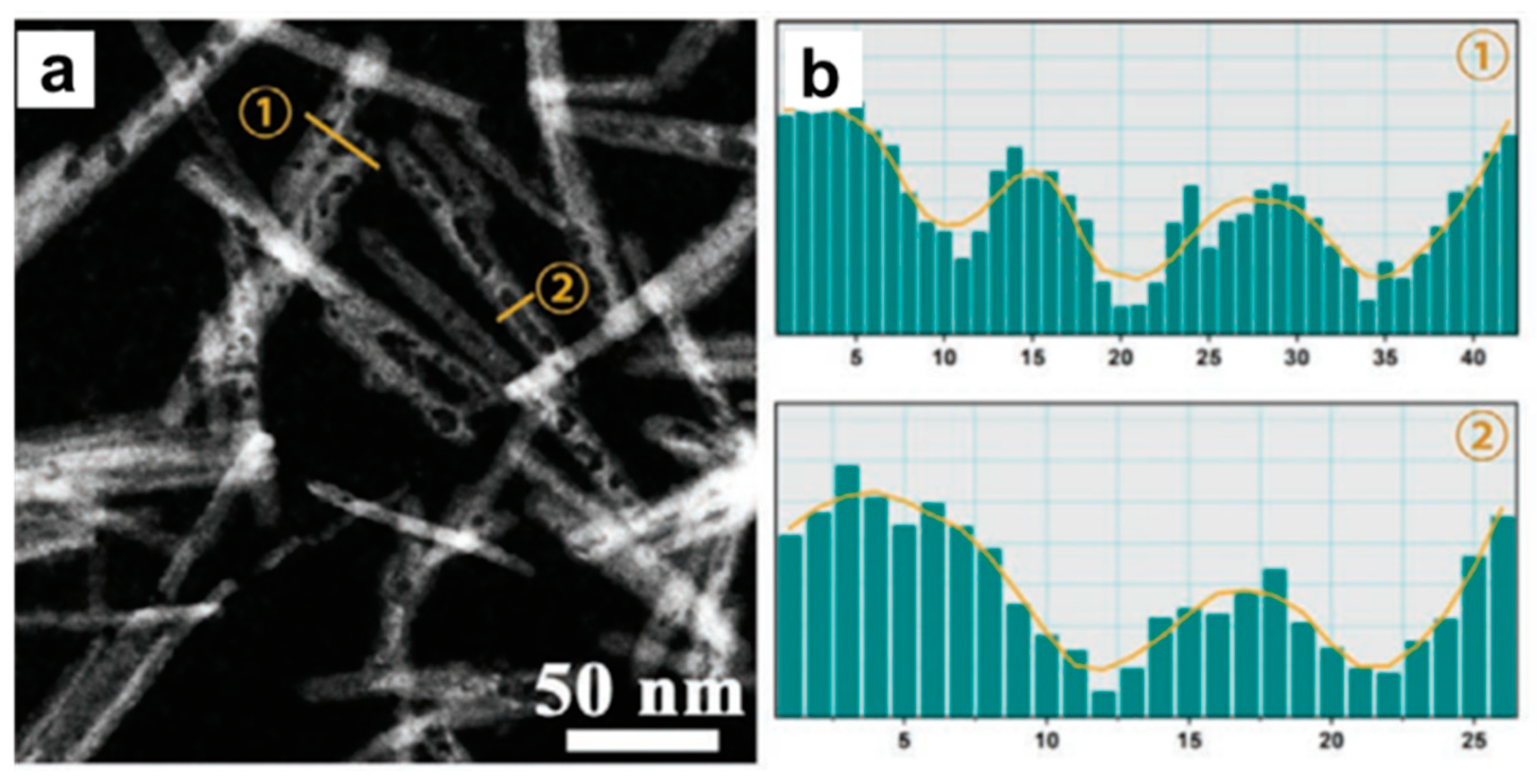
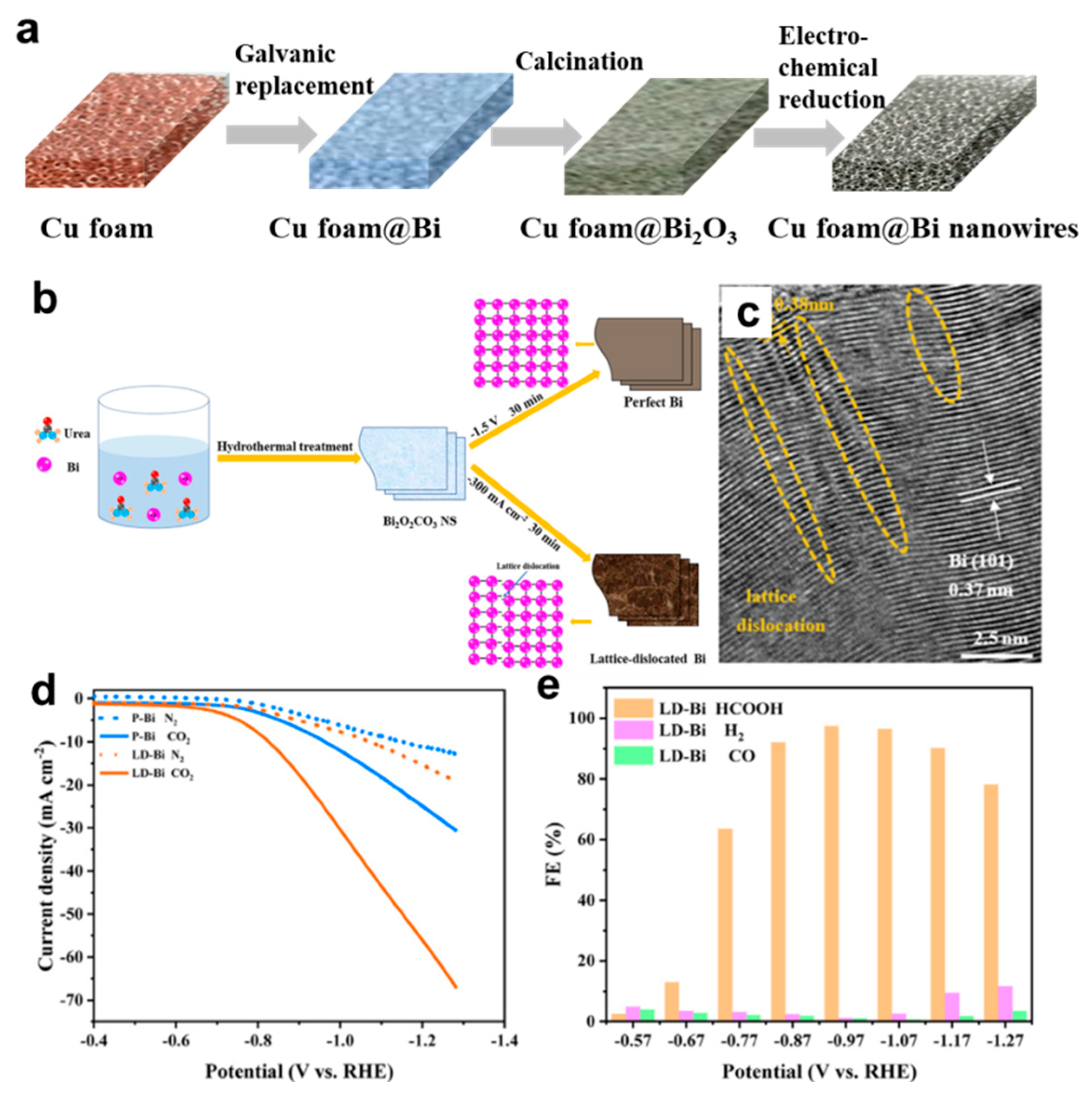
- Wang, Y.; Gong, H.; Wang, Y.; Gao, L. Lattice-dislocated Bi nanosheets for electrocatalytic reduction of carbon dioxide to formate over a wide potential window. J. Colloid Interf. Sci. 2022, 611, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Ciston, J.; Kanan, M.W. Electroreduction of carbon monoxide to liquid fuel on oxide–derived nanocrystalline copper. Nature 2014, 508, 504–507. [Google Scholar] [CrossRef]
- Cheng, T.; Huang, Y.; Xiao, H.; Goddard, W.A., 3rd. Predicted Structures of the Active Sites Responsible for the Improved Reduction of Carbon Dioxide by Gold Nanoparticles. J. Phys. Chem. Lett. 2017, 8, 3317–3320. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Fu, J.; Liu, H.; Ling, T.; Yang, J.; Qiao, S.Z.; Du, X.-W. Tuning the selectivity and activity of Au catalysts for carbon dioxide electroreduction via grain boundary engineering: A DFT study. J. Mater. Chem. A 2017, 5, 7184–7190. [Google Scholar] [CrossRef]
