Ag Nanoparticles Decorated ZnO Nanorods as Multifunctional SERS Substrates for Ultrasensitive Detection and Catalytic Degradation of Rhodamine B
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
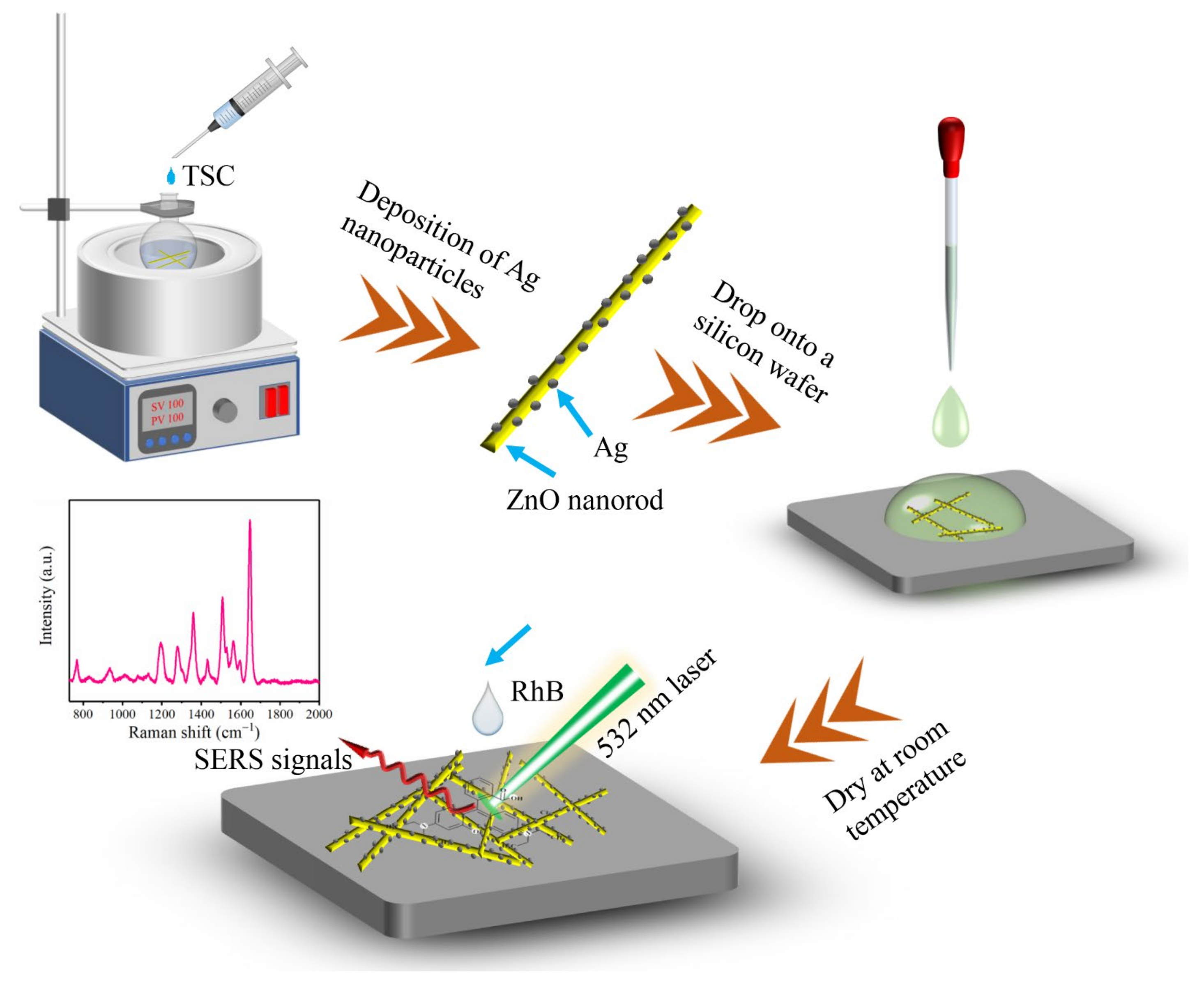
2.3. Synthesis of ZnO/Ag
2.4. SERS Detection
3. Results
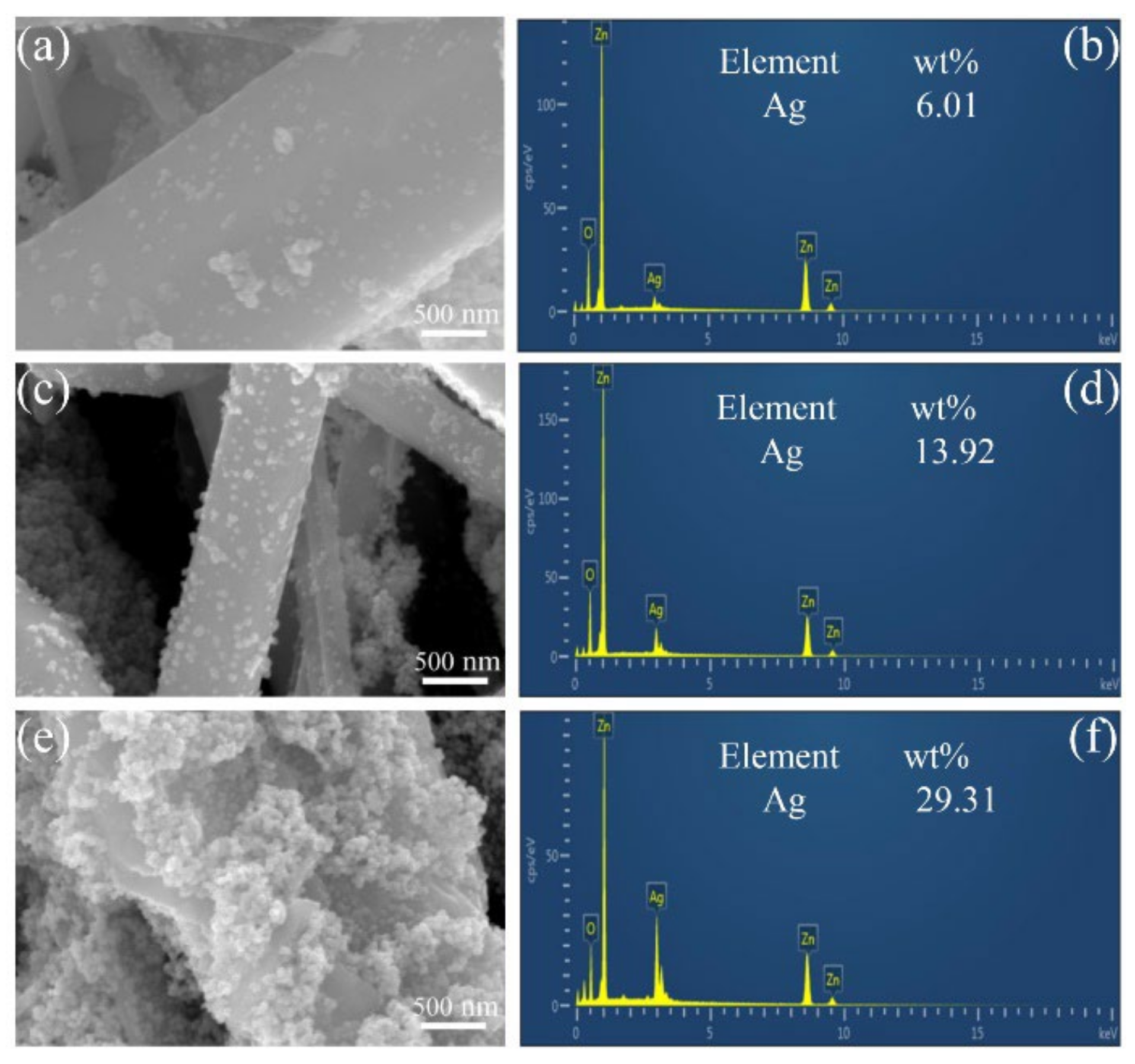
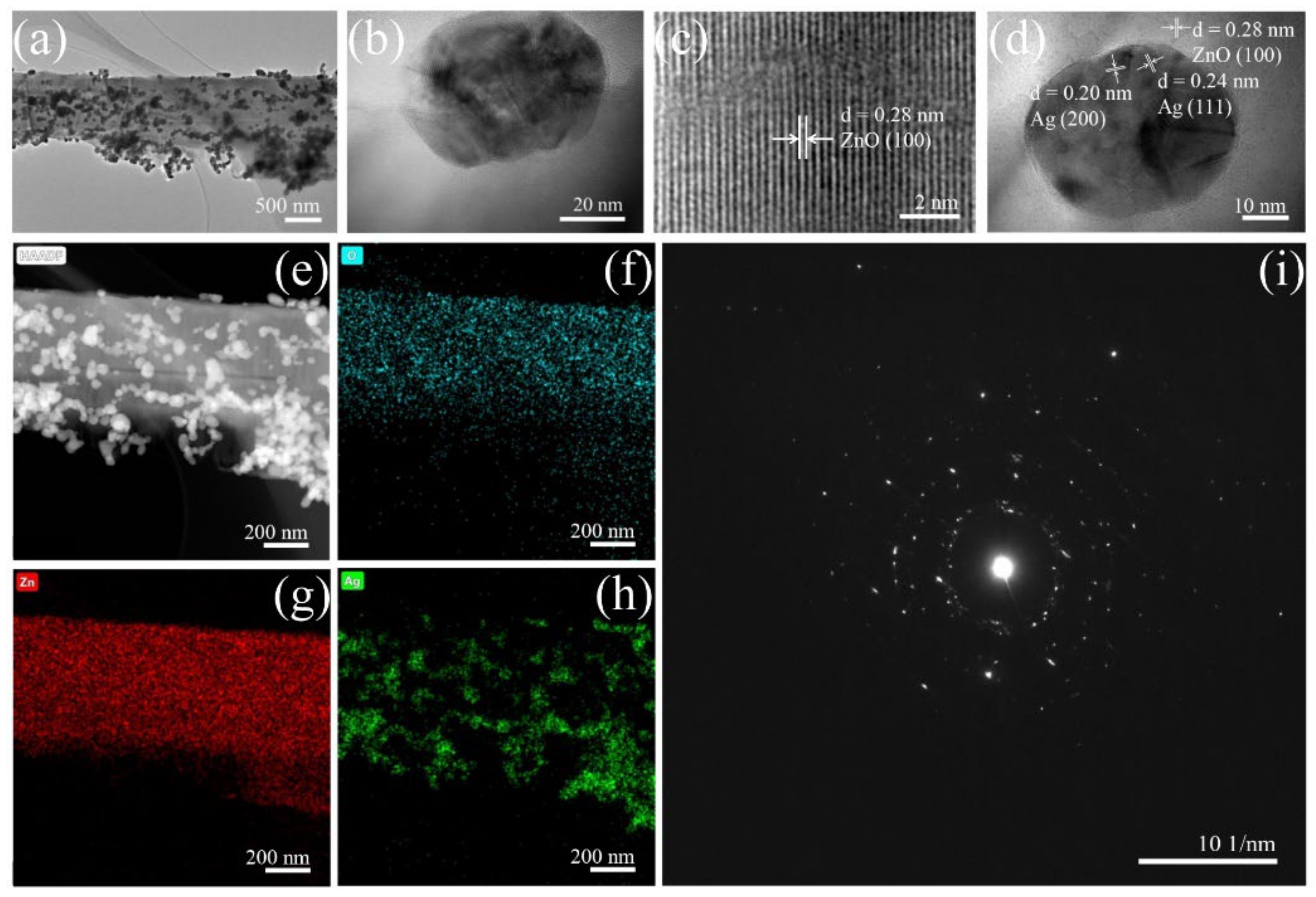
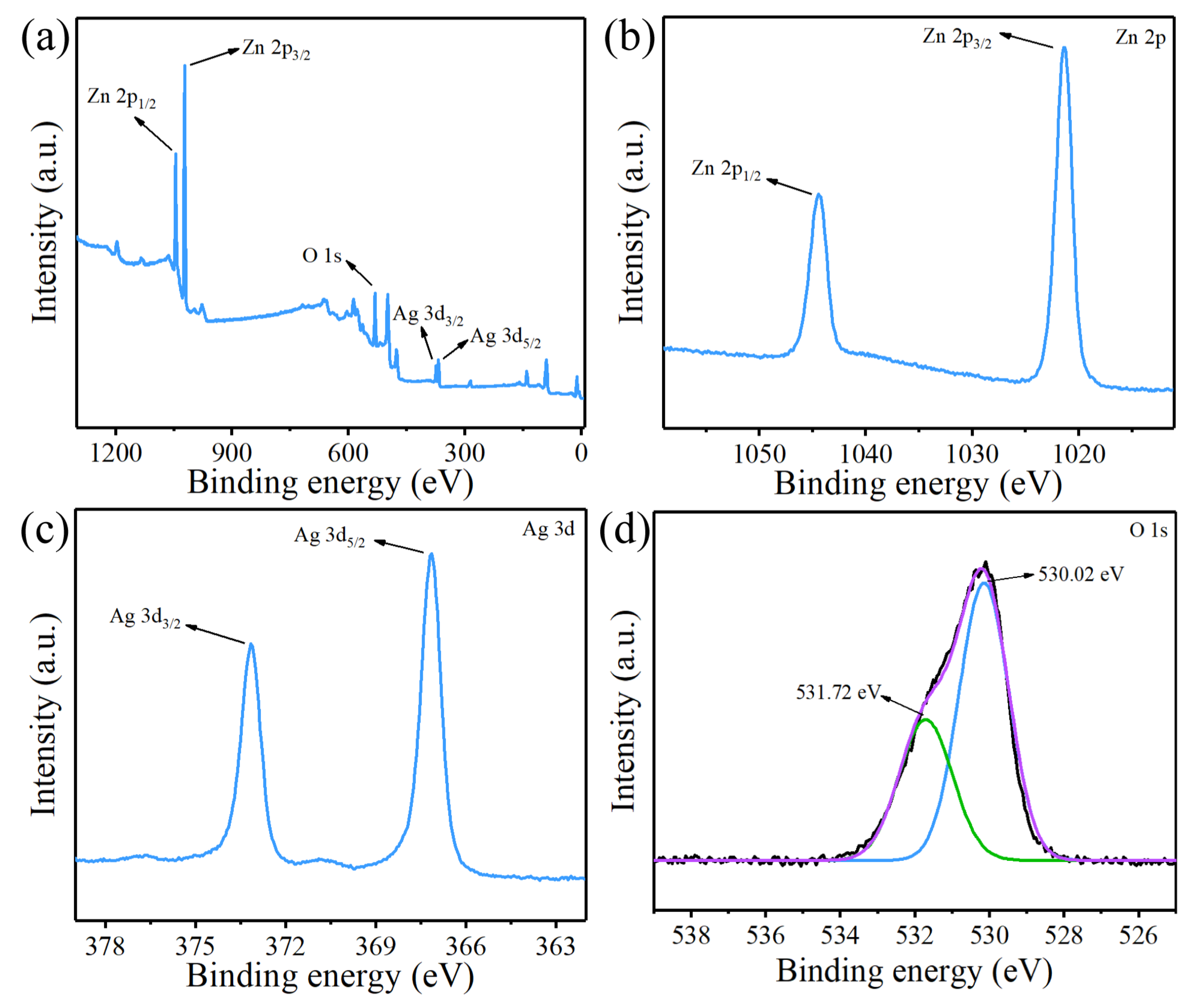
3.1. Characterisation of ZnO/Ag
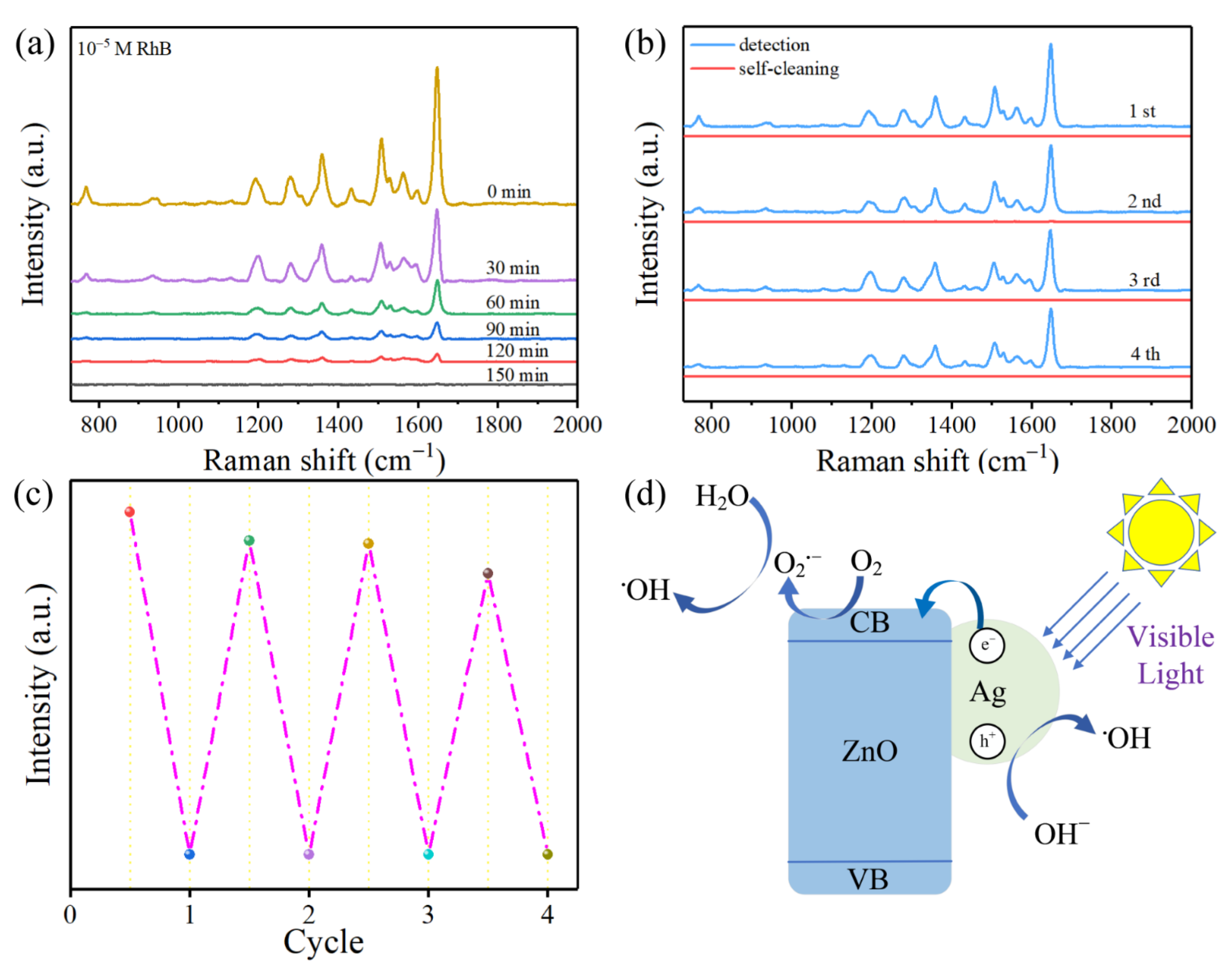
3.2. SERS Detection of Rhodamine B
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, W.; Huang, Q. Rapid fabrication of silver nanoparticle-coated filter paper as SERS substrate for low-abundance molecules detection. Spectrochim. Acta Part A 2017, 179, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Senapati, S.; Nanda, K.K. “Rinse, Repeat”: An efficient and reusable SERS and catalytic platform fabricated by controlled deposition of silver nanoparticles on cellulose paper. ACS Sustain. Chem. Eng. 2019, 7, 14089–14101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Duoerkun, G.; Shi, Z.; Cao, W.; Liu, T.; Liu, J.; Zhang, L.; Li, M.; Chen, Z. Construction of TiO2/Ag3PO4 nanojunctions on carbon fiber cloth for photocatalytically removing various organic pollutants in static or flowing wastewater. J. Colloid Interface Sci. 2020, 571, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Gao, W.; Lian, Y.; Du, J.; Tie, X. The transfer of natural rhodamine B contamination from raw paprika fruit to capsicum oleoresin during the extraction process. Food Chem. 2017, 237, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Sukriti; Anand, P.; Pruthi, V.; Chaddha, A.S.; Bhatia, J.; Kaith, B.S. RSM-CCD optimized adsorbent for the sequestration of carcinogenic rhodamine-B: Kinetics and equilibrium studies. Mater. Chem. Phys. 2017, 196, 270–283. [Google Scholar] [CrossRef]
- Fu, D.S.; Wu, P.P.; Zhong, X.D.; Liu, Q.; Luo, H.D.; Li, Y.Q. A simple synchronous fluorescence approach for rapid and sensitive determination of rhodamine B in chilli products. Food Anal. Methods 2015, 8, 189–194. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Tsai, T.H. A validated LC–MS/MS determination method for the illegal food additive rhodamine B: Applications of a pharmacokinetic study in rats. J. Pharm. Biomed. Anal. 2016, 125, 394–399. [Google Scholar] [CrossRef]
- Chiang, T.L.; Wang, Y.C.; Ding, W.H. Trace determination of rhodamine B and rhodamine 6G dyes in aqueous samples by solid-phase extraction and high-performance liquid chromatography coupled with fluorescence detection. J. Chin. Chem. Soc. 2012, 59, 515–519. [Google Scholar] [CrossRef]
- Li, C.; Xu, S.; Yu, J.; Li, Z.; Li, W.; Wang, J.; Liu, A.; Man, B.; Yang, S.; Zhang, C. Local hot charge density regulation: Vibration-free pyroelectric nanogenerator for effectively enhancing catalysis and in-situ surface enhanced Raman scattering monitoring. Nano Energy 2021, 81, 105585. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Fan, Y.; Lai, K. Rapid detection of flusilazole in pears with Au@Ag nanoparticles for surface-enhanced Raman scattering. Nanomaterials 2018, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Huang, S.C.; Hu, S.; Yan, S.; Ren, B. Fundamental understanding and applications of plasmon-enhanced Raman spectroscopy. Nat. Rev. Phys. 2020, 2, 253–271. [Google Scholar] [CrossRef]
- Ding, Q.; Wang, J.; Chen, X.; Liu, H.; Li, Q.; Wang, Y.; Yang, S. Quantitative and sensitive SERS platform with analyte enrichment and filtration function. Nano Lett. 2020, 20, 7304–7312. [Google Scholar] [CrossRef]
- Zhu, C.; Meng, G.; Zheng, P.; Huang, Q.; Li, Z.; Hu, X.; Wang, X.; Huang, Z.; Li, F.; Wu, N. A hierarchically ordered array of silver-nanorod bundles for surface-enhanced Raman scattering detection of phenolic pollutants. Adv. Mater. 2016, 28, 4871–4876. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Chen, B.; Hu, M.; Zhou, N.; Huang, Z.; Meng, G. In-situ monitoring the SERS spectra of para-aminothiophenol adsorbed on plasmon-tunable Au@Ag core-shell nanostars. Nanomaterials 2022, 12, 1156. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, X.; Wang, C.; Qiu, G.; Ye, W.; Li, Y.; Wang, D. ZnO/Ag nanorods as a prominent SERS substrate contributed by synergistic charge transfer effect for simultaneous detection of oral antidiabetic drugs pioglitazone and phenformin. Sens. Actuators B 2020, 307, 127634. [Google Scholar] [CrossRef]
- Lv, W.; Liu, C.; Ma, Y.; Wang, X.; Luo, J.; Ye, W. Multi-hydrogen bond assisted SERS detection of adenine based on multifunctional graphene oxide/poly (diallyldimethyl ammonium chloride)/Ag nanocomposites. Talanta 2019, 204, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Yao, J.; Yang, S.; Chen, L.; Liu, Y.; Lang, J.; Zeng, H.; Yang, J.; Gao, M. Detect, remove and re-use: Sensing and degradation pesticides via 3D tilted ZMRs/Ag arrays. J. Hazard. Mater. 2020, 391, 122222. [Google Scholar] [CrossRef]
- Ashok Kumar, E.; Jiann Wang, T.; Chang, Y.H. Ultrasensitive SERS substrates based on Au nanoparticles photo-decorated on Cu2O microspheres for the detection of rhodamine B and methylene blue. Appl. Surf. Sci. 2022, 585, 152696. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, H.; Ying, G.; Zhang, J.; Wu, Y.; Wu, S.; Yang, Y. Flexible, fouling-resistant and self-cleaning Ti3C2Tx-derivated hydrophilic nanofiltration membrane for highly efficient rejection of organic molecules from wastewater. J. Mater. Res. Technol. 2020, 9, 11675–11686. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.J. Recent advances in TiO2 films prepared by sol-gel methods for photocatalytic degradation of organic pollutants and antibacterial activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef] [Green Version]
