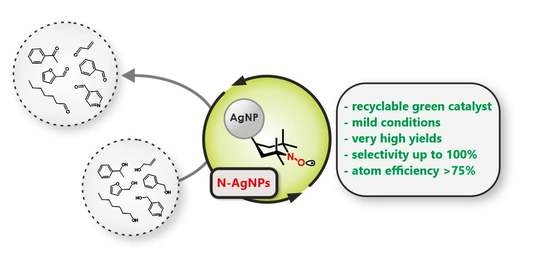
Silver Nanoparticles Densely Grafted with Nitroxides as a Recyclable Green Catalyst in the Selective Oxidation of Alcohols
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Techniques
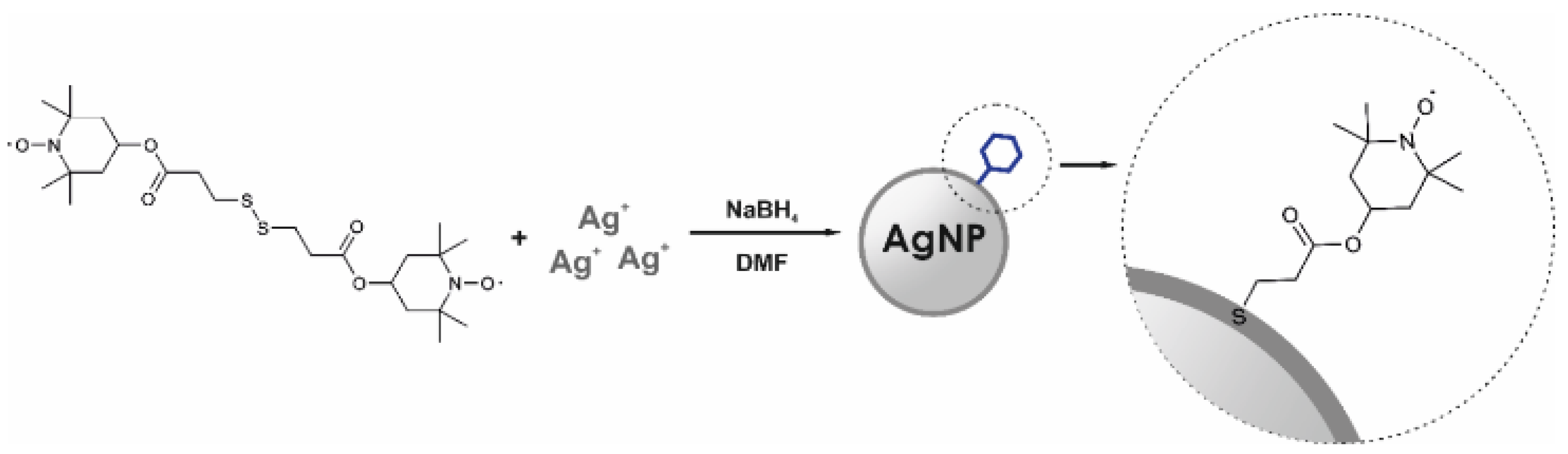
2.3. Preparation of Nitroxide-Coated Silver Nanoparticles (N-AgNPs)
2.4. Representative Procedure for the Oxidation of Alcohols by (bpy)CuI/N-AgNPs Catalytic System
3. Results and Discussion
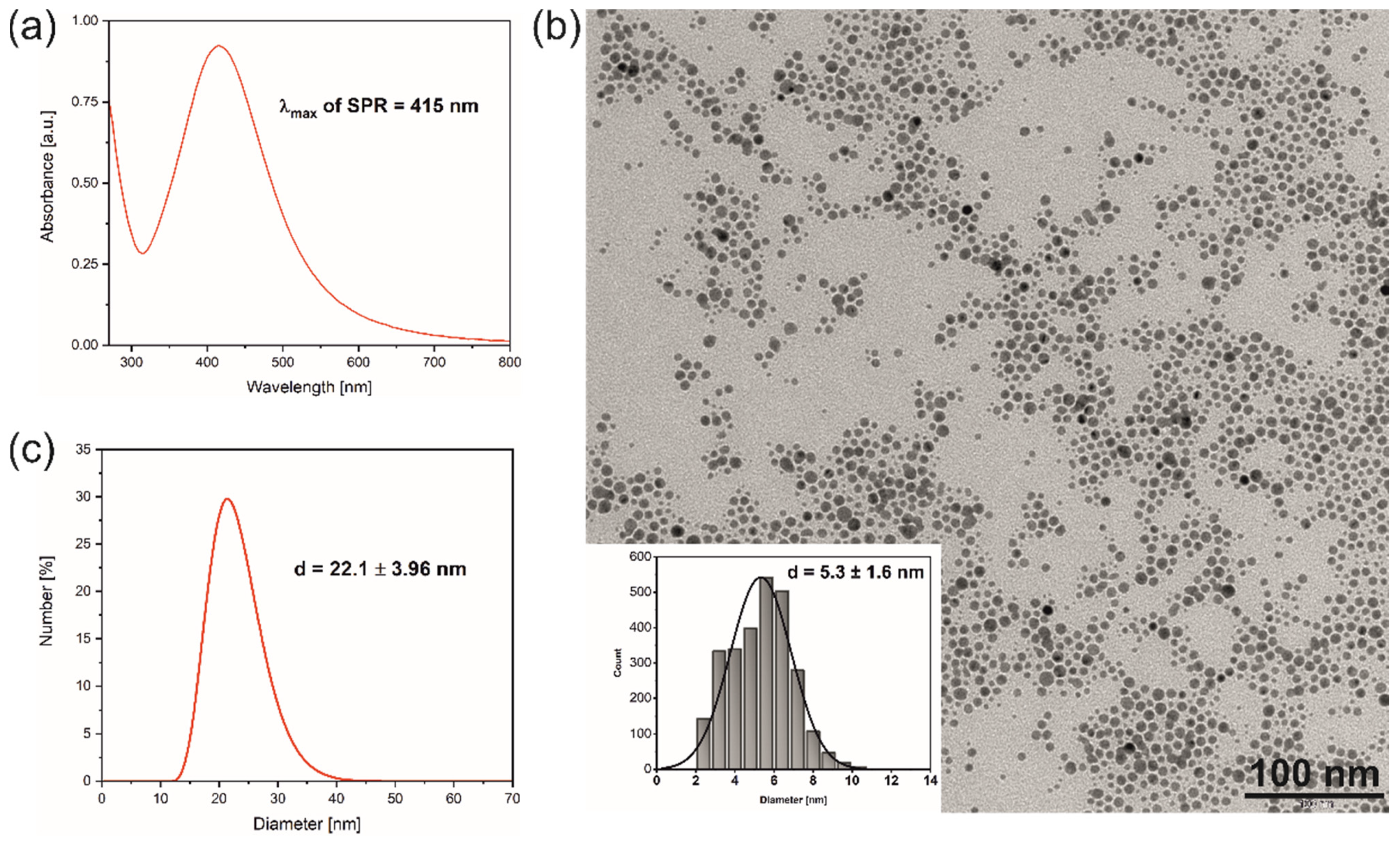
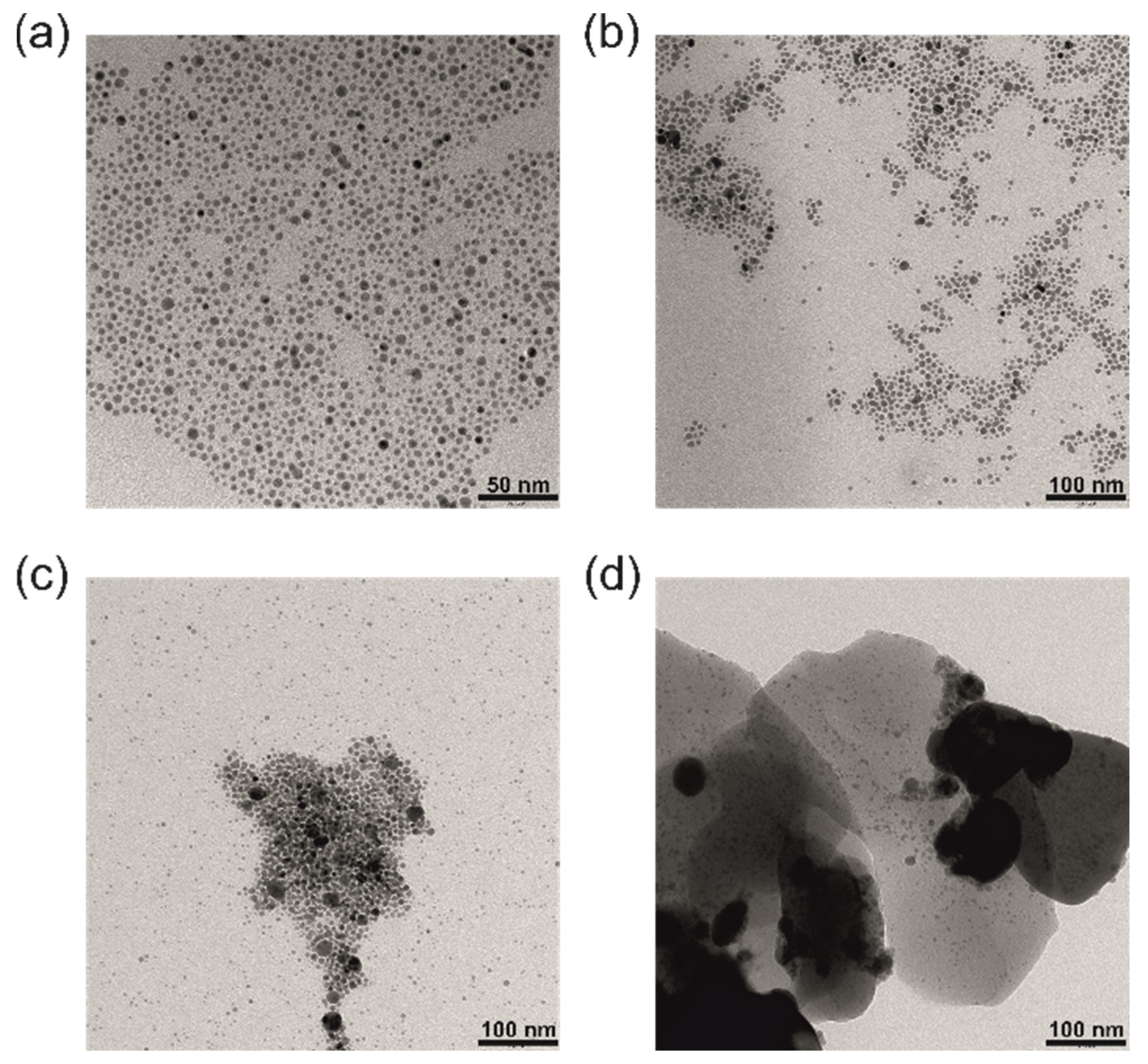
3.1. Synthesis and Characterisation of N-AgNPs
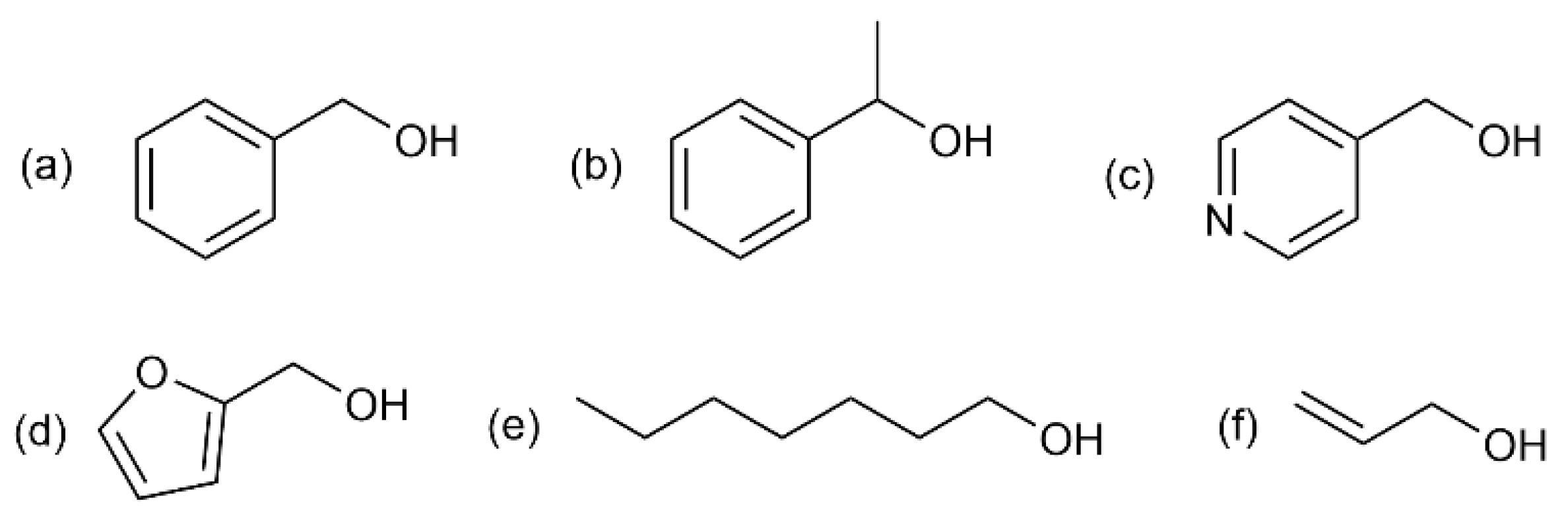
3.2. Catalytic Oxidation of Alcohols
3.2.1. Catalytic Activity of N-AgNPs in the Consecutive Cycles of Oxidation of Benzyl Alcohol with the Various Source of Oxygen
3.2.2. Catalytic Activity of N-AgNPs in the Consecutive Cycles of Oxidation of Selected Alcohols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corey, E.J.; Suggs, J.W. Chlorochromate; an Efficient Reagent for Oxidation of Primary and Secondary Alcohols to Carbonyl Compounds. Tetrahedron Lett. 1975, 16, 2647–2650. [Google Scholar] [CrossRef]