- Hu, F.; Abeyweera, S.C.; Yu, J.; Zhang, D.; Wang, Y.; Yan, Q.; Sun, Y. Quantifying Electrocatalytic Reduction of CO2 on Twin Boundaries. Chem 2020, 6, 3007–3021. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, J.; Li, M.; Züttel, A. Boosting CO Production in Electrocatalytic CO2 Reduction on Highly Porous Zn Catalysts. ACS Catal. 2019, 9, 3783–3791. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zou, J.; Hu, X.; Ning, S.; Wang, X.; Kang, X.; Chen, S. Heterostructured intermetallic CuSn catalysts: High performance towards the electrochemical reduction of CO2 to formate. J. Mater. Chem. A 2019, 7, 27514–27521. [Google Scholar] [CrossRef]
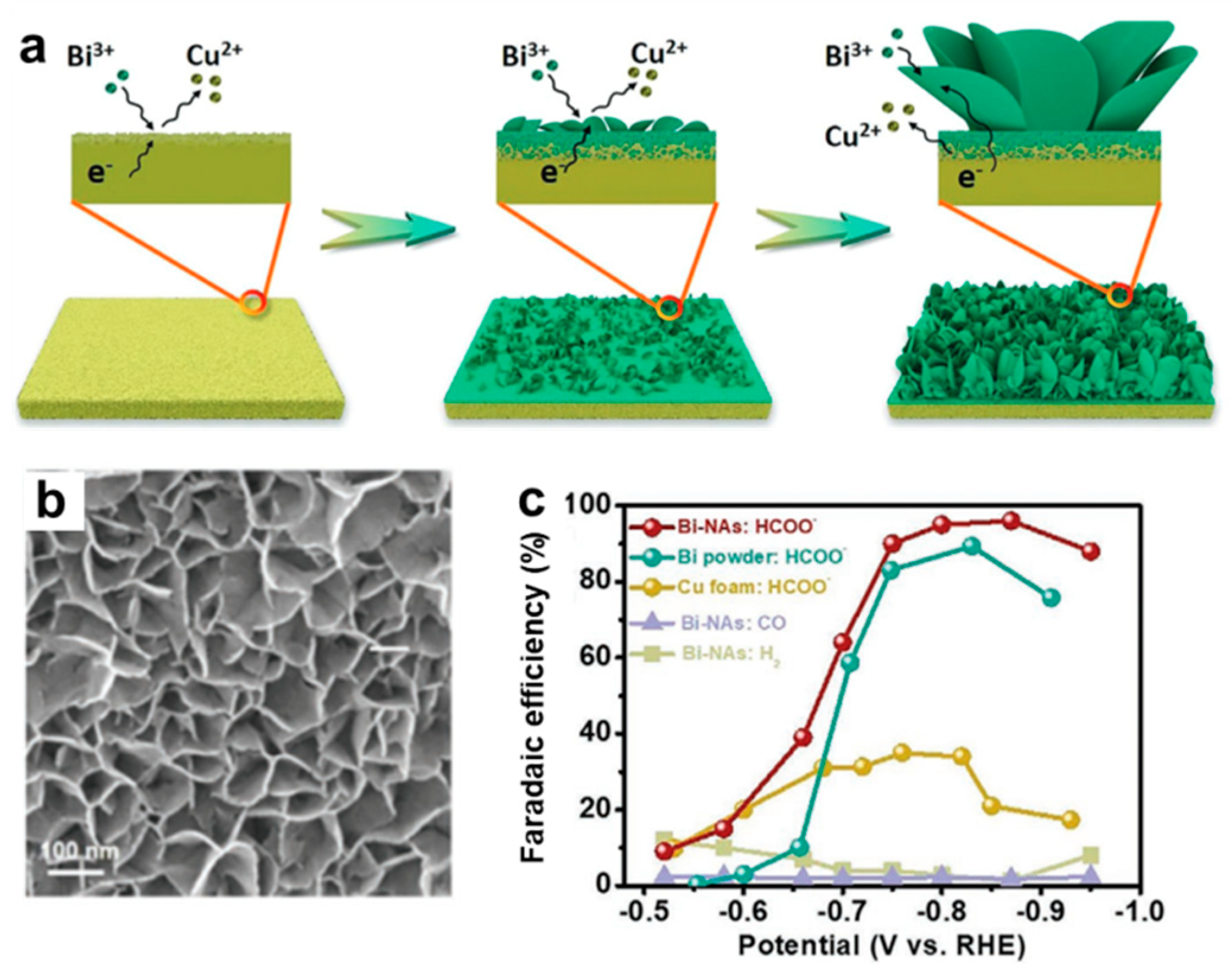
