Pd-Modified LaFeO3 as a High-Efficiency Gas-Sensing Material for H2S Gas Detection
Abstract
:1. Introduction
2. Materials and Methods
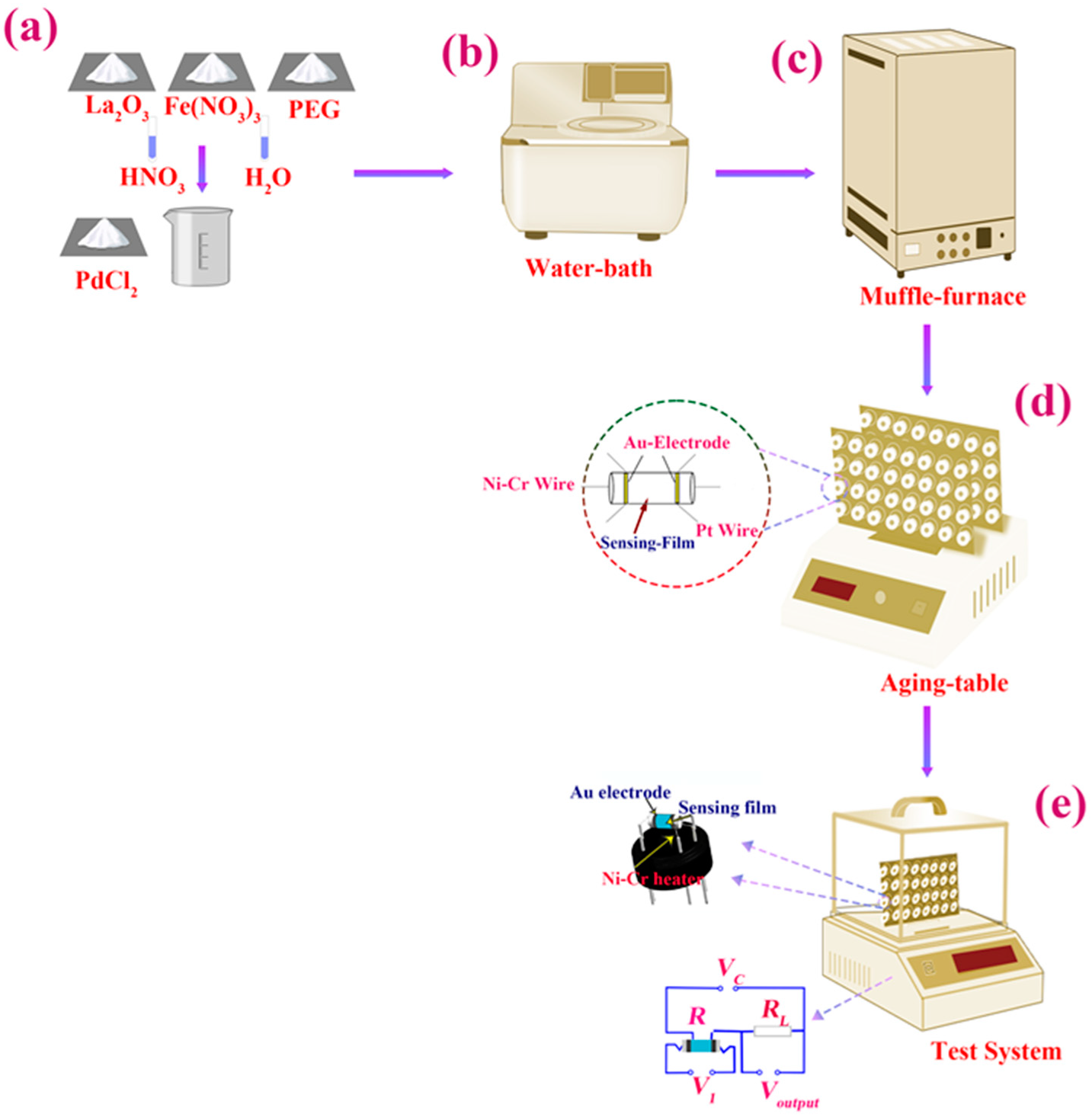
2.1. Preparation and Characterization of Nanocrystalline Pd–LaFeO3
2.2. Fabrication and Measurement of Sensor
2.3. Ready-Made Sensor
3. Results
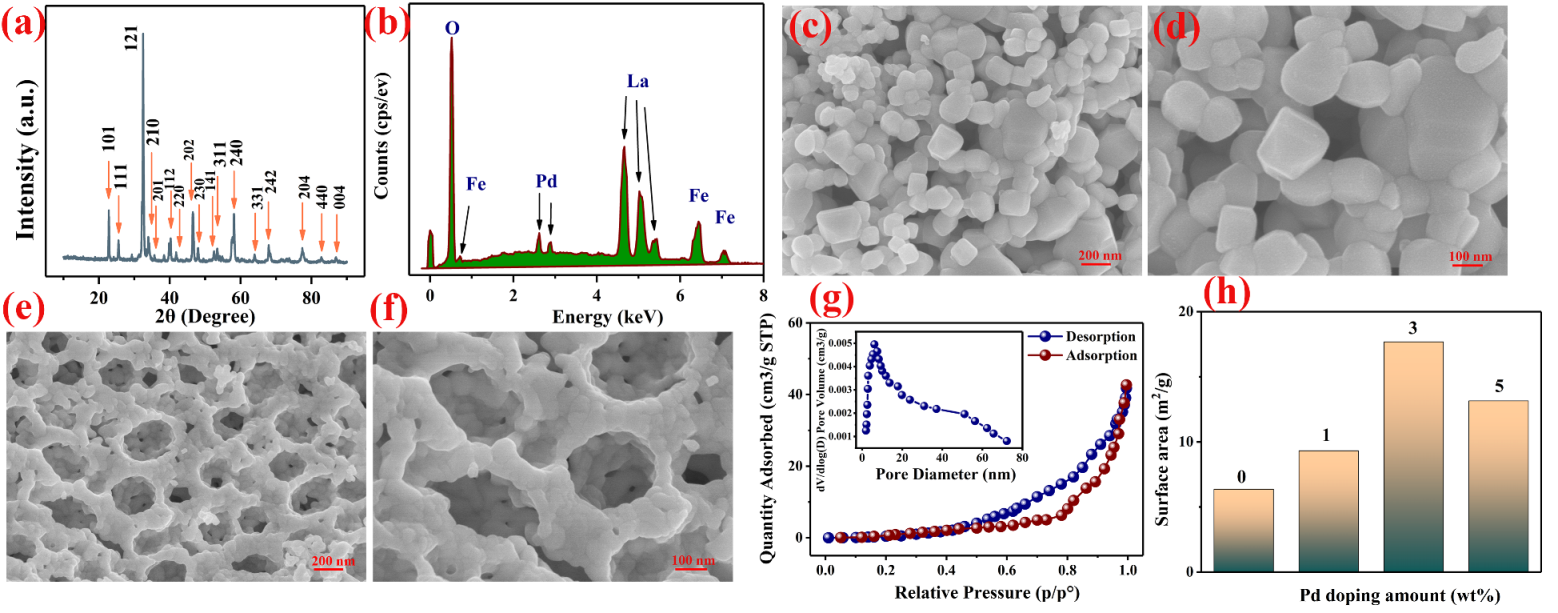
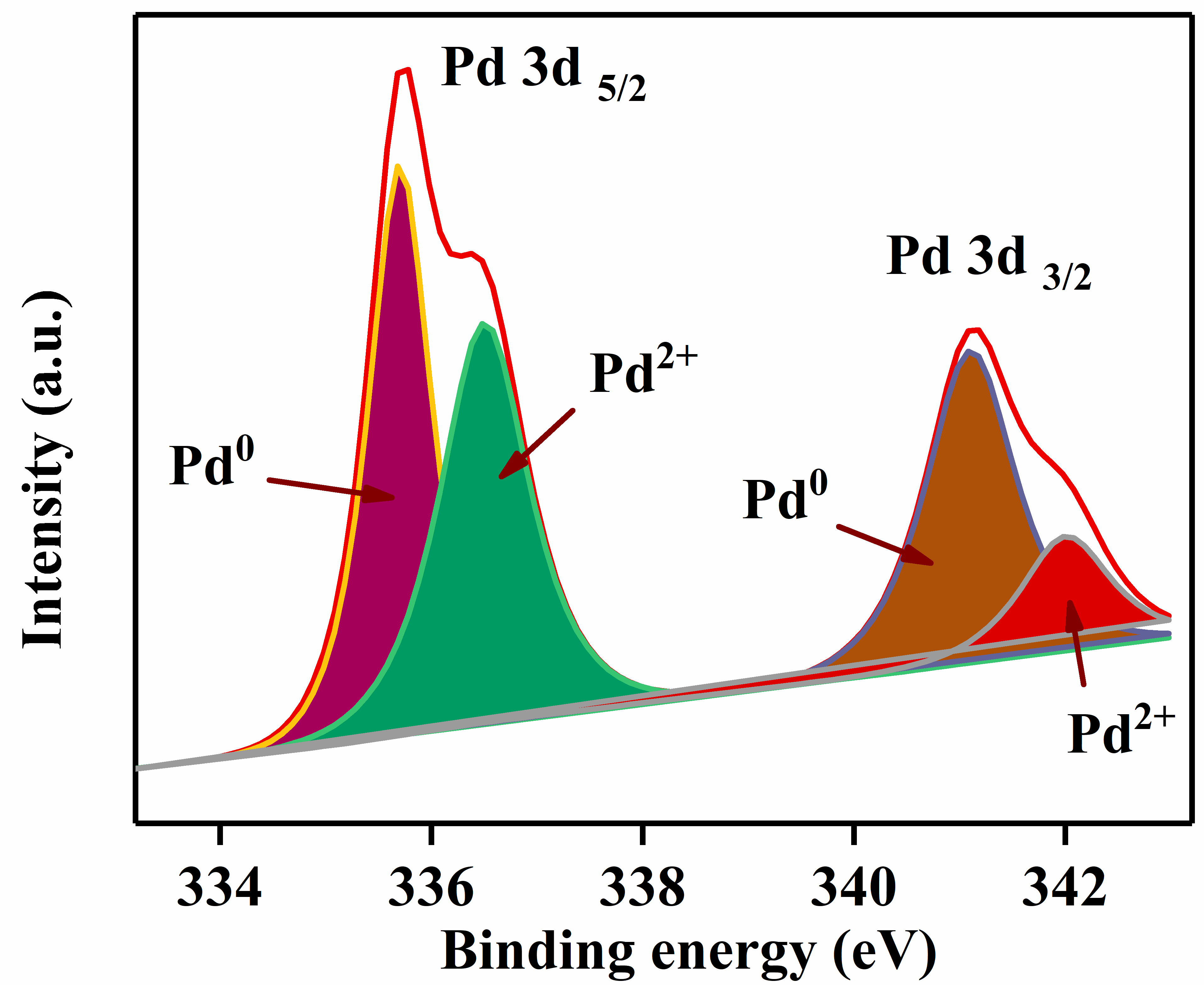
3.1. Material Characterization
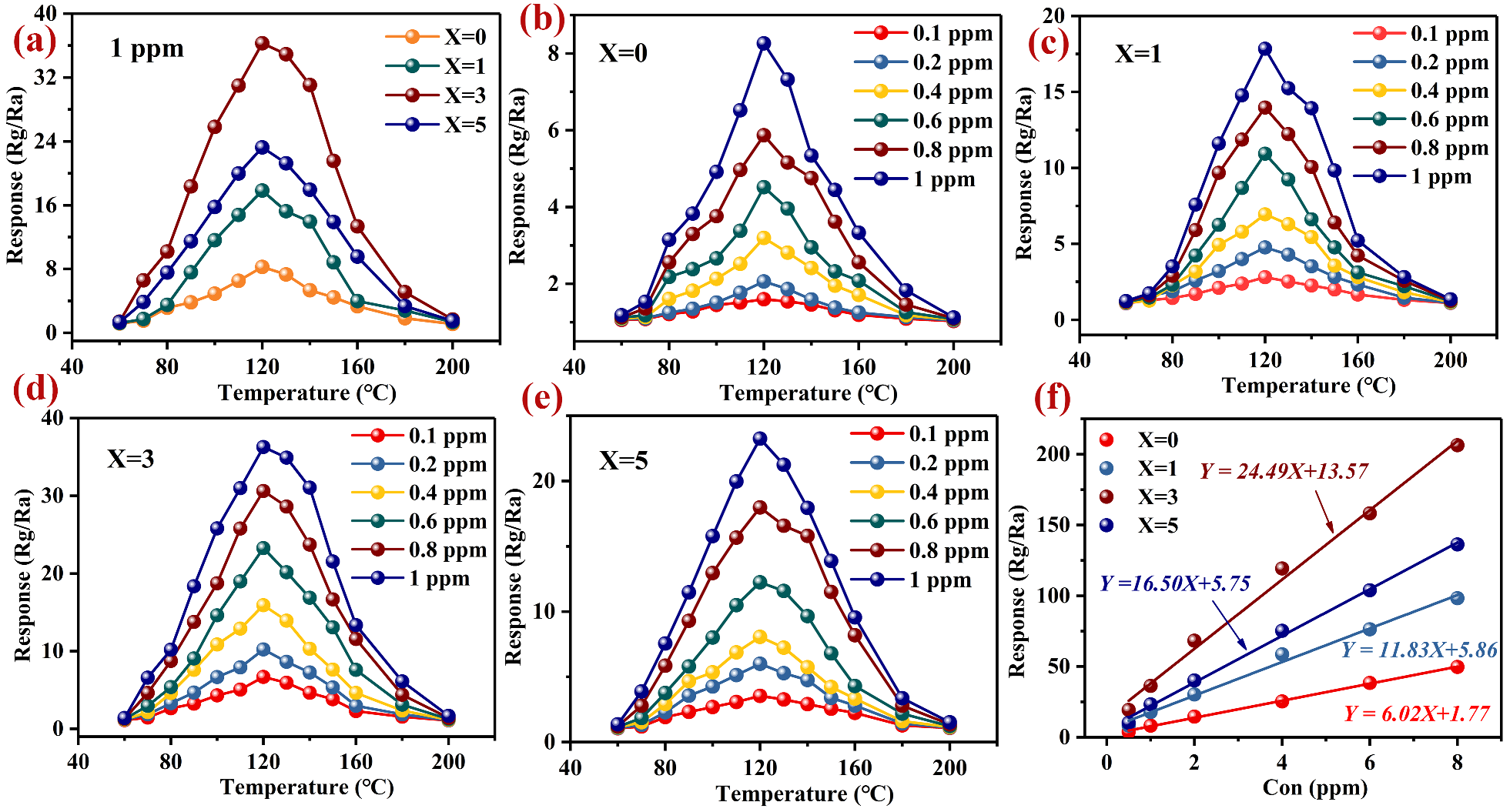
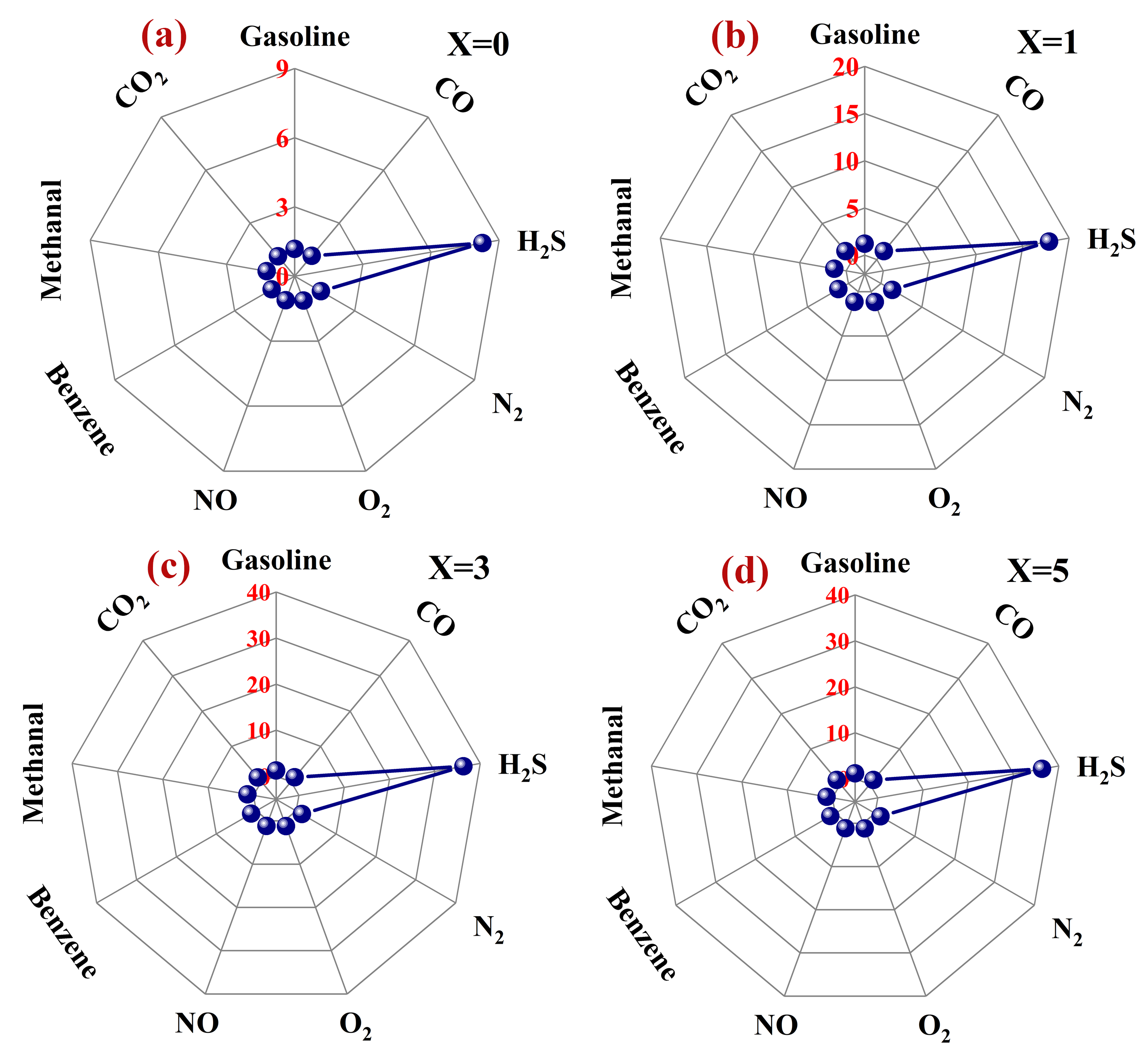
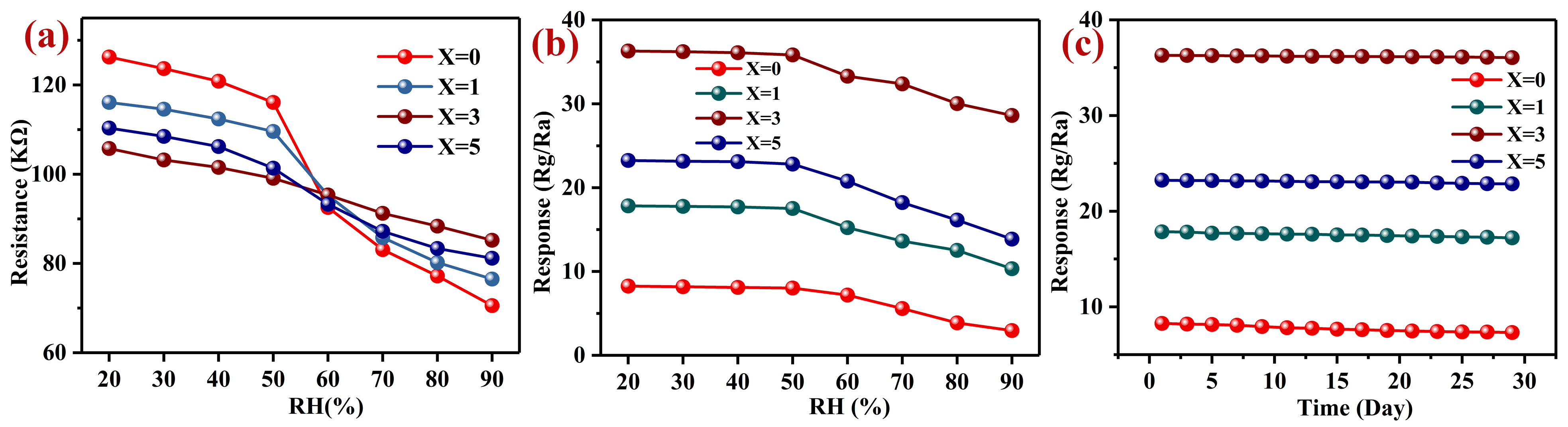
3.2. Gas-Sensing Performance
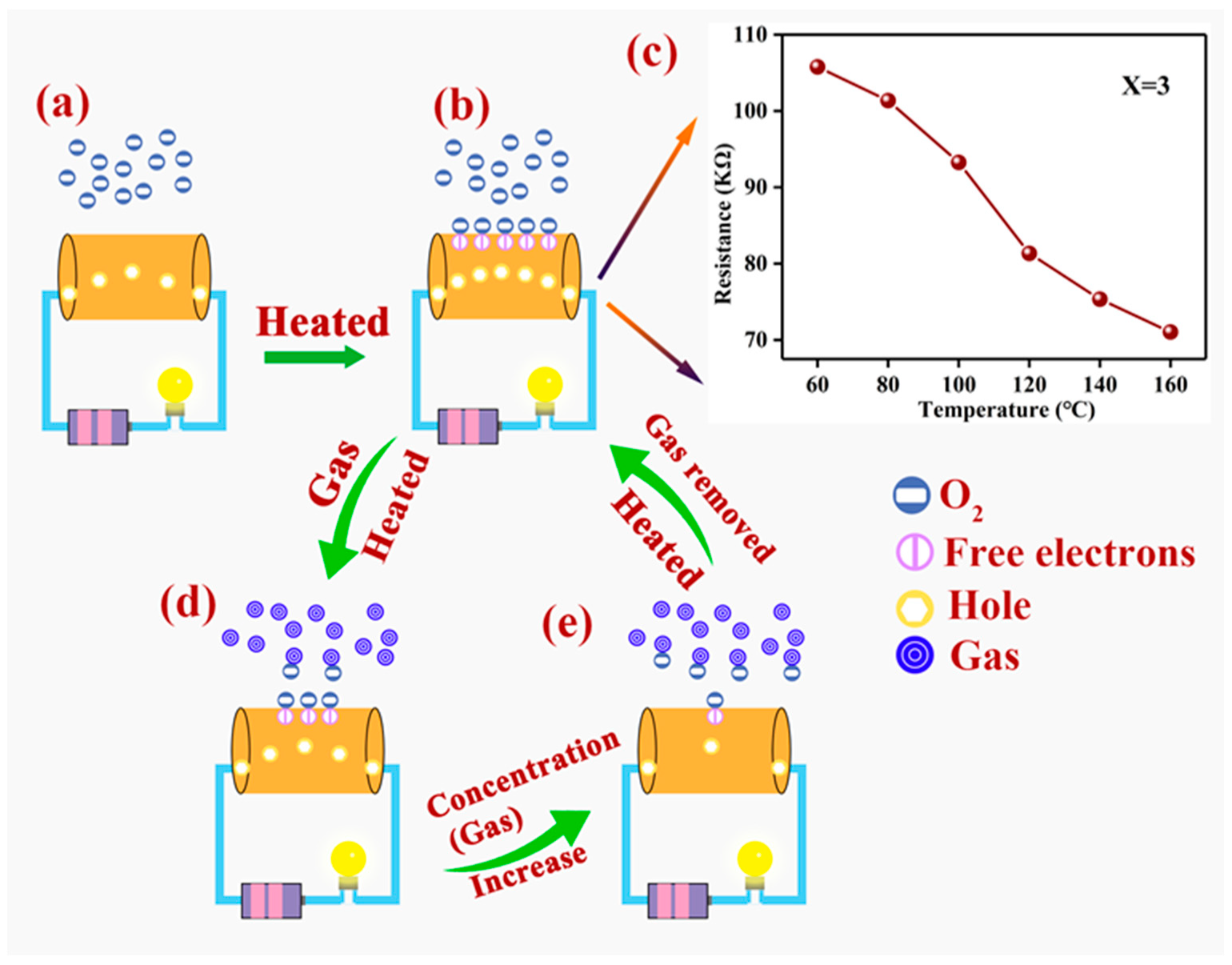
4. Sensing-Mechanism Analysis
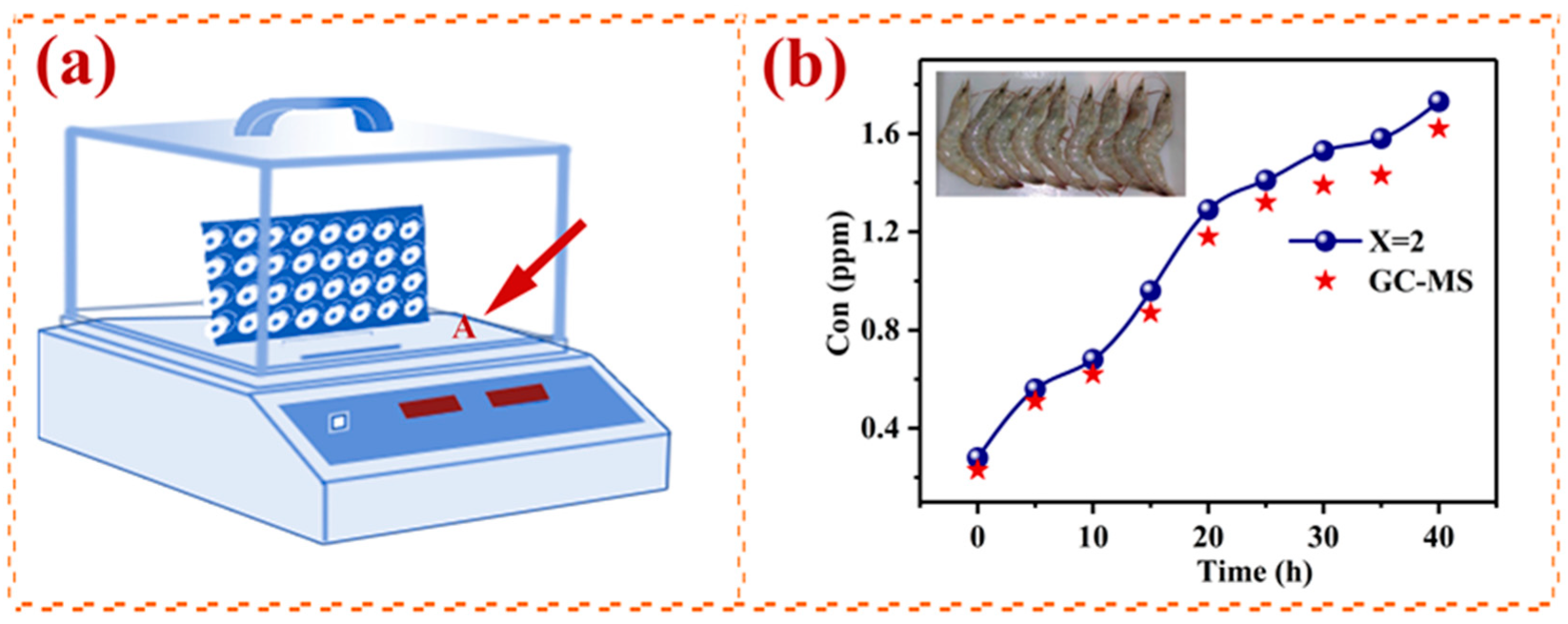
5. Application in the Detection of H2S
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuo, P.; Wang, R.; Li, F.; Wu, F.; Xu, G.; Niu, W. A Trace Ppb-Level Electrochemical H2S Sensor Based on Ultrathin Pt Nanotubes. Talanta 2021, 233, 122539. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Majhi, S.M.; Kim, K.-H.; Kim, H.W.; Kwon, E.E. Advances in In2O3-Based Materials for the Development of Hydrogen Sulfide Sensors. Chem. Eng. J. 2021, 404, 126472. [Google Scholar] [CrossRef]
- Li, X.; Hu, J.; Ban, J.; He, S.; Zheng, N.; Shao, G.; Cao, G. Mechanism of Enhanced H2S Sensor Ability Based on Emerging Li0.5La0.5TiO3-SnO2 Core-Shell Structure. Sens. Actuators B Chem. 2022, 352, 131054. [Google Scholar] [CrossRef]
- Priya, A.K.; Suresh, R.; Kumar, P.S.; Rajendran, S.; Vo, D.-V.N.; Soto-Moscoso, M. A Review on Recent Advancements in Photocatalytic Remediation for Harmful Inorganic and Organic Gases. Chemosphere 2021, 284, 131344. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, L.; Luo, Y.; Gao, L.; Duan, G. The Dehydrogenation of H-S Bond into Sulfur Species on Supported Pd Single Atoms Allows Highly Selective and Sensitive Hydrogen Sulfide Detection. Small 2021, 17, 2105643. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, G.; Yao, Z.; Zheng, Y.; Shen, L.; Liu, F.; Cao, Y.; Liang, S.; Xiao, Y.; Jiang, L. Engineering of Crystal Phase over Porous MnO2 with 3D Morphology for Highly Efficient Elimination of H2S. J. Hazard. Mater. 2021, 411, 125180. [Google Scholar] [CrossRef]