- Jing, H.; Keqiang, S.; Daiping, H.; Boqing, X. Amorphous Manganese Oxide for Catalytic Aerobic Oxidation of Benzyl Alcohol. Chin. J. Catal. 2007, 28, 1025–1027. [Google Scholar]
- Lou, J.D.; Ma, Y.C.; Gao, C.L.; Li, L. A New Efficient Oxidation of Alcohols with Jones Reagent Supported on Kieselguhr. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2006, 36, 381–383. [Google Scholar] [CrossRef]
- Pfitzner, K.; Moffatt, J. A New and Selective Oxidation of Alcohols. J. Am. Chem. Soc. 1963, 85, 3027–3028. [Google Scholar] [CrossRef]
- Tidwell, T.T. Oxidation of Alcohols by Activated Dimethyl Sulfoxide and Related Reactions: An Update. Synthesis 1990, 1990, 857–870. [Google Scholar] [CrossRef]
- Frigerio, M.; Santagostino, M. A mild oxidizing reagent for alcohols and 1,2-diols: O-iodoxybenzoic acid (IBX) in DMSO. Tetrahedron Lett. 1994, 35, 8019–8022. [Google Scholar] [CrossRef]
- Dess, D.; Martin, J. Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J. Org. Chem. 1983, 48, 4155–4156. [Google Scholar] [CrossRef]
