Atomic Layer Deposition of Cobalt Catalyst for Fischer–Tropsch Synthesis in Silicon Microchannel Microreactor
Abstract
:1. Introduction
2. Experimentation
2.1. Materials
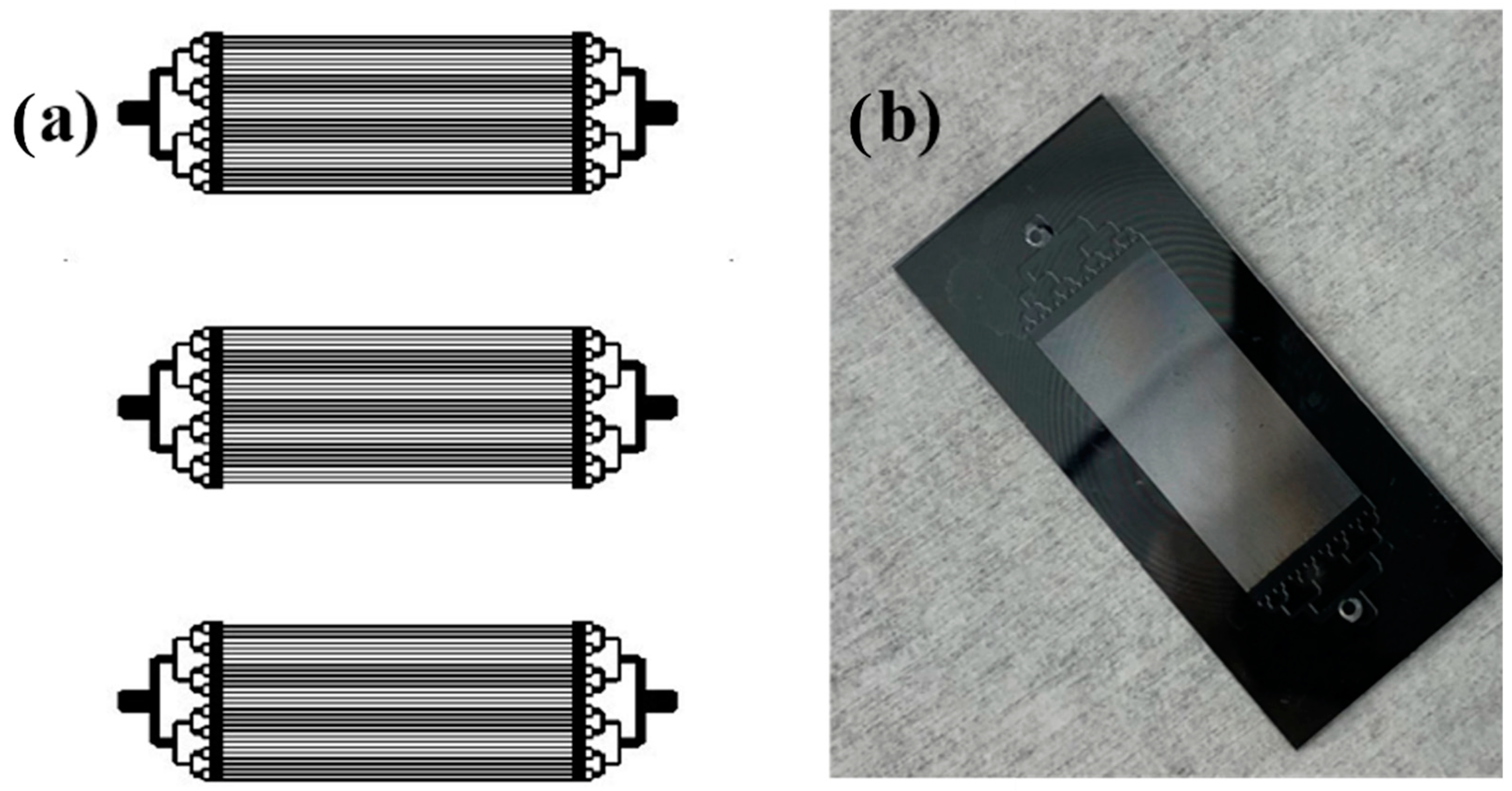
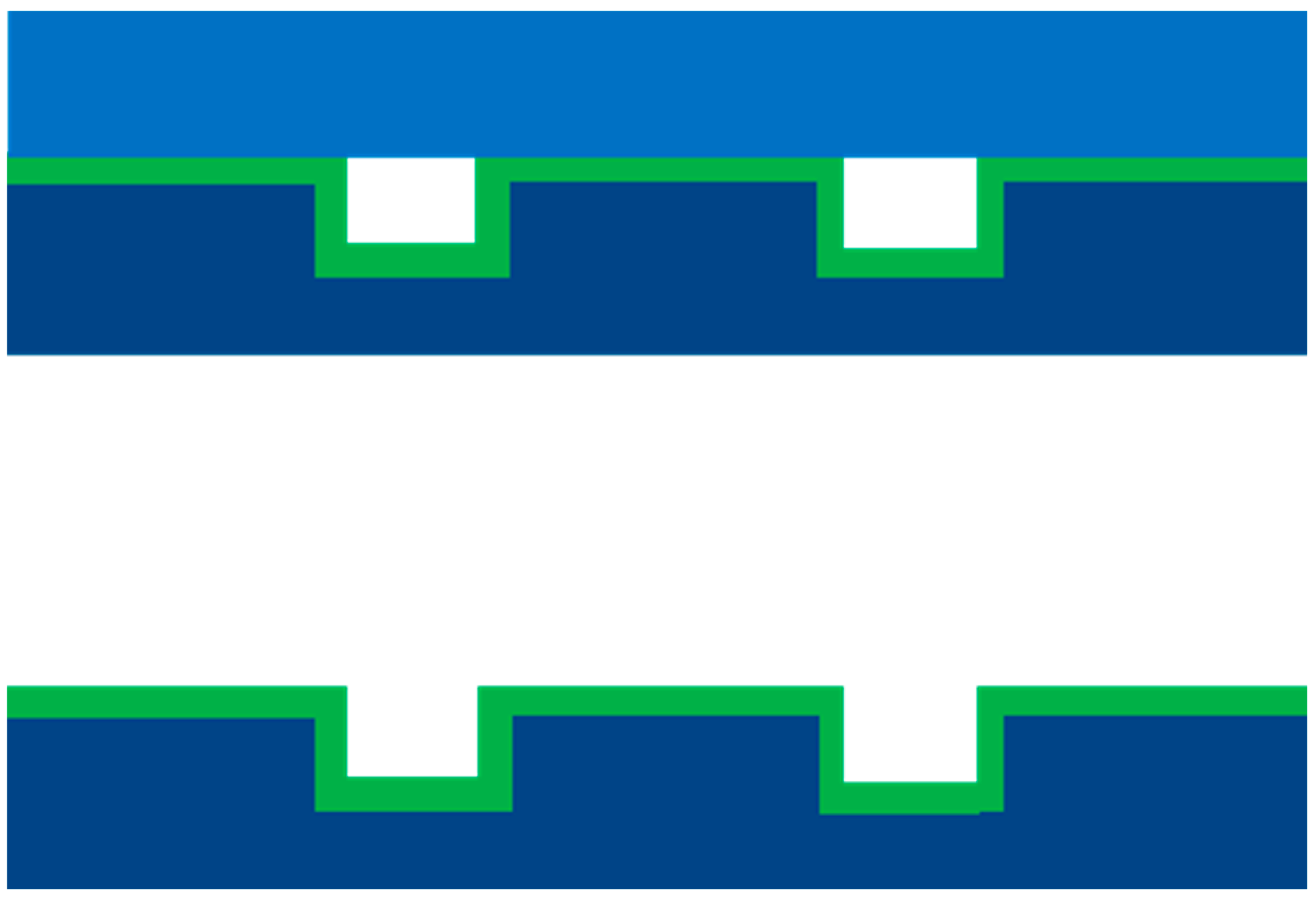
2.2. Fabrication of Silicon Microreactors
2.3. Deposition of Cobalt Catalyst Using ALD Technique
2.4. Experimental Setup for Fischer–Tropsch Synthesis in Silicon Microchannel Microreactor
3. Characterization
4. Results and Discussion
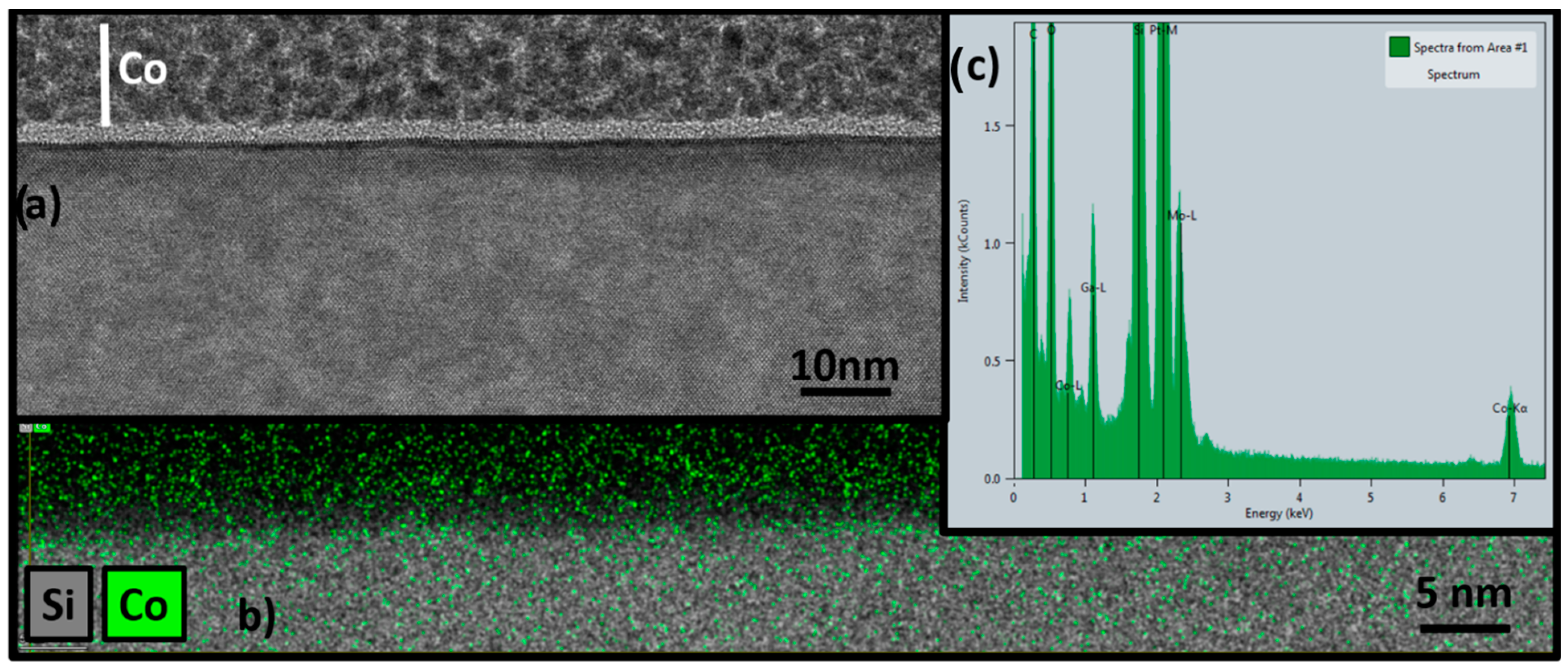
4.1. TEM
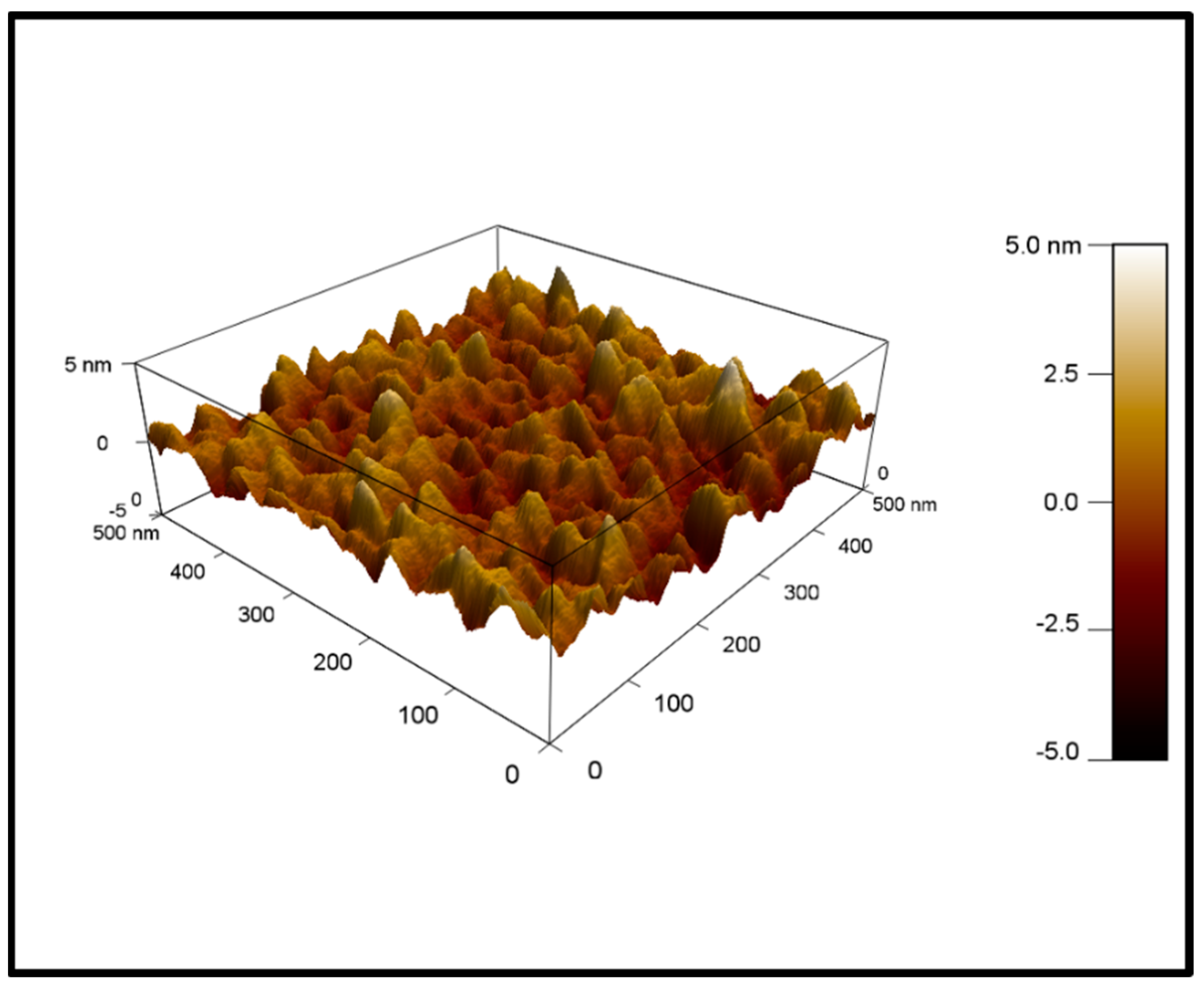
4.2. AFM
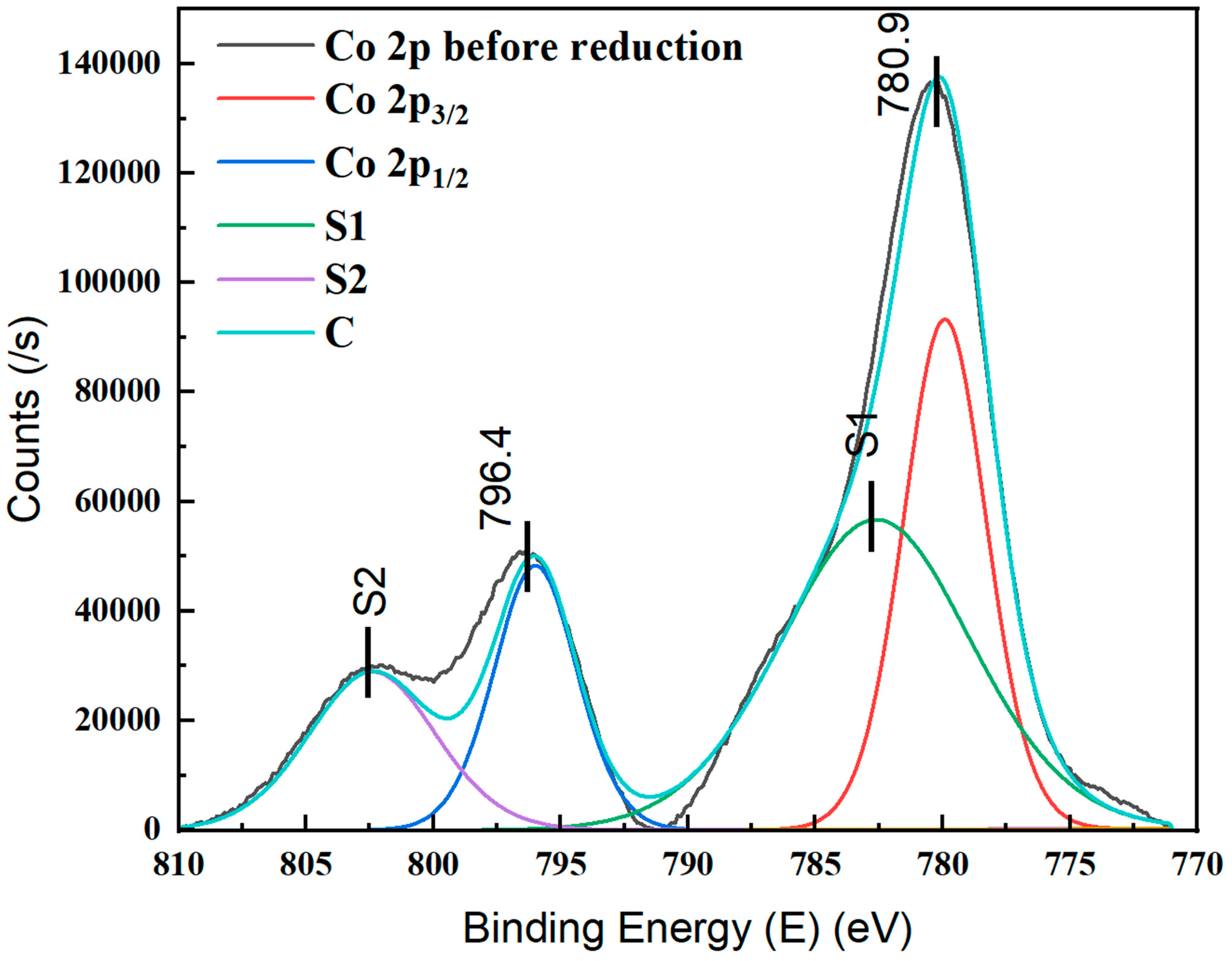
4.3. Chemical Oxidation States of ALD-Deposited Catalyst
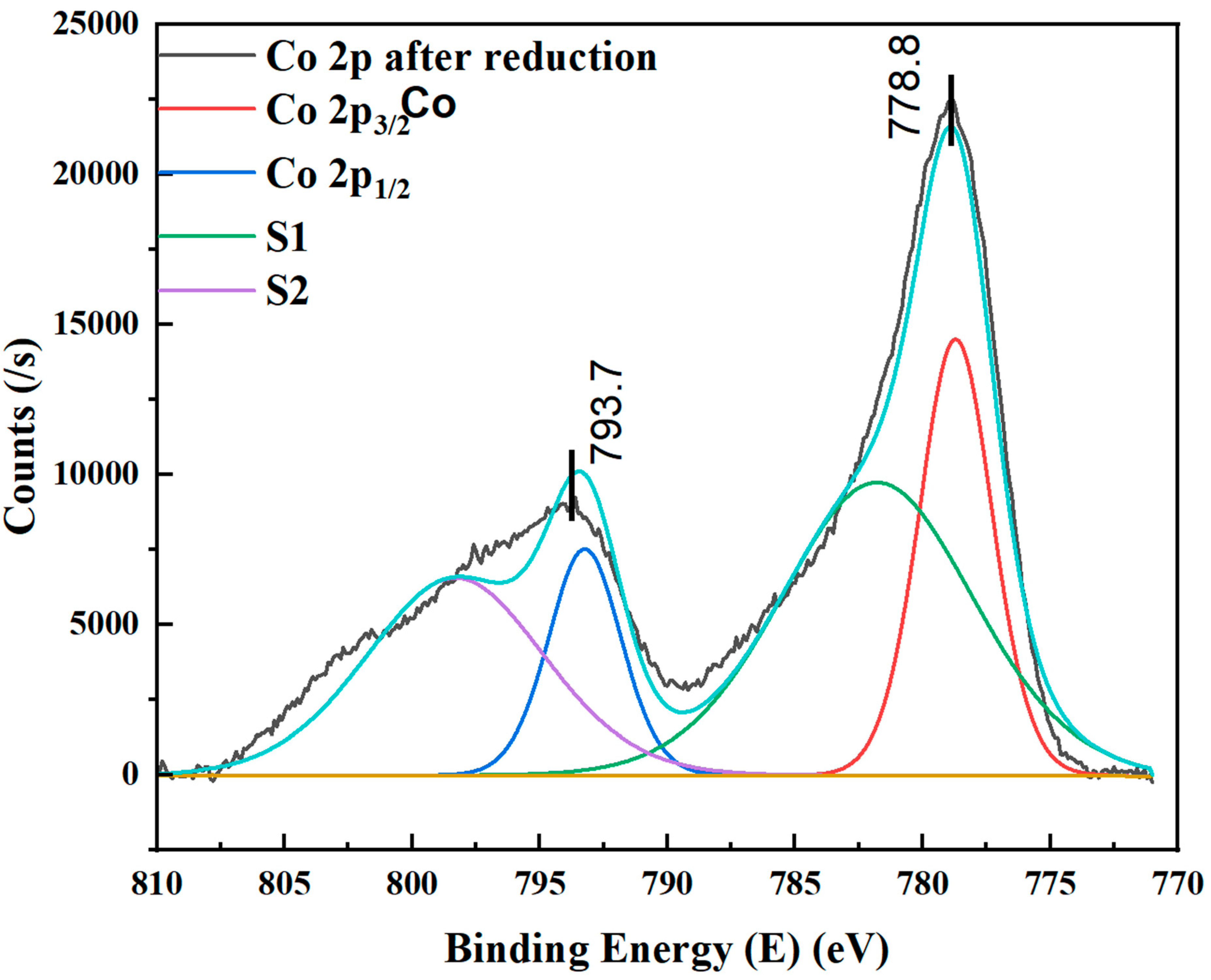
4.4. XPS Studies of Reduced Cobalt Metal in Microreactors
4.5. Atmospheric Pressure Fischer–Tropsch Synthesis in Silicon Microchannel Microreactors
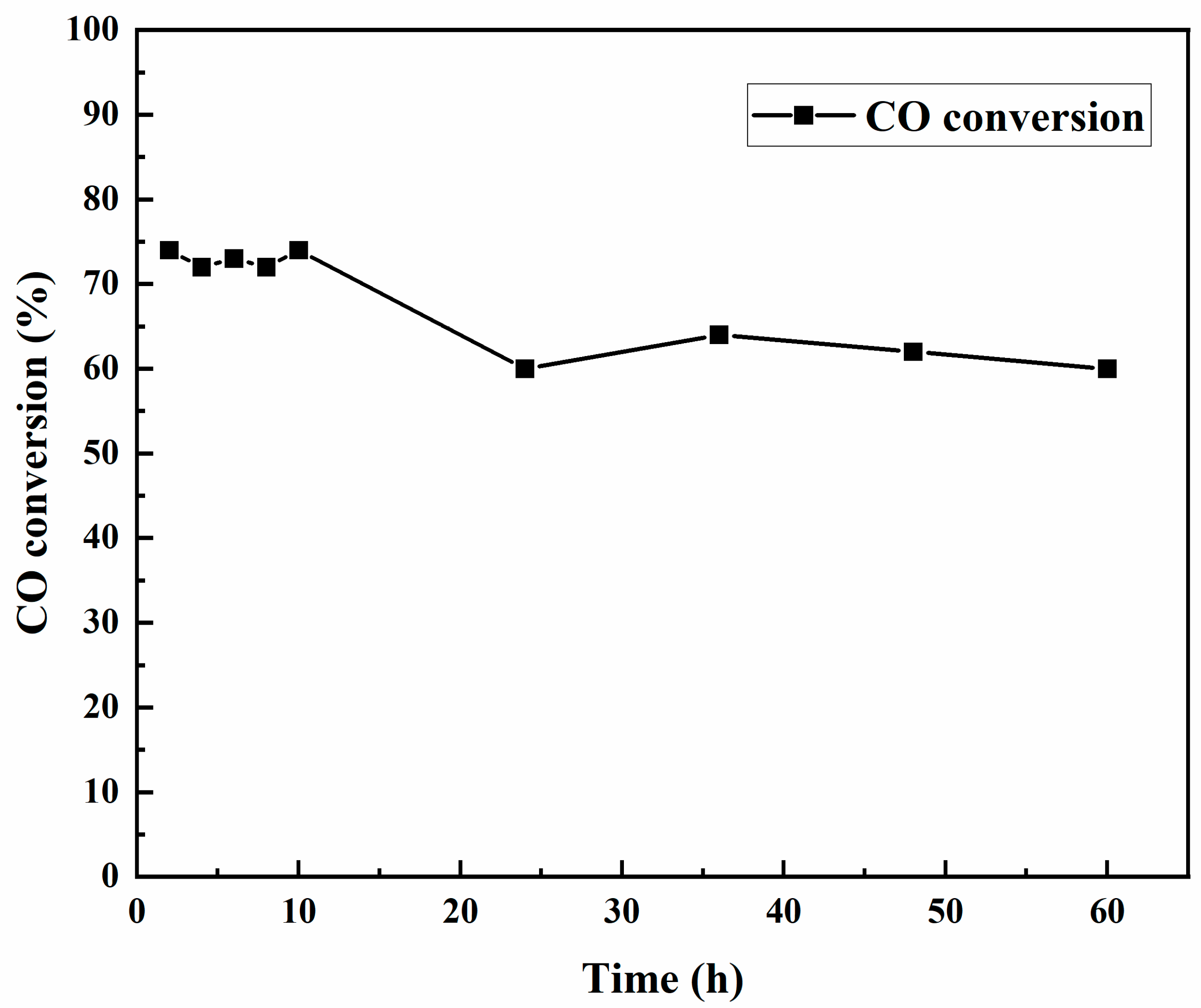
4.6. Deactivation Studies of Co-Catalysts Used for Fischer–Tropsch Synthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steynberg, A. Introduction to fischer-tropsch technology. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2004; Volume 152, pp. 1–63. [Google Scholar]
