Expired Cefalexin Loaded into Mesoporous Nanosilica for Self-Healing Epoxy Coating on 304 Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Synthesis of MSNs
2.3. Preparation of Cefalexin@MSNs and EP-Cefalexin@MSNs Coatings
2.4. Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
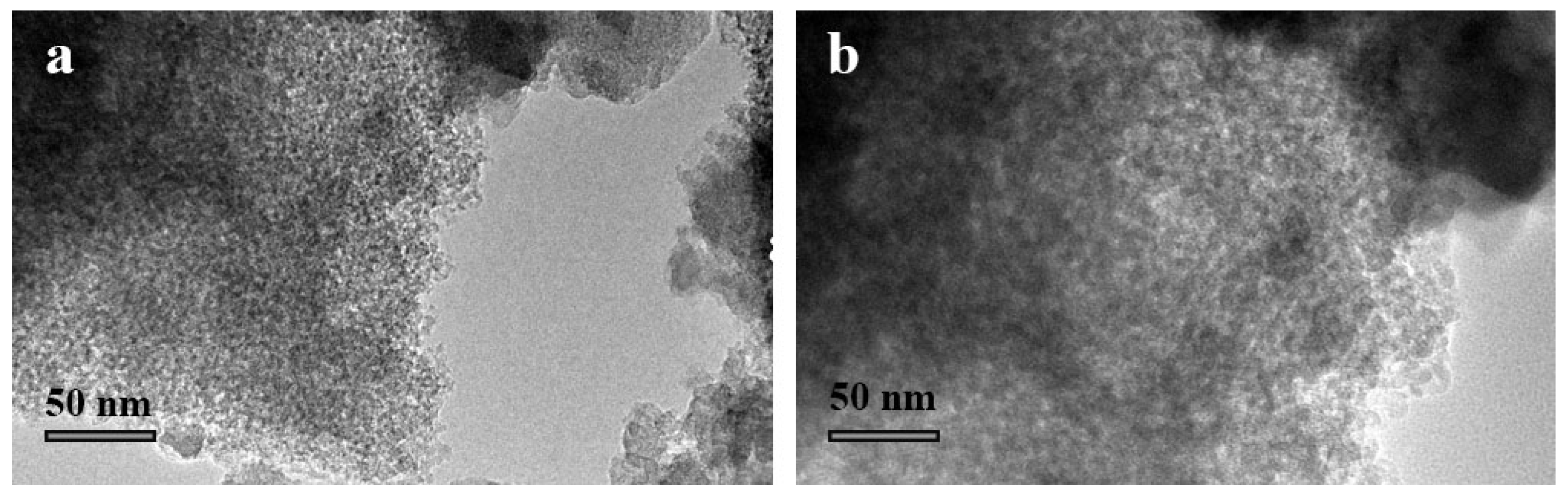
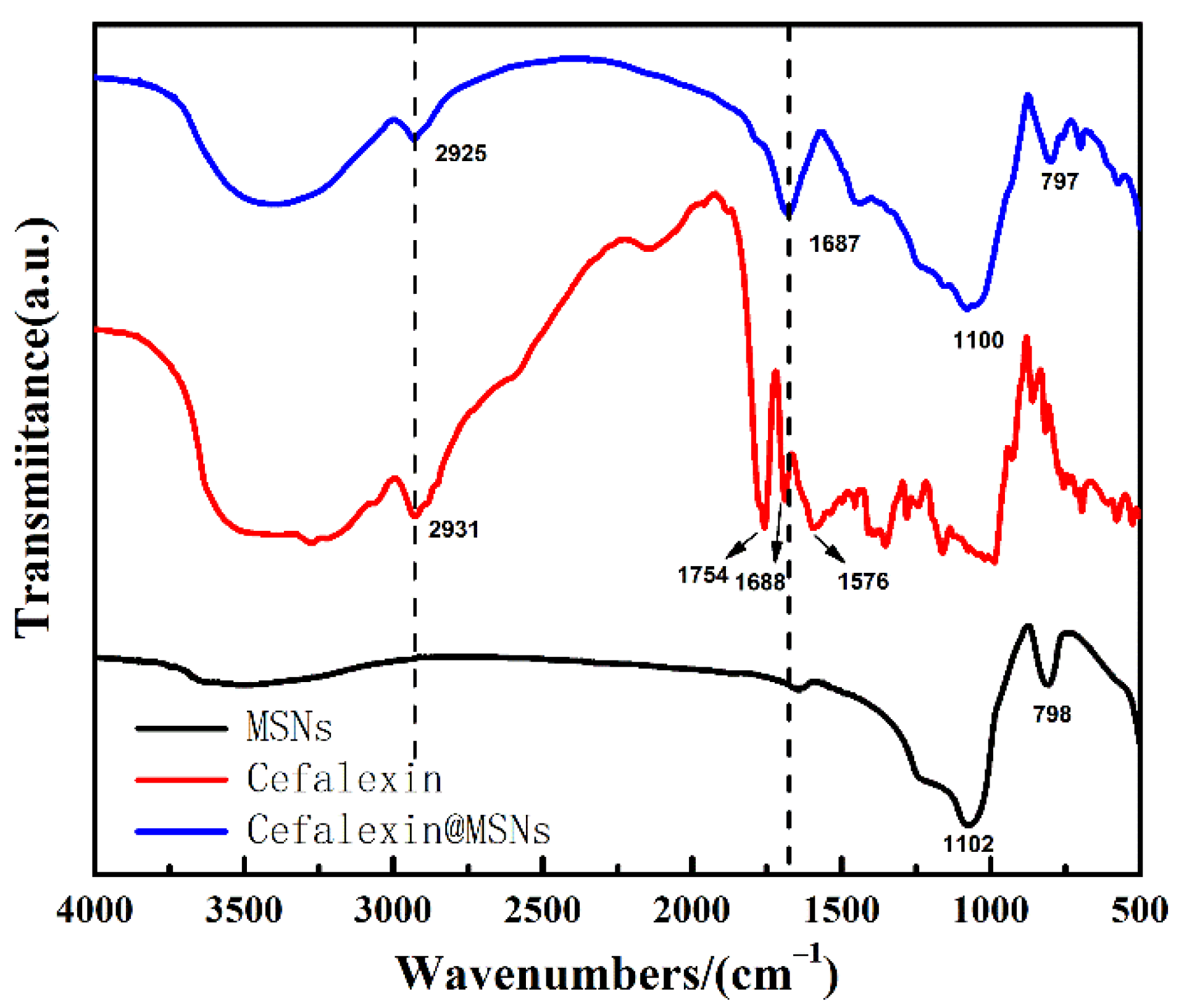
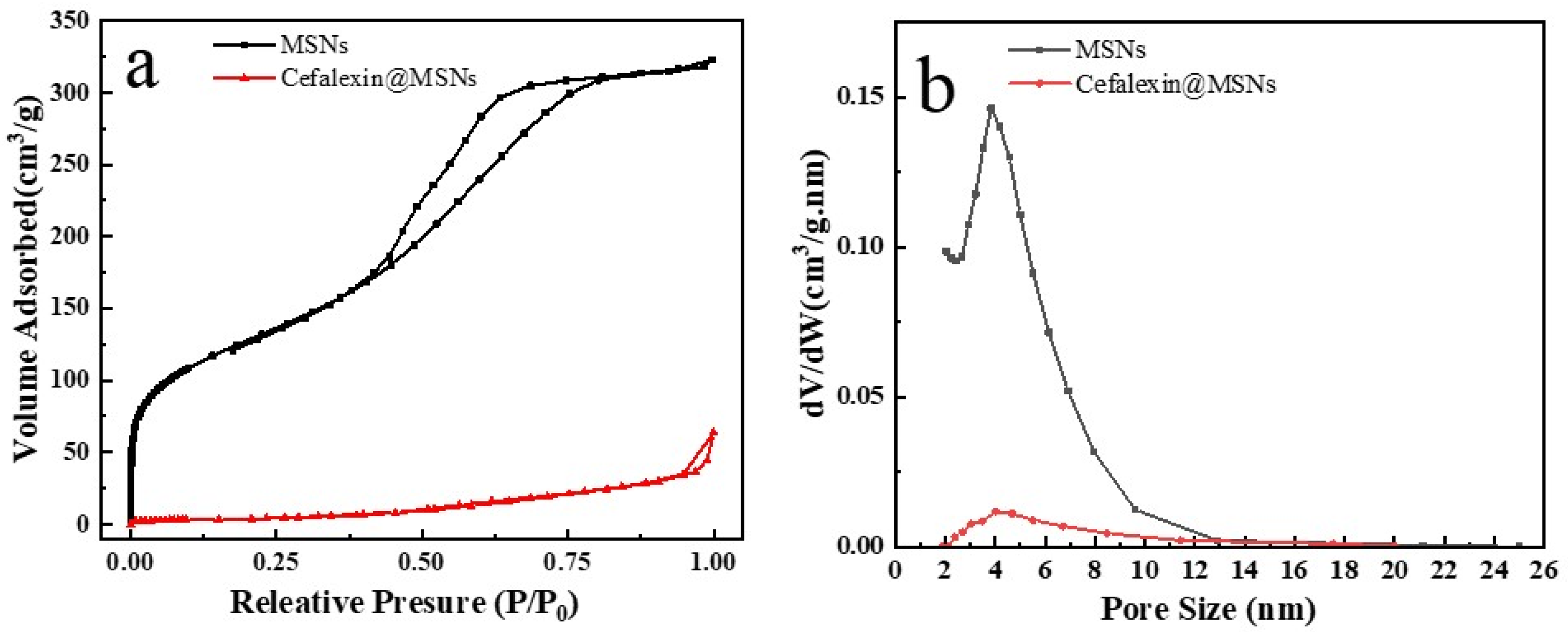
3.1. Characterization of MSNs and Cefalexin@MSNs
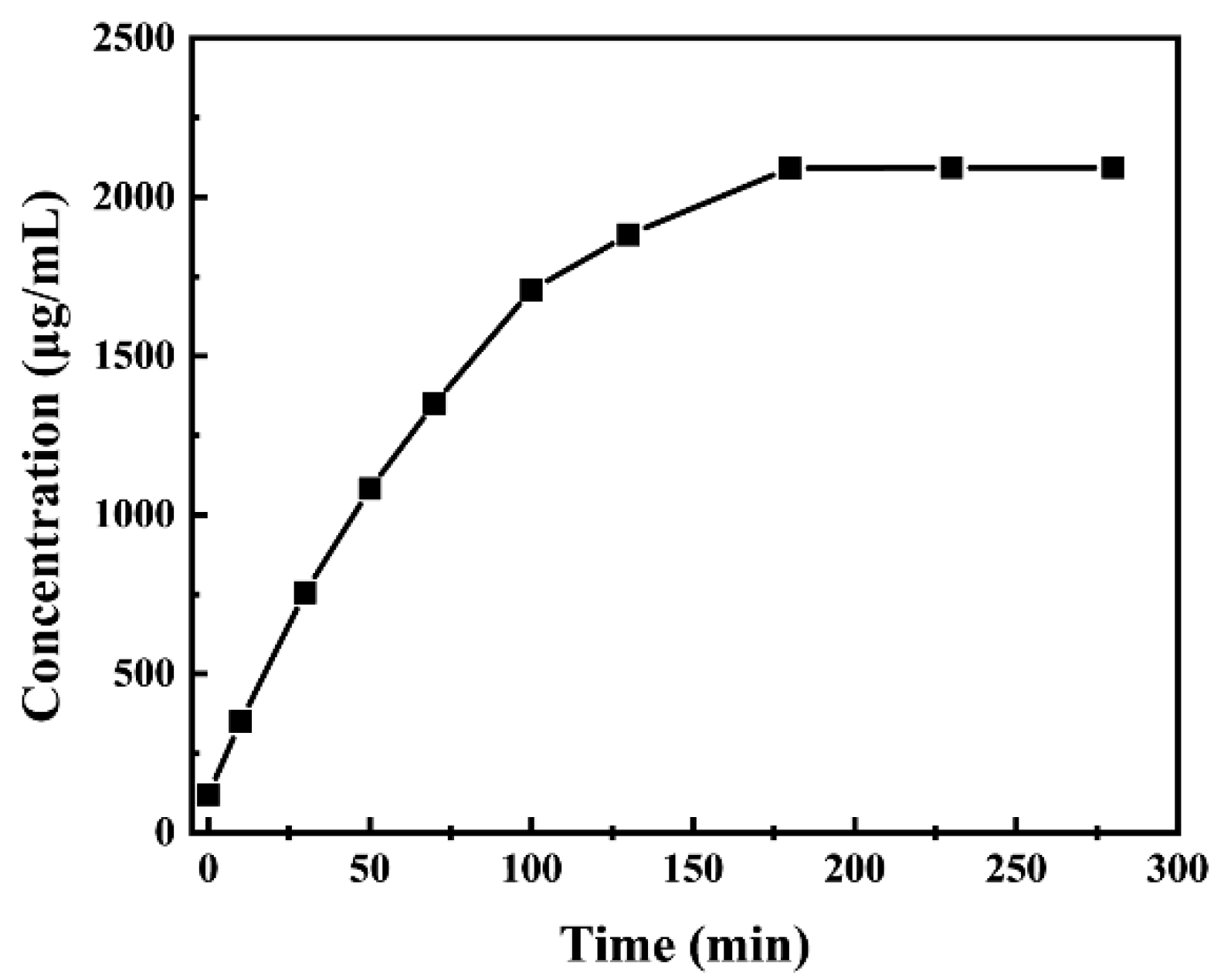
3.2. Slow Release of Cefalexin@MSNs
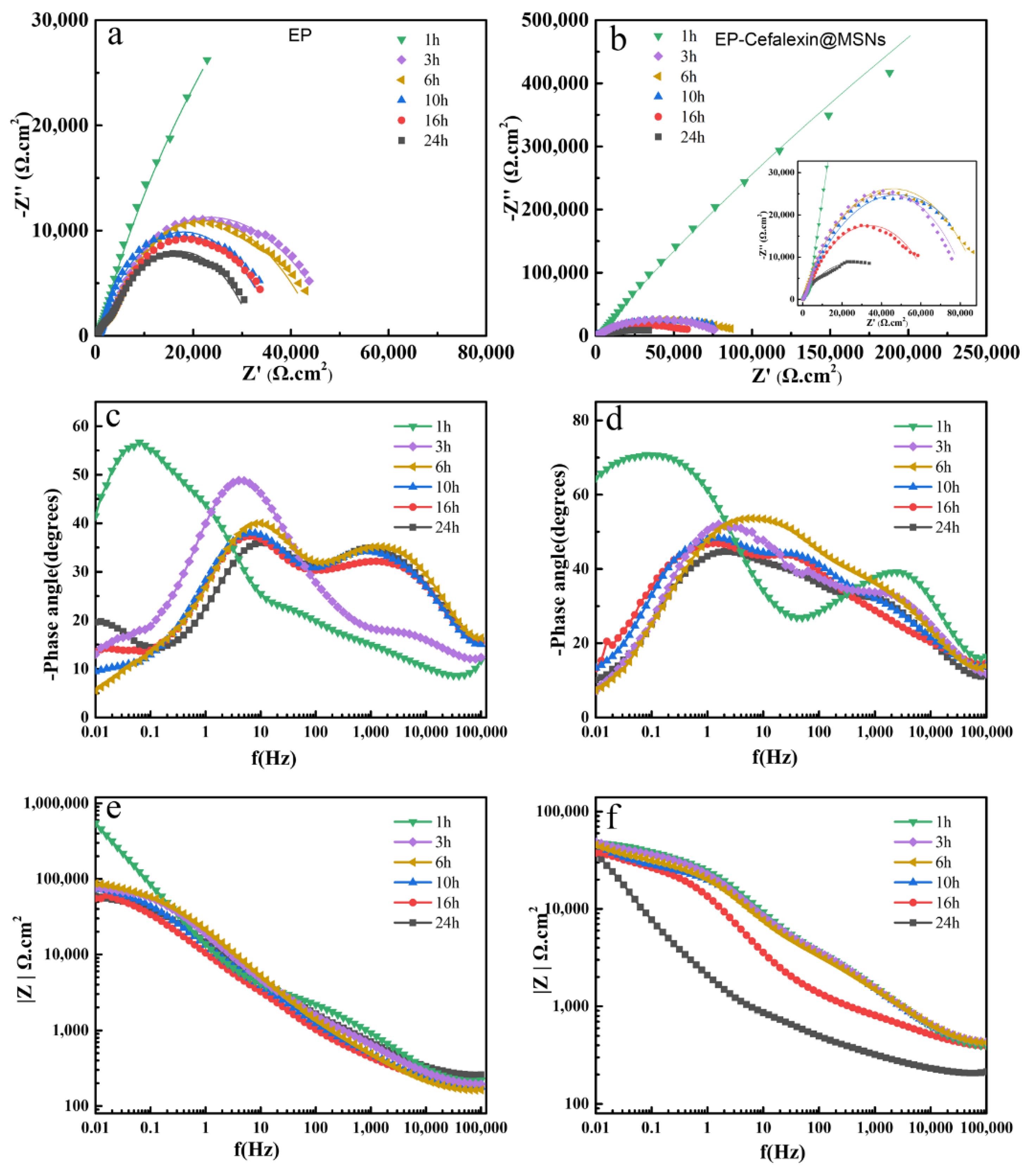
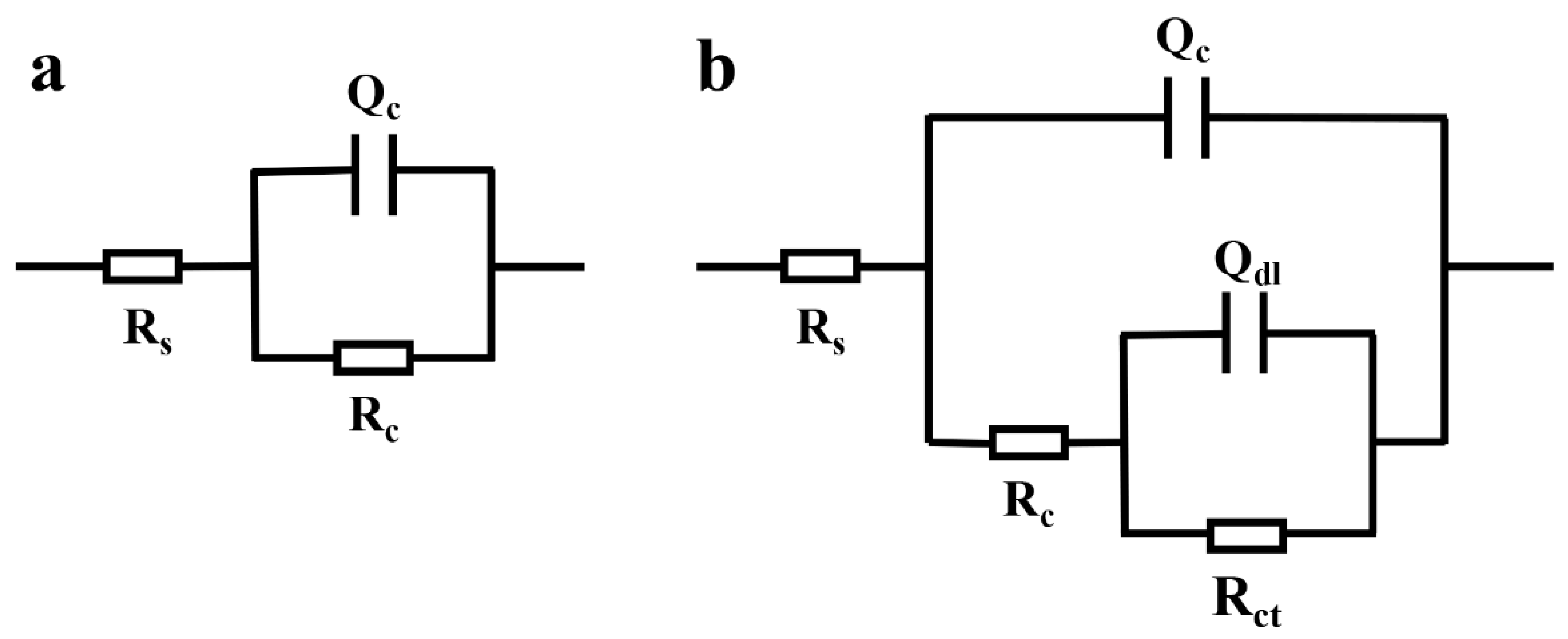
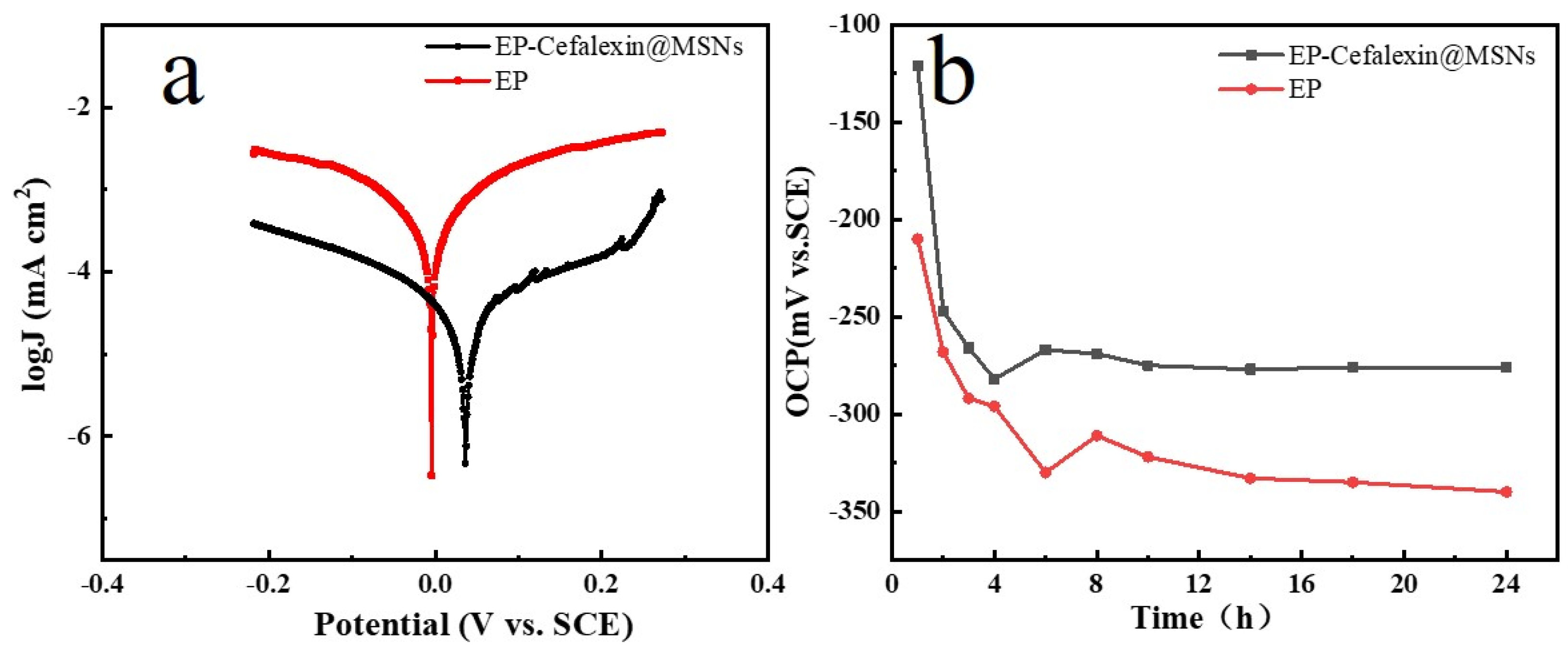
3.3. Corrosion Resistance
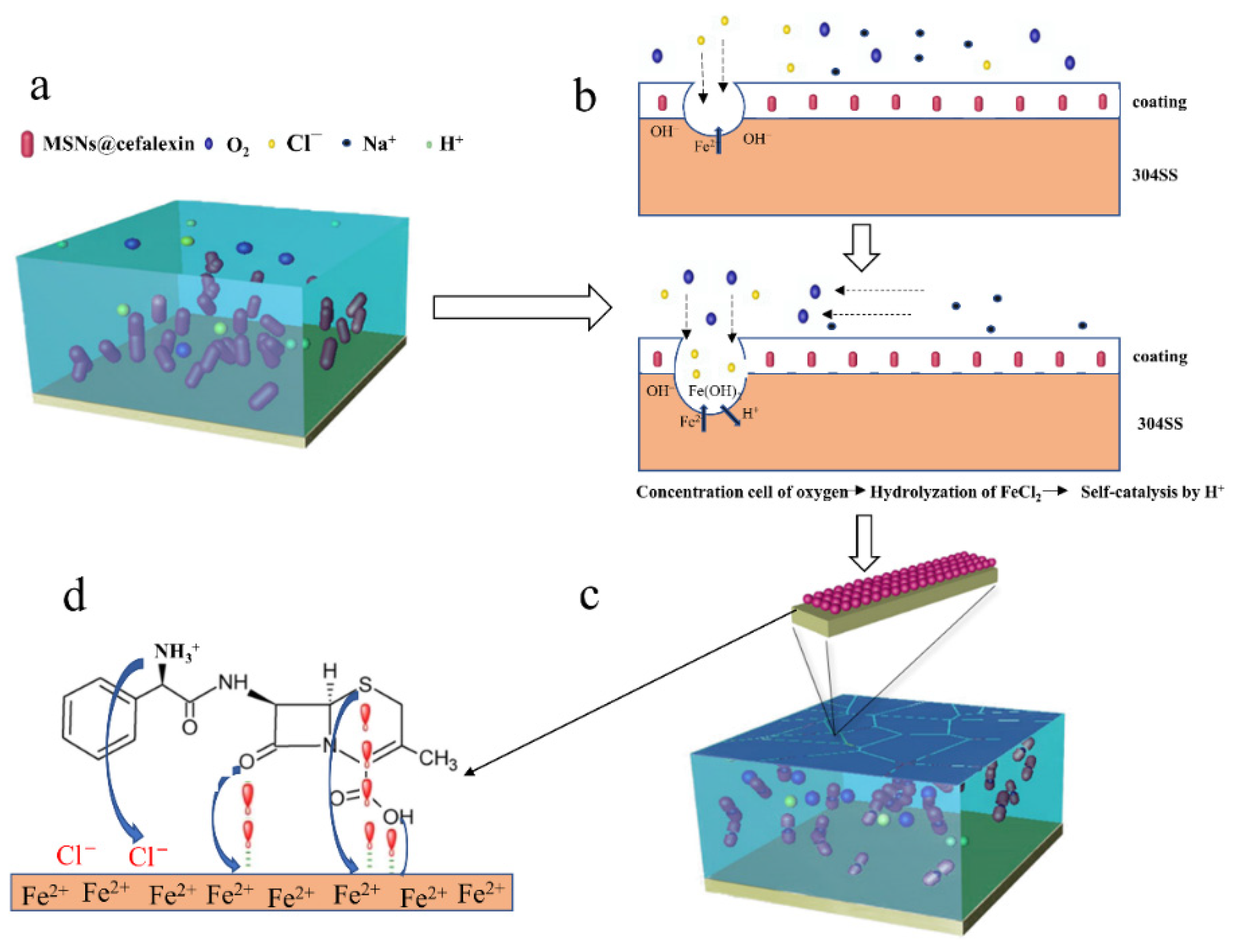
3.4. Self-Healing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutierrez, E.; Rodriguez, J.A.; Cruz-Borbolla, J.; Alvarado-Rodriguez, J.G.; Thangarasu, P. Development of a predictive model for corrosion inhibition of carbon steel by imidazole and benzimidazole derivatives. Corros. Sci. 2016, 108, 23–35. [Google Scholar] [CrossRef]
- Mousavi, M.; Mohammadalizadeh, M.; Khosravan, A. Theoretical investigation of corrosion inhibition effect of imidazole and its derivatives on mild steel using cluster model. Corros. Sci. 2011, 53, 3086–3091. [Google Scholar] [CrossRef]
- He, X.; Song, R.G.; Kong, D.J. Microstructure and corrosion behaviors of composite coatings on S355 offshore steel prepared by laser cladding combined with micro-arc oxidation. Appl. Surf. Sci. 2019, 497, 143703. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Shchukin, D.G.; Andreeva, D.V.; Zheludkevich, M.L.; Mohwald, H.; Ferreira, M.G.S. Sol-gel/polyelectrolyte active corrosion protection system. Adv. Funct. Mater. 2008, 18, 3137–3147. [Google Scholar]