- Nutting, J.E.; Rafiee, M.; Stahl, S.S. Tetramethylpiperidine N-Oxyl (TEMPO), Phthalimide N-oxyl (PINO), and Related N-Oxyl Species: Electrochemical Properties and Their Use in Electrocatalytic Reactions. Chem. Rev. 2018, 188, 4834–4885. [Google Scholar] [CrossRef]
- Likhtenshtein, G.I.; Yamauchi, J.; Nakatsuji, S.I.; Smirnov, A.I.; Tamura, R. Nitroxides: Applications in Chemistry, Biomedicine, and Materials Science; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Studer, A.; Vogler, T. Applications of TEMPO in Synthesis. Synthesis 2008, 2008, 1979–1993. [Google Scholar] [CrossRef]
- Tebben, L.; Studer, A. Nitroxides: Applications in synthesis and in polymer chemistry. Angew. Chem. Int. Ed. Engl. 2011, 50, 5034–5068. [Google Scholar] [CrossRef]
- Megiel, E. Surface modification using TEMPO and its derivatives. Adv. Colloid Interface Sci. 2017, 250, 158–184. [Google Scholar] [CrossRef] [PubMed]
- Bobbitt, J.M. Oxoammonium Salts. 6.1 4-Acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium Perchlorate: A Stable and Convenient Reagent for the Oxidation of Alcohols. Silica Gel Catalysis. J. Org. Chem. 1998, 63, 9367–9374. [Google Scholar] [CrossRef]
- Merbouh, N.; Bobbitt, J.M.; Brückner, C. Preparation of Tetramethylpiperidine-1-oxoammonium Salts and their Use as Oxidants in Organic Chemistry. Org. Prep. Proced. Int. 2004, 36, 1–31. [Google Scholar] [CrossRef]
- Gamez, P.; Arends, I.W.; Reedijk, J.; Sheldon, R.A. Copper(ii)-catalysed aerobic oxidation of primary alcohols to aldehydes. Chem. Commun. 2003, 2003, 2414–2415. [Google Scholar] [CrossRef] [PubMed]
- Gamez, P.; Arends, I.W.; Sheldon, R.A.; Reedijk, J. Room temperature aerobic copper–catalysed selective oxidation of primary alcohols to aldehydes. Adv. Synth. Catal. 2004, 346, 805–811. [Google Scholar] [CrossRef]
- Hoover, J.M.; Stahl, S.S. Highly Practical Copper(I)/TEMPO Catalyst System for Chemoselective Aerobic Oxidation of Primary Alcohols. J. Am. Chem. Soc. 2011, 133, 16901–16910. [Google Scholar] [CrossRef] [PubMed]