- Jahangiri, H.; Bennett, J.; Mahjoubi, P.; Wilson, K.; Gu, S. A review of advanced catalyst development for Fischer–Tropsch synthesis of hydrocarbons from biomass derived syn-gas. Catal. Sci. Technol. 2014, 4, 2210–2229. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, N.; Abrokwah, R.Y.; Stevens-Boyd, R.G.; Aravamudhan, S.; Kuila, D. Fischer-Tropsch studies in a 3D-printed stainless steel microchannel microreactor coated with cobalt-based bimetallic-MCM-41 catalysts. Catal. Today 2020, 358, 303–315. [Google Scholar] [CrossRef]
- Mohammad, N.; Bepari, S.; Aravamudhan, S.; Kuila, D.J. Kinetics of Fischer–Tropsch Synthesis in a 3-D Printed Stainless Steel Microreactor Using Different Mesoporous Silica Supported Co-Ru Catalysts. Catalysts 2019, 9, 872. [Google Scholar] [CrossRef] [Green Version]
- Gorimbo, J.; Muleja, A.; Liu, X.; Hildebrandt, D. Fischer–Tropsch synthesis: Product distribution, operating conditions, iron catalyst deactivation and catalyst speciation. Int. J. Ind. Chem. 2018, 9, 317–333. [Google Scholar] [CrossRef]