- Leal, D.A.; Riegel-Vidotti, I.C.; Ferreira, M.G.S.; Marino, C.E.B. Smart coating based on double stimuli-responsive microcapsules containing linseed oil and benzotriazole for active corrosion protection. Corros. Sci. 2018, 130, 56–63. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Seifzadeh, D.; Raghibi-Boroujeni, M. Analysis of electrochemical noise data in both time and frequency domains to evaluate the effect of ZnO nanopowder addition on the corrosion protection performance of epoxy coatings. Arab. J. Chem. 2016, 9, S1320–S1327. [Google Scholar] [CrossRef] [Green Version]
- Nazeer, A.A.; Madkour, M. Potential use of smart coatings for corrosion protection of metals and alloys: A review. J. Mol. Liq. 2018, 253, 11–22. [Google Scholar] [CrossRef]
- Nezamdoust, S.; Seifzadeh, D.; Rajabalizadeh, Z. PTMS/OH-MWCNT sol-gel nanocomposite for corrosion protection of magnesium alloy. Surf. Coat. Tech. 2018, 335, 228–240. [Google Scholar]
- Tarzanagh, Y.J.; Seifzadeh, D.; Rajabalizadeh, Z.; Habibi-Yangjeh, A.; Khodayari, A.; Sohrabnezhad, S. Sol-gel/MOF nanocomposite for effective protection of 2024 aluminum alloy against corrosion. Surf. Coat. Tech. 2019, 380, 125038. [Google Scholar] [CrossRef]
- Sun, D.W.; Zheng, Y.; Lan, M.Z.; Wang, Z.M.; Cui, S.P.; Yang, J.L. Robust and impermeable metal shell microcapsules for one-component self-healing coatings. Appl. Surf. Sci. 2021, 546, 149114. [Google Scholar]