- Hoover, J.M.; Ryland, B.L.; Stahl, S.S. Mechanism of Copper(I)/TEMPO-Catalyzed Aerobic Alcohol Oxidation. J. Am. Chem. Soc. 2013, 135, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xiao, W.; Wang, Z.; Xie, T.; Yi, C.; Xu, Z. Engineering of polystyrene-supported acid–base catalysts for aldol condensation in water. Mater. Today Chem. 2022, 24, 100872. [Google Scholar] [CrossRef]
- Tsubokawa, N.; Kimoto, T.; Endo, T. Oxidation of alcohols with copper(II) salts mediated by nitroxyl radicals immobilized on ultrafine silica and ferrite surface. J. Mol. Catal. A Chem. 1995, 101, 45–50. [Google Scholar] [CrossRef]
- Fey, T.; Fischer, H.; Bachmann, S.; Albert, K.; Bolm, C. Silica-Supported TEMPO Catalysts: Synthesis and Application in the Anelli Oxidation of Alcohols. J. Org. Chem. 2001, 66, 8154–8159. [Google Scholar] [CrossRef]
- Brunel, D.; Fajula, F.; Nagy, J.; Deroide, B.; Verhoef, M.; Veum, L.; Peters, J.; van Bekkum, H. Comparison of two MCM-41 grafted TEMPO catalysts in selective alcohol oxidation. Appl. Catal. A 2001, 213, 73–82. [Google Scholar] [CrossRef]
- Karimi, B.; Biglari, A.; Clark, J.H.; Budarin, V. