1. Introduction
Enzymatic biofuel cells that convert chemical energy into electrical energy by electroenzymatic reactions offer attractive potentialities for powering disposable electronic systems [
1,
2]. However, the development of enzymatic biofuel cells is confronted with two major technological obstacles, namely their short operational and storage lifetime in solution and, to a lesser extent, their low power output [
3,
4]. The low stability of the bioelectrodes of the enzymatic fuel cells is linked to the deactivation of the immobilized enzymes and seems inevitable. Regarding the power of the biofuel cells, the latter is partly related to the quantity of enzyme immobilized per unit of conductive surface and to the efficiency of the electrical connection between the enzyme and the electrode.
The enzyme electrodes of biofuel cells result from the immobilization of different redox enzymes on the surfaces of the electrodes for their electrical connection. This fixation of enzyme can be obtained by chemical grafting or affinity interactions or by physical entrapment. Regarding the immobilization by covalent or non-covalent bond, this configuration offers good access of the substrate and redox mediators to the immobilized enzyme, but the quantity of biocatalyst is limited to a quasi-monolayer at the modified electrode–solution interface, thus strongly limiting the power.
Although immobilization in a 3D matrix increases the surface density of enzymes, this entrapment process induces a denaturation process due to the non-biocompatible environment. Additionally, the activity of the entrapped enzyme can be affected by the permeability and hydrophobicity of the host structure. Steric constraints can drastically reduce the permeation of substrates and redox mediators or even block the conformational flexibility of the protein. One way to increase the power of biofuel cells is to develop porous electrodes that increase the effective surface area of the electrodes. However, this approach does not solve the storage stability problem.
Recently, a new type of biofuel cell based on the non-immobilization of catalysts has been developed based on compartments comprising a dialysis membrane and containing enzymes, redox nanoparticles and an electrode [
5]. As enzymes and mediators in solution can freely diffuse and rotate, this approach allows good orientation favoring electrical wiring of the enzymes. However, this process requires a peristaltic pump to provide a constant flow of fuel and hence is difficult to miniaturize. More recently, Li et al. reported a novel design of a bioelectrode based on a carbon felt electrode and an aqueous slurry of glucose oxidase, electron mediator and dispersed nanomaterial (graphene-like Ti
3C
2 MXene) confined in an acrylic shell and a dialysis membrane [
6]. The maximum current density of this bioanode is maintained at 25% of its initial value after 9 days, illustrating the relatively good storage stability of the bioanode. In addition, for a low continuous current discharge (5 µA), the resulting hybrid biofuel cell exhibits good operational stability, losing only 34% of its initial power (2.75 µW) after 19 days. However, the biofuel cell configuration based on an acrylic container wrapped in a dialysis membrane and the stability of the dispersion of the nanomaterial can be limiting factors for the miniaturization of the biofuel cell and its stability.
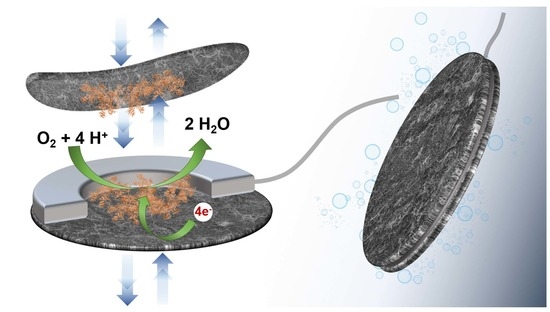
Recently, we have demonstrated that a deposit of a thin layer of carbon nanotube (CNT) on a non-conductive support could play the role of an electrode while offering good permeation to low molecular weight compounds in an aqueous medium [
7]. Taking into account this property, we report here the original creation of a hollow planar bioelectrode of very low thickness and large surface area containing the enzyme in powder form. The enzyme is trapped during the bonding of two conductive sheets made up of carbon nanotubes (buckypaper), the volume of the microcavity being defined by the thickness of the glue film binding these buckypapers. The buckypapers being permeable to water and small molecules but not allowing the permeation of enzymes, the bioelectrode presents a high density of protein in a microvolume. Moreover, the bioelectrode can be stored dry and the enzyme is only solubilized during the use of the bioelectrode.
