Noble Metal-Based Catalysts with Core-Shell Structure for Oxygen Reduction Reaction: Progress and Prospective
Abstract
:1. Introduction
2. ORR Testing Technology
2.1. Testing Technology of Thin-Film Rotating Disk Electrode (TF-RDE)
2.2. Testing Technology of Gas Diffusion Electrode (GDE)
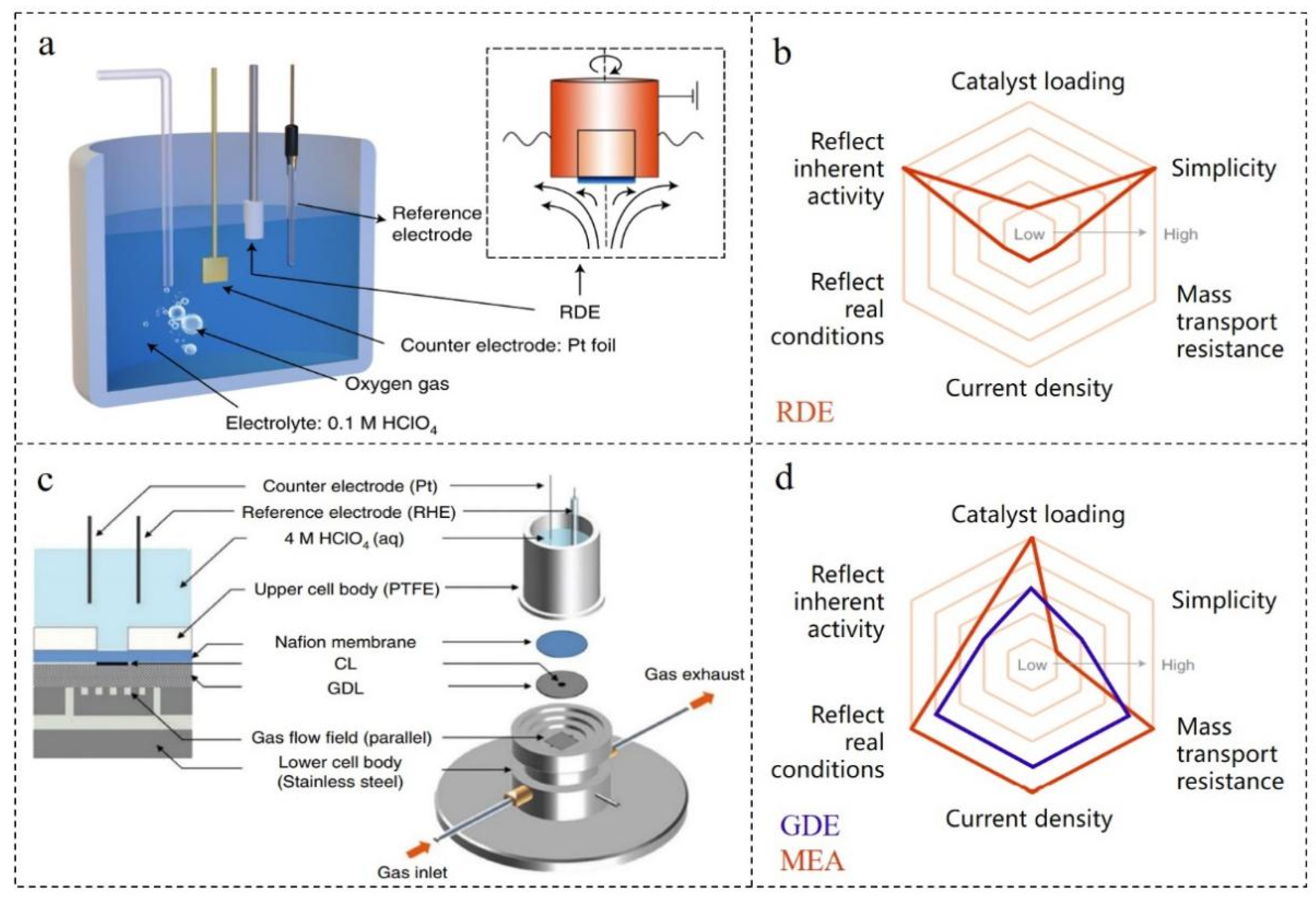
3. Factors Affecting the ORR Performances of the Core-Shell Structural Noble Metal-Based Nanomaterials
3.1. Synthesis Method
3.1.1. Electrodeposition Method
3.1.2. Chemical Reduction Method
3.1.3. Other Methods
3.2. Effect of Temperature on ORR Performances
3.3. Influence of Strain Effect
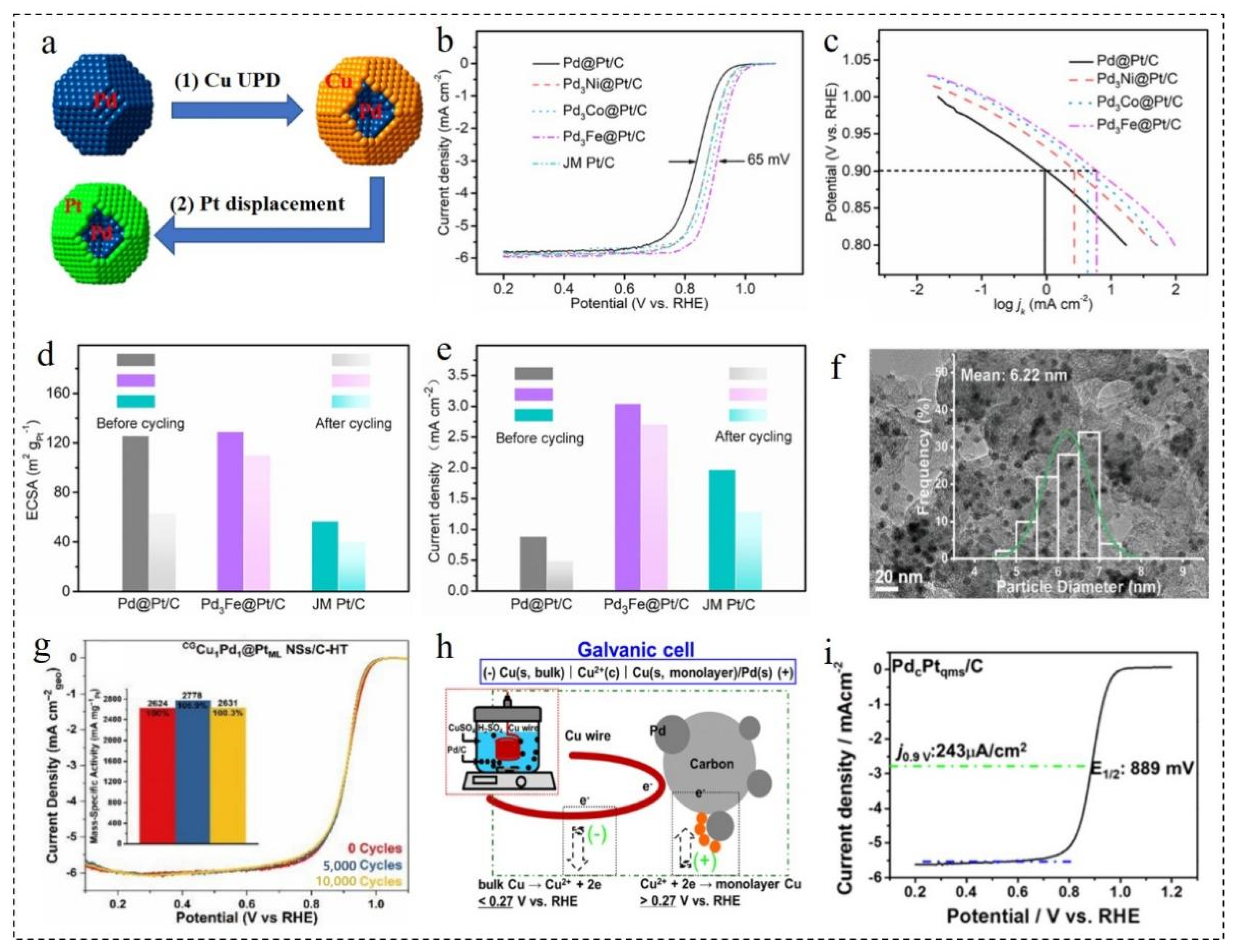
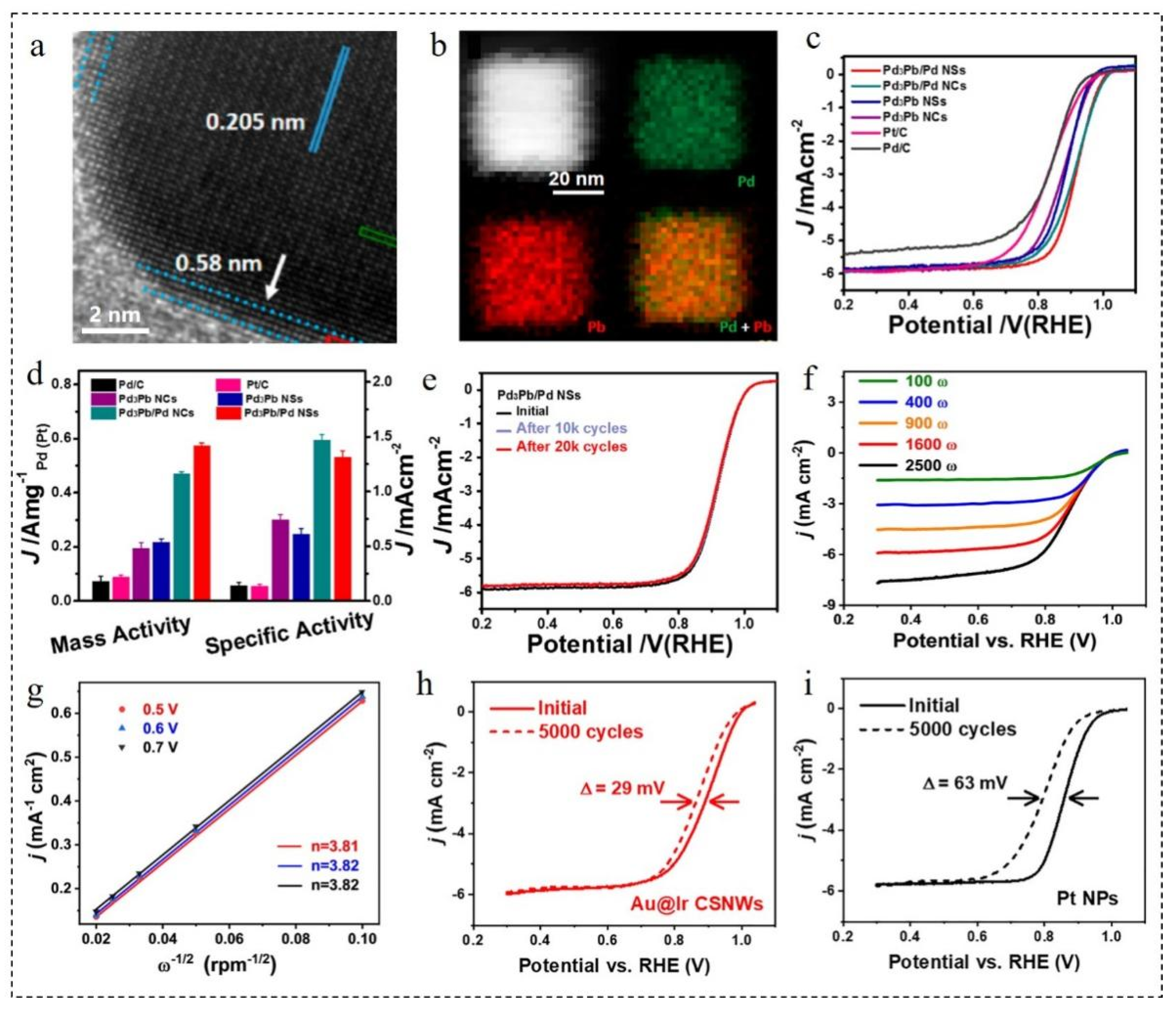
3.3.1. Effect of Core Doping
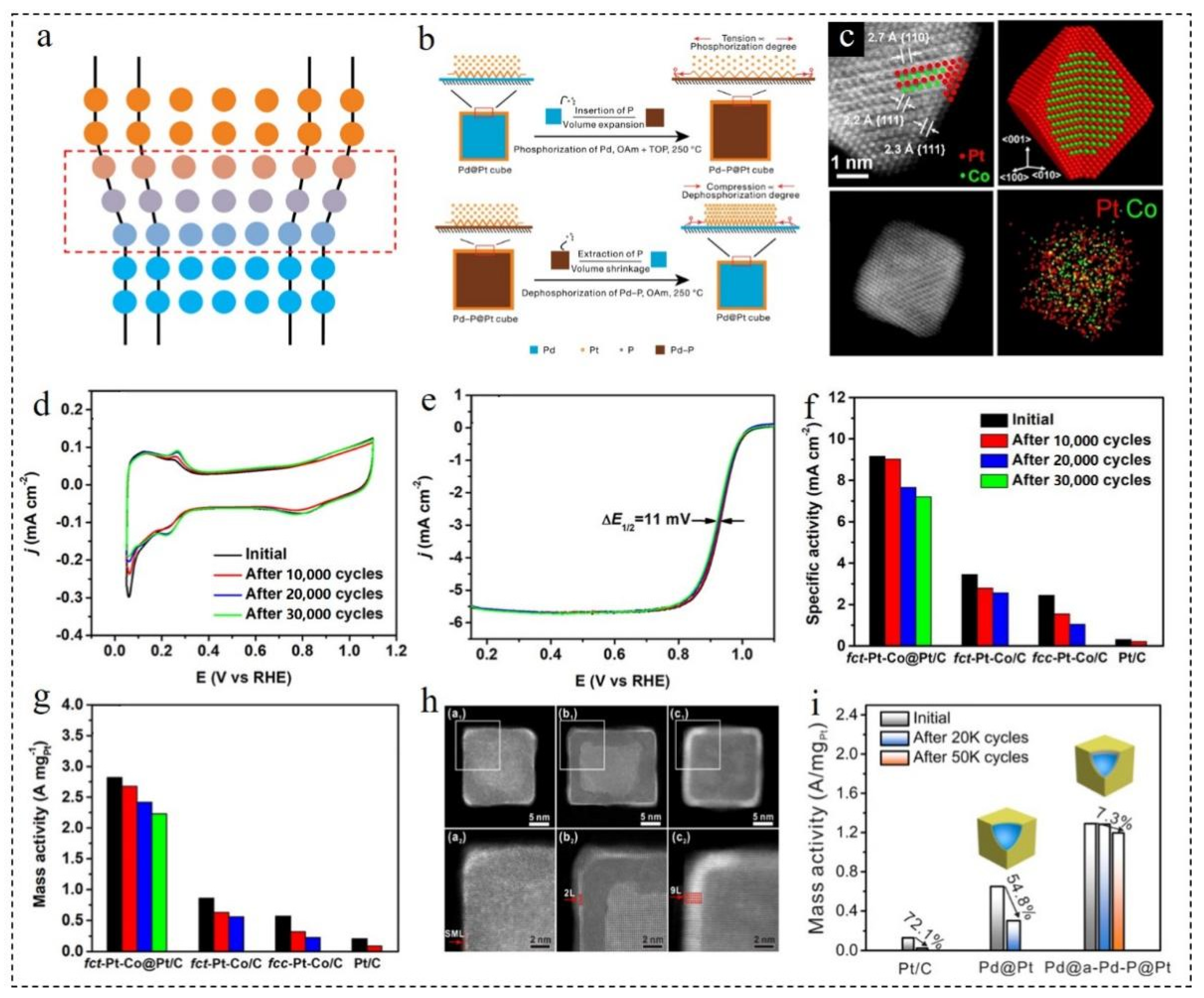
3.3.2. Effect of Shell Doping
3.4. Effect of Shell Layer Number on the Performance of ORR
4. Conclusion and Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, Q.B.; Jia, H.X.; Yang, B.; Qi, Z.P.; Zhang, Z.Y.; Lee, A.; Hu, N. The electro-chemo-mechanical coupling at the solid-liquid interface and its application into electrocatalysis. Adv. Mech. 2022, 52, 221–252. [Google Scholar]
- Chhetri, K.; Muthurasu, A.; Dahal, B.; Kim, T.; Mukhiya, T.; Chae, S.H.; Ko, T.H.; Choi, Y.C.; Kim, H.Y. Engineering the abundant heterointerfaces of integrated bimetallic sulfide-coupled 2D MOF-derived mesoporous CoS2 nanoarray hybrids for electrocatalytic water splitting. Mater. Today Nano 2022, 17, 100146. [Google Scholar] [CrossRef]
- Chhetri, K.; Dahal, B.; Mukhiya, T.; Tiwari, A.P.; Muthurasu, A.; Kim, T.; Kim, H.; Kim, H.Y. Integrated hybrid of graphitic carbon-encapsulated CuxO on multilayered mesoporous carbon from copper MOFs and polyaniline for asymmetric supercapacitor and oxygen reduction reactions. Carbon 2021, 179, 89–99. [Google Scholar] [CrossRef]
- Puangsombut, P.; Tantavichet, N. Effect of plating bath composition on chemical composition and oxygen reduction reaction activity of electrodeposited Pt-Co catalysts. Rare Met. 2019, 38, 95–106. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.G. Two-electron oxygen reduction reaction by high-loading molybdenum single-atom catalysts. Rare Met. 2020, 39, 455–457. [Google Scholar] [CrossRef]
- Han, Z.F.; Qi, Z.P.; Wei, Q.; Deng, Q.B.; Wang, K. The Mechanical Effect of MnO2 Layers on Electrochemical Actuation Performance of Nanoporous Gold. Nanomaterials 2020, 10, 2056. [Google Scholar] [CrossRef]
- Qiu, W.J.; An, C.H.; Yan, Y.W.; Xu, J.; Zhang, Z.J.; Guo, W.; Wang, Z.; Zheng, Z.J.; Wang, Z.B.; Deng, Q.B.; et al. Suppressed polysulfide shuttling and improved Li+ transport in Li-S batteries enabled by NbN modified PP separator. J. Power Sources 2019, 423, 98–105. [Google Scholar] [CrossRef]
- Wang, A.X.; Deng, Q.B.; Deng, L.J.; Guan, X.Z.; Luo, J.Y. Eliminating Tip Dendrite Growth by Lorentz Force for Stable Lithium Metal Anodes. Adv. Funct. Mater. 2019, 29, 1902630. [Google Scholar] [CrossRef]
- Wang, K.; Deng, Q.B. Constructing Core-Shell Co@N-Rich Carbon Additives toward Enhanced Hydrogen Storage Performance of Magnesium Hydride. Front. Chem. 2020, 8, 223. [Google Scholar] [CrossRef]
- An, C.H.; Kang, W.; Deng, Q.B.; Hu, N. Pt and Te codoped ultrathin MoS2 nanosheets for enhanced hydrogen evolution reaction with wide pH range. Rare Met. 2022, 41, 378–384. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Liu, X.; Wang, X.; Tian, H.; Waterhouse, G.I.N.; Kruger, P.E.; Telfer, S.G.; Ma, S. Large-scale synthesis of N-doped carbon capsules supporting atomically dispersed iron for efficient oxygen reduction reaction electrocatalysis. eScience 2022, 2, 227–234. [Google Scholar] [CrossRef]
- Deng, Q.B.; Gopal, V.; Weissmuller, J. Less Noble or More Noble: How Strain Affects the Binding of Oxygen on Gold. Angew. Chem. Int. Ed. 2015, 54, 12981–12985. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Shen, Y.; Yang, M.; Zhang, H.; Deng, Q.; Ding, Y. The effect of surface strain on the CO-poisoned surface of Pt electrode for hydrogen adsorption. J. Catal. 2017, 350, 212–217. [Google Scholar] [CrossRef]
- Xu, X.; Liang, T.; Kong, D.; Wang, B.; Zhi, L. Strain engineering of two-dimensional materials for advanced electrocatalysts. Mater. Today Nano 2021, 14, 100111. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, H.; Deng, Q. Understanding the copper underpotential deposition process at strained gold surface. Electrochem. Commun. 2017, 82, 125–128. [Google Scholar] [CrossRef]
- Deng, Q.B.; Weissmuller, J. Electrocapillary Coupling during Electrosorption. Langmuir 2014, 30, 10522–10530. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.B.; Smetanin, M.; Weissmuller, J. Mechanical modulation of reaction rates in electrocatalysis. J. Catal. 2014, 309, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.J.; Zhang, L.M.; Li, L.; Deng, Q.B.; Jiang, G.X.; Wang, J.Q.; Cao, B.Q.; Wang, Y.J. A ternary FeS2/Fe7S8@nitrogen-sulfur co-doping reduced graphene oxide hybrid towards superior-performance lithium storage. Prog. Nat. Sci. 2021, 31, 207–214. [Google Scholar] [CrossRef]
- Zhang, H.X.; Han, Z.F.; Deng, Q.B. The Effect of an External Magnetic Field on the Electrochemical Capacitance of Nanoporous Nickel for Energy Storage. Nanomaterials 2019, 9, 694. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.L.; Zhang, Z.J.; Qiu, W.J.; Wei, J.K.; Fang, Z.H.; Deng, Q.B.; Guo, W.; Liu, D.; Xie, Z.Z.; Qu, D.Y.; et al. Multifunctional Polypropylene Separator via Cooperative Modification and Its Application in the Lithium-Sulfur Battery. Langmuir 2020, 36, 11147–11153. [Google Scholar] [CrossRef]
- Li, K.; Xu, J.; Chen, C.; Xie, Z.Z.; Liu, D.; Qu, D.Y.; Tang, H.L.; Wei, Q.; Deng, Q.B.; Li, J.S.; et al. Activating the hydrogen evolution activity of Pt electrode via synergistic interaction with NiS2. J. Colloid Interface Sci. 2021, 582, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.F.; Lv, Q.Y.; Liu, L.F.; Liu, B.H.; Wang, Y.R.; Liu, A.M.; Wu, G. Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustain. Energy Fuels 2020, 4, 15–30. [Google Scholar] [CrossRef]
- Song, G.T.; Wang, Y.; Qi, Y.; Li, W.M.; Zhang, L.X. Fabrication of titanium nitride nanoparticles onto carbon nanotubes by atomic layer deposition for utilization as Pt electrocatalyst supports. Rare Met. 2020, 39, 784–791. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.H.; Priest, C.; Li, S.W.; Xu, P.; Wu, G. Advanced Electrocatalysis for Energy and Environmental Sustainability via Water and Nitrogen Reactions. Adv. Mater. 2021, 33, 2000381. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, S.; Priest, C.; Shi, Q.; Wu, G. Atomically dispersed metal-nitrogen-carbon catalysts for fuel cells: Advances in catalyst design, electrode performance, and durability improvement. Chem. Soc. Rev. 2020, 49, 3484–3524. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.S.; Pathak, B. Computational Screening for ORR Activity of 3d Transition Metal Based M@Pt Core-Shell Clusters. J. Phys. Chem. C 2019, 123, 3634–3644. [Google Scholar] [CrossRef]
- Lu, Y.L.; Zhang, H.P.; Wang, Y.F.; Chen, Z. First principles study on the oxygen reduction reaction of Ir@Pt core-shell structure. Chem. Phys. 2022, 552, 111356. [Google Scholar] [CrossRef]
