CO2 Activation and Hydrogenation on Cu-ZnO/Al2O3 Nanorod Catalysts: An In Situ FTIR Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation
- (i)
- Nanorods Al2O3 preparation.
- (ii)
- Catalyst preparation.
2.2. Characterization
2.3. Catalytic Performance Evaluation
3. Results and Discussion
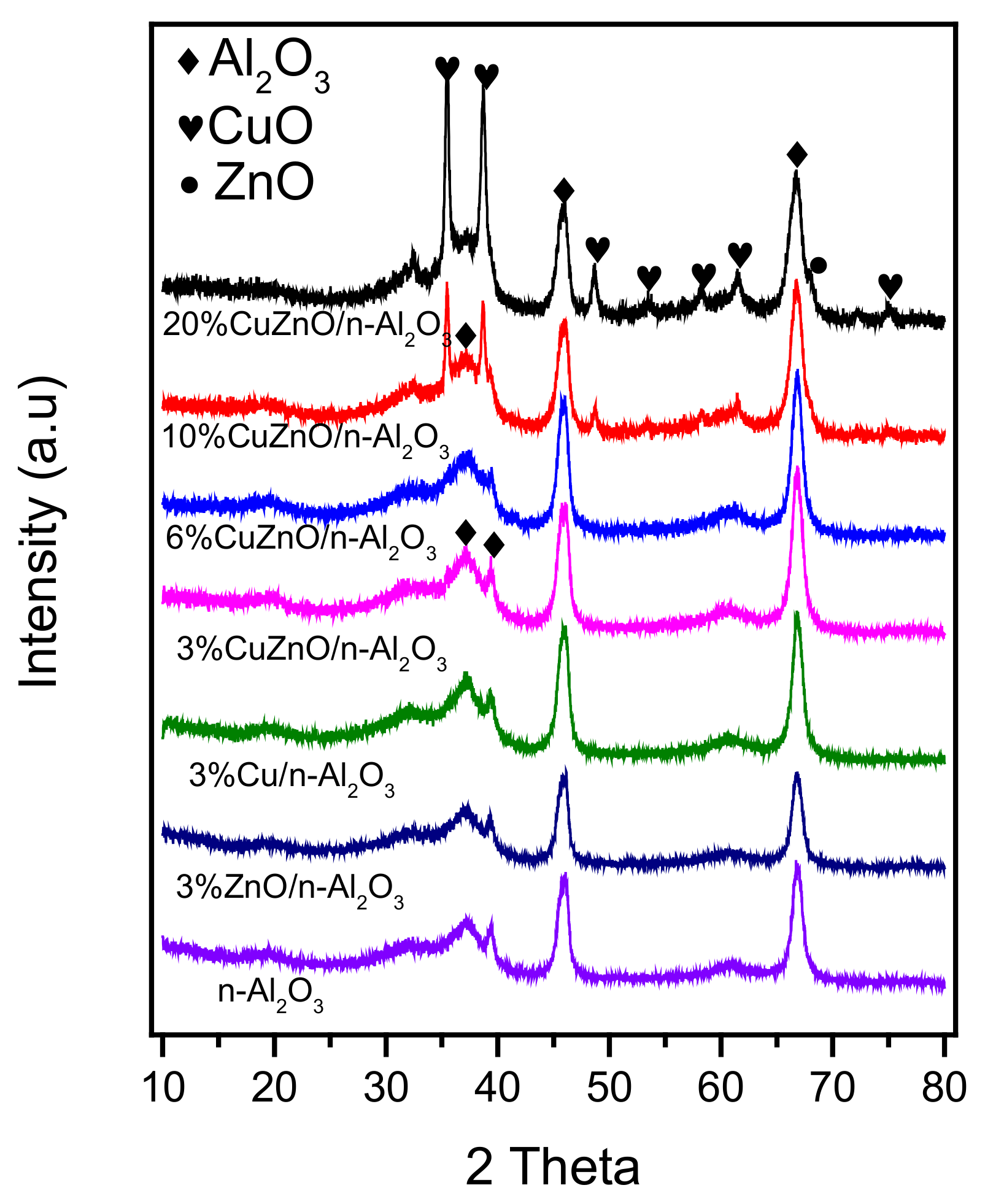
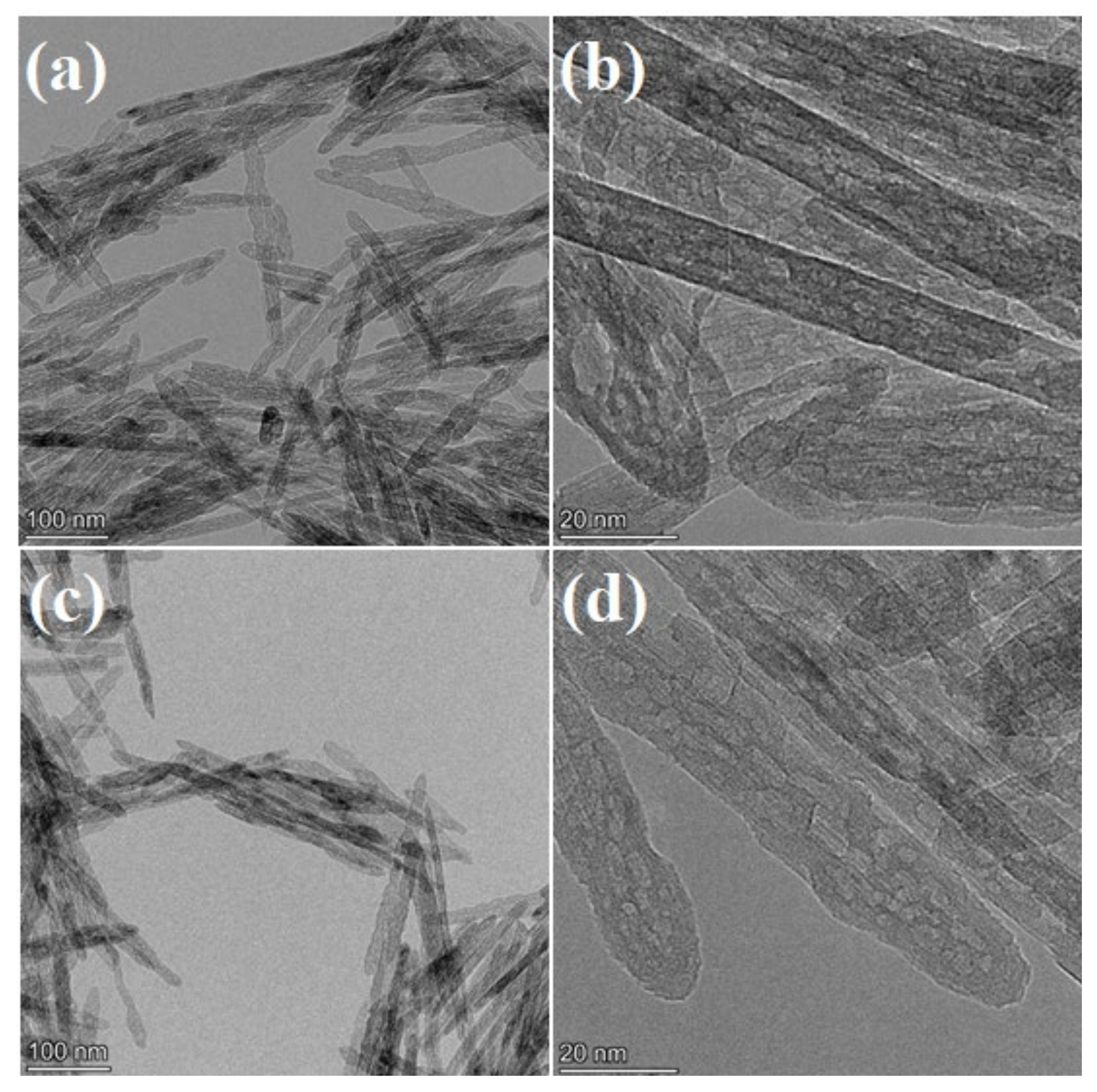
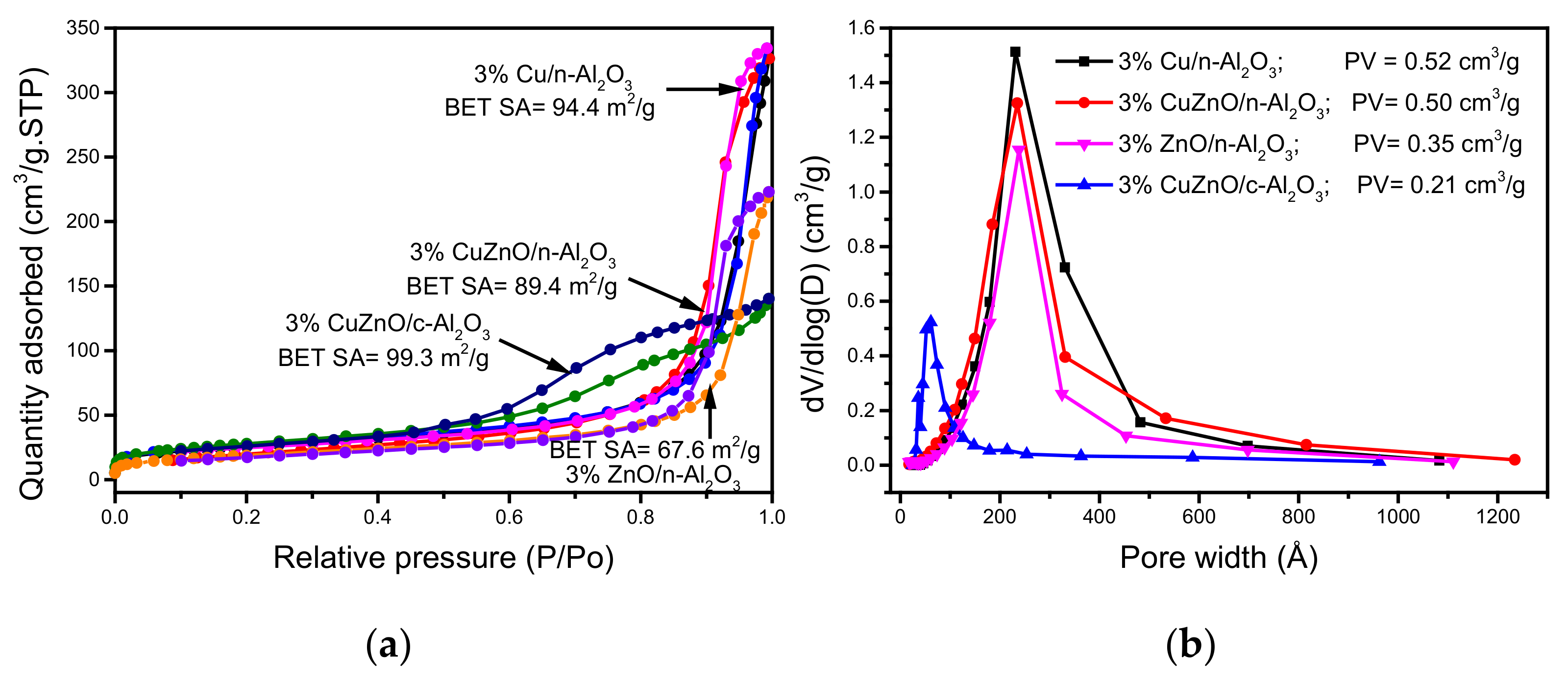
3.1. Catalyst Structure, Morphology and Textural Properties
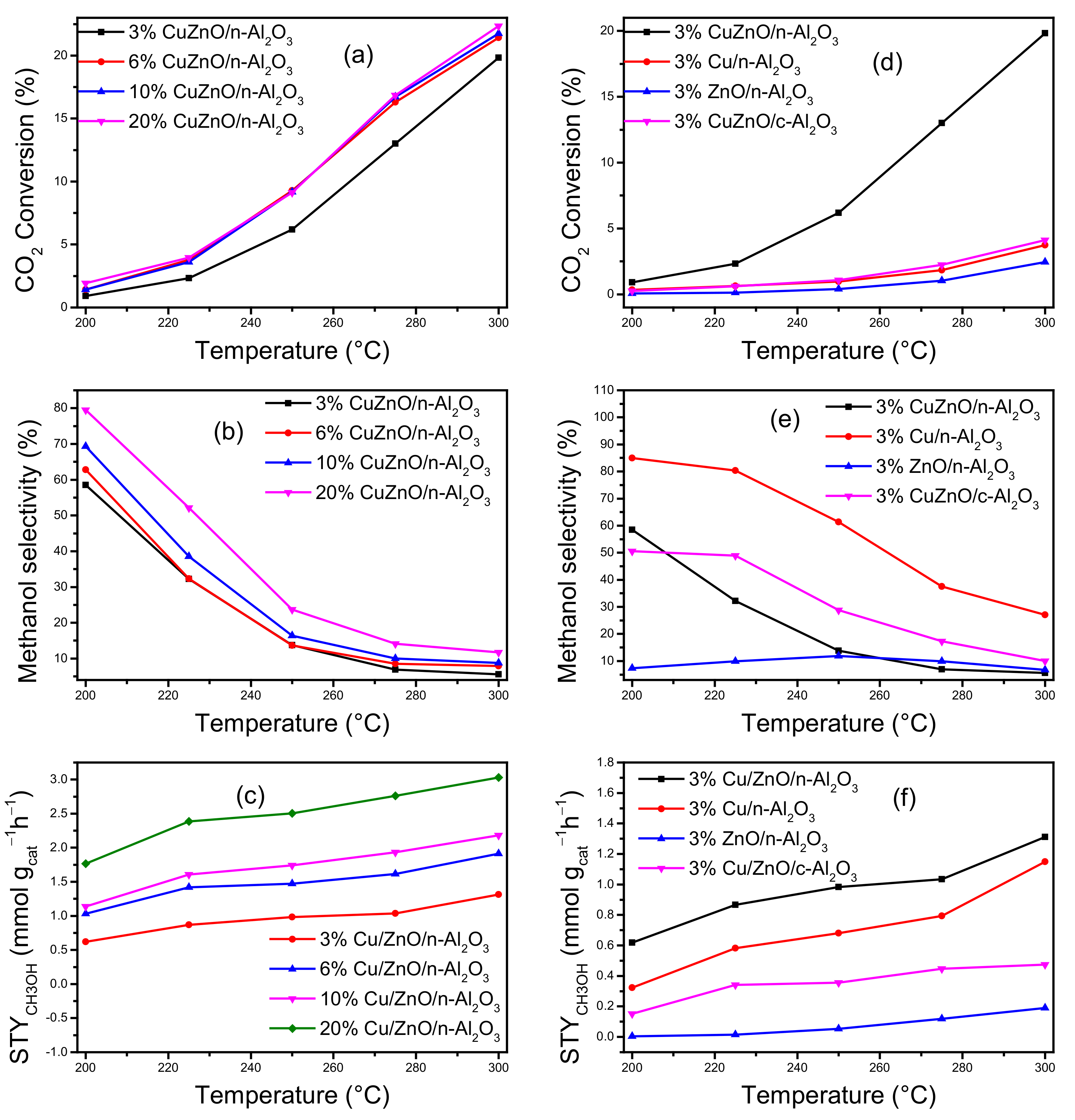
3.2. Catalytic Performance Evaluation
3.2.1. CO2 Hydrogenation to Methanol
3.2.2. DRIFTS Study on the CO2 Activation
- (i)
- CO2 activation on n-Al2O3
- (ii)
- CO2 activation on Cu/Al2O3 and ZnO/n-Al2O3
- (iii)
- CO2 activation on CuZnO/Al2O3.
3.2.3. DRIFTS Study of Methanol Formation from CO2 Hydrogenation
3.3. Roles of Al2O3, ZnO, and Cu in CO2 Hydrogenation to Methanol
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yang, X.; Boscoboinik, J.A.; Chen, J.G. Molybdenum Carbide as Alternative Catalysts to Precious Metals for Highly Selective Reduction of CO2 to CO. Angew. Chem. Int. Ed. 2014, 53, 6705–6709. [Google Scholar] [CrossRef]
- Studt, F.; Sharafutdinov, I.; Abild-Pedersen, F.; Elkjær, C.F.; Hummelshøj, J.S.; Dahl, S.; Chorkendorff, I.; Nørskov, J.K. Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nat. Chem. 2014, 6, 320–324. [Google Scholar] [CrossRef]
- Sehested, J. Industrial and scientific directions of methanol catalyst development. J. Catal. 2019, 371, 368–375. [Google Scholar] [CrossRef]
- De, S.; Dokania, A.; Ramirez, A.; Gascon, J. Advances in the Design of Heterogeneous Catalysts and Thermocatalytic Processes for CO2 Utilization. ACS Catal. 2020, 10, 14147–14185. [Google Scholar] [CrossRef]
- Kattel, S.; Liu, P.; Chen, J.G. Tuning selectivity of CO2 hydrogenation reactions at the metal/oxide interface. J. Am. Chem. Soc. 2017, 139, 9739–9754. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Zhu, J.; Zhang, X.; Ding, F.; Zhang, A.; Guo, X.; Song, C. CO2 hydrogenation to methanol over In2O3-based catalysts: From mechanism to catalyst development. ACS Catal. 2021, 11, 1406–1423. [Google Scholar] [CrossRef]
- Lin, L.; Wang, G.; Zhao, F. CO2 Hydrogenation to Methanol on ZnO/ZrO2 Catalysts: Effects of Zirconia Phase. ChemistrySelect 2021, 6, 2119–2125. [Google Scholar] [CrossRef]
- Etim, U.; Song, Y.; Zhong, Z. Improving the Cu/ZnO-Based Catalysts for Carbon Dioxide Hydrogenation to Methanol, and the Use of Methanol as a Renewable Energy Storage Media. Front. Energy Res. 2020, 8. [Google Scholar] [CrossRef]
- Etim, U.J.; Semiat, R.; Zhong, Z. CO2 Valorization Reactions over Cu-Based Catalysts: Characterization and the Nature of Active Sites. Am. J. Chem. Eng. 2021, 9, 53–78. [Google Scholar] [CrossRef]
- Dong, X.; Li, F.; Zhao, N.; Xiao, F.; Wang, J.; Tan, Y. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts prepared by precipitation-reduction method. Appl. Catal. B Environ. 2016, 191, 8–17. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Lekse, J.W.; Baltrus, J.P.; Ohodnicki, P.R., Jr.; Howard, B.H.; Deng, X.; Matranga, C. Active sites and structure–activity relationships of copper-based catalysts for carbon dioxide hydrogenation to methanol. ACS Catal. 2012, 2, 1667–1676. [Google Scholar] [CrossRef]