To illustrate this innovative concept, a hollow bioelectrode configuration with entrapped bilirubin oxidase (BOx) was fabricated and applied to electroenzymatic reduction of O2. The electrocatalytic performance of the BOx bioelectrode was described as a function of pH, temperature and the amount of entrapped enzyme. The operational and storage stability of the bioelectrode in solution have been determined via the evolution of the catalytic current.
In addition, the influence of the iron-protoporphyrin (hemin) adsorbed on buckypaper on the orientation of BOx and therefore on the direct electron transfer (DET) was studied. Moreover, the effect of adding 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) as a redox mediator for mediated electron transfer (MET) on the electrical wiring of the BOx was also investigated.
3. Results and Discussion
The hollow electrode was created by assembling a disk (diameter 30 mm) of a commercial buckypaper (C
bp) with a similar disk of buckypaper (L
bp) made by filtration of an organic dispersion of multiwalled carbon nanotubes (MWCNTs) by sticking the periphery of the discs with carbon paste. C
bp presents a more porous structure than L
bp which facilitates the penetration of water and substrates inside the inner cavity. In contrast, L
bp exhibits a denser and compact structure composed of significantly smaller nanotubes (diameters 10–20 nm) which allow direct electron transfer (DET) with redox enzymes [
9]. The electrical connection of the two glued disks was carried out by insertion of a metallic wire into the carbon paste, the thickness of which defines the volume of the microcavity.
Figure 1A shows an image of the resulting hollow electrode as well as a section of the inner cavity that corresponds to about 28 ± 4 µL.
With the aim to illustrate the concept of entrapment and electrical wiring of an enzyme within a hollow bioelectrode, bilirubin oxidase (BOx) was chosen as a model enzyme. BOx is a multicopper oxidase typically employed as the biocatalyst for the four-electron reduction of O
2 to H
2O and widely used for the production of biofuel cells [
10,
11,
12,
13]. Thus, 2 mg of BOx powder was deposited on one disk before the formation of the cavity (diameter 12 mm). The resulting bioelectrode was then immersed into a in 0.1 mol L
−1 phosphate buffer (pH 6.5) and the potential electroactivity of BOx towards the reduction of O
2 was investigated by cyclic voltammetry.
Figure 1B shows the cyclic voltammograms recorded at 1 mV s
−1 under argon and O
2-saturated conditions. In presence of O
2, a catalytic current clearly appears with onset potential
ca. 0.55 V which corresponds to the conventional catalytic phenomena observed for bioelectrodes based on a DET with immobilized BOx [
14]. This catalytic current confirms the penetration of water and substrate, the solubilization of the enzyme powder in the inner microcavity and the ability of the hollow electrode to establish an electrical communication with BOx.
As previously reported, the presence of different types of porphyrins adsorbed on MWCNTs coatings or buckypapers generally induces a BOx orientation favorable to a DET between its T1 Cu center and carbon nanotubes and thus enhances the intensity of the catalytic current for O
2 reduction [
14]. The improvement of the electrocatalytic activity of the hollow bioelectrode was therefore studied via the modification of the L
bp by an iron-protoporphyrin. For this purpose, hemin was solubilized in the MWCNTs dispersion and firmly adsorbed on MWCNTs walls by strong π–π interactions before the filtration step. The resulting bioelectrode based on hemin-L
bp glued with C
bp was tested towards O
2 reduction. As expected, a marked increase in current intensity (+52% at 0.5 V) and an improved onset potential (
ca. 0.77 V) were recorded corroborating the efficient orientation of BOx induced by the adsorbed hemin (
Figure 1B).
In addition to the orientation of the BOx by electrostatic interactions beneficial to the DET, the presence of carboxylic groups on the hemin confers a less hydrophobic character to the MWCNTs and therefore to the buckypaper, thus facilitating its wettability. This hypothesis has been corroborated by contact angle measurements made with the different buckypapers. The water contact angles of L
bp and C
bp were 128 ± 7° and 131 ± 3°, respectively. In contrast, the contact angle of hemin-L
bp could not be measured, confirming that modification of MWCNTs by hemin rendered the surface hydrophilic (
Figure S1). Moreover, top-down SEM images of buckypapers (
Figure S1) showed a subtle morphologic change of hemin-L
bp compared to L
bp due to the presence of hemin layers. Furthermore, the amount of immobilized hemin on L
bp was estimated via the charge recorded under the Fe
2+/3+ redox couple of hemin at E
1/2= −0.140 V vs. RHE. A resulting surface coverage with hemin of 2.66 × 10
−8 mol cm
−2 (
Figure S2) theoretically corresponds to the formation of 380 compact monolayers on flat surface illustrating the 3D functionalization of L
BP [
15].