- Bharadwaj, N.; Nair, A.S.; Pathak, B. Dimensional-Dependent Effects in Platinum Core-Shell-Based Catalysts for Fuel Cell Applications. ACS Appl. Nano Mater. 2021, 4, 9697–9708. [Google Scholar] [CrossRef]
- Zhou, W.P.; Sasaki, K.; Su, D.; Zhu, Y.; Wang, J.X.; Adzic, R.R. Gram-Scale-Synthesized Pd2Co-Supported Pt Monolayer Electrocatalysts for Oxygen Reduction Reaction. J. Phys. Chem. C 2010, 114, 8950–8957. [Google Scholar] [CrossRef]
- Price, S.W.T.; Speed, J.D.; Kannan, P.; Russell, A.E. Exploring the First Steps in Core-Shell Electrocatalyst Preparation: In Situ Characterization of the Underpotential Deposition of Cu on Supported Au Nanoparticles. J. Am. Chem. Soc. 2011, 133, 19448–19458. [Google Scholar] [CrossRef]
- Wang, X.; Orikasa, Y.; Inaba, M.; Uchimototo, Y. Reviving Galvanic Cells to Synthesize Core-Shell Nanoparticles with a Quasi-Monolayer Pt Shell for Electrocatalytic Oxygen Reduction. ACS Catal. 2020, 10, 430–434. [Google Scholar] [CrossRef]
- Gamler, J.T.L.; Leonardi, A.; Sang, X.H.; Koczkur, K.M.; Unocic, R.R.; Engel, M.; Skrabalak, S.E. Effect of lattice mismatch and shell thickness on strain in core@shell nanocrystals. Nanoscale Adv. 2020, 2, 1105–1114. [Google Scholar] [CrossRef]
- Sasaki, K.; Kuttiyiel, K.A.; Adzic, R.R. Designing high performance Pt monolayer core-shell electrocatalysts for fuel cells. Curr. Opin. Electrochem. 2020, 21, 368–375. [Google Scholar] [CrossRef]
- Keith, J.A.; Jerkiewicz, G.; Jacob, T. Theoretical Investigations of the Oxygen Reduction Reaction on Pt(111). ChemPhysChem 2010, 11, 2779–2794. [Google Scholar] [CrossRef]
- Raj, C.R.; Samanta, A.; Noh, S.H.; Mondal, S.; Okajima, T.; Ohsaka, T. Emerging new generation electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2016, 4, 11156–11178. [Google Scholar] [CrossRef]
- Hansen, H.A.; Viswanathan, V.; Norskov, J.K. Unifying Kinetic and Thermodynamic Analysis of 2 e− and 4 e− Reduction of Oxygen on Metal Surfaces. J. Phys. Chem. C 2014, 118, 6706–6718. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.T.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Raciti, D.; Kubal, J.; Ma, C.; Barclay, M.; Gonzalez, M.; Chi, M.F.; Greeley, J.; More, K.L.; Wang, C. Pt3Re alloy nanoparticles as electrocatalysts for the oxygen reduction reaction. Nano Energy 2016, 20, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Malacrida, P.; Casalongue, H.G.S.; Masini, F.; Kaya, S.; Hernandez-Fernandez, P.; Deiana, D.; Ogasawara, H.; Stephens, I.E.L.; Nilsson, A.; Chorkendorff, I. Direct observation of the dealloying process of a platinum-yttrium nanoparticle fuel cell cathode and its oxygenated species during the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2015, 17, 28121–28128. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Renault, C.; Nioradze, N.; Arroyo-Curras, N.; Leonard, K.C.; Bard, A.J. Electrocatalytic Activity of Individual Pt Nanoparticles Studied by Nanoscale Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2016, 138, 8560–8568. [Google Scholar] [CrossRef]
- Yue, J.; Du, Z.; Shao, M.H. Mechanisms of Enhanced Electrocatalytic Activity for Oxygen Reduction Reaction on High-Index Platinum n(111)-(111) Surfaces. J. Phys. Chem. Lett. 2015, 6, 3346–3351. [Google Scholar] [CrossRef] [PubMed]
- Bhalothia, D.; Krishnia, L.; Yang, S.S.; Yan, C.; Hsiung, W.H.; Wang, K.W.; Chen, T.Y. Recent Advancements and Future Prospects of Noble Metal-Based Heterogeneous Nanocatalysts for Oxygen Reduction and Hydrogen Evolution Reactions. Appl. Sci. 2020, 10, 7708. [Google Scholar] [CrossRef]