- Huš, M.; Kopač, D.; Štefančič, N.S.; Jurković, D.L.; Dasireddy, V.D.; Likozar, B. Unravelling the mechanisms of CO2 hydrogenation to methanol on Cu-based catalysts using first-principles multiscale modelling and experiments. Catal. Sci. Technol. 2017, 7, 5900–5913. [Google Scholar] [CrossRef] [Green Version]
- Kuld, S.; Thorhauge, M.; Falsig, H.; Elkjær, C.F.; Helveg, S.; Chorkendorff, I.; Sehested, J. Quantifying the promotion of Cu catalysts by ZnO for methanol synthesis. Science 2016, 352, 969–974. [Google Scholar] [CrossRef] [Green Version]
- Nitta, Y.; Suwata, O.; Ikeda, Y.; Okamoto, Y.; Imanaka, T. Copper-zirconia catalysts for methanol synthesis from carbon dioxide: Effect of ZnO addition to Cu-ZrO2 catalysts. Catal. Lett. 1994, 26, 345–354. [Google Scholar] [CrossRef]
- An, B.; Zhang, J.; Cheng, K.; Ji, P.; Wang, C.; Lin, W. Confinement of Ultrasmall Cu/ZnOx Nanoparticles in Metal–Organic Frameworks for Selective Methanol Synthesis from Catalytic Hydrogenation of CO2. J. Am. Chem. Soc. 2017, 139, 3834–3840. [Google Scholar] [CrossRef]
- Dasireddy, V.D.; Likozar, B. The role of copper oxidation state in Cu/ZnO/Al2O3 catalysts in CO2 hydrogenation and methanol productivity. Renew. Energ. 2019, 140, 452–460. [Google Scholar] [CrossRef]
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B.-L. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 2012, 336, 893–897. [Google Scholar] [CrossRef]
- Liang, B.; Ma, J.; Su, X.; Yang, C.; Duan, H.; Zhou, H.; Deng, S.; Li, L.; Huang, Y. Investigation on Deactivation of Cu/ZnO/Al2O3 Catalyst for CO2 Hydrogenation to Methanol. Ind. Eng. Chem. Res. 2019, 58, 9030–9037. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, R.; El-Sayed, M.A. Catalysis with Transition Metal Nanoparticles in Colloidal Solution: Nanoparticle Shape Dependence and Stability; ACS Publications: Washington, DC, USA, 2005; pp. 12663–12676. [Google Scholar]
- Sakpal, T.; Lefferts, L. Structure-dependent activity of CeO2 supported Ru catalysts for CO2 methanation. J. Catal. 2018, 367, 171–180. [Google Scholar] [CrossRef]
- Lei, H.; Nie, R.; Wu, G.; Hou, Z. Hydrogenation of CO2 to CH3OH over Cu/ZnO catalysts with different ZnO morphology. Fuel 2015, 154, 161–166. [Google Scholar] [CrossRef]
- Liao, F.; Huang, Y.; Ge, J.; Zheng, W.; Tedsree, K.; Collier, P.; Hong, X.; Tsang, S.C. Morphology—Dependent Interactions of ZnO with Cu Nanoparticles at the Materials’ Interface in Selective Hydrogenation of CO2 to CH3OH. Angew. Chem. Int. Ed. 2011, 50, 2162–2165. [Google Scholar] [CrossRef]
