Low-Dimensional Nanomaterial Systems Formed by IVA Group Elements Allow Energy Conversion Materials to Flourish
Abstract
:1. Introduction
2. Morphology and Properties of IVA-LD
2.1. 0D Quantum Dots
2.2. 1D Nanowires/Rods/Tubes
2.3. Monolayer
3. Preparation of IVA-LD
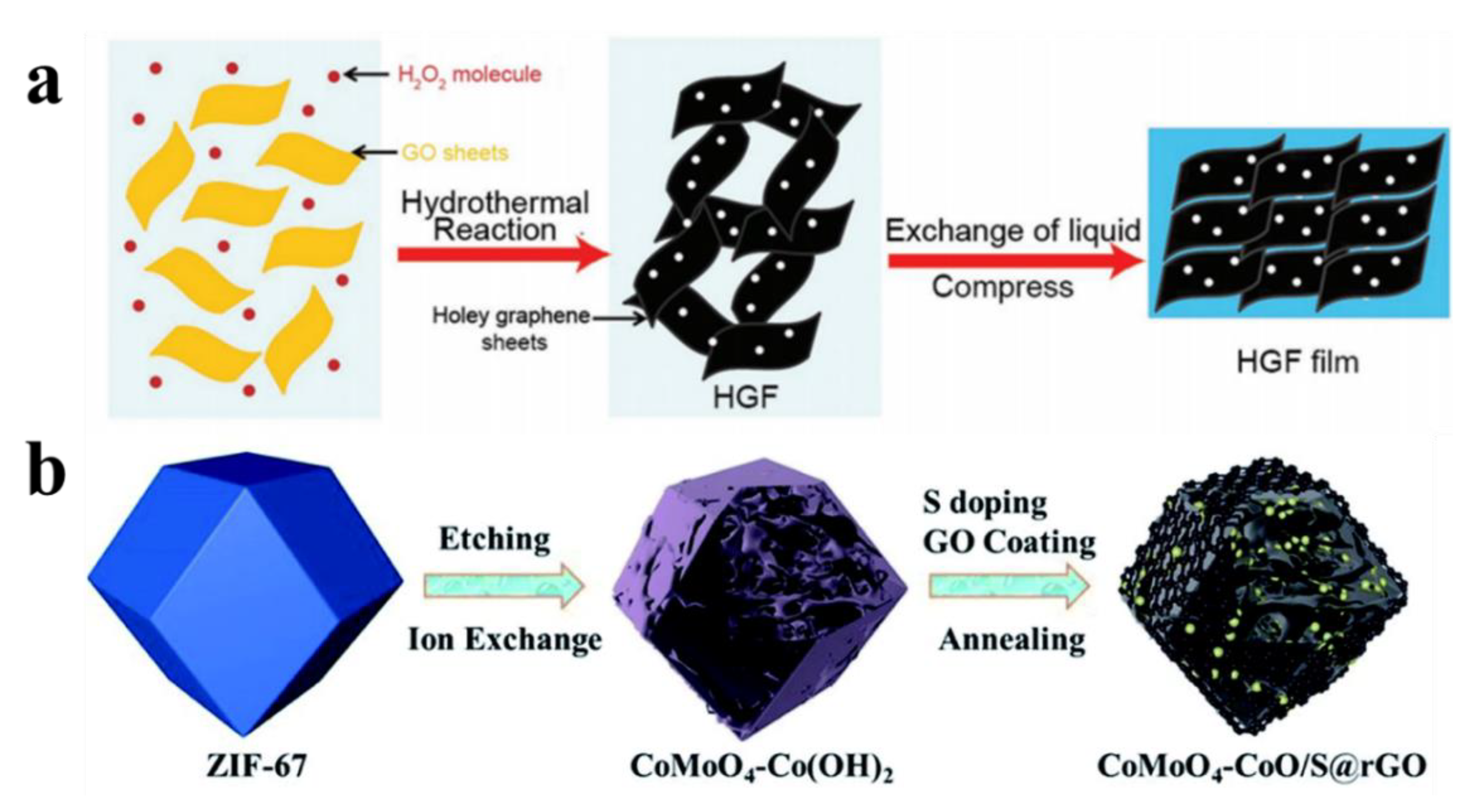
3.1. Hydrothermal Synthesis
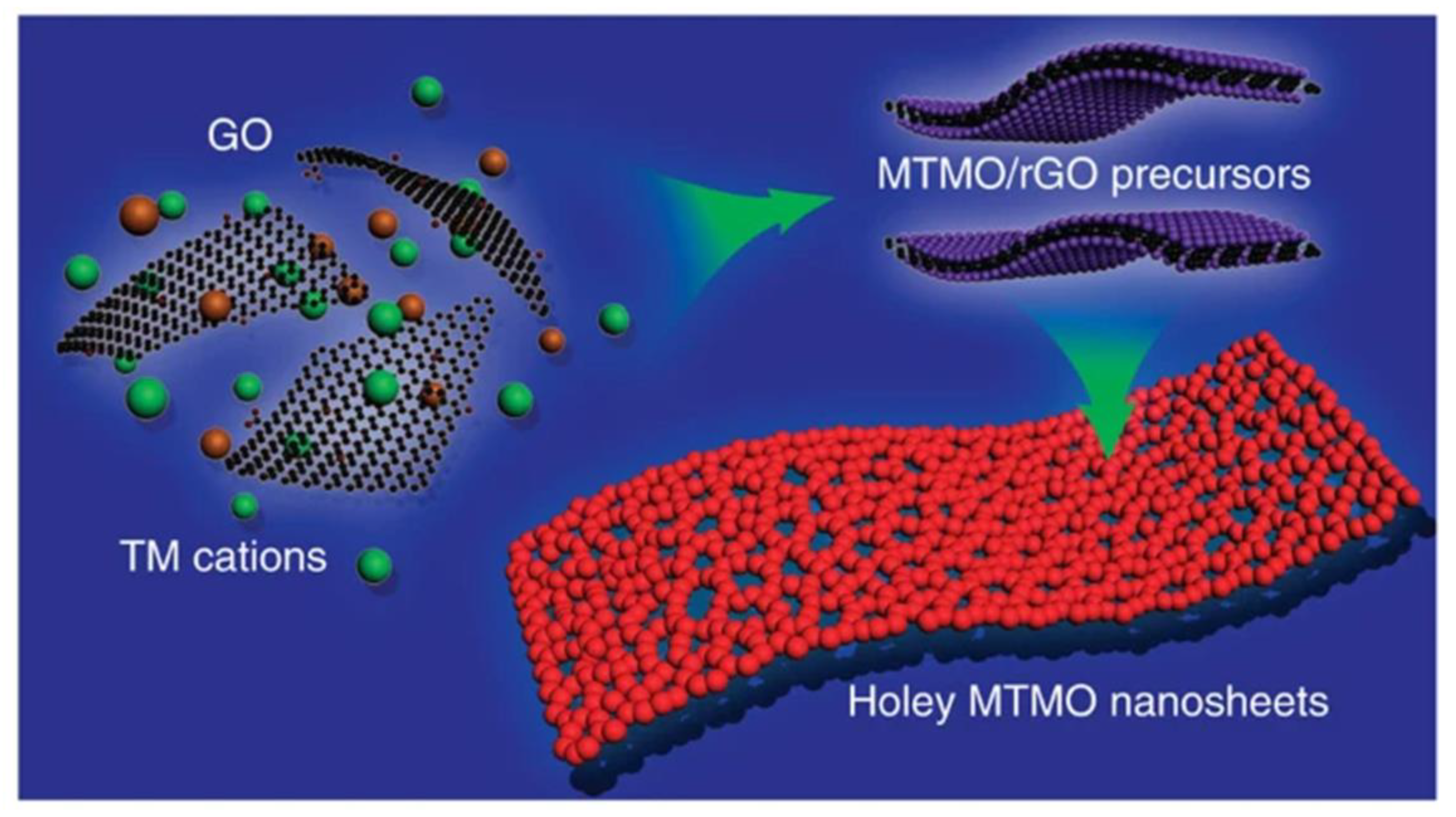
3.2. Template-Directed Synthesis
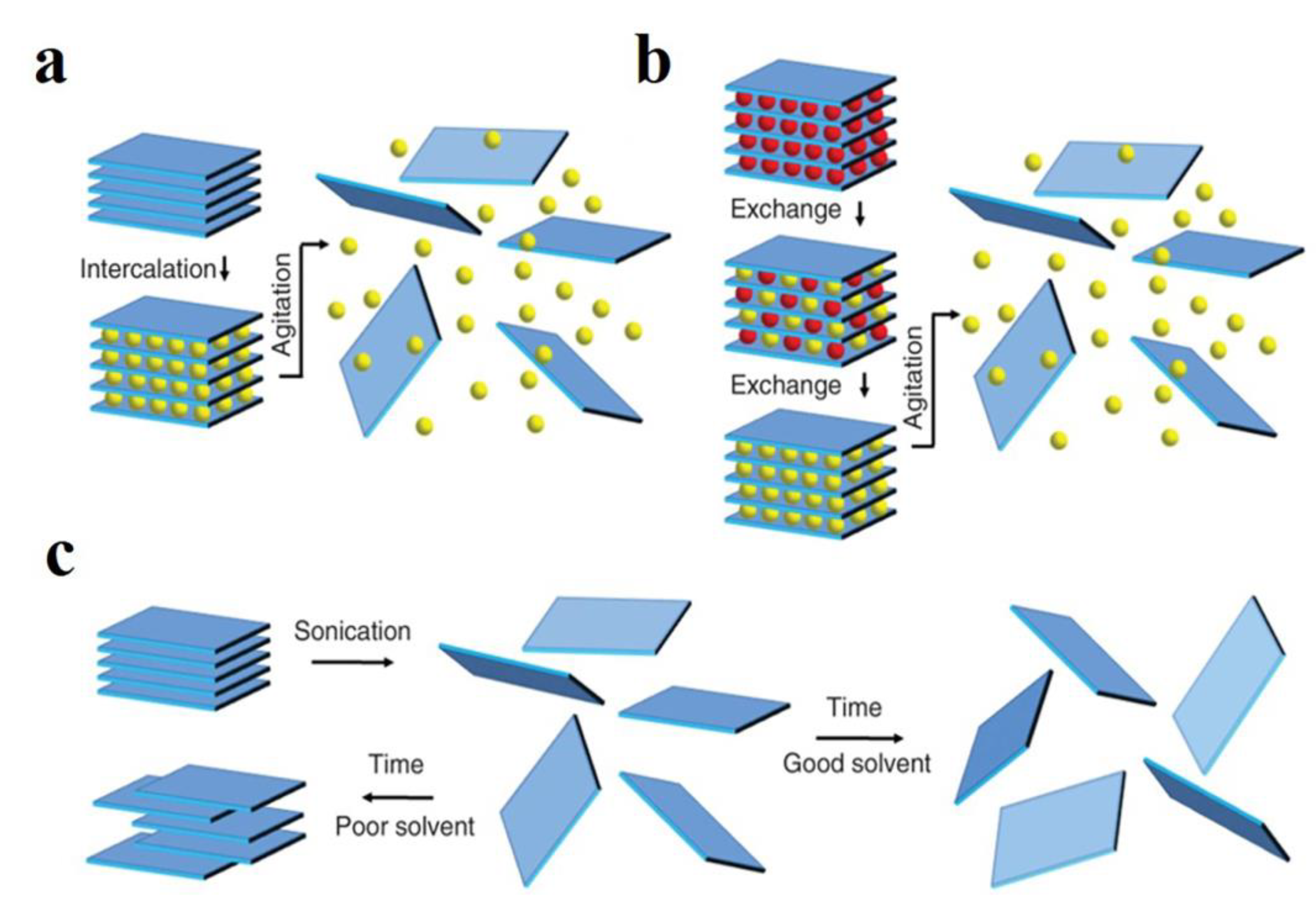
3.3. Liquid Phase Stripping Nanosheet Method
4. Applications of IVA-LD in Energy Conversion Materials
4.1. Battery
4.2. Transducer
- (1)
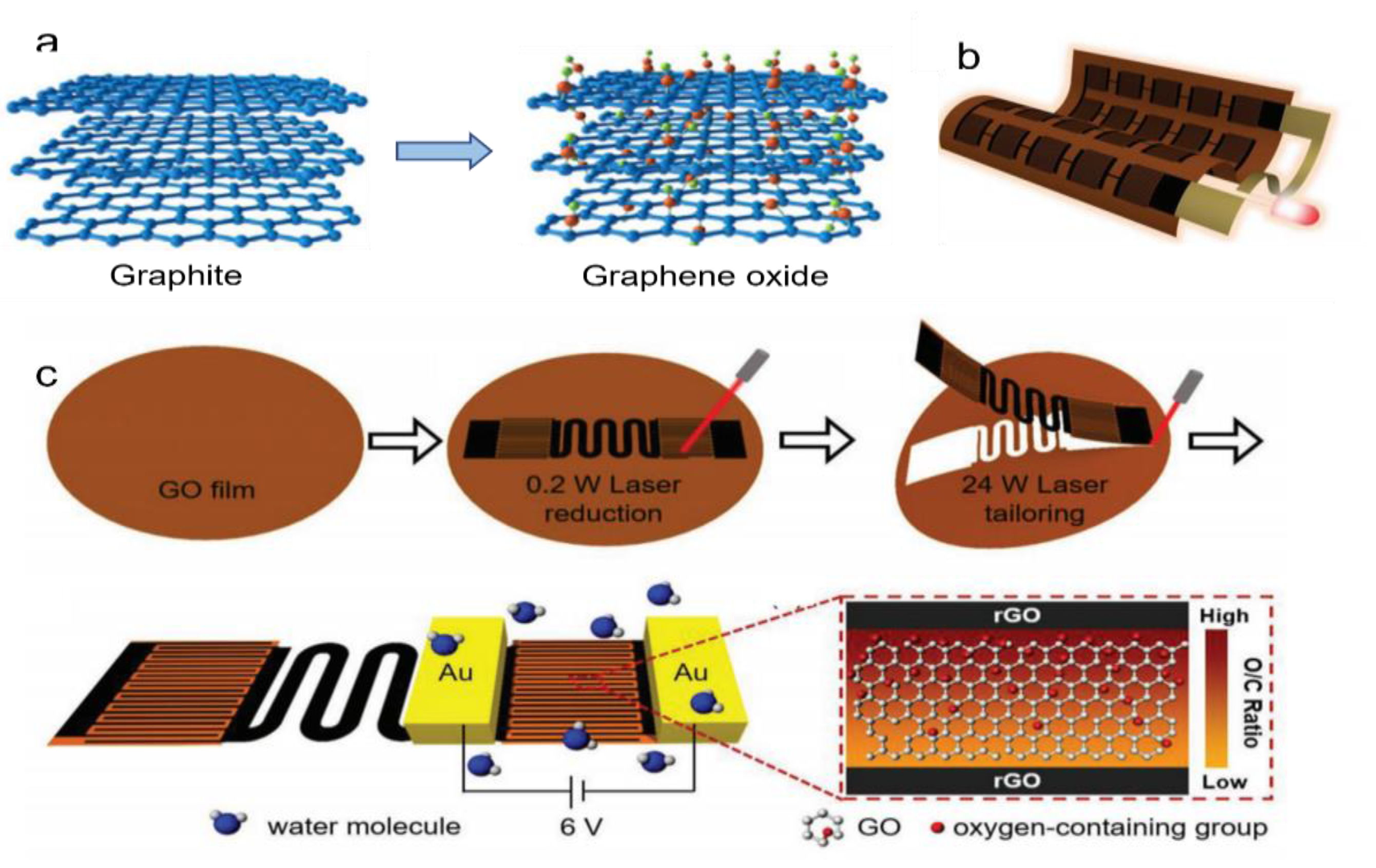
- Asymmetric treatment of material structure. As suggested by Yang et al., a potassium hydroxide (KOH) solution is added to the GO solution to introduce a large ionic gradient [81]. Most of the oxygen-containing functional groups of GO are destroyed by reaction with KOH. The structure of GO is destroyed, leaving potassium ions (K+) between the lamellar structures, forming rGO. GO and rGO come into contact through overlapping. An ionic solution is formed in the middle of the layered structure when exposed to moisture. So, the potassium ions (K+) are distributed asymmetrically throughout