- Voiry, D.; Chhowalla, M.; Gogotsi, Y.; Kotov, N.A.; Li, Y.; Penner, R.M.; Schaak, R.E.; Weiss, P.S. Best Practices for Reporting Electrocatalytic Performance of Nanomaterials. ACS Nano 2018, 12, 9635–9638. [Google Scholar] [CrossRef] [Green Version]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, R.; Cai, X.; Zeng, H.; Yu, Z.P. Stability of High-Performance Pt-Based Catalysts for Oxygen Reduction Reactions. Adv. Mater. 2018, 30, 1705332. [Google Scholar] [CrossRef]
- Zhang, J.W.; Yuan, Y.L.; Gao, L.; Zeng, G.M.; Li, M.F.; Huang, H.W. Stabilizing Pt-Based Electrocatalysts for Oxygen Reduction Reaction: Fundamental Understanding and Design Strategies. Adv. Mater. 2021, 33, 2006494. [Google Scholar] [CrossRef]
- Shinozaki, K.; Zack, J.W.; Richards, R.M.; Pivovar, B.S.; Kocha, S.S. Oxygen Reduction Reaction Measurements on Platinum Electrocatalysts Utilizing Rotating Disk Electrode Technique I. Impact of Impurities, Measurement Protocols and Applied Corrections. J. Electrochem. Soc. 2015, 162, F1144–F1158. [Google Scholar] [CrossRef]
- Kocha, S.S.; Shinozaki, K.; Zack, J.W.; Myers, D.J.; Kariuki, N.N.; Nowicki, T.; Stamenkovic, V.; Kang, Y.J.; Li, D.G.; Papageorgopoulos, D. Best Practices and Testing Protocols for Benchmarking ORR Activities of Fuel Cell Electrocatalysts Using Rotating Disk Electrode. Electrocatalysis 2017, 8, 366–374. [Google Scholar] [CrossRef]
- Shinozaki, K.; Zack, J.W.; Pylypenko, S.; Pivovar, B.S.; Kocha, S.S. Oxygen Reduction Reaction Measurements on Platinum Electrocatalysts Utilizing Rotating Disk Electrode Technique II. Influence of Ink Formulation, Catalyst Layer Uniformity and Thickness. J. Electrochem. Soc. 2015, 162, F1384–F1396. [Google Scholar] [CrossRef]
- Wei, C.; Rao, R.R.; Peng, J.Y.; Huang, B.T.; Stephens, I.E.L.; Risch, M.; Xu, Z.C.J.; Shao-Horn, Y. Recommended Practices and Benchmark Activity for Hydrogen and Oxygen Electrocatalysis in Water Splitting and Fuel Cells. Adv. Mater. 2019, 31, 1806296. [Google Scholar] [CrossRef]
- Ehelebe, K.; Seeberger, D.; Paul, M.T.Y.; Thiele, S.; Mayrhofer, K.J.J.; Cherevko, S. Evaluating Electrocatalysts at Relevant Currents in a Half-Cell: The Impact of Pt Loading on Oxygen Reduction Reaction. J. Electrochem. Soc. 2019, 166, F1259–F1268. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Pinaud, B.A.; Bonakdarpour, A.; Daniel, L.; Sharman, J.; Wilkinson, D.P. Key Considerations for High Current Fuel Cell Catalyst Testing in an Electrochemical Half-Cell. J. Electrochem. Soc. 2017, 164, F321–F327. [Google Scholar] [CrossRef]
- Fan, J.T.; Chen, M.; Zhao, Z.L.; Zhang, Z.; Ye, S.Y.; Xu, S.Y.; Wang, H.J.; Li, H. Bridging the gap between highly active oxygen reduction reaction catalysts and effective catalyst layers for proton exchange membrane fuel cells. Nat. Energy 2021, 6, 475–486. [Google Scholar] [CrossRef]
- Sievers, G.W.; Jensen, A.W.; Brüser, V.; Arenz, M.; Escudero-Escribano, M. Sputtered Platinum Thin-films for Oxygen Reduction in Gas Diffusion Electrodes: A Model System for Studies under Realistic Reaction Conditions. Surfaces 2019, 2, 336–348. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Jiang, Y.L.; Jensen, J.O.; Cleemann, L.N.; Li, Q.F. Catalyst evaluation for oxygen reduction reaction in concentrated phosphoric acid at elevated temperatures. J. Power Sources 2018, 375, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Wiberg, G.K.H.; Fleige, M.; Arenz, M. Gas diffusion electrode setup for catalyst testing in concentrated phosphoric acid at elevated temperatures. Rev. Sci. Instrum. 2015, 86, 024102. [Google Scholar] [CrossRef]