- Cui, X.; Yan, Y.G.; Huang, J.; Qiu, X.Y.; Zhang, P.P.; Chen, Y.; Hu, Z.F.; Liang, X.B. A substrate-independent isocyanate-modified polydimethylsiloxane coating harvesting mechanical durability, self-healing ability and low surface energy with anti-corrosion/biofouling potential. Appl. Surf. Sci. 2022, 579, 152186. [Google Scholar] [CrossRef]
- Wu, F.; Li, J.F.; Quan, H.; Han, J.; Liu, X.X.; Zhang, X.K.; Yang, J.L.; Xiang, Y. Robust polyurea/poly(urea-formaldehyde) hybrid microcapsules decorated with Al2O3 nano-shell for improved self-healing performance. Appl. Surf. Sci. 2021, 542, 148561. [Google Scholar] [CrossRef]
- Ni, X.X.; Gao, Y.J.; Zhang, X.H.; Lei, Y.; Sun, G.; You, B. An eco-friendly smart self-healing coating with NIR and pH dual-responsive superhydrophobic properties based on biomimetic stimuli-responsive mesoporous polydopamine microspheres. Chem. Eng. J. 2021, 406, 126725. [Google Scholar] [CrossRef]
- Adibzadeh, E.; Mirabedini, S.M.; Behzadnasab, M.; Farnood, R.R. A novel two-component self-healing coating comprising vinyl ester resin-filled microcapsules with prolonged anticorrosion performance. Prog. Org. Coat. 2021, 154, 106220. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Kuang, Y.; Li, Q.L.; Zheng, D.W.; Song, Q.F.; Chen, H.; Chen, X.Q.; Xu, Y.L.; Li, C.; et al. Ingenious pH-sensitive dextran/mesoporous silica nanoparticles based drug delivery systems for controlled intracellular drug release. Int. J. Biol. Macromol. 2017, 98, 691–700. [Google Scholar] [CrossRef]
- Borisova, D.; Mohwald, H.; Shchukin, D.G. Mesoporous Silica Nanoparticles for Active Corrosion Protection. ACS Nano 2011, 5, 1939–1946. [Google Scholar] [CrossRef]