- Fan, J.; Zhao, X.; Mao, X.; Xu, J.; Han, N.; Yang, H.; Pan, B.; Li, Y.; Wang, L.; Li, Y. Large–Area Vertically Aligned Bismuthene Nanosheet Arrays from Galvanic Replacement Reaction for Efficient Electrochemical CO2 Conversion. Adv. Mater. 2021, 33, e2100910. [Google Scholar] [CrossRef] [PubMed]
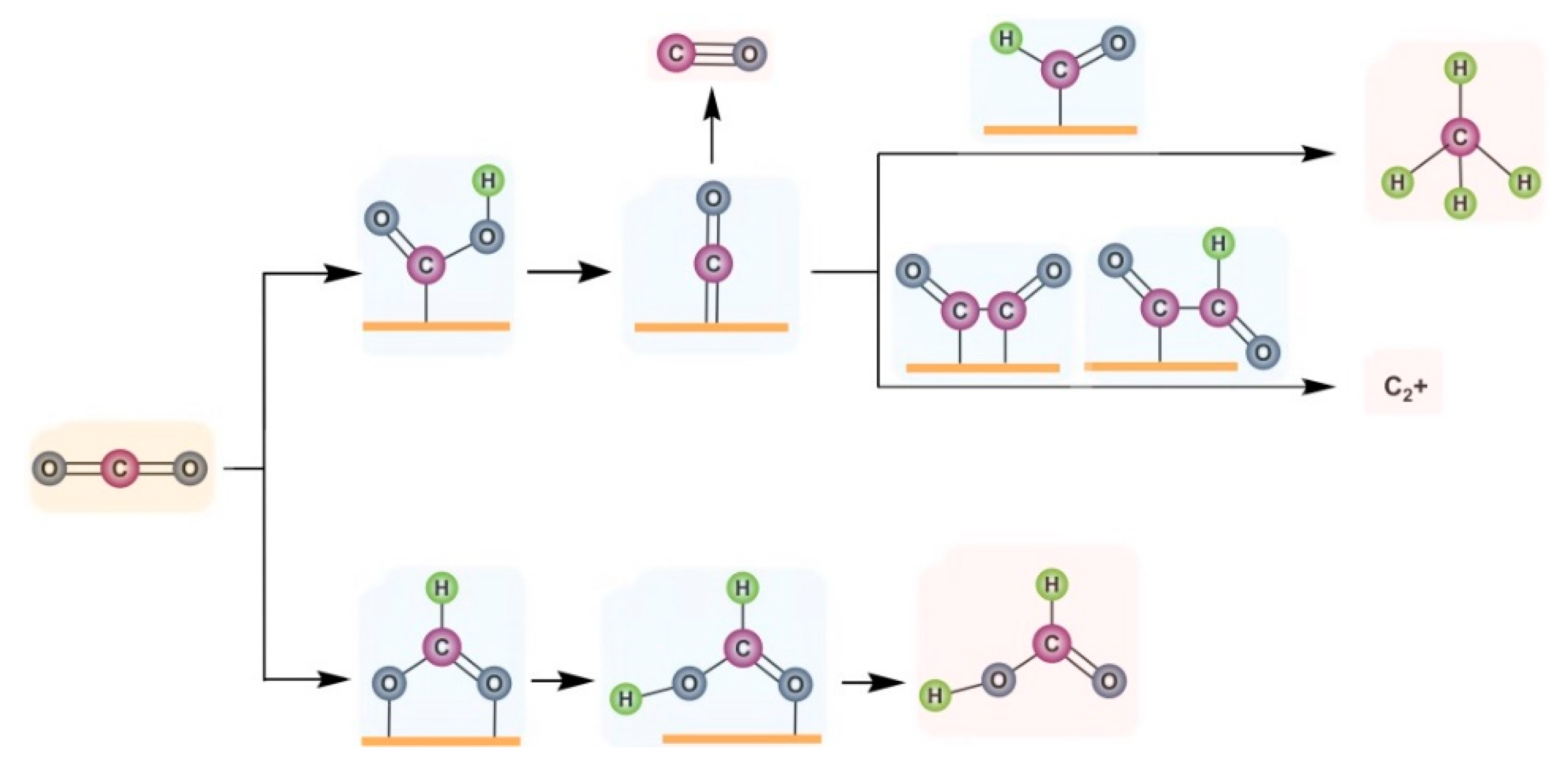
- Zhou, Y.; Che, F.; Liu, M.; Zou, C.; Liang, Z.; De Luna, P.; Yuan, H.; Li, J.; Wang, Z.; Xie, H.; et al. Dopant–induced electron localization drives CO2 reduction to C2 hydrocarbons. Nat. Chem. 2018, 10, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, Y.; Chen, J.; Lawrence, R.; Luo, W.; Sacchi, M.; Jiang, W.; Yang, J. Residual Chlorine Induced Cationic Active Species on a Porous Copper Electrocatalyst for Highly Stable Electrochemical CO2 Reduction to C2. Angew. Chem. Int. Ed. 2021, 60, 11487–11493. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, X.; Guo, S.-X.; Bond, A.M.; Zhang, J. Formation of lattice–dislocated bismuth nanowires on copper foam for enhanced electrocatalytic CO2 reduction at low overpotential. Energy Environ. Sci. 2019, 12, 1334–1340. [Google Scholar] [CrossRef]
- Tian, N.; Zhou, Z.Y.; Sun, S.G.; Ding, Y.; Wang, Z.L. Synthesis of tetrahexahedral platinum nanocrystals with high–index facets and high electro–oxidation activity. Science 2007, 316, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.X.; Lei, Z.C.; Jiang, Z.Y.; Hou, C.P.; Liu, D.Y.; Xu, M.M.; Tian, Z.Q.; Xie, Z.X. Supersaturation–dependent surface structure evolution: From ionic, molecular to metallic micro/nanocrystals. J. Am. Chem. Soc. 2013, 135, 9311–9314. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, H.; Lu, N.; Jin, M.; Wang, J.; Kim, M.J.; Xie, Z.; Xia, Y. Synthesis of rhodium concave tetrahedrons by collectively manipulating the reduction kinetics, facet–selective capping, and surface diffusion. Nano Lett. 2013, 13, 6262–6268. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Nosheen, F.; Zhang, J.C.; Yang, Y.; Wang, P.P.; Zhuang, J.; Wang, X. Growth of concave polyhedral Pd nanocrystals with 32 facets through in situ facet–selective etching. ChemSusChem 2013, 6, 1893–1897. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, Y.; Zhang, J.; Zhang, L.; Chen, Q.; Xie, Z.; Zheng, L. Unique excavated rhombic dodecahedral PtCu3 alloy nanocrystals constructed with ultrathin nanosheets of high–energy {110} facets. J. Am. Chem. Soc. 2014, 136, 3748–3751. [Google Scholar] [CrossRef]
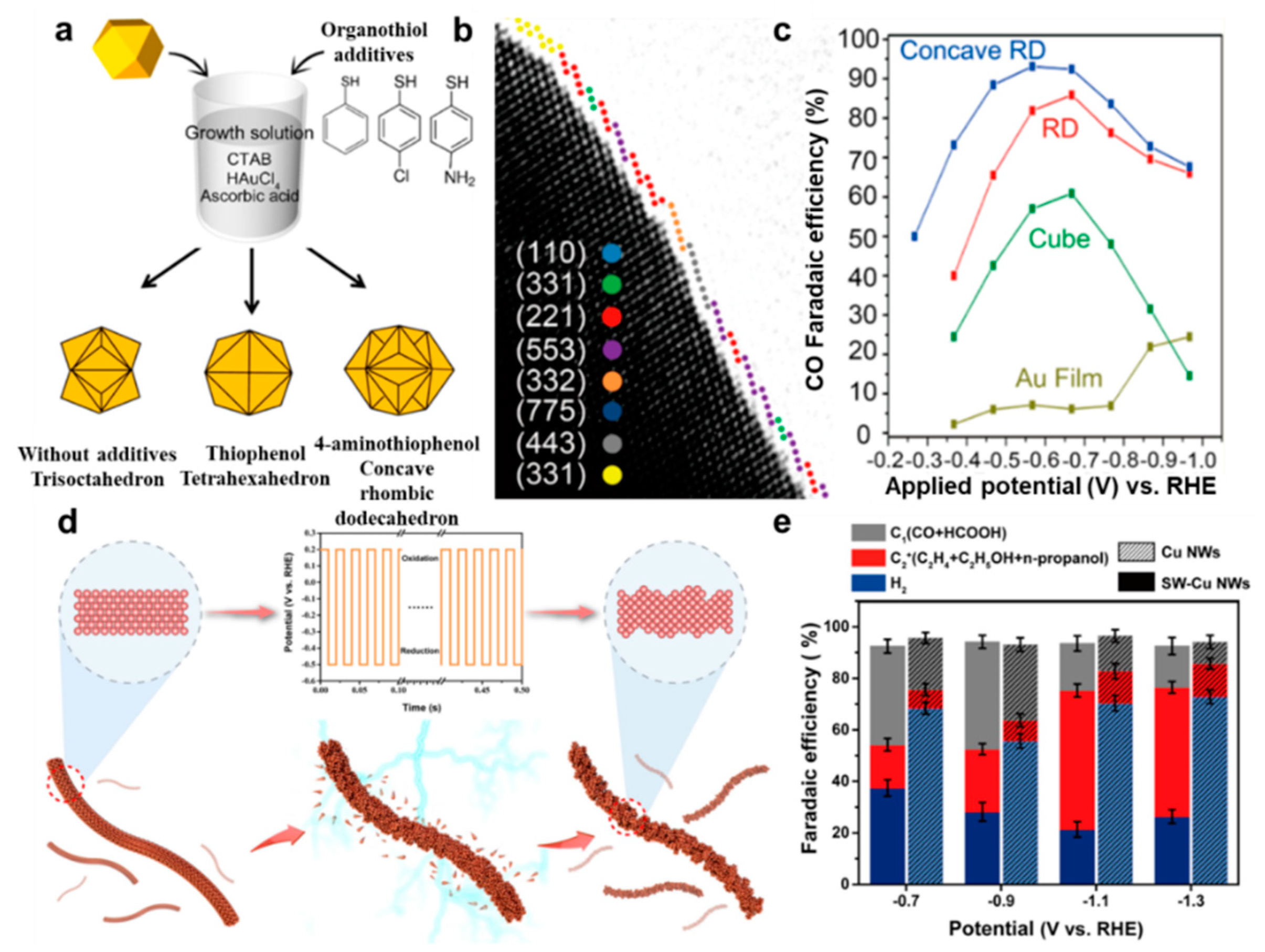
- Lee, H.E.; Yang, K.D.; Yoon, S.M.; Ahn, H.Y.; Lee, Y.Y.; Chang, H.; Jeong, D.H.; Lee, Y.S.; Kim, M.Y.; Nam, K.T. Concave Rhombic Dodecahedral Au Nanocatalyst with Multiple High–Index Facets for CO2 Reduction. ACS Nano 2015, 9, 8384–8393. [Google Scholar] [CrossRef]