the system. The potassium ions (K+) move spontaneously from the rGO side to the GO side, generating a stable voltage and current. A graphene hygroelectric generator also has been prepared by laser treatment of graphene. The graphene hygroelectric generators can be folded [82], stretched, or even stripped in three dimensions. The rGO is formed by engraving the GO film using a direct laser writing technology, as in Figure 6b,c. On this basis, the gradient distribution of oxygen-containing groups between the positive and negative poles is changed by a moisture–electric annealing polarization process. When the device encounters moisture, the free hydrogen ions released from the oxygen-containing groups in it form an ion gradient and create a concentration difference within.
- (2)
- Treatment of functional groups. GO can not only be reduced, but also acidified. After acidification, the density of functional groups on GO can be adjusted to make the functional groups dissociate more easily, resulting in a larger proton gradient difference between the upper and lower surfaces of the GO films. In zhu et al.’s. experiment [83], GO/PVA treated with 32% HCI could produce a voltage of 0.85 V. Apparently, acidification can greatly increase the voltage output, as shown in Figure 7a.
- (3)
- Composite with other materials. GOs are also frequently combined with other materials to enhance their MEG function. Huang et al. proposed a moisture electric generator based on porous GO and PAAS composites [84], as shown in Figure 7b. In this material, the large specific surface area and hydrophilic groups work together to enhance its water absorption, which substantially promotes ion dissociation and efficient transport. In addition, the heterogeneous structure of the material and the asymmetric metal electrode allow the system to construct Schottky contact, which facilitates unidirectional ion transport and significantly improves the device performance. Carbon nanotubes can also be combined with GO. A MEG is fabricated by the end-to-end connection of two equal asymmetric regional sandwich structural GO/CNT composite films [85]. Proper addition of CNT helps to create continuous CNT network channels and generates a voltage as water flows over the CNTs surface, thus improving output performance. The generator uses exhaled moisture to generate electricity. As a person continues to breathe, electricity can be continuously generated.
4.3. Water Evaporation
5. Summary and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kittner, N.; Lill, F.; Kammen, D.M. Energy storage deployment and innovation for the clean energy transition. Nat. Energy 2017, 2, 17125. [Google Scholar] [CrossRef]
- Arent, D.J.; Bragg-Sitton, S.M.; Miller, D.C.; Tarka, T.J.; Garfield, D.J. Multi-input, Multi-output Hybrid Energy Systems. Joule 2020, 5, 47–58. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ajayan, P.M. The nano-revolution spawned by carbon. Nature 2019, 575, 49–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrows, C.H. Silicon goes heavyweight. Nat. Mater. 2021, 20, 1177–1178. [Google Scholar] [CrossRef]
- Fadaly, E.M.T.; Dijkstra, A.; Suckert, J.R.; Ziss, D.; van Tilburg, M.A.J.; Mao, C.; Ren, Y.; van Lange, V.T.; Korzun, K.; Kölling, S.; et al. Direct-bandgap emission from hexagonal Ge and SiGe alloys. Nature 2020, 580, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Farquhar, A.K.; Supur, M.; Smith, S.R.; Dyck, C.V.; McCreery, R.L. Hybrid Graphene Ribbon/Carbon Electrodes for High-Performance Energy Storage. Adv. Energy Mater. 2018, 8, 1802439. [Google Scholar] [CrossRef]
- Zhang, X.S.; Kwon, K.; Henriksson, J.; Luo, J.; Wu, M.C. A large-scale microelectromechanical-systems-based silicon photonics LiDAR. Nature 2022, 603, 253–258. [Google Scholar] [CrossRef]
- Luo, W.; Gong, Y.H.; Zhu, Y.Z.; Li, Y.J.; Yao, Y.G.; Zhang, Y.; Fu, K.; Pastel, G.; Lin, C.F.; Mo, Y.J.; et al. Reducing Interfacial Resistance between Garnet-Structured Solid-State Electrolyte and Li-Metal Anode by a Germanium Layer. Adv. Mater. 2017, 29, 1606042. [Google Scholar] [CrossRef]
- Kang, L.T.; Cui, M.W.; Jiang, F.Y.; Gao, Y.F.; Luo, H.J.; Liu, J.J.; Liang, W.; Zhi, C.Y. Nanoporous CaCO3 Coatings Enabled Uniform Zn Stripping/Plating for Long-Life Zinc Rechargeable Aqueous Batteries. Adv. Energy Mater. 2018, 8, 801090. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chen, N.; Li, Z.L.; Shao, H.B.; Sun, X.T.; Liu, F.; Liu, X.T.; Guo, Q.; Qu, L.T. Reborn Three-Dimensional Graphene with Ultrahigh Volumetric Desalination Capacity. Adv. Mater. 2021, 33, 2105853. [Google Scholar] [CrossRef]
- Stetson, C.; Schnabel, M.; Li, Z.F.; Harvey, S.P.; Jiang, C.S.; Norman, A.; Decaluwe, S.C.; Al-Jassim, M.; Burrell, A. Microscopic Observation of Solid Electrolyte Interphase Bilayer Inversion on Silicon Oxide. ACS Energy Lett. 2020, 5, 3567. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Liang, S.X.; Liu, W.; Eickemeyer, F.T.; Cai, X.B.; Zhou, K.; Bian, J.; Zhu, H.W.; Zhu, C.; Wang, N.; et al. Ti1-graphene single-atom material for improved energy level alignment in perovskite solar cells. Nat. Energy 2021, 6, 1154–1163. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Li, T.; Sun, C.; Xia, J.; Jiao, Y.; Xu, H. Selenium-Doped Carbon Quantum Dots for Free-Radical Scavenging. Angew. Chem. Int. Ed. 2017, 56, 9910–9914. [Google Scholar] [CrossRef]
- Yu, T.; Wang, F.; Xu, Y.; Ma, L.; Pi, X.; Yang, D. Graphene Coupled with Silicon Quantum Dots for High-Performance Bulk-Silicon-Based Schottky-Junction Photodetectors. Adv. Mater. 2016, 28, 4912–4919. [Google Scholar] [CrossRef]
- Zhang, B.; Jie, J.; Zhang, X.; Ou, X.; Zhang, X. Large-Scale Fabrication of Silicon Nanowires for Solar Energy Applications. ACS Appl. Mater. Interf. 2017, 9, 34527–34543. [Google Scholar] [CrossRef]
- Peng, K.Q.; Lee, S.T. Silicon Nanowires for Photovoltaic Solar Energy Conversion. Adv. Mater. 2011, 23, 198–215. [Google Scholar] [CrossRef]
- Zeng, G.; Chen, W.; Chen, X.; Hu, Y.; Chen, Y.; Zhang, B.; Chen, H.; Sun, W.; Shen, Y.; Li, Y.; et al. Realizing 17.5% Efficiency Flexible Organic Solar Cells via Atomic-Level Chemical Welding of Silver Nanowire Electrodes. J. Am. Chem. Soc. 2022, 144, 8658–8668. [Google Scholar] [CrossRef]
- Pal, B.; Sarkar, K.J.; Banerji, P. Fabrication and studies on Si/InP core-shell nanowire based solar cell using etched Si nanowire arrays. Sol. Energy Mater Sol. Cells 2020, 204, 110217. [Google Scholar] [CrossRef]
- Park, C.; Samuel, E.; Joshi, B.; Kim, T.; Aldalbahi, A.; El-Newehy, M.; Yoon, W.Y.; Yoon, S.S. Supersonically sprayed Fe2O3/C/CNT composites for highly stable Li-ion battery anodes. Chem. Eng. J. 2020, 395, 125018. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z.; Boussaid, F.; Zhang, D.; Pan, X.; Zhao, H.; Bermak, A.; Tsui, C.Y.; Wang, X.; Fan, Z. Ultra-Low-Power Smart Electronic Nose System Based on Three-Dimensional Tin Oxide Nanotube Arrays. ACS Nano 2018, 12, 6079–6088. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, Z.; Zhao, Y.; Qu, L. Graphene Platforms for Smart Energy Generation and Storage. Joule 2018, 2, 245–268. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Zhao, F.; Cheng, Z.H.; Zhou, Q.H.; Shao, H.B.; Jiang, L.; Qu, L.T. Self-powered wearable graphene fiber for information expression. Nano Energy 2017, 32, 329–335. [Google Scholar] [CrossRef]
- Blanco, M.; Mosconi, D.; Otyepka, M.; Medved, M.; Bakandritsos, A.; Agnoli, S.; Granozzi, G. Combined high degree of carboxylation and electronic conduction in graphene acid sets new limits for metal free catalysis in alcohol oxidation. Chem Sci 2019, 10, 9438–9445. [Google Scholar] [CrossRef] [Green Version]
- Chronopoulos, D.D.; Bakandritsos, A.; Pykal, M.; Zboril, R.; Otyepka, M. Chemistry, properties, and applications of fluorographene. Appl Mater Today 2017, 9, 60–70. [Google Scholar] [CrossRef]
- Bakandritsos, A.; Pykal, M.; Blonski, P.; Jakubec, P.; Chronopoulos, D.D.; Polakova, K.; Georgakilas, V.; Cepe, K.; Tomanec, O.; Ranc, V.; et al. Cyanographene and Graphene Acid: Emerging Derivatives Enabling High-Yield and Selective Functionalization of Graphene. ACS Nano 2017, 11, 2982–2991. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.; Zhang, X.; Liang, X.; Zhang, F.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Few-layer methyl-terminated germanene–graphene nanocomposite with high capacity for stable lithium storage. Carbon 2020, 161, 287–298. [Google Scholar] [CrossRef]
- Dhungana, D.S.; Grazianetti, C.; Martella, C.; Achilli, S.; Fratesi, G.; Molle, A. Two-Dimensional Silicene–Stanene Heterostructures by Epitaxy. Adv. Funct. Mater. 2021, 31, 2102797. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.Y.; Zhao, Z.; Lin, Z.; Lee, C.; Xu, X.; Wang, C.; Huang, Y.; Shakir, M.I.; Duan, X. Solution Processable Holey Graphene Oxide and Its Derived Macrostructures for High-Performance Supercapacitors. Nano Lett 2015, 15, 4605–4610. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, K.; Liang, P.; Wu, M.; Rao, Y.; Zheng, H.; Liu, J.; Yan, K.; Wang, J. Ultrahigh reversible lithium storage of hierarchical porous Co–Mo oxides via graphene encapsulation and hydrothermal S-doping. J. Mater. Chem. A 2022, 10, 5373–5380. [Google Scholar] [CrossRef]
- Bols, P.S.; Anderson, H.L. Template-Directed Synthesis of Molecular Nanorings and Cages. Acc. Chem. Res. 2018, 51, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, Y.; Yan, J.; Ning, G.; Wang, Q.; Wei, T.; Zhi, L.; Wei, F. Template-Directed Synthesis of Pillared-Porous Carbon Nanosheet Architectures: High-Performance Electrode Materials for Supercapacitors. Adv. Energy Mater. 2012, 2, 419–424. [Google Scholar] [CrossRef]
- Peng, L.; Xiong, P.; Ma, L.; Yuan, Y.; Zhu, Y.; Chen, D.; Luo, X.; Lu, J.; Amine, K.; Yu, G. Holey two-dimensional transition metal oxide nanosheets for efficient energy storage. Nat. Commun. 2017, 8, 15139. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Backes, C.; Higgins, T.M.; Kelly, A.; Boland, C.; Harvey, A.; Hanlon, D.; Coleman, J.N. Guidelines for Exfoliation, Characterization and Processing of Layered Materials Produced by Liquid Exfoliation. Chem. Mater. 2017, 29, 243–255. [Google Scholar] [CrossRef]
- Walker, G.F.; Garrett, W.G. Chemical Exfoliation of Vermiculite and the Production of Colloidal Dispersions. Science 1967, 156, 385–387. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Zhang, L.; Hu, Z.; Yu, L.; Jiang, T.; Lu, C.; Yan, C.; Sun, J.; Liu, Z. Caging Nb2O5 Nanowires in PECVD-Derived Graphene Capsules toward Bendable Sodium-Ion Hybrid Supercapacitors. Adv. Mater. 2018, 30, 1800963. [Google Scholar] [CrossRef]
- Gao, L.; Cao, M.; Fu, Y.Q.; Zhong, Z.; Shen, Y.; Wang, M. Hierarchical TiO2 spheres assisted with graphene for a high performance lithium–sulfur battery. J. Mater. Chem. A 2016, 4, 16454–16461. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, H.; Zhang, D.; Ma, Y.; Bi, X.; Yang, S. Vertically oriented growth of MoO3 nanosheets on graphene for superior lithium storage. J. Mater. Chem. A 2018, 6, 672–679. [Google Scholar] [CrossRef]
- Ma, C.; Liao, Q.; Sun, H.; Lei, S.; Zheng, Y.; Yin, R.; Zhao, A.; Li, Q.; Wang, B. Tuning the Doping Types in Graphene Sheets by N Monoelement. Nano Lett. 2018, 18, 386–394. [Google Scholar] [CrossRef]
- Orangi, J.; Tetik, H.; Parandoush, P.; Kayali, E.; Lin, D.; Beidaghi, M. Conductive and highly compressible MXene aerogels with ordered microstructures as high-capacity electrodes for Li-ion capacitors. Mater. Today Adv. 2021, 9, 100135. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, B.; Hu, L.; Xu, Q.; Li, Y.; Liu, D.; Xu, M. Synthesis of SnS nanoparticle-modified MXene (Ti3C2Tx) composites for enhanced sodium storage. J. Alloys Compd. 2018, 732, 448–453. [Google Scholar] [CrossRef]
- Huang, H.; Cui, J.; Liu, G.; Bi, R.; Zhang, L. Carbon-Coated MoSe2/MXene Hybrid Nanosheets for Superior Potassium Storage. ACS Nano 2019, 13, 3448–3456. [Google Scholar] [CrossRef]
- Manthiram, A. An Outlook on Lithium Ion Battery Technology. An Outlook on Lithium Ion Battery Technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 1800561. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhou, G.; Boyle, D.; Wan, J.; Wang, H.; Lin, D.; Mackanic, D.; Zhang, Z.; Kim, S.C.; Lee, H.R.; et al. Electrode Design with Integration of High Tortuosity and Sulfur-Philicity for High-Performance Lithium-Sulfur Battery. Matter 2020, 2, 1605–1620. [Google Scholar] [CrossRef]
- Lei, J.; Fan, X.X.; Liu, T.; Xu, P.; Hou, Q.; Li, K.; Yuan, R.M.; Zheng, M.S.; Dong, Q.F.; Chen, J.J. Single-dispersed polyoxometalate clusters embedded on multilayer graphene as a bifunctional electrocatalyst for efficient Li-S batteries. Nat. Commun. 2022, 13, 202. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Chen, S.; Chen, P.; Wang, Y. Carbon nanotubes grown in situ on graphene nanosheets as superior anodes for Li-ion batteries. Nanoscale 2011, 3, 4323–4329. [Google Scholar] [CrossRef]
- Li, S.; Luo, Y.; Lv, W.; Yu, W.; Wu, S.; Hou, P.; Yang, Q.; Meng, Q.; Liu, C.; Cheng, H.-M. Vertically Aligned Carbon Nanotubes Grown on Graphene Paper as Electrodes in Lithium-Ion Batteries and Dye-Sensitized Solar Cells. Adv. Energy Mater. 2011, 1, 486–490. [Google Scholar] [CrossRef]
- Bae, S.-H.; Karthikeyan, K.; Lee, Y.-S.; Oh, I.-K. Microwave self-assembly of 3D graphene-carbon nanotube-nickel nanostructure for high capacity anode material in lithium ion battery. Carbon 2013, 64, 527–536. [Google Scholar] [CrossRef]
- Fang, S.; Shen, L.; Zheng, H.; Zhang, X. Ge–graphene–carbon nanotube composite anode for high performance lithium-ion batteries. J. Mater. Chem. A 2015, 3, 1498–1503. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, J.; Han, Y.; Gong, X.; Yang, Y.; Yang, G. The composite of carbon nanotube connecting SnO2/reduced graphene clusters as highly reversible anode material for lithium-/sodium-ion batteries and full cell. Compos. B. Eng. 2019, 169, 109–117. [Google Scholar] [CrossRef]
- Woo, H.; Wi, S.; Kim, J.; Kim, J.; Lee, S.; Hwang, T.; Kang, J.; Kim, J.; Park, K.; Gil, B.; et al. Complementary surface modification by disordered carbon and reduced graphene oxide on SnO2 hollow spheres as an anode for Li-ion battery. Carbon 2018, 129, 342–348. [Google Scholar] [CrossRef]
- Asiri, A.M.; Akhtar, K.; Khan, S.B. Cobalt oxide nanocomposites and their electrocatalytic behavior for oxygen evolution reaction. Ceram. Int. 2019, 45, 13340–13346. [Google Scholar] [CrossRef]
- Luo, J.; Li, F.; Zhou, Y.; Liu, S.; Ma, J.; Liu, J. Paper-like TiO2/graphene-carbon nanotube hybrid electrode with high mass loading: Toward high-performance lithium ion battery. J. Alloys Compd. 2018, 749, 697–704. [Google Scholar] [CrossRef]
- Ren, J.; Ren, R.-P.; Lv, Y.-K. A flexible 3D graphene@CNT@MoS2 hybrid foam anode for high-performance lithium-ion battery. Chem. Eng. J. 2018, 353, 419–424. [Google Scholar] [CrossRef]
- Zhai, X.; Mao, Z.; Zhao, G.; Rooney, D.; Zhang, N.; Sun, K. Nanoflake δ-MnO2 deposited on carbon nanotubes-graphene-Ni foam scaffolds as self-standing three-dimensional porous anodes for high-rate-performance lithium-ion batteries. J. Power Sources 2018, 402, 373–380. [Google Scholar] [CrossRef]
- Ma, H.; Du, S.; Tao, H.; Li, T.; Zhang, Y. Three-dimensionally integrated carbon tubes/MoS2 with reduced graphene oxide foam as a binder-free anode for lithium ion battery. J. Electroanal. Chem. 2018, 823, 307–314. [Google Scholar] [CrossRef]
- Tian, S.; Zhu, G.; Tang, Y.; Xie, X.; Wang, Q.; Ma, Y.; Ding, G.; Xie, X. Three-dimensional cross-linking composite of graphene, carbon nanotubes and Si nanoparticles for lithium ion battery anode. Nanotechnology 2018, 29, 125603. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, F.; Gollon, S.; Yuan, C. Micro Silicon–Graphene–Carbon Nanotube Anode for Full Cell Lithium-ion Battery. J. Electrochem. Energy Convers. Storage 2018, 16, 011009. [Google Scholar] [CrossRef]
- Wang, M.-S.; Wang, Z.-Q.; Jia, R.; Yang, Z.-L.; Yang, Y.; Zhu, F.-Y.; Huang, Y.; Li, X. Nano tin dioxide anchored onto carbon nanotube/graphene skeleton as anode material with superior lithium-ion storage capability. J. Electroanal. Chem. 2018, 815, 30–39. [Google Scholar] [CrossRef]
- Cheng, D.; Zhao, Y.; Tang, X.; An, T.; Wang, X.; Zhou, H.; Zhang, D.; Fan, T. Densely integrated Co, N-Codoped Graphene@Carbon nanotube porous hybrids for high-performance lithium-sulfur batteries. Carbon 2019, 149, 750–759. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, X.; Wu, Z.-S.; Dong, Y.; Lu, P.; Chen, J.; Ren, W.; Cheng, H.-M.; Bao, X. Free-standing integrated cathode derived from 3D graphene/carbon nanotube aerogels serving as binder-free sulfur host and interlayer for ultrahigh volumetric-energy-density lithiumsulfur batteries. Nano Energy 2019, 60, 743–751. [Google Scholar] [CrossRef]
- Wang, N.; Peng, S.; Chen, X.; Wang, J.; Wang, C.; Qi, X.; Dai, S.; Yan, S. Construction of ultrathin MnO2 decorated graphene/carbon nanotube nanocomposites as efficient sulfur hosts for high-performance lithium–sulfur batteries. RSC Adv. 2019, 9, 6346–6355. [Google Scholar] [CrossRef] [Green Version]
- Geng, X.; Yi, R.; Yu, Z.; Zhao, C.; Li, Y.; Wei, Q.; Liu, C.; Zhao, Y.; Lu, B.; Yang, L. Isothermal sulfur condensation into carbon nanotube/nitrogen-doped graphene composite for high performance lithium–sulfur batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 10071–10081. [Google Scholar] [CrossRef]
- Raji, A.-R.O.; Villegas Salvatierra, R.; Kim, N.D.; Fan, X.; Li, Y.; Silva, G.A.L.; Sha, J.; Tour, J.M. Lithium Batteries with Nearly Maximum Metal Storage. ACS Nano 2017, 11, 6362–6369. [Google Scholar] [CrossRef] [Green Version]
- Ozanam, F.; Rosso, M. Silicon as anode material for Li-ion batteries. Mater. Sci. Eng. B 2016, 213, 2–11. [Google Scholar] [CrossRef]
- Gu, M.; He, Y.; Zheng, J.; Wang, C. Nanoscale silicon as anode for Li-ion batteries: The fundamentals, promises, and challenges. Nano Energy 2015, 17, 366–383. [Google Scholar] [CrossRef] [Green Version]
- Du, F.-H.; Wang, K.-X.; Chen, J.-S. Strategies to succeed in improving the lithium-ion storage properties of silicon nanomaterials. J. Mater. Chem. A 2016, 4, 32–50. [Google Scholar] [CrossRef]
- Ko, M.; Chae, S.; Cho, J.J.C. Challenges in accommodating volume change of Si anodes for Li-ion batteries. ChemElectroChem 2015, 2, 1645–1651. [Google Scholar] [CrossRef] [Green Version]
- Grigoras, K.; Keskinen, J.; Grönberg, L.; Yli-Rantala, E.; Laakso, S.; Välimäki, H.; Kauranen, P.; Ahopelto, J.; Prunnila, M. Conformal titanium nitride in a porous silicon matrix: A nanomaterial for in-chip supercapacitors. Nano Energy 2016, 26, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Karuppiah, S.; Keller, C.; Kumar, P.; Jouneau, P.H.; Aldakov, D.; Ducros, J.B.; Lapertot, G.; Chenevier, P.; Haon, C. A Scalable Silicon Nanowires-Grown-On-Graphite Composite for High-Energy Lithium Batteries. ACS Nano 2020, 14, 12006–12015. [Google Scholar] [CrossRef]
- Jing, W.T.; Yang, C.C.; Jiang, Q. Recent progress on metallic Sn- and Sb-based anodes for sodium-ion batteries. J. Mater. Chem. A 2020, 8, 2913–2933. [Google Scholar] [CrossRef]
- Park, M.-G.; Lee, D.-H.; Jung, H.; Choi, J.-H.; Park, C.-M. Sn-Based Nanocomposite for Li-Ion Battery Anode with High Energy Density, Rate Capability, and Reversibility. ACS Nano 2018, 12, 2955–2967. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T.; Wei, S.; Gao, W. Nitrogen-doped carbon hollow spheres packed with multiple nano Sn particles for enhanced lithium storage. Chem. Eng. J. 2022, 446, 136768. [Google Scholar] [CrossRef]
- Wang, G.; Aubin, M.; Mehta, A.; Tian, H.; Chang, J.; Kushima, A.; Sohn, Y.; Yang, Y. Stabilization of Sn Anode through Structural Reconstruction of a Cu–Sn Intermetallic Coating Layer. Adv. Mater. 2020, 32, 2003684. [Google Scholar] [CrossRef]
- Chen, D.; Tang, J.; Zhang, X.; Fang, J.; Li, Y.; Zhuo, R. Detecting decompositions of sulfur hexafluoride using reduced graphene oxide decorated with Pt nanoparticles. J. Phys. D 2018, 51, 185304. [Google Scholar] [CrossRef]
- Yang, L.; Yang, F.; Liu, X.; Li, K.; Zhou, Y.; Wang, Y.; Yu, T.; Zhong, M.; Xu, X.; Zhang, L.; et al. A moisture-enabled fully printable power source inspired by electric eels. Proc. Natl. Acad. Sci. USA 2021, 118, 2023164118. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Y.; Cheng, H.; Jiang, L.; Qu, L. Hygroelectric Generators: Rollable, Stretchable, and Reconfigurable Graphene Hygroelectric Generators. Adv. Mater. 2019, 31, 1970013. [Google Scholar] [CrossRef]
- Zhu, R.; Zhu, Y.; Chen, F.; Patterson, R.; Zhou, Y.; Wan, T.; Hu, L.; Wu, T.; Joshi, R.; Li, M.; et al. Boosting moisture induced electricity generation from graphene oxide through engineering oxygen-based functional groups. Nano Energy 2022, 94, 106942. [Google Scholar] [CrossRef]
- Xu, T.; Ding, X.; Huang, Y.; Shao, C.; Song, L.; Gao, X.; Zhang, Z.; Qu, L. An efficient polymer moist-electric generator. Energy Environ. Sci. 2019, 12, 972–978. [Google Scholar] [CrossRef]
- Chen, S.; Xia, H.; Ni, Q.-Q. A sustainable, continuously expandable, wearable breath moisture-induced electricity generator. Carbon 2022, 194, 104–113. [Google Scholar] [CrossRef]
- Lu, Q.; Shi, W.; Yang, H.; Wang, X.J.A.M. Nanoconfined Water-Molecule Channels for High-Yield Solar Vapor Generation under Weaker Sunlight. Adv. Mater. 2020, 32, 2001544. [Google Scholar] [CrossRef]
- Liang, H.; Liao, Q.; Chen, N.; Liang, Y.; Lv, G.; Zhang, P.; Lu, B.; Qu, L.J.A.C. Thermal Efficiency of Solar Steam Generation Approaching 100% through Capillary Water Transport. Angew. Chem. Int. Ed. 2019, 58, 19041–19046. [Google Scholar] [CrossRef]
- Yao, J.; Zheng, Z.; Yang, G. Layered tin monoselenide as advanced photothermal conversion materials for efficient solar energy-driven water evaporation. Nanoscale 2018, 10, 2876–2886. [Google Scholar] [CrossRef]
- Cheng, S.; Yu, Z.; Lin, Z.; Li, L.; Mao, Z.J.C.E.J. A lotus leaf like vertical hierarchical solar vapor generator for stable and efficient evaporation of high-salinity brine. Chem. Eng. J. 2020, 401, 126108. [Google Scholar] [CrossRef]
- Soo Joo, B.; Soo Kim, I.; Ki Han, I.; Ko, H.; Gu Kang, J.; Kang, G. Plasmonic silicon nanowires for enhanced heat localization and interfacial solar steam generation. Appl. Surf. Sci. 2022, 583, 152563. [Google Scholar] [CrossRef]
- Xiao, C.; Hasi, Q.; Wang, S.; Zhang, Y.; Li, H.; Zhang, L.; Chen, L.; Li, A. Hollow SiO2 microspheres in-situ doped poly(ionicliquid)s gels as efficient solar steam generators for desalination. J. Colloid Interface Sci. 2022, 613, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhong, G.; Kübel, C.; Jelle, A.A.; Qian, C.; Wang, L.; Ebrahimi, M.; Reyes, L.M.; Helmy, A.S.; Ozin, G.A.J.A.C. Size-Tunable Photothermal Germanium Nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 6329–6334. [Google Scholar] [CrossRef] [PubMed]
- Gai, W.; Zhao, D.L.; Chung, T.S. Thin film nanocomposite hollow fiber membranes comprising Na+-functionalized carbon quantum dots for brackish water desalination. Water Res. 2019, 154, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Fathizadeh, M.; Tien, H.N.; Khivantsev, K.; Song, Z.; Zhou, F.; Yu, M. Polyamide/nitrogen-doped graphene oxide quantum dots (N-GOQD) thin film nanocomposite reverse osmosis membranes for high flux desalination. Desalination 2019, 451, 125–132. [Google Scholar] [CrossRef]
- Chao, W.; Li, Y.; Sun, X.; Cao, G.; Wang, C.; Ho, S.-H. Enhanced wood-derived photothermal evaporation system by in-situ incorporated lignin carbon quantum dots. Chem. Eng. J. 2021, 405, 126703. [Google Scholar] [CrossRef]
- Hamasaki, H.; Takimoto, S.; Hirahara, K.J.N.L. Visualization of Thermal Transport within and between Carbon Nanotubes. Nano Letters 2021, 21, 3134–3138. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Song, J.; Yang, Z.; Kuang, Y.; Hitz, E.; Jia, C.; Gong, A.; Jiang, F.; Zhu, J.Y.; et al. Highly Flexible and Efficient Solar Steam Generation Device. Adv. Mater. 2017, 29, 1701756. [Google Scholar] [CrossRef]
- Zhu, Y.; Tian, G.; Liu, Y.; Li, H.; Zhang, P.; Zhan, L.; Gao, R.; Huang, C. Low-Cost, Unsinkable, and Highly Efficient Solar Evaporators Based on Coating MWCNTs on Nonwovens with Unidirectional Water-Transfer. Adv. Sci. 2021, 8, 2101727. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Shi, J.; Hou, X.; Guo, C.F.J.A.N. Shape-Programmable Interfacial Solar Evaporator with Salt-Precipitation Monitoring Function. ACS Nano 2021, 15, 5752–5761. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, F.; Liao, Q.; Yao, H.; Geng, H.; Cheng, H.; Li, C.; Qu, L. A Microstructured Graphene/Poly(N-isopropylacrylamide) Membrane for Intelligent Solar Water Evaporation. Angew. Chem. Int. Ed. 2018, 57, 16343–16347. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Zhou, Z.; Wang, Z.; Ho, D.T.; Sheng, G.; Chen, L.; Zhao, Y.; Li, X.; Cao, L.; Schwingenschlögl, U.; et al. An Ultrahigh-Flux Nanoporous Graphene Membrane for Sustainable Seawater Desalination using Low-Grade Heat. Adv. Mater. 2022, 34, 2109718. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Lv, J.; Shi, M.; Wang, L.; Yang, T.; Yang, Y.; Chen, N. Low-Dimensional Nanomaterial Systems Formed by IVA Group Elements Allow Energy Conversion Materials to Flourish. Nanomaterials 2022, 12, 2521. https://doi.org/10.3390/nano12152521
Li D, Lv J, Shi M, Wang L, Yang T, Yang Y, Chen N. Low-Dimensional Nanomaterial Systems Formed by IVA Group Elements Allow Energy Conversion Materials to Flourish. Nanomaterials. 2022; 12(15):2521. https://doi.org/10.3390/nano12152521
Chicago/Turabian StyleLi, Dan, Jinsheng Lv, Mengfan Shi, Liru Wang, Tian Yang, Ya’nan Yang, and Nan Chen. 2022. "Low-Dimensional Nanomaterial Systems Formed by IVA Group Elements Allow Energy Conversion Materials to Flourish" Nanomaterials 12, no. 15: 2521. https://doi.org/10.3390/nano12152521
APA StyleLi, D., Lv, J., Shi, M., Wang, L., Yang, T., Yang, Y., & Chen, N. (2022). Low-Dimensional Nanomaterial Systems Formed by IVA Group Elements Allow Energy Conversion Materials to Flourish. Nanomaterials, 12(15), 2521. https://doi.org/10.3390/nano12152521