- An, X.; Li, J.; Zuo, Y.; Zhang, Q.; Wang, D.; Wang, J. A Cu/Zn/Al/Zr fibrous catalyst that is an improved CO2 hydrogenation to methanol catalyst. Catal. Lett. 2007, 118, 264–269. [Google Scholar] [CrossRef]
- Huttunen, P.K.; Labadini, D.; Hafiz, S.S.; Gokalp, S.; Wolff, E.P.; Martell, S.M.; Foster, M. DRIFTS investigation of methanol oxidation on CeO2 nanoparticles. Appl. Surf. Sci. 2021, 554, 149518. [Google Scholar] [CrossRef]
- Reddy, K.P.; Choi, H.; Kim, D.; Choi, M.; Ryoo, R.; Park, J.Y. The facet effect of ceria nanoparticles on platinum dispersion and catalytic activity of methanol partial oxidation. Chem Commun 2021, 57, 7382–7385. [Google Scholar] [CrossRef]
- Chen, K.; Fang, H.; Wu, S.; Liu, X.; Zheng, J.; Zhou, S.; Duan, X.; Zhuang, Y.; Tsang, S.C.E.; Yuan, Y. CO2 hydrogenation to methanol over Cu catalysts supported on La-modified SBA-15: The crucial role of Cu–LaOx interfaces. Appl. Catal. B Environ. 2019, 251, 119–129. [Google Scholar] [CrossRef]
- Zhu, J.; Su, Y.; Chai, J.; Muravev, V.; Kosinov, N.; Hensen, E.J. Mechanism and nature of active sites for methanol synthesis from CO/CO2 on Cu/CeO2. ACS Catal. 2020, 10, 11532–11544. [Google Scholar] [CrossRef]
- Shen, S.; Chen, Q.; Chow, P.; Tan, G.; Zeng, X.; Wang, Z.; Tan, R.B. Steam-assisted solid wet-gel synthesis of high-quality nanorods of boehmite and alumina. J. Phy. Chem. C 2007, 111, 700–707. [Google Scholar] [CrossRef]
- Bai, P.; Su, F.; Wu, P.; Wang, L.; Lee, F.Y.; Lv, L.; Yan, Z.-f.; Zhao, X.S. Copolymer-Controlled Homogeneous Precipitation for the Synthesis of Porous Microfibers of Alumina. Langmuir 2007, 23, 4599–4605. [Google Scholar] [CrossRef]
- Martínez, A.; Prieto, G.; Rollán, J. Nanofibrous γ-Al2O3 as support for Co-based Fischer–Tropsch catalysts: Pondering the relevance of diffusional and dispersion effects on catalytic performance. J. Catal. 2009, 263, 292–305. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, L.; Etim, U.J.; Song, Y.; Gazit, O.M.; Zhong, Z. Oxygen vacancies in Cu/TiO2 boost strong metal-support interaction and CO2 hydrogenation to methanol. J. Catal. 2022, 413, 284–296. [Google Scholar] [CrossRef]
- Wang, L.-X.; Guan, E.; Wang, Z.; Wang, L.; Gong, Z.; Cui, Y.; Yang, Z.; Wang, C.; Zhang, J.; Meng, X. Dispersed nickel boosts catalysis by copper in CO2 hydrogenation. ACS Catal. 2020, 10, 9261–9270. [Google Scholar] [CrossRef]
- Kattel, S.; Yan, B.; Yang, Y.; Chen, J.G.; Liu, P. Optimizing binding energies of key intermediates for CO2 hydrogenation to methanol over oxide-supported copper. J. Am. Chem. Soc. 2016, 138, 12440–12450. [Google Scholar] [CrossRef]