- Chu, Q.; Han, B.; Jin, Y.; Guo, S.; Jin, S.; Park, E.; Chen, L.; Jung, Y.M. Surface plasmon resonance induced charge transfer effect on the Ag-ZnSe-PATP system. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119167. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Kutsanedzie, F.Y.H.; Xu, J.; Viswadevarayalu, A.; Hassan, M.M.; Li, H.; Xu, Y.; Chen, Q. SERS-signal optimised AgNPs-plated-ZnO nanoflower-like structure synthesised for sensing applications. Phys. Lett. A 2019, 383, 1312–1317. [Google Scholar] [CrossRef]
- Yang, B.; Jin, S.; Guo, S.; Park, Y.; Chen, L.; Zhao, B.; Jung, Y.M. Recent development of SERS technology: Semiconductor-based study. ACS Omega 2019, 4, 20101–20108. [Google Scholar] [CrossRef]
- Yang, J.; Chen, B.; Peng, J.; Huang, B.; Deng, W.; Xie, W.; Luo, Z. Preparation of CuO nanowires/Ag composite substrate and study on SERS activity. Plasmonics 2021, 16, 1059–1070. [Google Scholar] [CrossRef]
- Xue, X.; Chen, L.; Wang, L.; Wang, C.; Qiao, Y.; Zhao, C.; Wang, H.; Nie, P.; Shi, J.; Chang, L. Facile fabrication of PS/Cu2S/Ag sandwich structure as SERS substrate for ultra-sensitive detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 265, 120370. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, H.; Zang, L.; Jin, S.; Guo, S.; Park, E.; Mao, Z.; Jung, Y.M. Flexible and reusable Ag coated TiO2 nanotube arrays for highly sensitive SERS detection of formaldehyde. Molecules 2020, 25, 1199. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Wei, Z.; Li, C.; Ma, J.; Li, L. Photocatalytic degradation of methyl orange by noble metal Ag modified semiconductor Zn2SnO4. Mater. Sci. Semicond. Process. 2022, 138, 106290. [Google Scholar] [CrossRef]
- Li, S.; Cai, J.; Wu, X.; Zheng, F. Sandwich-like TiO2@ZnO-based noble metal (Ag, Au, Pt, or Pd) for better photo-oxidation performance: Synergistic effect between noble metal and metal oxide phases. Appl. Surf. Sci. 2018, 443, 603–612. [Google Scholar] [CrossRef]
- Bowker, M.; O’Rourke, C.; Mills, A. The role of metal nanoparticles in promoting photocatalysis by TiO2. Top. Curr. Chem. 2022, 380, 17. [Google Scholar] [CrossRef]
- Zhou, W.; Fu, H. Defect-mediated electron–hole separation in semiconductor photocatalysis. Inorg. Chem. Front. 2018, 5, 1240–1254. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Lee, T.K.; Park, J.; Ha, M.; Kwak, S.K.; Ko, H. Particle-on-film gap plasmons on antireflective ZnO nanocone arrays for molecular-level surface-enhanced Raman scattering sensors. ACS Appl. Mater. Interfaces 2015, 7, 26421–26429. [Google Scholar] [CrossRef] [PubMed]
- Barveen, N.R.; Wang, T.-J.; Chang, Y.-H.; Yuan-Liu, Z. Ultrasensitive and reusable SERS probe for the detection of synthetic dyes in food industry through hybrid flower-shaped ZnO@Ag nanostructures. J. Alloy Compd. 2021, 861, 157952. [Google Scholar] [CrossRef]
- Subash, B.; Krishnakumar, B.; Swaminathan, M.; Shanthi, M. Highly efficient, solar active, and reusable photocatalyst: Zr-loaded Ag–ZnO for reactive red 120 dye degradation with synergistic effect and dye-sensitized mechanism. Langmuir 2013, 29, 939–949. [Google Scholar] [CrossRef]
- Wang, C.; Xu, X.; Qiu, G.; Ye, W.; Li, Y.; Harris, R.A.; Jiang, C. Group-targeting SERS screening of total benzodiazepines based on large-size (111) faceted silver nanosheets decorated with zinc oxide nanoparticles. Anal. Chem. 2021, 93, 3403–3410. [Google Scholar] [CrossRef] [PubMed]
- Godad, S.P.; Kamal, S. Multifunctional Ag2CO3 microrods as SERS-active substrate for the detection and degradation of rhodamine B dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120176. [Google Scholar] [CrossRef]
- Yi, Z.; Xu, X.; Kang, X.; Zhao, Y.; Zhang, S.; Yao, W.; Yi, Y.; Luo, J.; Wang, C.; Yi, Y.; et al. Fabrication of well-aligned ZnO@Ag nanorod arrays with effective charge transfer for surface-enhanced Raman scattering. Surf. Coat. Technol. 2017, 324, 257–263. [Google Scholar] [CrossRef]
- Macias-Montero, M.; Peláez, R.J.; Rico, V.J.; Saghi, Z.; Midgley, P.; Afonso, C.N.; González-Elipe, A.R.; Borras, A. Laser treatment of Ag@ZnO nanorods as long-life-span SERS surfaces. ACS Appl. Mater. Interfaces 2015, 7, 2331–2339. [Google Scholar] [CrossRef] [Green Version]
- Sivashanmugan, K.; Liao, J.D.; Liu, B.H.; Yao, C.K.; Luo, S.C. Ag nanoclusters on ZnO nanodome array as hybrid SERS-active substrate for trace detection of malachite green. Sens. Actuators B Chem. 2015, 207, 430–436. [Google Scholar] [CrossRef]
- Yang, M.; Yu, J.; Lei, F.; Zhou, H.; Wei, Y.; Man, B.; Zhang, C.; Li, C.; Ren, J.; Yuan, X. Synthesis of low-cost 3D-porous ZnO/Ag SERS-active substrate with ultrasensitive and repeatable detectability. Sens. Actuators B Chem. 2018, 256, 268–275. [Google Scholar] [CrossRef]
- Huang, J.; Chen, F.; Zhang, Q.; Zhan, Y.; Ma, D.; Xu, K.; Zhao, Y. 3D silver nanoparticles decorated zinc oxide/silicon heterostructured nanomace arrays as high-performance surface-enhanced Raman scattering substrates. ACS Appl. Mater. Interfaces 2015, 7, 5725–5735. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hassan, M.M.; Ahmad, W.; Jiao, T.; Xu, Y.; Li, H.; Ouyang, Q.; Guo, Z.; Chen, Q. A highly structured hollow ZnO@ Ag nanosphere SERS substrate for sensing traces of nitrate and nitrite species in pickled food. Sens. Actuators B Chem. 2019, 285, 302–309. [Google Scholar] [CrossRef]
- Chin, H.K.; Lin, P.Y.; Chen, J.; Kirankumar, R.; Wen, Z.H.; Hsieh, S. Polydopamine-Mediated Ag and ZnO as an active and recyclable SERS substrate for Rhodamine B with significantly improved enhancement factor and efficient photocatalytic degradation. Appl. Sci. 2021, 11, 4914. [Google Scholar] [CrossRef]
- Huang, C.; Xu, C.; Lu, J.; Li, Z.; Tian, Z. 3D Ag/ZnO hybrids for sensitive surface-enhanced Raman scattering detection. Appl. Surf. Sci. 2016, 365, 291–295. [Google Scholar] [CrossRef]
- Zhang, G.; Deng, C.; Shi, H.; Zou, B.; Li, Y.; Liu, T.; Wang, W. ZnO/Ag composite nanoflowers as substrates for surface-enhanced Raman scattering. Appl. Surf. Sci. 2017, 402, 154–160. [Google Scholar] [CrossRef]