- Davis, B.H. Fischer–Tropsch synthesis: Overview of reactor development and future potentialities. Top. Catal. 2005, 32, 143–168. [Google Scholar] [CrossRef]
- Okabe, K.; Li, X.; Wei, M.; Arakawa, H. Fischer–Tropsch synthesis over Co–SiO2 catalysts prepared by the sol–gel method. Catal. Today 2004, 89, 431–438. [Google Scholar] [CrossRef]
- Kim, H.-J.; Ryu, J.-H.; Joo, H.; Yoon, J.; Jung, H.; Yang, J.-I. Mass- and heat-transfer-enhanced catalyst system for Fischer-Tropsch synthesis in fixed-bed reactors. Res. Chem. Intermed. 2008, 34, 811–816. [Google Scholar] [CrossRef]
- Hardt, S.; Ehrfeld, W.; Hessel, V.; Bussche, K.M. Strategies for size reduction of microreactors by heat transfer enhancement effects. Chem. Eng. Commun. 2003, 190, 540–559. [Google Scholar] [CrossRef]
- van Male, P.; de Croon, M.H.J.M.; Tiggelaar, R.M.; van den Berg, A.; Schouten, J.C. Heat and mass transfer in a square microchannel with asymmetric heating. Int. J. Heat Mass Transf. 2004, 47, 87–99. [Google Scholar] [CrossRef] [Green Version]
- Ying, X.; Zhang, L.; Xu, H.; Ren, Y.; Xuan, J. An Experimental Study on a Microchannel Reactor for Fischer-tropsch Synthesis. Energy Procedia 2014, 61, 1394–1397. [Google Scholar] [CrossRef] [Green Version]