- Chen, T.R.; Wang, C. Rapid fault isolation for a class of nonlinear lipschitz systems via deterministic learning. In Proceedings of the 2017 29th Chinese Control And Decision Conference (CCDC), Chongqing, China, 28–30 May 2017; pp. 4199–4204. [Google Scholar]
- Jiang, X.M.; Cheng, Y.S.; Smyth, H.D.C. Nanostructured Aerosol Particles: Fabrication, Pulmonary Drug Delivery, and Controlled Release. J. Nanomater. 2011, 2011, 760237. [Google Scholar] [CrossRef]
- Skorb, E.V.; Fix, D.; Andreeva, D.V.; Mohwald, H.; Shchukin, D.G. Surface-Modified Mesoporous SiO2 Containers for Corrosion Protection. Adv. Funct. Mater. 2009, 19, 2373–2379. [Google Scholar] [CrossRef]
- Yeganeh, M.; Saremi, M.; Rezaeyan, H. Corrosion inhibition of steel using mesoporous silica nanocontainers incorporated in the polypyrrole. Prog. Org. Coat. 2014, 77, 1428–1435. [Google Scholar] [CrossRef]
- Falcon, J.M.; Otubo, L.M.; Aoki, I.V. Highly ordered mesoporous silica loaded with dodecylamine for smart anticorrosion coatings. Surf. Coat. Tech. 2016, 303, 319–329. [Google Scholar] [CrossRef]
- Zea, C.; Barranco-Garcia, R.; Alcantara, J.; Simancas, J.; Morcillo, M.; de la Fuente, D. pH-dependent release of environmentally friendly corrosion inhibitor from mesoporous silica nanoreservoirs. Micropor. Mesopor. Mat. 2018, 255, 166–173. [Google Scholar] [CrossRef]
- Recloux, I.; Mouanga, M.; Druart, M.E.; Paint, Y.; Olivier, M.G. Silica mesoporous thin films as containers for benzotriazole for corrosion protection of 2024 aluminium alloys. Appl. Surf. Sci. 2015, 346, 124–133. [Google Scholar] [CrossRef]