- Du, J.; Quinson, J.; Zana, A.; Arenz, M. Elucidating Pt-Based Nanocomposite Catalysts for the Oxygen Reduction Reaction in Rotating Disk Electrode and Gas Diffusion Electrode Measurements. ACS Catal. 2021, 11, 7584–7594. [Google Scholar] [CrossRef]
- Shi, Y.; Lee, C.; Tan, X.; Yang, L.; Zhu, Q.; Loh, X.; Xu, J.; Yan, Q. Atomic-Level Metal Electrodeposition: Synthetic Strategies, Applications, and Catalytic Mechanism in Electrochemical Energy Conversion. Small Struct. 2022, 3, 2100185. [Google Scholar] [CrossRef]
- Zhang, L.; Doyle-Davis, K.; Sun, X.L. Pt-Based electrocatalysts with high atom utilization efficiency: From nanostructures to single atoms. Energy Environ. Sci. 2019, 12, 492–517. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [Green Version]
- Nan, H.; Su, Y.Q.; Tang, C.; Cao, R.; Li, D.; Yu, J.; Liu, Q.; Deng, Y.; Tian, X. Engineering the electronic and strained interface for high activity of PdMcore@Ptmonolayer electrocatalysts for oxygen reduction reaction. Sci. Bull. 2020, 65, 1396–1404. [Google Scholar] [CrossRef]
- Luo, L.; Fu, C.; Shen, S.; Zhu, F.; Zhang, J. Probing structure-designed Cu–Pd nanospheres and their Pt-monolayer-shell derivatives as high-performance electrocatalysts for alkaline and acidic oxygen reduction reactions. J. Mater. Chem. A 2020, 8, 22389–22400. [Google Scholar] [CrossRef]
- Loichet Torres, P.A.; El-Sayed, H.A.; Schwaemmlein, J.N.; Friedrich, F.; Gasteiger, H.A. Hydrogen Gas Promoted Self-Limiting Copper Monolayer Deposition on Platinum. J. Electrochem. Soc. 2021, 168, 052508. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, Y.X.; Niu, Z. Recent Advances in Earth-Abundant Core/Noble-Metal Shell Nanoparticles for Electrocatalysis. ACS Catal. 2020, 10, 10886–10904. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, J.; Fang, J. Nanoscale Design of Pd-Based Electrocatalysts for Oxygen Reduction Reaction Enhancement in Alkaline Media. Small Struct. 2022, 3, 2100188. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, D.; Liu, H.; Cui, P.; Hu, C.; Chen, D.; Xu, L.; Wu, X.; Yang, J. Electronic and lattice strain dual tailoring for boosting Pd electrocatalysis in oxygen reduction reaction. Iscience 2021, 24, 103332. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, Q.; Liu, H.; Hu, C.; Chen, D.; Xu, L.; Yang, J. Combining the core-shell construction with an alloying effect for high efficiency ethanol electrooxidation. Cell Rep. Phys. Sci. 2021, 2, 100357. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, N.; Ji, Y.; Shao, Q.; Li, Y.; Xiao, X.; Huang, X. Fully Tensile Strained Pd3Pb/Pd Tetragonal Nanosheets Enhance Oxygen Reduction Catalysis. Nano Lett. 2019, 19, 1336–1342. [Google Scholar] [CrossRef]
- Xue, Q.; Sun, H.Y.; Li, Y.N.; Zhong, M.J.; Li, F.M.; Tian, X.; Chen, P.; Yin, S.B.; Chen, Y. Au@Ir core-shell nanowires towards oxygen reduction reaction. Chem. Eng. J. 2021, 421, 129760. [Google Scholar] [CrossRef]
- Wang, Y.F.; Gong, T.Y.; Lee, M.; Hall, A.S. Structural transformations of metal alloys under electrocatalytic conditions. Curr. Opin. Electrochem. 2021, 30, 100796. [Google Scholar] [CrossRef]
- Peng, L.Y.; Gan, L.; Wei, Y.P.; Yang, H.; Li, J.; Du, H.D.; Kang, F.Y. Pt Submonolayers on Au Nanoparticles: Coverage-Dependent Atomic Structures and Electrocatalytic Stability on Methanol Oxidation. J. Phys. Chem. C 2016, 120, 28664–28671. [Google Scholar] [CrossRef]