- Perego, C.; Bortolo, R.; Zennaro, R. Gas to liquids technologies for natural gas reserves valorization: The Eni experience. Catal. Today 2009, 142, 9–16. [Google Scholar] [CrossRef]
- Mohammad, N.; Chukwudoro, C.; Bepari, S.; Basha, O.; Aravamudhan, S.; Kuila, D. Scale-up of high-pressure F-T synthesis in 3D printed stainless steel microchannel microreactors: Experiments and modeling. Catal. Today 2022, 397–399, 182–196. [Google Scholar] [CrossRef]
- Abrokwah, R.Y.; Rahman, M.M.; Deshmane, V.G.; Kuila, D. Effect of titania support on Fischer-Tropsch synthesis using cobalt, iron, and ruthenium catalysts in silicon-microchannel microreactor. Mol. Catal. 2019, 478, 110566. [Google Scholar] [CrossRef]
- Myrstad, R.; Eri, S.; Pfeifer, P.; Rytter, E.; Holmen, A. Fischer–Tropsch synthesis in a microstructured reactor. Catal. Today 2009, 147, S301–S304. [Google Scholar] [CrossRef]
- Jensen, K.F. Silicon-Based Microreactors; ACS Publications: Washington, DC, USA, 2005. [Google Scholar]
- Zhao, S.; Nagineni, V.S.; Seetala, N.V.; Kuila, D. Microreactors for Syngas Conversion to Higher Alkanes: Effect of Ruthenium on Silica-Supported Iron−Cobalt Nanocatalysts. Ind. Eng. Chem. Res. 2008, 47, 1684–1688. [Google Scholar] [CrossRef]
- Mehta, S.; Deshmane, V.; Zhao, S.; Kuila, D. Comparative Studies of Silica-Encapsulated Iron, Cobalt, and Ruthenium Nanocatalysts for Fischer–Tropsch Synthesis in Silicon-Microchannel Microreactors. Ind. Eng. Chem. Res. 2014, 53, 16245–16253. [Google Scholar] [CrossRef]
- Nagineni, V.S.; Zhao, S.; Potluri, A.; Liang, Y.; Siriwardane, U.; Seetala, N.V.; Fang, J.; Palmer, J.; Kuila, D. Microreactors for Syngas Conversion to Higher Alkanes: Characterization of Sol−Gel-Encapsulated Nanoscale Fe−Co Catalysts in the Microchannels. Ind. Eng. Chem. Res. 2005, 44, 5602–5607. [Google Scholar] [CrossRef]
- Zhao, S.; Kuila, D. Nanocatalysis in Microreactor for Fuels; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Mohammad, N.; Basha, O.M.; Bepari, S.; Abrokwah, R.Y.; Deshmane, V.; Wang, L.; Aravamudhan, S.; Kuila, D. Fischer-Tropsch Synthesis in Silicon and 3D Printed Stainless Steel Microchannel Microreactors. In Catalysis for Clean Energy and Environmental Sustainability; Springer: Cham, Switzerland, 2021; pp. 429–457. [Google Scholar] [CrossRef]