- Ma, X.R.; Dang, R.; Kang, Y.H.; Gong, Y.; Luo, J.; Zhang, Y.Y.; Fu, J.W.; Li, C.Y.; Ma, Y.J. Electrochemical Studies of Expired Drug (Formoterol) as Oilfield Corrosion Inhibitor for Mild Steel in H2SO4 Media. Int. J. Electrochem. Sci. 2020, 15, 1964–1981. [Google Scholar] [CrossRef]
- Alfakeer, M.; Abdallah, M.; Fawzy, A. Corrosion Inhibition Effect of Expired Ampicillin and Flucloxacillin Drugs for Mild Steel in Aqueous Acidic Medium. Int. J. Electrochem. Sci. 2020, 15, 3283–3297. [Google Scholar] [CrossRef]
- Abdallah, M.; Fawzy, A.; Al Bahir, A. The Effect of Expired Acyclovir and Omeprazole Drugs on the Inhibition of Sabic Iron Corrosion in HCl Solution. Int. J. Electrochem. Sci. 2020, 15, 4739–4753. [Google Scholar] [CrossRef]
- Li, X.H.; Deng, S.D.; Fu, H. Allyl thiourea as a corrosion inhibitor for cold rolled steel in H3PO4 solution. Corros. Sci. 2012, 55, 280–288. [Google Scholar] [CrossRef]
- Shukla, S.K.; Quraishi, M.A. Cefalexin drug: A new and efficient corrosion inhibitor for mild steel in hydrochloric acid solution. Mater. Chem. Phys. Sci. 2010, 120, 142–147. [Google Scholar] [CrossRef]
- Fayomi, O.S.I.; Akande, I.G.; Daramola, D.; Oluwadare, G.A.; Popoola, A.P.I. Inhibitive characteristics of cefalexin drug addition on corrosion evolution of mild steel in a chloride medium. Mater. Chem. Phys. 2021, 39, 155–163. [Google Scholar]
- Basaldella, E.I.; Legnoverde, M.S. Functionalized silica matrices for controlled delivery of cephalexin. J. Sol-Gel. Sci. Technol. 2010, 56, 191–196. [Google Scholar] [CrossRef]
- Llusar, M.; Monros, G.; Roux, C.; Pozzo, J.L.; Sanchez, C. One-pot synthesis of phenyl- and amine-functionalized silica fibers through the use of anthracenic and phenazinic organogelators. J. Mater. Chem. 2003, 13, 2505–2514. [Google Scholar] [CrossRef]