- Guo, W.; Yao, X.; Peng, L.; Lin, B.; Kang, Y.; Gan, L. Platinum monolayers stabilized on dealloyed AuCu core-shell nanoparticles for improved activity and stability on methanol oxidation reaction. Chin. Chem. Lett. 2020, 31, 836–840. [Google Scholar] [CrossRef]
- Lu, X.Y.; Ahmadi, M.; DiSalvo, F.J.; Abruna, H.D. Enhancing the Electrocatalytic Activity of Pd/M (M = Ni, Mn) Nanoparticles for the Oxygen Reduction Reaction in Alkaline Media through Electrochemical Dealloying. ACS Catal. 2020, 10, 5891–5898. [Google Scholar] [CrossRef]
- Lyu, X.; Jia, Y.; Mao, X.; Li, D.; Li, G.; Zhuang, L.; Wang, X.; Yang, D.; Wang, Q.; Du, A.; et al. Gradient-Concentration Design of Stable Core-Shell Nanostructure for Acidic Oxygen Reduction Electrocatalysis. Adv. Mater. 2020, 32, 2003493. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N.; Inoue, H.; Yoshiura, R.; Hasegawa, Y.; Miyazaki, S.; Suzuki, A.; Daimon, H.; Doi, T.; Inaba, M.; Higashi, K.; et al. Electrochemical Properties and Single Cell Performance of Pd Core-Pt Shell Structured Catalyst Synthesized by a Simple Direct Displacement Reaction. J. Electrochem. Soc. 2020, 167, 044513. [Google Scholar] [CrossRef]
- Park, A.H.; Shi, W.; Jung, J.U.; Kwon, Y.U. Mechanism study of Single-Step synthesis of Fe(core)@Pt(shell) nanoparticles by sonochemistry. Ultrason. Sonochem. 2021, 77, 105679. [Google Scholar] [CrossRef]
- Kongkanand, A.; Subramanian, N.P.; Yu, Y.C.; Liu, Z.Y.; Igarashi, H.; Muller, D.A. Achieving High-Power PEM Fuel Cell Performance with an Ultralow-Pt-Content Core-Shell Catalyst. ACS Catal. 2016, 6, 1578–1583. [Google Scholar] [CrossRef]
- Pedersen, C.M.; Escudero-Escribano, M.; Velazquez-Palenzuela, A.; Christensen, L.H.; Chorkendorff, I.; Stephens, I.E.L. Benchmarking Pt-based electrocatalysts for low temperature fuel cell reactions with the rotating disk electrode: Oxygen reduction and hydrogen oxidation in the presence of CO (review article). Electrochim. Acta 2015, 179, 647–657. [Google Scholar] [CrossRef]
- Tse, E.C.M.; Gewirth, A.A. Effect of Temperature and Pressure on the Kinetics of the Oxygen Reduction Reaction. J. Phys. Chem. A 2015, 119, 1246–1255. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Liu, Q.T.; Liu, X.F.; Shui, J.L. Temperature Impacts on Oxygen Reduction Reaction Measured by the Rotating Disk Electrode Technique. J. Phys. Chem. C 2020, 124, 3069–3079. [Google Scholar] [CrossRef]
- Liu, C.; Uchiyama, T.; Yamamoto, K.; Watanabe, T.; Gao, X.; Imai, H.; Matsumoto, M.; Sugawara, S.; Shinohara, K.; Oshima, K.; et al. Effect of Temperature on Oxygen Reduction Reaction Kinetics for Pd Core-Pt Shell Catalyst with Different Core Size. ACS Appl. Energy Mater. 2021, 4, 810–818. [Google Scholar] [CrossRef]
- Cai, X.; Lin, R.; Liu, X.; Zhao, Y. Effect of heat treatment on the surface structure of Pd@Pt–Ni core-shell catalysts for the oxygen reduction reaction. J. Alloys Compd. 2021, 884, 161059. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, S.; Zhang, L.; Su, D.; Shao, M. Interatomic diffusion in Pd-Pt core-shell nanoparticles. Chin. J. Catal. 2020, 41, 807–812. [Google Scholar] [CrossRef]
- Luo, M.; Guo, S. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Mater. 2017, 2, 17059. [Google Scholar] [CrossRef]
- He, T.O.; Wang, W.C.; Yang, X.L.; Cao, Z.M.; Kuang, Q.; Wang, Z.; Shan, Z.W.; Jin, M.S.; Yin, Y.D. Inflating hollow nanocrystals through a repeated Kirkendall cavitation process. Nat. Commun. 2017, 8, 1261. [Google Scholar]
- Liu, G.G.; Zhou, W.; Ji, Y.R.; Chen, B.; Fu, G.T.; Yun, Q.B.; Chen, S.M.; Lin, Y.X.; Yin, P.F.; Cui, X.Y.; et al. Hydrogen-Intercalation-Induced Lattice Expansion of Pd@Pt Core-Shell Nanoparticles for Highly Efficient Electrocatalytic Alcohol Oxidation. J. Am. Chem. Soc. 2021, 143, 11262–11270. [Google Scholar] [CrossRef]
- He, T.O.; Wang, W.C.; Shi, F.L.; Yang, X.L.; Li, X.; Wu, J.B.; Yin, Y.D.; Jin, M.S. Mastering the surface strain of platinum catalysts for efficient electrocatalysis. Nature 2021, 598, 76–81. [Google Scholar] [CrossRef]
- Xie, M.H.; Lyu, Z.H.; Chen, R.H.; Shen, M.; Cao, Z.M.; Xia, Y.N. Pt-Co@Pt Octahedral Nanocrystals: Enhancing Their Activity and Durability toward Oxygen Reduction with an Intermetallic Core and an Ultrathin Shell. J. Am. Chem. Soc. 2021, 143, 8509–8518. [Google Scholar] [CrossRef]
- Li, W.Z.; Lu, B.A.; Gan, L.; Tian, N.; Zhang, P.Y.; Yan, W.; Chen, W.X.; Chen, Y.H.; Zhou, Z.Y.; Sun, S.G. High activity and durability of carbon-supported core-shell PtPx@Pt/C catalyst for oxygen reduction reaction. Chin. J. Catal. 2021, 42, 2173–2180. [Google Scholar] [CrossRef]
- Wang, X.; Vara, M.; Luo, M.; Huang, H.; Ruditskiy, A.; Park, J.; Bao, S.; Liu, J.; Howe, J.; Chi, M.; et al. Pd@Pt Core-Shell Concave Decahedra: A Class of Catalysts for the Oxygen Reduction Reaction with Enhanced Activity and Durability. J. Am. Chem. Soc. 2015, 137, 15036–15042. [Google Scholar] [CrossRef]
- Xie, S.; Choi, S.I.; Lu, N.; Roling, L.T.; Herron, J.A.; Zhang, L.; Park, J.; Wang, J.; Kim, M.J.; Xie, Z.; et al. Atomic Layer-by-Layer Deposition of Pt on Pd Nanocubes for Catalysts with Enhanced Activity and Durability toward Oxygen Reduction. Nano Lett. 2014, 14, 3570–3576. [Google Scholar] [CrossRef]
- Hunt, S.T.; Milina, M.; Alba-Rubio, A.C.; Hendon, C.H.; Dumesic, J.A.; Roman-Leshkov, Y. Self-assembly of noble metal monolayers on transition metal carbide nanoparticle catalysts. Science 2016, 352, 974–978. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Wu, L.; Kuttiyiel, K.A.; Goodman, K.R.; Zhang, C.; Zhu, Y.; Vukmirovic, M.B.; White, M.G.; Sasaki, K.; Adzic, R.R. Increasing Stability and Activity of Core-Shell Catalysts by Preferential Segregation of Oxide on Edges and Vertexes: Oxygen Reduction on Ti-Au@Pt/C. J. Am. Chem. Soc. 2016, 138, 9294–9300. [Google Scholar] [CrossRef]
- He, T.O.; Wang, W.C.; Yang, X.L.; Shi, F.L.; Ye, Z.Y.; Zheng, Y.Z.; Li, F.; Wu, J.B.; Yin, Y.D.; Jin, M.S. Deposition of Atomically Thin Pt Shells on Amorphous Palladium Phosphide Cores for Enhancing the Electrocatalytic Durability. ACS Nano 2021, 15, 7348–7356. [Google Scholar] [CrossRef]
- Wang, Z.X.; Yao, X.Z.; Kang, Y.Q.; Miao, L.Q.; Xia, D.S.; Gan, L. Structurally Ordered Low-Pt Intermetallic Electrocatalysts toward Durably High Oxygen Reduction Reaction Activity. Adv. Funct. Mater. 2019, 29, 1902987. [Google Scholar] [CrossRef]
- Li, J.R.; Sharma, S.; Liu, X.M.; Pan, Y.T.; Spendelow, J.S.; Chi, M.F.; Jia, Y.K.; Zhang, P.; Cullen, D.A.; Xi, Z.; et al. Hard-Magnet L1(0)-CoPt Nanoparticles Advance Fuel Cell Catalysis. Joule 2019, 3, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ye, K.; Gu, Q.; Jiang, Q.; Qin, J.; Leng, D.; Liu, Q.; Yang, B.; Yin, F. Optimized oxygen reduction activity by tuning shell component in Pd@Pt-based core-shell electrocatalysts. J. Colloid Interface Sci. 2021, 604, 301–309. [Google Scholar] [CrossRef]
- Zhu, J.W.; Elnabawy, A.O.; Lyu, Z.H.; Xie, M.H.; Murray, E.A.; Chen, Z.T.; Jin, W.Q.; Mavrikakis, M.; Xia, Y.N. Facet-controlled Pt-Ir nanocrystals with substantially enhanced activity and durability towards oxygen reduction. Mater. Today 2020, 35, 69–77. [Google Scholar] [CrossRef]
- Strickler, A.L.; Jackson, A.; Jaramillo, T.F. Active and Stable Ir@Pt Core-Shell Catalysts for Electrochemical Oxygen Reduction. ACS Energy Lett. 2017, 2, 244–249. [Google Scholar] [CrossRef]
- Ramirez-Caballero, G.E.; Ma, Y.; Callejas-Tovar, R.; Balbuena, P.B. Surface segregation and stability of core-shell alloy catalysts for oxygen reduction in acid medium. Phys. Chem. Chem. Phys. 2010, 12, 2209–2218. [Google Scholar] [CrossRef]
- Guan, J.; Yang, S.; Liu, T.; Yu, Y.; Niu, J.; Zhang, Z.; Wang, F. Intermetallic FePt@PtBi Core-Shell Nanoparticles for Oxygen Reduction Electrocatalysis. Angew. Chem. Int. Ed. Engl. 2021, 60, 21899–21904. [Google Scholar] [CrossRef]
- Feng, Y.G.; Shao, Q.; Lv, F.; Bu, L.Z.; Guo, J.; Guo, S.J.; Huang, X.Q. Intermetallic PtBi Nanoplates Boost Oxygen Reduction Catalysis with Superior Tolerance over Chemical Fuels. Adv. Sci. 2020, 7, 1800178. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.C.; Sun, Y.J.; Wang, L.; Guo, S.J. Tuning Multimetallic Ordered Intermetallic Nanocrystals for Efficient Energy Electrocatalysis. Adv. Energy Mater. 2017, 7, 1602073. [Google Scholar] [CrossRef]
- Xia, Z.H.; Guo, S.J. Strain engineering of metal-based nanomaterials for energy electrocatalysis. Chem. Soc. Rev. 2019, 48, 3265–3278. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Li, X.; Yan, Y.; Huang, J.; Li, J.; Shen, R.; Tian, H.; Yang, D.; Zhang, H. A unique ligand effect in Pt-based core–shell nanocubes to boost oxygen reduction electrocatalysis. J. Mater. Chem. A 2021, 9, 22653–22659. [Google Scholar] [CrossRef]
- Asano, M.; Kawamura, R.; Sasakawa, R.; Todoroki, N.; Wadayama, T. Oxygen Reduction Reaction Activity for Strain-Controlled Pt-Based Model Alloy Catalysts: Surface Strains and Direct Electronic Effects Induced by Alloying Elements. ACS Catal. 2016, 6, 5285–5289. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef]


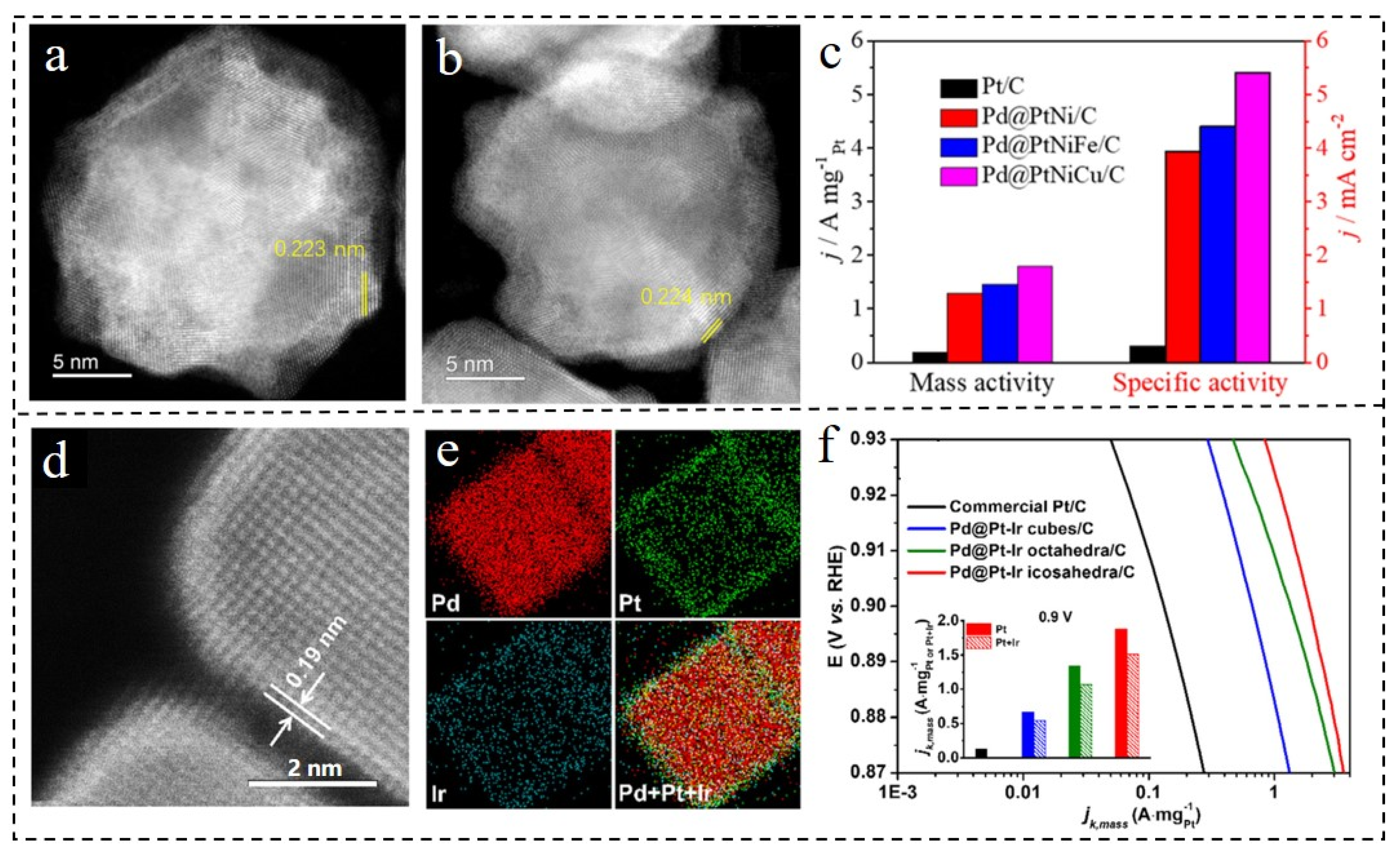

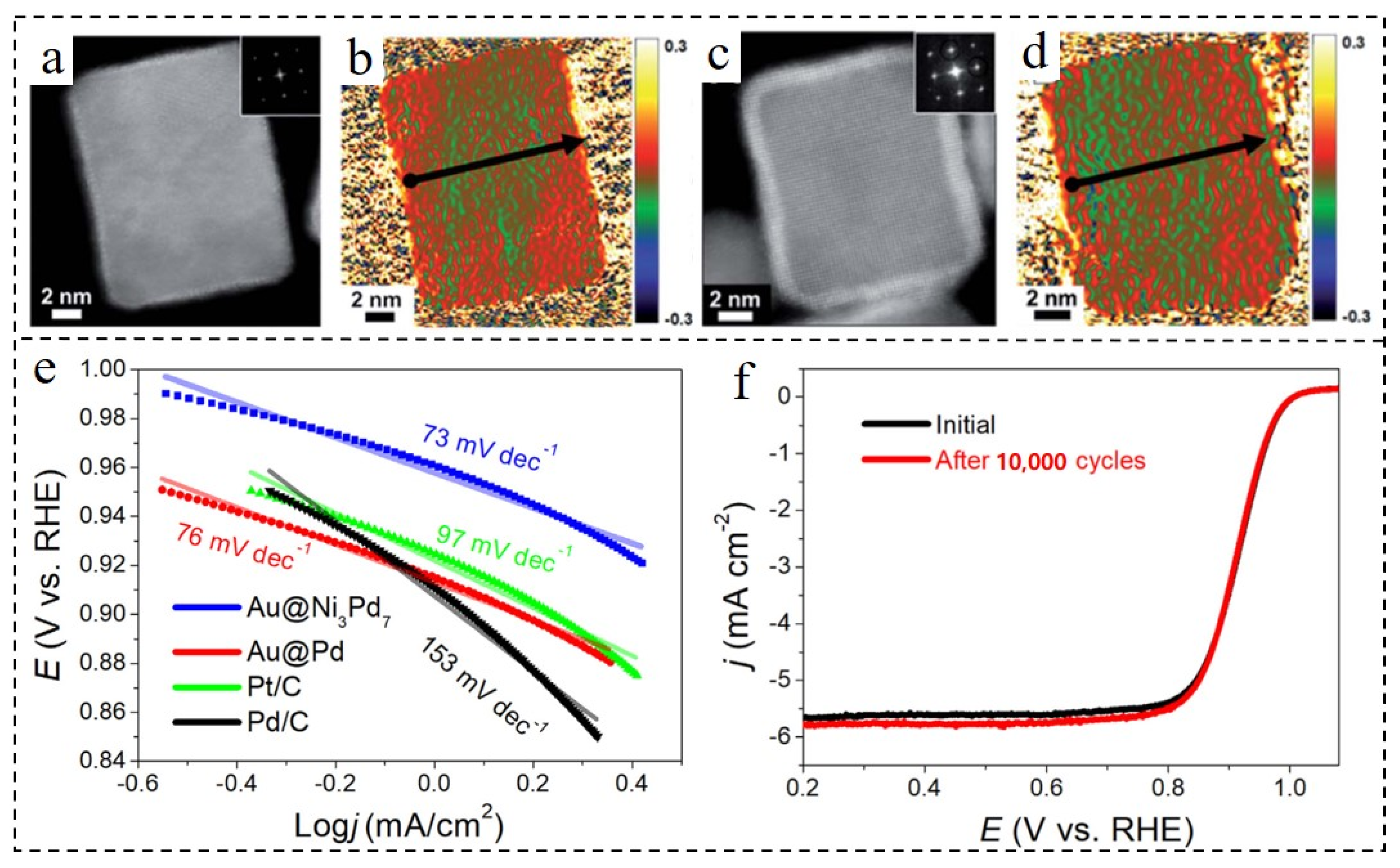

| Core-Shell Materials | Electrolyte | Mass Activity [A·mgPt−1] | Specific Activity [mA·cm−2] | Refs |
|---|---|---|---|---|
| Pt@Pd2Co/C NPs | 0.1 M HClO4 | 0.72 | 0.5 | [29] |
| Pd3Fe@Pt/C NPs | 0.1 M HClO4 | 1.14 | 0.88 | [62] |
| CGCuPd/C NPs | 0.1 M HClO4 | 2.624 | 1.11 | [63] |
| PtNi@PtD/G NPs | 0.1 M HClO4 | 0.061 | 0.098 | [75] |
| PtCo@Pt NPs | 0.1 M HClO4 | 2.82 | 9.16 | [89] |
| PtP1.4@Pt/C NPs | 0.1 M HClO4 | 0.31 | 0.62 | [90] |
| Pd@PtNiCu NPs | 0.1 M HClO4 | 1.79 | 5.4 | [98] |
| Pd@PtIr NPs | 0.1 M HClO4 | 1.88 | 1.27 | [99] |
| FePt@PtBi NPs | 0.1 M HClO4 | 0.96 | 2.06 | [102] |
| Pd3Pb@Pt3Pb NPs | 0.1 M KOH | 4.69 | 6.69 | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; An, C.; Qin, C.; Gomaa, H.; Deng, Q.; Wu, S.; Hu, N. Noble Metal-Based Catalysts with Core-Shell Structure for Oxygen Reduction Reaction: Progress and Prospective. Nanomaterials 2022, 12, 2480. https://doi.org/10.3390/nano12142480
Wang C, An C, Qin C, Gomaa H, Deng Q, Wu S, Hu N. Noble Metal-Based Catalysts with Core-Shell Structure for Oxygen Reduction Reaction: Progress and Prospective. Nanomaterials. 2022; 12(14):2480. https://doi.org/10.3390/nano12142480
Chicago/Turabian StyleWang, Chao, Cuihua An, Chunling Qin, Hassanien Gomaa, Qibo Deng, Shuai Wu, and Ning Hu. 2022. "Noble Metal-Based Catalysts with Core-Shell Structure for Oxygen Reduction Reaction: Progress and Prospective" Nanomaterials 12, no. 14: 2480. https://doi.org/10.3390/nano12142480
APA StyleWang, C., An, C., Qin, C., Gomaa, H., Deng, Q., Wu, S., & Hu, N. (2022). Noble Metal-Based Catalysts with Core-Shell Structure for Oxygen Reduction Reaction: Progress and Prospective. Nanomaterials, 12(14), 2480. https://doi.org/10.3390/nano12142480