- Mohammad, N. Catalyst and Microreactor Development for Atmospheric and High-Pressure Fischer-Tropsch Synthesis. Ph.D. Thesis, North Carolina Agricultural and Technical State University, Greensboro, NC, USA, 2020. [Google Scholar]
- Parakh, M.; Ramaswamy, P.; Devkota, S.; Kuchoor, H.; Dawkins, K.; Iyer, S. Passivation efficacy study of Al(2)O(3)dielectric on self-catalyzed molecular beam epitaxially grown GaAs(1-x)Sb(x)nanowires. Nanotechnology 2022, 33, 315602. (In English) [Google Scholar] [CrossRef]
- Ramaswamy, P.; Devkota, S.; Pokharel, R.; Nalamati, S.; Stevie, F.; Jones, K.; Reynolds, L.; Iyer, S. A study of dopant incorporation in Te-doped GaAsSb nanowires using a combination of XPS/UPS, and C-AFM/SKPM. Sci. Rep. 2021, 11, 8329. [Google Scholar] [CrossRef]
- Elko-Hansen, T.D.M.; Ekerdt, J.G. XPS Investigation of the Atomic Layer Deposition Half Reactions of Bis(N-tert-butyl-N′-ethylpropionamidinato) Cobalt(II). Chem. Mater. 2014, 26, 2642–2646. [Google Scholar] [CrossRef]