- Mani, S.P.; Rajendran, N. Corrosion and interfacial contact resistance behavior of electrochemically nitrided 316L SS bipolar plates for proton exchange membrane fuel cells. Energy 2017, 133, 1050–1062. [Google Scholar] [CrossRef]
- Dinodi, N.; Shetty, A.N. Alkyl carboxylates as efficient and green inhibitors of magnesium alloy ZE41 corrosion in aqueous salt solution. Corr. Sci. 2014, 85, 411–427. [Google Scholar]
- Feng, Y.; Cheng, Y.F. An intelligent coating doped with inhibitor-encapsulated nanocontainers for corrosion protection of pipeline steel. Chem. Eng. J. 2017, 315, 537–551. [Google Scholar] [CrossRef]
- Yoganandan, G.; Balaraju, J.N.; Low, C.H.C.; Qi, G.J.; Chen, Z. Electrochemical and long term corrosion behavior of Mn and Mo oxyanions sealed anodic oxide surface developed on aerospace aluminum alloy (AA2024). Surf. Coat. Technol. 2016, 288, 115–125. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, M.D.; Wang, C.; Feng, J.; Li, J.S.; Wang, L.J.; Fu, J.J. Facile Synthesis of Smart Nanocontainers as Key Components for Construction of Self-Healing Coating with Superhydrophobic Surfaces. Nanoscale. Res. Lett. 2016, 11, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Ma, Z.; Li, A.; Zhu, G.; Zhang, Y. Anticorrosive performance of polyaniline/waterborne epoxy/poly (methylhydrosiloxane) composite coatings. Prog. Org. Coat. 2020, 139, 105462. [Google Scholar] [CrossRef]