- Shan, Y.; Yang, Y.; Cao, Y.; Fu, C.; Huang, Z. Synthesis of wheatear-like ZnO nanoarrays decorated with Ag nanoparticles and its improved SERS performance through hydrogenation. Nanotechnology 2016, 27, 145502. [Google Scholar] [CrossRef] [PubMed]
- Tieu, D.T.; Quynh Trang, T.N.; Tuan Hung, L.V.; Hanh Thu, V.T. Assembly engineering of Ag@ZnO hierarchical nanorod arrays as a pathway for highly reproducible surface-enhanced Raman spectroscopy applications. J. Alloys Compd. 2019, 808, 151735. [Google Scholar] [CrossRef]
- Xu, J.; Teng, Y.; Teng, F. Effect of surface defect states on valence band and charge separation and transfer efficiency. Sci. Rep. 2016, 6, 32457. [Google Scholar] [CrossRef] [Green Version]
- Abdel Messih, M.F.; Ahmed, M.A.; Soltan, A.; Anis, S.S. Synthesis and characterization of novel Ag/ZnO nanoparticles for photocatalytic degradation of methylene blue under UV and solar irradiation. J. Phys. Chem. Solids 2019, 135, 109086. [Google Scholar] [CrossRef]
- Kadam, A.N.; Bhopate, D.P.; Kondalkar, V.V.; Majhi, S.M.; Bathula, C.D.; Tran, A.V.; Lee, S.-W. Facile synthesis of Ag-ZnO core–shell nanostructures with enhanced photocatalytic activity. J. Ind. Eng. Chem. 2018, 61, 78–86. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Seifi, A. The green synthesis of Ag/ZnO in montmorillonite with enhanced photocatalytic activity. Appl. Surf. Sci. 2016, 386, 33–40. [Google Scholar] [CrossRef]
- She, P.; Xu, K.; He, Q.; Zeng, S.; Sun, H.; Liu, Z. Controlled preparation and visible light photocatalytic activities of corn cob-like Au–ZnO nanorods. J. Mater. Sci. 2017, 52, 3478–3489. [Google Scholar] [CrossRef]
- Fauzia, V.; Yudiana, A.; Yulizar, Y.; Dwiputra, M.A.; Roza, L.; Soegihartono, I. The impact of the Au/Ag ratio on the photocatalytic activity of bimetallic alloy AuAg nanoparticle-decorated ZnO nanorods under UV irradiation. J. Phys. Chem. Solids 2021, 154, 110038. [Google Scholar] [CrossRef]

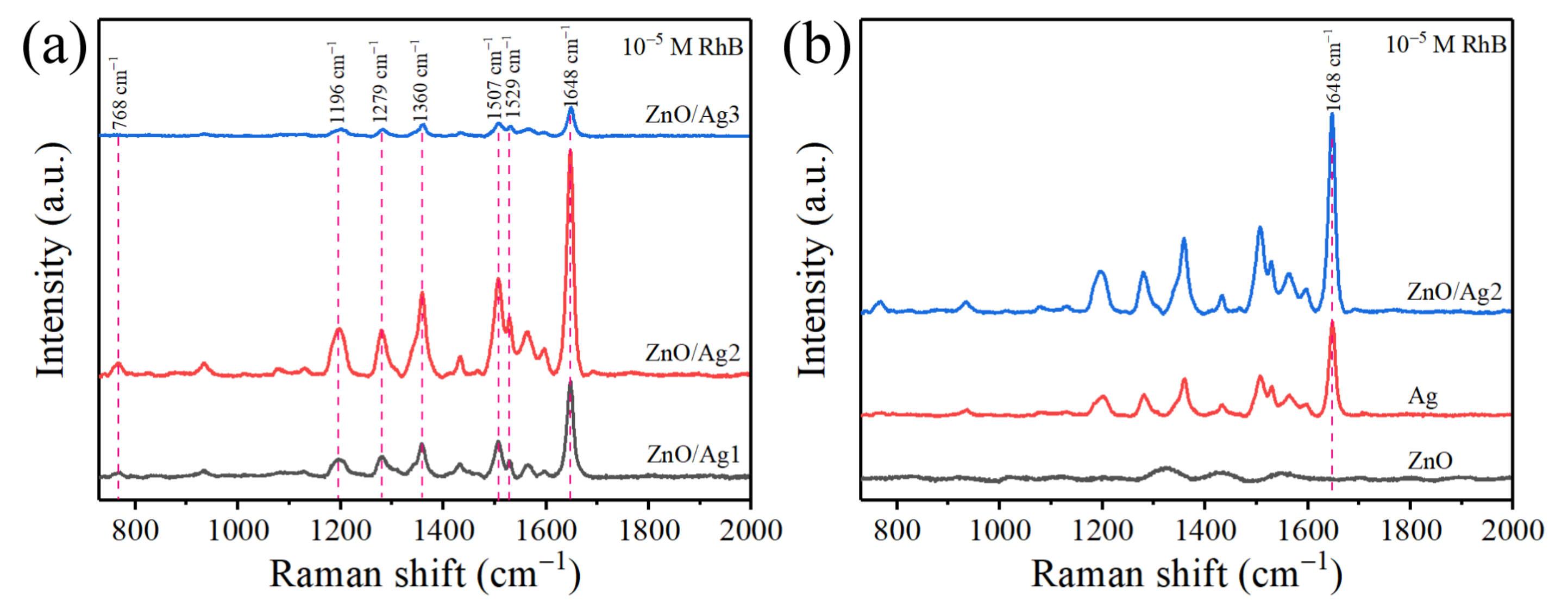
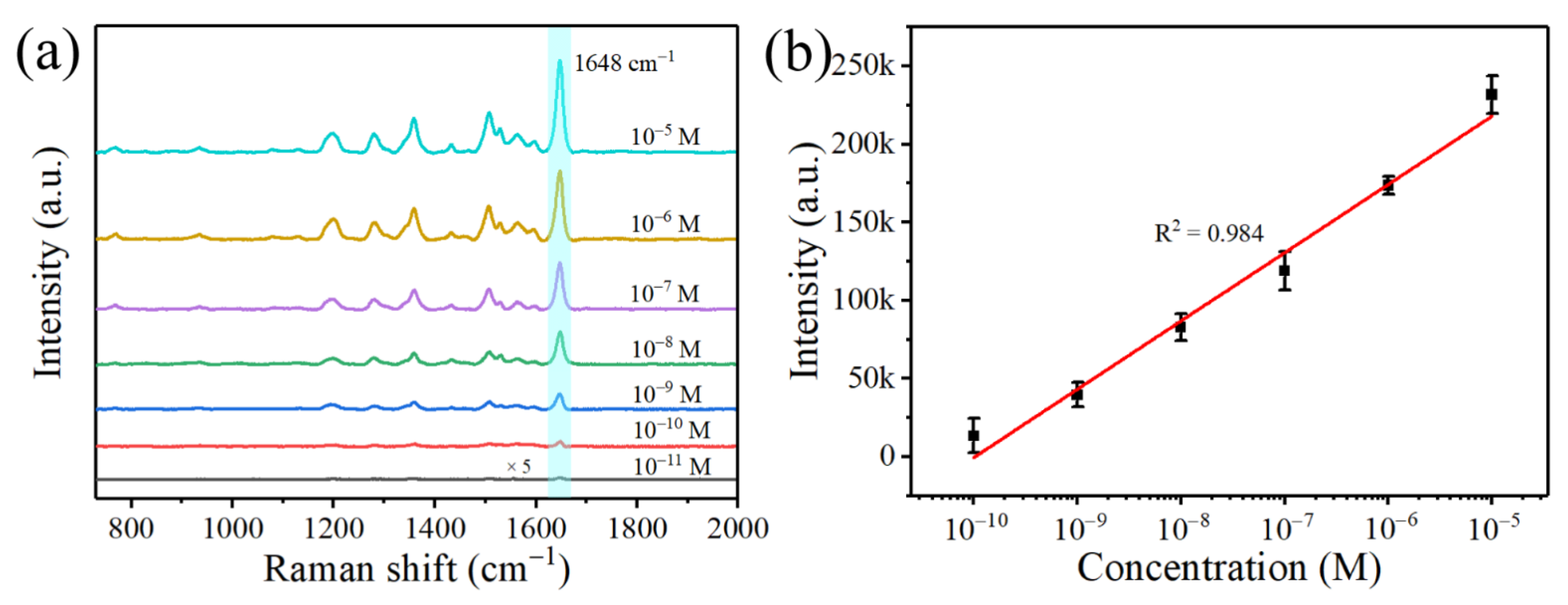
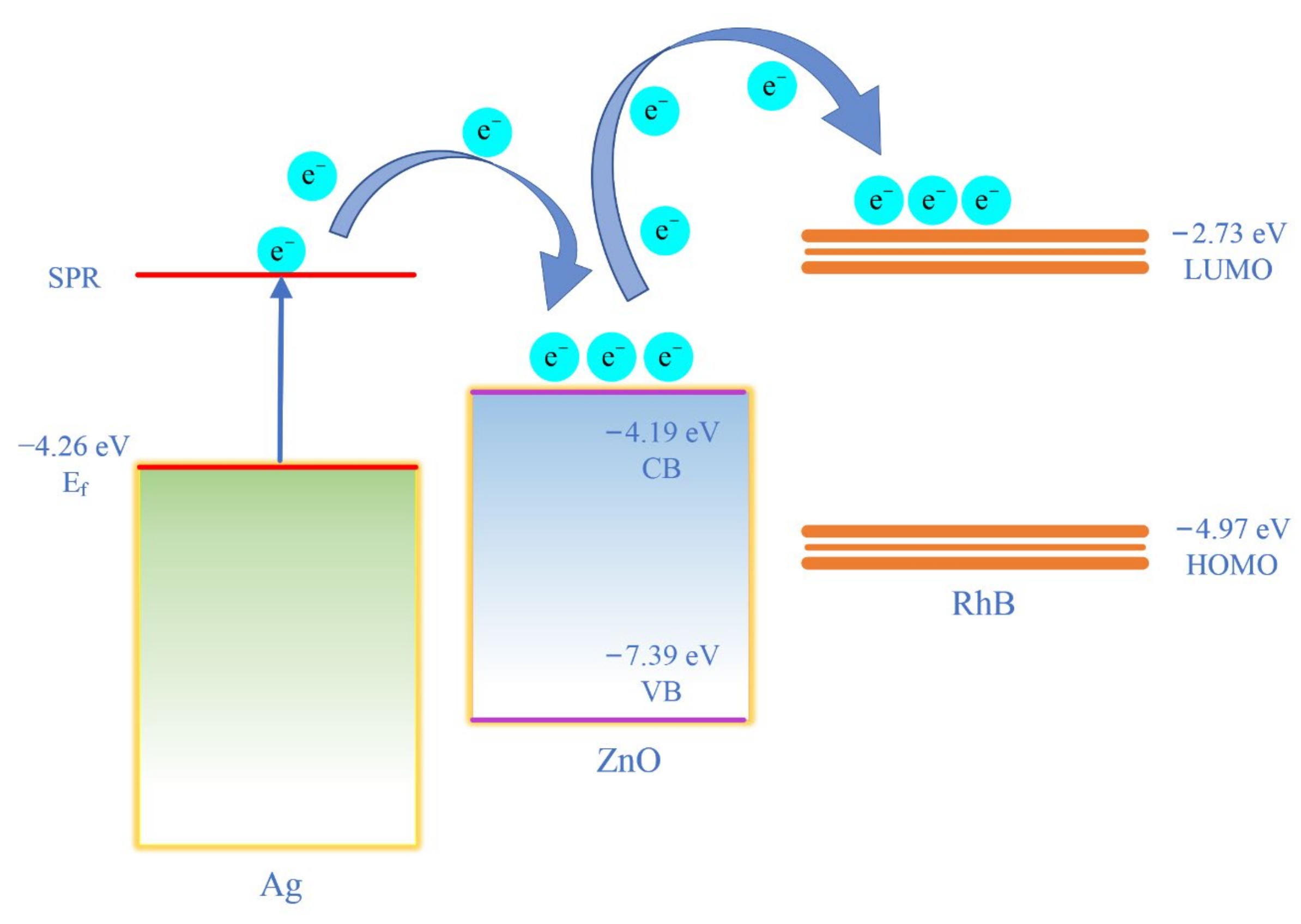
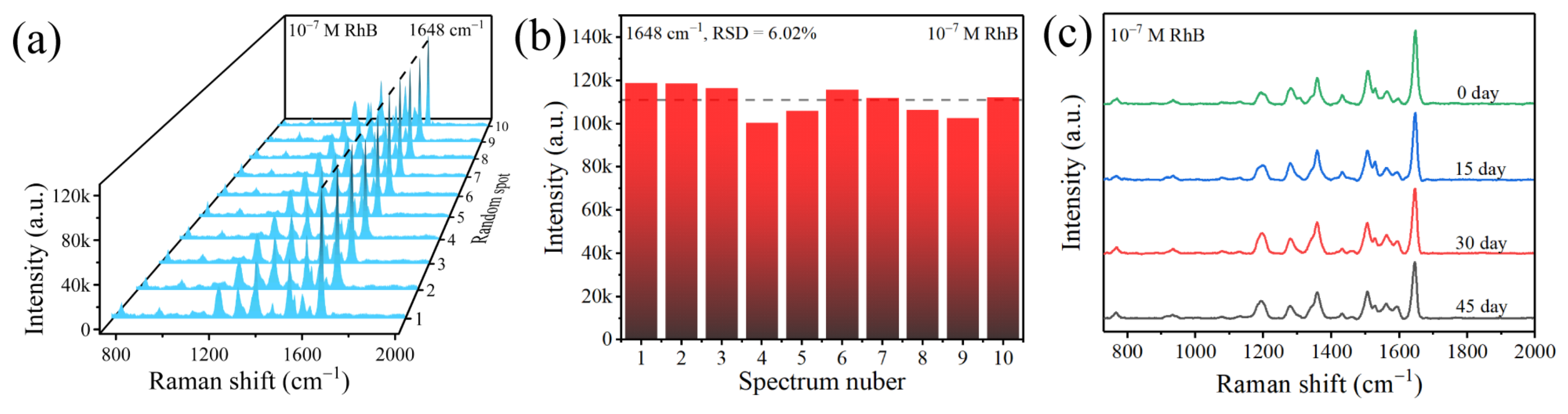
| Raman Shift (cm−1) | Peak Assignment |
|---|---|
| 768 | xanthene ring puckering |
| 1196 | aromatic C–C stretching |
| 1279 | C–H bending |
| 1360 | stretching vibration of bridge C–C aromatic bonds |
| 1507 | aromatic C–C bending |
| 1529 | C–H stretching |
| 1648 | the symmetric bending C–C and C=C bonds of benzene ring |
| SERS Substrate Based on ZnO | EF | Reference |
|---|---|---|
| Ag@ZnO Nanorods arrays | 3.87 × 108 | [37] |
| Ag@ZnO Nanorods | 1.6 × 106 | [38] |
| Ag@ZnO Nanodomes | 106 | [39] |
| 3D-porous ZnO/Ag | 2 × 108 | [40] |
| Ag/ZnO/Si | 8.7 × 107 | [41] |
| Ag@ZnO Nanosphere | 3.17 × 108 | [42] |
| Ag/PDA/ZnO@GMF | 1010 | [43] |
| 3D ZnO/Ag | 3.18 × 109 | [44] |
| ZnO/Ag Nanoflowers | 3.41 × 107 | [45] |
| ZnO/Ag Nanorods | 9.2 × 108 | In this work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhu, L.; Ma, Z.; Wang, M.; Zhao, R.; Zou, Y.; Fan, Y. Ag Nanoparticles Decorated ZnO Nanorods as Multifunctional SERS Substrates for Ultrasensitive Detection and Catalytic Degradation of Rhodamine B. Nanomaterials 2022, 12, 2394. https://doi.org/10.3390/nano12142394
Chen X, Zhu L, Ma Z, Wang M, Zhao R, Zou Y, Fan Y. Ag Nanoparticles Decorated ZnO Nanorods as Multifunctional SERS Substrates for Ultrasensitive Detection and Catalytic Degradation of Rhodamine B. Nanomaterials. 2022; 12(14):2394. https://doi.org/10.3390/nano12142394
Chicago/Turabian StyleChen, Xingang, Lei Zhu, Zhipeng Ma, Meilin Wang, Rui Zhao, Yueyue Zou, and Yijie Fan. 2022. "Ag Nanoparticles Decorated ZnO Nanorods as Multifunctional SERS Substrates for Ultrasensitive Detection and Catalytic Degradation of Rhodamine B" Nanomaterials 12, no. 14: 2394. https://doi.org/10.3390/nano12142394
APA StyleChen, X., Zhu, L., Ma, Z., Wang, M., Zhao, R., Zou, Y., & Fan, Y. (2022). Ag Nanoparticles Decorated ZnO Nanorods as Multifunctional SERS Substrates for Ultrasensitive Detection and Catalytic Degradation of Rhodamine B. Nanomaterials, 12(14), 2394. https://doi.org/10.3390/nano12142394