- Ethiraj, J.; Bonino, F.; Lamberti, C.; Bordiga, S. H2S Interaction with HKUST-1 and ZIF-8 MOFs: A Multitechnique Study. Microporous Mesoporous Mater. 2015, 207, 90–94. [Google Scholar] [CrossRef]
- Wu, X.; Xiong, S.; Gong, Y.; Gong, Y.; Wu, W.; Mao, Z.; Liu, Q.; Hu, S.; Long, X. MOF-SMO Hybrids as a H2S Sensor with Superior Sensitivity and Selectivity. Sens. Actuators B Chem. 2019, 292, 32–39. [Google Scholar] [CrossRef]
- Chen, L.; Luque, R.; Li, Y. Controllable Design of Tunable Nanostructures inside Metal–Organic Frameworks. Chem. Soc. Rev. 2017, 46, 4614–4630. [Google Scholar] [CrossRef]
- Lopez, J.D.; Keley, M.; Dante, A.; Werneck, M.M. Optical Fiber Sensor Coated with Copper and Iron Oxide Nanoparticles for Hydrogen Sulfide Sensing. Opt. Fiber Technol. 2021, 67, 102731. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Fang, T.-H.; Hsiao, Y.-J.; Li, Z.-J. Rapid Detection of Low Concentrations of H2S Using CuO-Doped ZnO Nanofibers. J. Alloys Compd. 2021, 852, 157014. [Google Scholar] [CrossRef]
- Yaegaki, K.; Sanada, K. Volatile Sulfur Compounds in Mouth Air from Clinically Healthy Subjects and Patients with Periodontal Disease. J. Periodontal Res. 1992, 27, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Jaouali, I.; Hamrouni, H.; Moussa, N.; Nsib, M.F.; Centeno, M.A.; Bonavita, A.; Neri, G.; Leonardi, S.G. LaFeO3 Ceramics as Selective Oxygen Sensors at Mild Temperature. Ceram. Int. 2018, 44, 4183–4189. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, K.; Xiao, C.; Jia, L. C-Doped LaFeO3 Porous Nanostructures for Highly Selective Detection of Formaldehyde. Sens. Actuators B Chem. 2021, 347, 130550. [Google Scholar] [CrossRef]
- Xiangfeng, C.; Siciliano, P. CH3SH-Sensing Characteristics of LaFeO3 Thick-Film Prepared by Co-Precipitation Method. Sens. Actuators B Chem. 2003, 94, 197–200. [Google Scholar] [CrossRef]
- Song, P.; Zhang, H.; Han, D.; Li, J.; Yang, Z.; Wang, Q. Preparation of Biomorphic Porous LaFeO3 by Sorghum Straw Biotemplate Method and Its Acetone Sensing Properties. Sens. Actuators B Chem. 2014, 196, 140–146. [Google Scholar] [CrossRef]