The storage stability of two hollow bioelectrodes maintained in an aqueous solution and containing 2 mg of BOx, was investigated by recording periodically their faradaic catalytic current for O
2 reduction at 0.5 V as a function of time (
Figure 1C). After an initial drastic decrease in catalytic current for approximately 20 days for both bioelectrodes, a near stabilization of the catalytic process occurs, exhibiting a small continuous decrease in catalytic current of 1.9 ± 0.3 and 2.2 ± 0.1 µA cm
−2 day
−1 for bioelectrode based on L
bp and hemin-L
bp, respectively. It should be noted that the bioelectrode without hemin loses almost totally its electroactivity after 75 days whereas the bioelectrode based on hemin-L
bp retains 30% and 11% of its initial activity after 3 and 6 months respectively.
The manufacturing process of the hollow electrode makes it possible to easily modulate the quantity of enzyme trapped inside the hollow electrode. Thus, the influence of immobilized amount of BOx (0.16–4 mg) on the electrocatalytic properties of the resulting bioelectrodes based on hemin-L
bp was studied (
Figure 2A). As expected, the catalytic current increases with increasing amounts of BOx up to 1 mg and reaches a plateau for higher amounts. Above 1 mg of trapped enzyme, the electroenzymatic reaction could be limited by the electroactive surface or the diffusion of oxygen inside the cavity. In this context, the experiments were continued with 2 mg of BOx in the microcavity in order to have a reserve of catalyst.
The effect of temperature and pH on the functioning of the trapped enzyme has been studied. The current response of the hollow bioelectrode was measured in the temperature range of 15 to 60 °C. The catalytic current increases to a maximum at 25–37 °C and then decreases sharply, reflecting enzyme deactivation (
Figure 2B). Regarding the pH dependence in the range 4–8.5, it appears that the bioelectrocatalytic response is good at pH values ranging from 6 to 7 (83% of activity), the maximum current being recorded at pH 6.0. Taking into account that a strong decrease in activity is observed between pH 5 and 6, the experiments were carried out at pH 6.5 to avoid pH effects (
Figure 2C). With the aim to estimate the turnover frequency for BOx in solution within the microcavity, the charge related to the chronoamperometric response of the bioelectrode based on hemin-L
bp at 0.5 V for 15 min was recorded (
Figure 2D). By the integration of the anodic current area to give the transferred charges from the chronoamperometric measurement and taking into account 4 e
− for O
2 reduction, a TOF of 0.3 s
−1 was calculated which represents 14% of the specific activity of the enzyme (1.96 U mg
−1 BOx).
If the electroenzymatic reaction is limited by the need to have contact between enzyme and buckypaper surface (DET), one possibility to improve the catalytic current would be to introduce a freely diffusing redox mediator into the confined solution to electrically connect BOx in solution via mediated electron transfer (MET). As ABTS has already been successfully used for MET with BOx [
16], 0.5 mg of ABTS and 2.0 mg of BOx were trapped in the cavity of the hollow electrode based on hemin-L
bp.
Figure 3A shows a clear increase (+40%) in the value of the faradaic catalytic current from −1.35 mA cm
−2 to −1.9 mA cm
−2 at 0.5 V, corroborating the electrical wiring of BOx by two phenomena: DET and MET by ABTS.
In addition, the long-term operational stability of the bioelectrode containing BOx and ABTS was studied by chronoamperometry at 0.5 V. A remarkable stability of the cathodic current (loss of 2% after 8 h) seems to indicate the absence of ABTS release (
Figure 3B). After 23 h of intermittent operation over 7 days, 218.6 C were recorded corresponding to the reduction of 5.66 × 10
−4 mol of O
2 and leading to a turnover number of 19245, the loss of catalytic current being only 54% after 7 days.