- Bansode, A.; Tidona, B.; von Rohr, P.R.; Urakawa, A. Impact of K and Ba promoters on CO2 hydrogenation over Cu/Al2O3 catalysts at high pressure. Catal. Sci. Technol. 2013, 3, 767–778. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Zhen, Y.; Chen, M.; Wan, H. In situ FTIR and ex situ XPS/HS-LEIS study of supported Cu/Al2O3 and Cu/ZnO catalysts for CO2 hydrogenation. Chin. J. Catal. 2021, 42, 367–375. [Google Scholar] [CrossRef]
- Bando, K.K.; Sayama, K.; Kusama, H.; Okabe, K.; Arakawa, H. In-situ FT-IR study on CO2 hydrogenation over Cu catalysts supported on SiO2, Al2O, and TiO2. Appl. Catal. A Gen. 1997, 165, 391–409. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Jensen, J.H.; Grassian, V.H. FTIR spectroscopy combined with isotope labeling and quantum chemical calculations to investigate adsorbed bicarbonate formation following reaction of carbon dioxide with surface hydroxyl groups on Fe2O3 and Al2O3. J. Phys. Chem. B 2006, 110, 12005–12016. [Google Scholar] [CrossRef] [PubMed]
- Sakong, S.; Groß, A. Dissociative adsorption of hydrogen on strained Cu surfaces. Surf. Sci. 2003, 525, 107–118. [Google Scholar] [CrossRef]
- Fujita, S.-I.; Usui, M.; Ohara, E.; Takezawa, N. Methanol synthesis from carbon dioxide at atmospheric pressure over Cu/ZnO catalyst. Role of methoxide species formed on ZnO support. Catal. Lett. 1992, 13, 349–358. [Google Scholar] [CrossRef]
- Bailey, S.; Froment, G.; Snoeck, J.-W.; Waugh, K. A DRIFTS study of the morphology and surface adsorbate composition of an operating methanol synthesis catalyst. Catal. Lett. 1994, 30, 99–111. [Google Scholar] [CrossRef]
- Kim, Y.; Trung, T.S.B.; Yang, S.; Kim, S.; Lee, H. Mechanism of the surface hydrogen induced conversion of CO2 to methanol at Cu (111) step sites. ACS Catal. 2016, 6, 1037–1044. [Google Scholar] [CrossRef]
- Liu, C.; Liu, P. Mechanistic study of methanol synthesis from CO2 and H2 on a modified model Mo6S8 cluster. ACS Catal. 2015, 5, 1004–1012. [Google Scholar] [CrossRef]

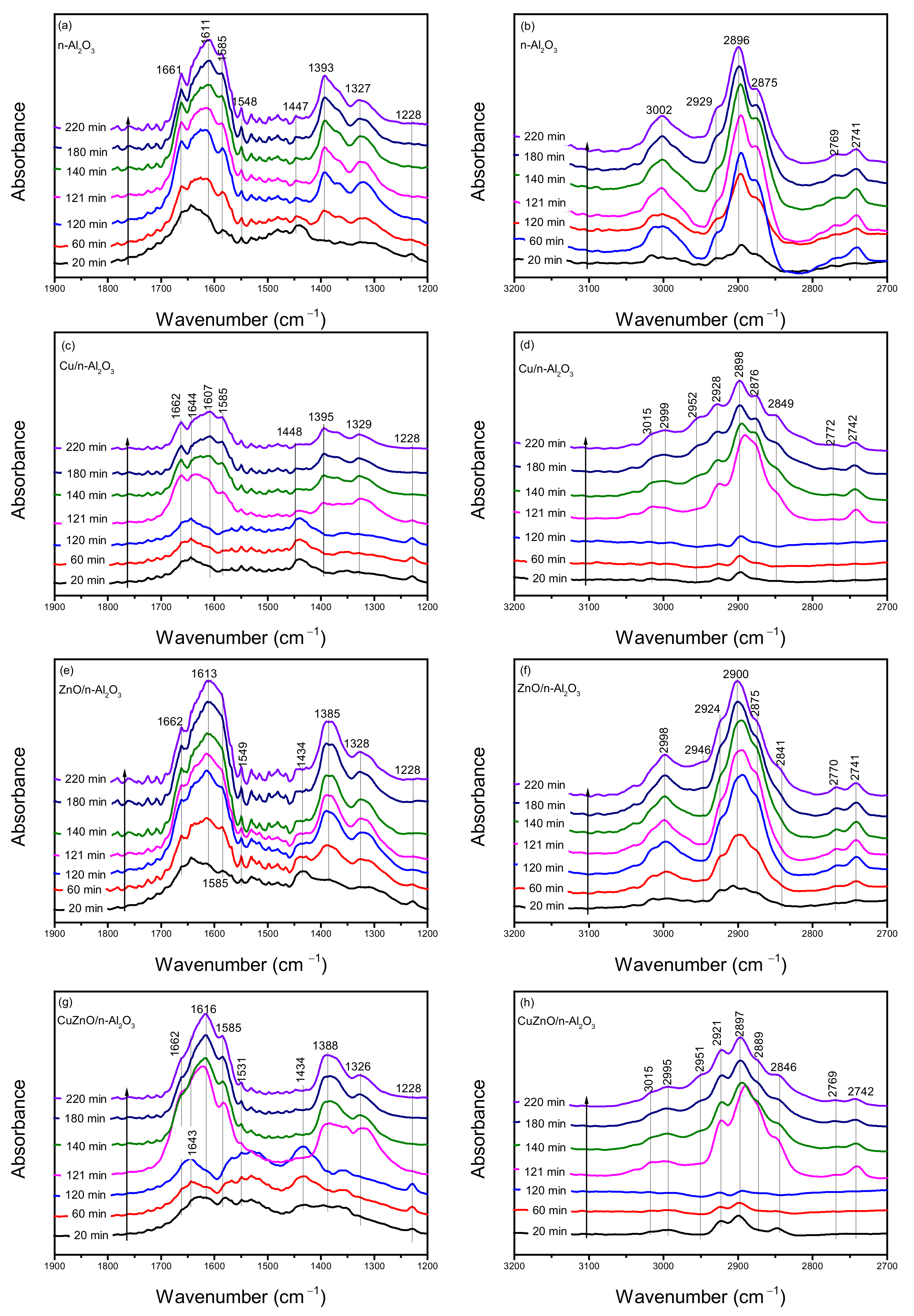

 bicarbonate,
bicarbonate,  formate,
formate,  methoxy,
methoxy,  hydrogen).
hydrogen).
 bicarbonate,
bicarbonate,  formate,
formate,  methoxy,
methoxy,  hydrogen).
hydrogen).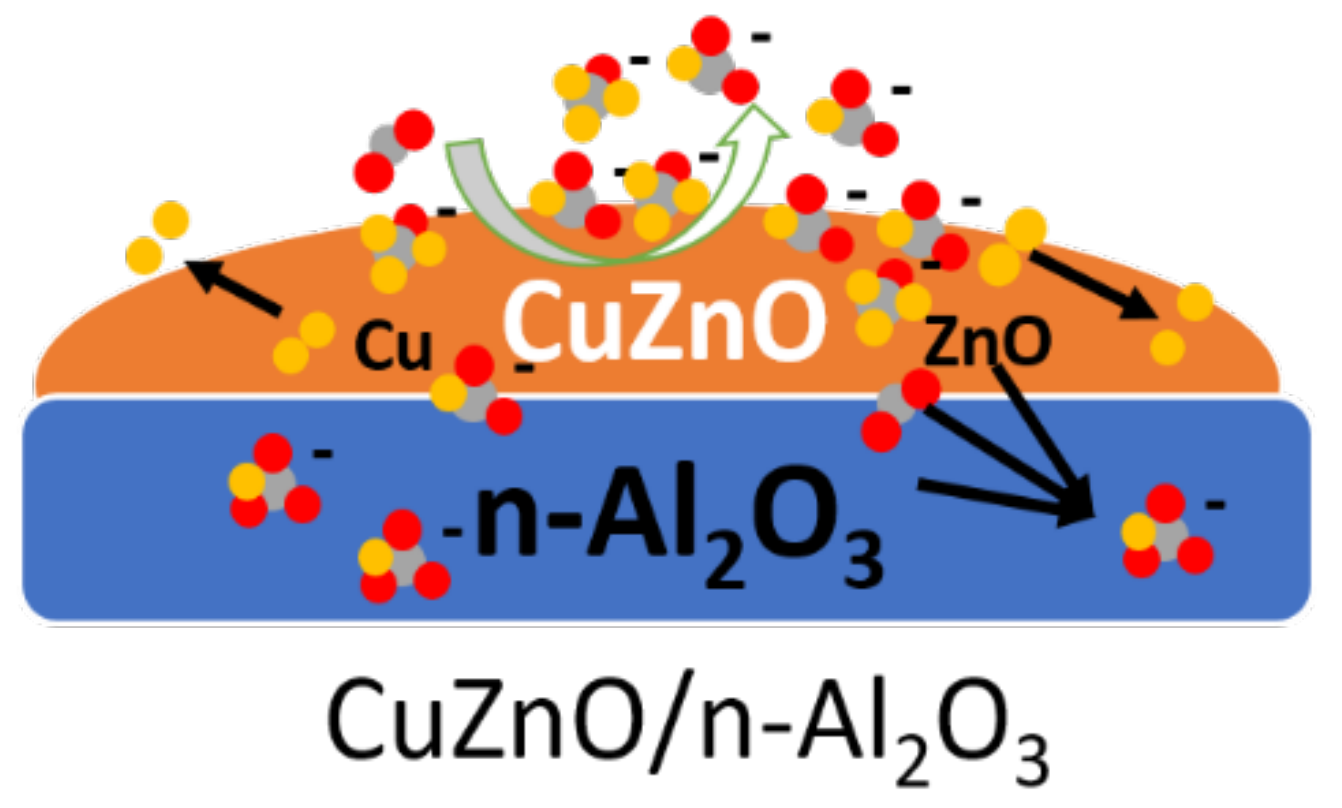
| Element | Cu | Zn | Al | O |
|---|---|---|---|---|
| (At%) | 0.81 | 0.29 | 40.57 | 58.33 |
| (Wt%) | 2.46 | 0.88 | 52.18 | 44.48 |
| Temperature (°C) | n-Al2O3 | 3% ZnO/n-Al2O3 | 3% Cu/n-Al2O3 | 3% CuZnO/n-Al2O3 |
|---|---|---|---|---|
| 50 | Bicarbonate | Bicarbonate (ZnO), bicarbonate (Al2O3) | Bicarbonate (Al2O3) | Bicarbonate (ZnO) |
| 100 | Bicarbonate ↓, monodentate, carbonate bidentate formate | Bidentate carbonate (ZnO) ↓, bicarbonate (Al2O3) ↓, polydentate carbonate (Al2O3 or ZnO), bidentate formate (ZnO and Al2O3) | Bicarbonate (Al2O3) ↓, bidentate formate (Al2O3), polydentate carbonate | Bicarbonate (ZnO) ↓, polydentate carbonate (Al2O3) |
| 150 | Bicarbonate ↓, polydentate carbonate, bidentate formate ↑ | Bidendentate formate (ZnO and Al2O3) ↑, monodentate carbonate (Al2O3) ↓, bicarbonate (Al2O3) ↓, polydentate carbonate (Al2O3) ↑ | Bicarbonate (Al2O3) ↓, bidentate formate (Cu), bidendentate formate (Al2O3) ↑ | Bicarbonate (ZnO) ↑, polydentate carbonate (Al2O3), bidentate formate (Al2O3 and ZnO), bidentate formate (Cu), bidendate formate (ZnO) ↑, methoxy |
| 200 | Bicarbonate ↓, monodentate carbonate ↓, bidentate formate ↑ | Bicarbonate (ZnO) ↑, bidentate carbonate (ZnO) ↑, bidentate formate (ZnO and Al2O3) ↑, polydentate carbonate (ZnO) ↑ | Bicarbonate (Al2O3) ↓, bidentate formate (Cu), bidentate formate (Al2O3) ↑, methoxy | Bidentate formate (Al2O3 and ZnO) ↑, bidentate formate (Cu) ↑, bidentate formate (ZnO) ↑, bidentate formate (Al2O3), methoxy |
| 250 | Bicarbonate ↓, nonodentate carbonate ↓, polydentate carbonate ↓, bidentate formate ↑ | Bicarbonate (ZnO) ↑, bidentate carbonate (ZnO) ↑, bidentate formate (ZnO and Al2O3) ↑, formate (Al2O3 ↑) | Bidendate formate (Cu) ↑, bidentate formate (Al2O3) ↑, methoxy ↑ | Bidentate formate (Al2O3 and ZnO) ↑, bidentate formate (Cu) ↑, bidentate formate (ZnO) ↑, bidentate formate (Al2O3) ↑, methoxy ↑ |
| 300 | Same as in 250 °C | Bicarbonate (ZnO) ↑, bidentate carbonate (ZnO) ↑, bidentate formate (ZnO and Al2O3) ↑, formate (Al2O3) ↑ | Same as in 250°C | Bidendate formate (Al2O3 and ZnO) ↑, bidentate formate (Cu) ↑, Bidentate formate (ZnO) ↑, bidentate formate (Al2O3) ↑, methoxy ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Etim, U.J.; Zhang, C.; Amirav, L.; Zhong, Z. CO2 Activation and Hydrogenation on Cu-ZnO/Al2O3 Nanorod Catalysts: An In Situ FTIR Study. Nanomaterials 2022, 12, 2527. https://doi.org/10.3390/nano12152527
Wang L, Etim UJ, Zhang C, Amirav L, Zhong Z. CO2 Activation and Hydrogenation on Cu-ZnO/Al2O3 Nanorod Catalysts: An In Situ FTIR Study. Nanomaterials. 2022; 12(15):2527. https://doi.org/10.3390/nano12152527
Chicago/Turabian StyleWang, Letian, Ubong Jerome Etim, Chenchen Zhang, Lilac Amirav, and Ziyi Zhong. 2022. "CO2 Activation and Hydrogenation on Cu-ZnO/Al2O3 Nanorod Catalysts: An In Situ FTIR Study" Nanomaterials 12, no. 15: 2527. https://doi.org/10.3390/nano12152527