- Hosoya, Y.; Itagaki, Y.; Aono, H.; Sadaoka, Y. Ozone Detection in Air Using SmFeO3 Gas Sensor. Sens. Actuators B Chem. 2005, 108, 198–201. [Google Scholar] [CrossRef]
- Huang, H.T.; Zhang, W.L.; Zhang, X.D.; Guo, X. NO2 Sensing Properties of SmFeO3 Porous Hollow Microspheres. Sens. Actuators B Chem. 2018, 265, 443–451. [Google Scholar] [CrossRef]
- Tomoda, M.; Okano, S.; Itagaki, Y.; Aono, H.; Sadaoka, Y. Air Quality Prediction by Using Semiconducting Gas Sensor with Newly Fabricated SmFeO3 Film. Sens. Actuators B Chem. 2004, 97, 190–197. [Google Scholar] [CrossRef]
- Han, T.; Ma, S.Y.; Xu, X.L.; Xu, X.H.; Pei, S.T.; Tie, Y.; Cao, P.F.; Liu, W.W.; Wang, B.J.; Zhang, R.; et al. Rough SmFeO3 Nanofibers as an Optimization Ethylene Glycol Gas Sensor Prepared by Electrospinning. Mater. Lett. 2020, 268, 127575. [Google Scholar] [CrossRef]
- Gaiardo, A.; Zonta, G.; Gherardi, S.; Malagù, C.; Fabbri, B.; Valt, M.; Vanzetti, L.; Landini, N.; Casotti, D.; Cruciani, G.; et al. Nanostructured SmFeO3 Gas Sensors: Investigation of the Gas Sensing Performance Reproducibility for Colorectal Cancer Screening. Sensors 2020, 20, 5910. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ma, S.Y.; Shen, X.F.; Wang, T.T.; Jiang, X.H.; Chen, Q.; Qiang, Z.; Yang, H.M.; Chen, H. PrFeO3 Hollow Nanofibers as a Highly Efficient Gas Sensor for Acetone Detection. Sens. Actuators B Chem. 2018, 255, 2546–2554. [Google Scholar] [CrossRef]
- Pei, S.; Ma, S.; Xu, X.; Xu, X.; Almamoun, O. Modulated PrFeO3 by Doping Sm3+ for Enhanced Acetone Sensing Properties. J. Alloys Compd. 2021, 856, 158274. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Y.; Ma, M.; Ren, J.; Liu, C.; Tan, J. Visible Light-Assisted Formaldehyde Sensor Based on HoFeO3 Nanoparticles with Sub-Ppm Detection Limit. Ceram. Int. 2020, 46, 16337–16344. [Google Scholar] [CrossRef]
- Sheng, H.; Ma, S.; Han, T.; Yun, P.; Yang, T.; Ren, J. A Highly Sensitivity and Anti-Humidity Gas Sensor for Ethanol Detection with NdFeO3 Nano-Coral Granules. Vacuum 2022, 195, 110642. [Google Scholar] [CrossRef]
- Addabbo, T.; Bertocci, F.; Fort, A.; Gregorkiewitz, M.; Mugnaini, M.; Spinicci, R.; Vignoli, V. Gas Sensing Properties and Modeling of YCoO3 Based Perovskite Materials. Sens. Actuators B Chem. 2015, 221, 1137–1155. [Google Scholar] [CrossRef]
- Cerdà, J.; Arbiol, J.; Dezanneau, G.; Díaz, R.; Morante, J.R. Perovskite-Type BaSnO3 Powders for High Temperature Gas Sensor Applications. Sens. Actuators B Chem. 2002, 84, 21–25. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, Y.; Zhou, P.; Lu, R.; Li, A.; Zhao, S.; Liu, W.; Wei, D.; Wei, K. Fabrication, Characterization and n-Propanol Sensing Properties of Perovskite-Type ZnSnO3 Nanospheres Based Gas Sensor. Appl. Surf. Sci. 2020, 509, 145335. [Google Scholar] [CrossRef]
- Balamurugan, C.; Lee, D.-W. Perovskite Hexagonal YMnO3 Nanopowder as P-Type Semiconductor Gas Sensor for H2S Detection. Sens. Actuators B Chem. 2015, 221, 857–866. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, L.; Kan, Z.; Yang, L.; Chang, Z.; Dong, B.; Bai, X.; Lu, G.; Song, H. A Multi-Platform Sensor for Selective and Sensitive H2S Monitoring: Three-Dimensional Macroporous ZnO Encapsulated by MOFs with Small Pt Nanoparticles. J. Hazard. Mater. 2022, 426, 128075. [Google Scholar] [CrossRef]
- Bae, G.; Kim, M.; Lee, A.; Ji, S.; Jang, M.; Yim, S.; Song, W.; Lee, S.S.; Yoon, D.H.; An, K.S. Nanometric Lamination of Zinc Oxide Nanofilms with Gold Nanoparticles for Self-Perceived Periodontal Disease Sensors. Compos. Part B Eng. 2022, 230, 109490. [Google Scholar] [CrossRef]
- Fan, J.; Liu, P.; Chen, X.; Zhou, H.; Fu, S.; Wu, W. Carbon Nanotubes-CuO/SnO2 Based Gas Sensor for Detecting H2S in Low Concentration. Nanotechnology 2019, 30, 475501. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhao, J.; Liu, J.; Wang, F.; Wu, L.; Meng, F.; Zhang, D.; Wang, R.; Ahmed, J.; Ojha, K. H2S Sensing Material Pt-WO3 Nanorods with Excellent Comprehensive Performance. J. Alloys Compd. 2022, 900, 163398. [Google Scholar] [CrossRef]