- Kretzschmar, B.S.M.; Assim, K.; Preuß, A.; Heft, A.; Korb, M.; Pügner, M.; Lampke, T.; Grünler, B.; Lang, H. Cobalt and manganese carboxylates for metal oxide thin film deposition by applying the atmospheric pressure combustion chemical vapour deposition process. RSC Adv. 2018, 8, 15632–15640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bepari, S.; Li, X.; Abrokwah, R.; Mohammad, N.; Arslan, M.; Kuila, D. Co-Ru catalysts with different composite oxide supports for Fischer–Tropsch studies in 3D-printed stainless steel microreactors. Appl. Catal. A Gen. 2020, 608, 117838. [Google Scholar] [CrossRef]
- Najafabadi, A.T.; Khodadadi, A.A.; Parnian, M.J.; Mortazavi, Y. Atomic layer deposited Co/γ-Al2O3 catalyst with enhanced cobalt dispersion and Fischer–Tropsch synthesis activity and selectivity. Appl. Catal. A Gen. 2016, 511, 31–46. [Google Scholar] [CrossRef]
- Bepari, S.; Stevens-Boyd, R.; Mohammad, N.; Li, X.; Abrokwah, R.; Kuila, D. Composite mesoporous SiO2-Al2O3 supported Fe, FeCo and FeRu catalysts for Fischer-Tropsch studies in a 3-D printed stainless-steel microreactor. Mater. Today Proc. 2020, 35, 221–228. [Google Scholar] [CrossRef]