- Alhumade, H.; Yu, A.; Elkamel, A.; Simon, L.; Abdala, A. Enhanced protective properties and UV stability of epoxy/graphene nanocomposite coating on stainless steel. Express. Poly. Lett. 2016, 10, 1034–1046. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, W.; Chen, Y.; Yin, X.; Liu, Y. Smart coatings embedded with polydopamine-decorated layer-by-layer assembled SnO2 nanocontainers for the corrosion protection of 304 stainless steels. J. Colloid Interface Sci. 2020, 579, 741–753. [Google Scholar] [CrossRef]
- Chen, M.H.; Zheng, Y.H.; Gao, J.W.; Li, C.; Yu, C.F.; Wang, Q.M. Fluorometric determination of dopamine by using a terbium (III) inorganic-organic network. Microchim. Acta 2017, 184, 2275–2280. [Google Scholar] [CrossRef]
- Ramkumar, S.; Nalini, D.; Quraishi, M.A.; Ebenso, E.E.; Verma, C. Anti-corrosive property of bioinspired environmental benign imidazole and isoxazoline heterocyclics: A cumulative studies of experimental and DFT methods. J. Heterocyclic. Chem. 2020, 57, 103–119. [Google Scholar] [CrossRef]
| Cr | Mn | Si | Ni | Mo | C | P | S | Fe |
|---|---|---|---|---|---|---|---|---|
| 18.50 | 0.88 | 0.59 | 8.12 | 0.30 | 0.05 | 0.015 | 0.028 | Balance |
| Sample | SBET (m2 g−1) | Vp (cm3 g−1) | DBJH (nm) |
|---|---|---|---|
| Pure-MSNs | 593 | 0.64 | 4.1 |
| Cefalexin-MSNs | 28 | 0.03 | 3.9 |
| Sample | Time(h) | Rc(Ω cm2) | Qc | Qdl | Rct(Ω cm2) | ||
|---|---|---|---|---|---|---|---|
| Cc(F/cm2) | n | Cdl(F/cm2) | n | ||||
| EP | 1 | 262,880 | 5.42 × 10−7 | 0.7235 | - | - | - |
| 3 | 6662 | 3.08 × 10−6 | 0.7522 | 4.05 × 10−6 | 0.6358 | 35,398 | |
| 6 | 6394 | 4.94 × 10−6 | 0.7633 | 4.29 × 10−6 | 0.7756 | 34,164 | |
| 10 | 5971 | 5.17 × 10−6 | 0.7521 | 4.44 × 10−6 | 0.7855 | 33,665 | |
| 16 | 5396 | 6.02 × 10−6 | 0.7825 | 6.63 × 10−6 | 0.7542 | 29,476 | |
| 24 | 991 | 8.70 × 10−6 | 0.7963 | 9.68 × 10−6 | 0.7865 | 23,649 | |
| EP-Cefalexin@ MSNs | 1 | 542,235 | 4.52 × 10−7 | 0.7562 | - | - | - |
| 3 | 99,685 | 2.44 × 10−6 | 0.769 | 3.42 × 10−6 | 0.7564 | 88,067 | |
| 6 | 100,675 | 2.05 × 10−6 | 0.7848 | 3.12 × 10−6 | 0.7756 | 90,659 | |
| 10 | 99,002 | 2.55 × 10−6 | 0.7935 | 3.50 × 10−6 | 0.7867 | 88,652 | |
| 16 | 81,003 | 3.59 × 10−6 | 0.7252 | 4.52 ×10−6 | 0.7683 | 64,562 | |
| 24 | 70,236 | 4.95 × 10−6 | 0.7741 | 5.30 × 10−6 | 0.7848 | 58,961 | |
| Sample | Ecorr/mV VS. SCE | Icorr/μA/cm2 |
|---|---|---|
| EP | −19 | 0.5236 |
| EP-Cefalexin@ MSNs | 58 | 0.04275 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Dong, J.; Bian, H.; Lu, H.; Bin, D.; Tang, S.; Song, Y.; Lu, H. Expired Cefalexin Loaded into Mesoporous Nanosilica for Self-Healing Epoxy Coating on 304 Stainless Steel. Nanomaterials 2022, 12, 2406. https://doi.org/10.3390/nano12142406
Yang B, Dong J, Bian H, Lu H, Bin D, Tang S, Song Y, Lu H. Expired Cefalexin Loaded into Mesoporous Nanosilica for Self-Healing Epoxy Coating on 304 Stainless Steel. Nanomaterials. 2022; 12(14):2406. https://doi.org/10.3390/nano12142406
Chicago/Turabian StyleYang, Beibei, Jiayu Dong, Haifeng Bian, Haimin Lu, Duan Bin, Shaochun Tang, Yaqiong Song, and Hongbin Lu. 2022. "Expired Cefalexin Loaded into Mesoporous Nanosilica for Self-Healing Epoxy Coating on 304 Stainless Steel" Nanomaterials 12, no. 14: 2406. https://doi.org/10.3390/nano12142406
APA StyleYang, B., Dong, J., Bian, H., Lu, H., Bin, D., Tang, S., Song, Y., & Lu, H. (2022). Expired Cefalexin Loaded into Mesoporous Nanosilica for Self-Healing Epoxy Coating on 304 Stainless Steel. Nanomaterials, 12(14), 2406. https://doi.org/10.3390/nano12142406