- Akamatsu, T.; Itoh, T.; Tsuruta, A.; Masuda, Y. CH3SH and H2S Sensing Properties of V2O5/WO3/TiO2 Gas Sensor. Chemosensors 2021, 9, 113. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.; Wang, X.; Qin, Z.; Hu, X.; Wang, X.; Xie, C.; Zeng, D. Exposed Edges of Porous Ultrathin WO3 Nanosheets Determined High-Performance Sensing for Hydrogen Sulfide. Appl. Surf. Sci. 2022, 571, 151327. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.; Zhou, J.; Liu, Z.; Geng, Y.; Liang, Z.; Du, Y.; Tian, X. Different Nanostructured Tungsten Oxides Synthesized by Facile Solvothermal Route for Chlorine Gas Sensing. Sens. Actuators B Chem. 2018, 275, 306–311. [Google Scholar] [CrossRef]
- Guo, L.; Xie, N.; Wang, C.; Kou, X.; Ding, M.; Zhang, H.; Sun, Y.; Song, H.; Wang, Y.; Lu, G. Enhanced Hydrogen Sulfide Sensing Properties of Pt-Functionalized α-Fe2O3 Nanowires Prepared by One-Step Electrospinning. Sens. Actuators B Chem. 2018, 255, 1015–1023. [Google Scholar] [CrossRef]
- Lim, K.; Jo, Y.M.; Kim, S.; Yoon, J.W.; Jeong, S.Y.; Kim, J.S.; Choi, H.J.; Cho, Y.; Park, J.; Jeong, Y.W.; et al. Selective Dual Detection of Hydrogen Sulfide and Methyl Mercaptan Using CuO/CuFe2O4 Nanopattern Chemiresistors. Sens. Actuators B Chem. 2021, 348, 130665. [Google Scholar] [CrossRef]
- Senapati, M.; Sahu, P.P. Meat Quality Assessment Using Au Patch Electrode Ag-SnO2/SiO2/Si MIS Capacitive Gas Sensor at Room Temperature. Food Chem. 2020, 324, 126893. [Google Scholar] [CrossRef]
- Gao, H.; Wei, D.; Lin, P.; Liu, C.; Sun, P.; Shimanoe, K.; Yamazoe, N.; Lu, G. The Design of Excellent Xylene Gas Sensor Using Sn-Doped NiO Hierarchical Nanostructure. Sens. Actuators B Chem. 2017, 253, 1152–1162. [Google Scholar] [CrossRef]
- Guo, L.; Shen, Z.; Ma, C.; Ma, C.; Wang, J.; Yuan, T. Gas Sensor Based on MOFs-Derived Au-Loaded SnO2 Nanosheets for Enhanced Acetone Detection. J. Alloys Compd. 2022, 906, 164375. [Google Scholar] [CrossRef]
- Fan, X.; Xu, Y.; Ma, C.; He, W. In-Situ Growth of Co3O4 Nanoparticles Based on Electrospray for an Acetone Gas Sensor. J. Alloys Compd. 2021, 854, 157234. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.; Liu, W.; Dong, H.; Wang, D.; Liu, J.; Liu, Q.; Chen, X. MOF-Based Nanoscale Pt Catalyst Decorated SnO2 Porous Nanofibers for Acetone Gas Detection. J. Alloys Compd. 2022, 893, 162322. [Google Scholar] [CrossRef]
- Zhu, H.; Haidry, A.A.; Wang, Z.; Ji, Y. Improved Acetone Sensing Characteristics of TiO2 Nanobelts with Ag Modification. J. Alloys Compd. 2021, 887, 161312. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, L.; Yu, Y.; Qiu, M.; Gao, H.; Chen, D. WO3 Nanoplates for Sensitive and Selective Detections of Both Acetone and NH3 Gases at Different Operating Temperatures. J. Alloys Compd. 2021, 858, 157638. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, S.; Qu, F.; Gong, S.; Wang, C.; Qiu, L.; Yang, M.; Cheng, W. High Performance Acetone Sensor Based on ZnO Nanorods Modified by Au Nanoparticles. J. Alloys Compd. 2019, 797, 246–252. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, Z.; Xiong, X.; Hu, X.; Wang, X.; Li, N.; Jin, T.; Chen, Y. ZnO/ZnSe Heterojunction Nanocomposites with Oxygen Vacancies for Acetone Sensing. J. Alloys Compd. 2022, 906, 164316. [Google Scholar] [CrossRef]
- Yuan, Z.; Feng, Z.; Kong, L.; Zhan, J.; Ma, X. Simple Synthesis of Porous ZnO Nanoplates Hyper-Doped with Low Concentration of Pt for Efficient Acetone Sensing. J. Alloys Compd. 2021, 865, 158890. [Google Scholar] [CrossRef]
- Zhang, H.J.; Liu, L.Z.; Zhang, X.R.; Zhang, S.; Meng, F.N. Microwave-Assisted Solvothermal Synthesis of Shape-Controlled CoFe2O4 Nanoparticles for Acetone Sensor. J. Alloys Compd. 2019, 788, 1103–1112. [Google Scholar] [CrossRef]
- Hanh, N.H.; Van Duy, L.; Hung, C.M.; Xuan, C.T.; Van Duy, N.; Hoa, N.D. High-Performance Acetone Gas Sensor Based on Pt–Zn2SnO4 Hollow Octahedra for Diabetic Diagnosis. J. Alloys Compd. 2021, 886, 161284. [Google Scholar] [CrossRef]
- Wang, X.; Qin, H.; Pei, J.; Chen, Y.; Li, L.; Xie, J.; Hu, J. Sensing Performances to Low Concentration Acetone for Palladium Doped LaFeO3 Sensors. J. Rare Earths 2016, 34, 704–710. [Google Scholar] [CrossRef]
- Ghasdi, M.; Alamdari, H. Highly Sensitive Pure and Pd-Doped LaFeO3 Nanocrystalline Perovskitebased Sensor Prepared by High Energy Ball Milling. Adv. Mater. Res. 2012, 409, 486–491. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, H.; Peng, J.; Duan, Z.; Ma, M.; Xin, X.; Li, W.; Zheng, X. Enhanced Humidity Sensing Properties of SmFeO3-Modified MoS2 Nanocomposites Based on the Synergistic Effect. Sens. Actuators B Chem. 2018, 272, 459–467. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, H.; Lv, W.; Zhang, H.; Hu, J. Gas Sensors Based on Ytterbium Ferrites Nanocrystalline Powders for Detecting Acetone with Low Concentrations. Sens. Actuators B Chem. 2017, 246, 9–19. [Google Scholar] [CrossRef]
- Wang, J.; Wu, F.Q.; Shi, K.H.; Wang, X.H.; Sun, P.P. Humidity Sensitivity of Composite Material of Lanthanum Ferrite/Polymer Quaternary Acrylic Resin. Sens. Actuators B Chem. 2004, 99, 586–591. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Li, X.; Lu, G.; You, L.; Liang, X.; Liu, F.; Zhang, T.; Du, Y. Highly Sensitive Humidity Sensor Based on High Surface Area Mesoporous LaFeO3 Prepared by a Nanocasting Route. Sens. Actuators B Chem. 2013, 181, 802–809. [Google Scholar] [CrossRef]
- Fan, S.X.; Tang, W. Synthesis, Characterization and Mechanism of Electrospun Carbon Nanofibers Decorated with ZnO Nanoparticles for Flexible Ammonia Gas Sensors at Room Temperature. Sens. Actuators B Chem. 2022, 362, 131789. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, H.; Song, P.; Hu, J.; Jiang, M. Electric Properties and Acetone-Sensing Characteristics of La1−xPbxFeO3 Perovskite System. Mater. Chem. Phys. 2006, 98, 358–362. [Google Scholar] [CrossRef]
- Liu, X.; Hu, J.; Cheng, B.; Qin, H.; Jiang, M. Acetone Gas Sensing Properties of SmFe1−xMgxO3 Perovskite Oxides. Sens. Actuators B Chem. 2008, 134, 483–487. [Google Scholar] [CrossRef]
- Wang, X.; Ma, W.; Sun, K.; Hu, J.; Qin, H. Nanocrystalline Gd1−xCaxFeO3 Sensors for Detection of Methanol Gas. J. Rare Earths 2017, 35, 690–696. [Google Scholar] [CrossRef]


| Pd | La | Fe | O | |
|---|---|---|---|---|
| 0 | 0 | 19.73 | 23.68 | 56.59 |
| 1 | 0.45 | 19.85 | 19.99 | 59.71 |
| 3 | 1.40 | 19.78 | 19.70 | 59.12 |
| 5 | 2.35 | 19.46 | 19.56 | 58.63 |
| Con (ppm) | 0.1 | 0.2 | 0.4 | 0.6 | 0.8 | 1 | |
|---|---|---|---|---|---|---|---|
| Pd (wt%) | |||||||
| 0 | 1.59 | 2.06 | 3.19 | 4.52 | 5.87 | 8.26 | |
| 1 | 2.8 | 4.76 | 6.94 | 10.93 | 13.97 | 17.85 | |
| 3 | 6.69 | 10.22 | 15.93 | 23.27 | 30.62 | 36.29 | |
| 5 | 3.53 | 5.98 | 8.06 | 12.25 | 17.98 | 23.25 | |
| T (°C) | 60 | 80 | 100 | 120 | 140 | 160 | Pd (wt%) | |
|---|---|---|---|---|---|---|---|---|
| Time (s) | ||||||||
| Response | 22.5 | 28.15 | 31.88 | 34.61 | 32.86 | 29.68 | 0 | |
| Recovery | 18.26 | 22.31 | 25.88 | 26.63 | 25.5 | 23.37 | 0 | |
| Response | 20.58 | 23.7 | 26.93 | 28.52 | 27.61 | 25.58 | 1 | |
| Recovery | 18.01 | 20.56 | 21.83 | 22.97 | 21.86 | 19.51 | 1 | |
| Response | 13.87 | 17.13 | 18.95 | 19.62 | 17.89 | 16.39 | 3 | |
| Recovery | 10.68 | 12.75 | 14.26 | 15.22 | 14.5 | 13.16 | 3 | |
| Response | 21.55 | 24.13 | 25.83 | 26.66 | 25.37 | 23.36 | 5 | |
| Recovery | 15.65 | 16.92 | 18.16 | 18.83 | 17.76 | 15.33 | 5 | |
| Time (h) | 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Method | ||||||||||
| Gas sensor | 0.28 | 0.56 | 0.68 | 0.96 | 1.29 | 1.41 | 1.53 | 1.58 | 1.73 | |
| GC–MS | 0.23 | 0.51 | 0.62 | 0.87 | 1.18 | 1.32 | 1.39 | 1.43 | 1.62 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xiao, J.; Chen, J.; Wang, Y.; Zhang, L.; Yue, S.; Li, S.; Huang, T.; Sun, D. Pd-Modified LaFeO3 as a High-Efficiency Gas-Sensing Material for H2S Gas Detection. Nanomaterials 2022, 12, 2460. https://doi.org/10.3390/nano12142460
Zhang H, Xiao J, Chen J, Wang Y, Zhang L, Yue S, Li S, Huang T, Sun D. Pd-Modified LaFeO3 as a High-Efficiency Gas-Sensing Material for H2S Gas Detection. Nanomaterials. 2022; 12(14):2460. https://doi.org/10.3390/nano12142460
Chicago/Turabian StyleZhang, Heng, Jing Xiao, Jun Chen, Yan Wang, Lian Zhang, Shuai Yue, Suyan Li, Tao Huang, and Da Sun. 2022. "Pd-Modified LaFeO3 as a High-Efficiency Gas-Sensing Material for H2S Gas Detection" Nanomaterials 12, no. 14: 2460. https://doi.org/10.3390/nano12142460
APA StyleZhang, H., Xiao, J., Chen, J., Wang, Y., Zhang, L., Yue, S., Li, S., Huang, T., & Sun, D. (2022). Pd-Modified LaFeO3 as a High-Efficiency Gas-Sensing Material for H2S Gas Detection. Nanomaterials, 12(14), 2460. https://doi.org/10.3390/nano12142460