| Catalyst | Type of Reactor | Optimum Temperature | % Co Conversion | Hydrocarbon Selectivity % | Reference | |||
|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | |||||
| 12% Co TiO2 | Silicon | 250 °C | ~95% | 95 | 4 | - | - | [14] |
| 12% Co SiO2 | Silicon | 250 °C | ~80 | 45 | 50 | <5 | <5 | [18] |
| 15% Co MCM-41 | 3D printed | 240 °C | ~60% | ~75 | ~15 | ~6 | ~4 | [3] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad, N.; Aravamudhan, S.; Kuila, D. Atomic Layer Deposition of Cobalt Catalyst for Fischer–Tropsch Synthesis in Silicon Microchannel Microreactor. Nanomaterials 2022, 12, 2425. https://doi.org/10.3390/nano12142425
Mohammad N, Aravamudhan S, Kuila D. Atomic Layer Deposition of Cobalt Catalyst for Fischer–Tropsch Synthesis in Silicon Microchannel Microreactor. Nanomaterials. 2022; 12(14):2425. https://doi.org/10.3390/nano12142425
Chicago/Turabian StyleMohammad, Nafeezuddin, Shyam Aravamudhan, and Debasish Kuila. 2022. "Atomic Layer Deposition of Cobalt Catalyst for Fischer–Tropsch Synthesis in Silicon Microchannel Microreactor" Nanomaterials 12, no. 14: 2425. https://doi.org/10.3390/nano12142425