- Han, L.; Tian, B.; Gao, X.; Zhong, Y.; Wang, S.; Song, S.; Wang, Z.; Zhang, Y.; Kuang, Y.; Sun, X. Copper nanowire with enriched high–index facets for highly selective CO2 reduction. SmartMat 2022, 3, 142–150. [Google Scholar] [CrossRef]
- Yao, D.; Tang, C.; Vasileff, A.; Zhi, X.; Jiao, Y.; Qiao, S.Z. The Controllable Reconstruction of Bi–MOFs for Electrochemical CO2 Reduction through Electrolyte and Potential Mediation. Angew. Chem. Int. Ed. 2021, 60, 18178–18184. [Google Scholar] [CrossRef]
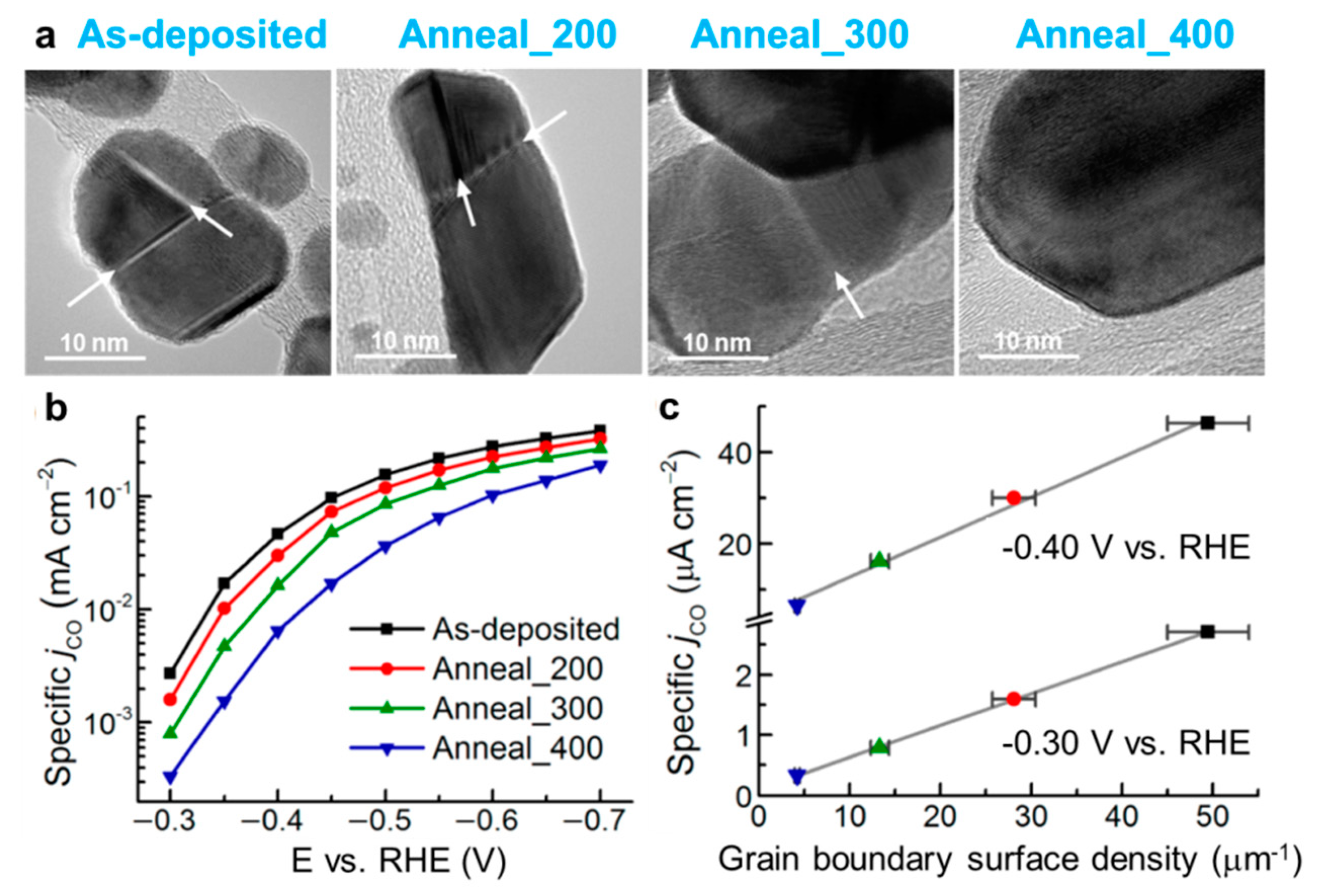
- Feng, X.; Jiang, K.; Fan, S.; Kanan, M.W. Grain–boundary–dependent CO2 electroreduction activity. J. Am. Chem. Soc. 2015, 137, 4606–4609. [Google Scholar] [CrossRef]
- Huang, H.; Jia, H.; Liu, Z.; Gao, P.; Zhao, J.; Luo, Z.; Yang, J.; Zeng, J. Understanding of Strain Effects in the Electrochemical Reduction of CO2: Using Pd Nanostructures as an Ideal Platform. Angew. Chem. Int. Ed. 2017, 56, 3594–3598. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Zhu, S.; Xu, L.; Chen, Z.; Zhang, Y.; Xie, M.; Li, T.; Zhou, S.; Liu, J.; Chi, M.; et al. Kinetically Controlled Synthesis of Pd-Cu Janus Nanocrystals with Enriched Surface Structures and Enhanced Catalytic Activities toward CO2 Reduction. J. Am. Chem. Soc. 2021, 143, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Liu, D.; Palmore, G.T.R. Electrochemical Reduction of CO2 at Copper Nanofoams. ACS Catal. 2014, 4, 3091–3095. [Google Scholar] [CrossRef]
- Yang, K.D.; Ko, W.R.; Lee, J.H.; Kim, S.J.; Lee, H.; Lee, M.H.; Nam, K.T. Morphology–Directed Selective Production of Ethylene or Ethane from CO2 on a Cu Mesopore Electrode. Angew. Chem. Int. Ed. 2017, 56, 796–800. [Google Scholar] [CrossRef]
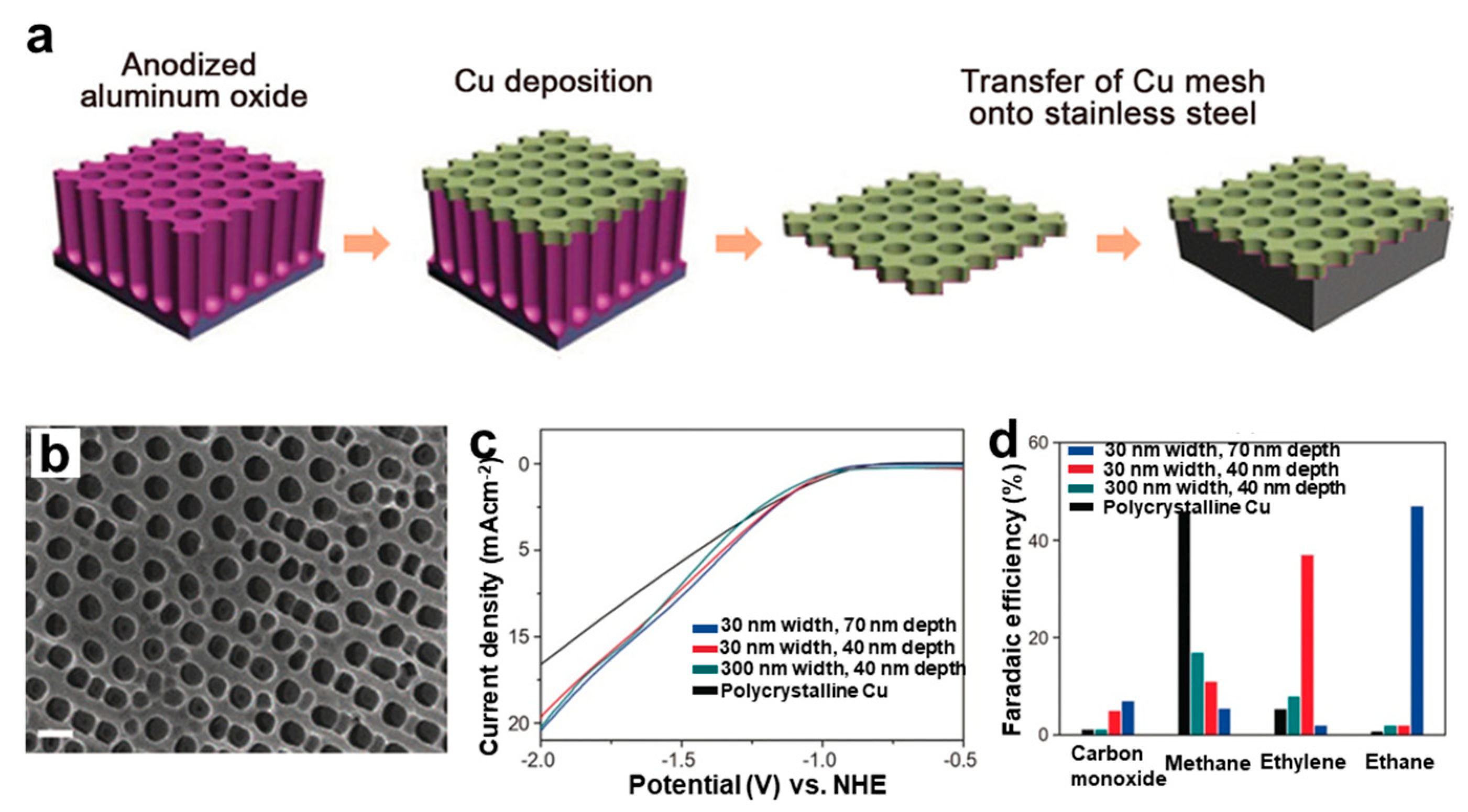
- Goyal, A.; Bondue, C.J.; Graf, M.; Koper, M.T.M. Effect of pore diameter and length on electrochemical CO2 reduction reaction at nanoporous gold catalysts. Chem. Sci. 2022, 13, 3288–3298. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Liu, A.; Li, Y.; Wen, Y.; Gao, X.; Chen, Q. Boosting the Electrocatalytic CO2 Reduction Reaction by Nanostructured Metal Materials via Defects Engineering. Nanomaterials 2022, 12, 2389. https://doi.org/10.3390/nano12142389
Zhao S, Liu A, Li Y, Wen Y, Gao X, Chen Q. Boosting the Electrocatalytic CO2 Reduction Reaction by Nanostructured Metal Materials via Defects Engineering. Nanomaterials. 2022; 12(14):2389. https://doi.org/10.3390/nano12142389
Chicago/Turabian StyleZhao, Shuangyang, Aihua Liu, Yonghe Li, Yanyan Wen, Xiaoqian Gao, and Qiaoli Chen. 2022. "Boosting the Electrocatalytic CO2 Reduction Reaction by Nanostructured Metal Materials via Defects Engineering" Nanomaterials 12, no. 14: 2389. https://doi.org/10.3390/nano12142389





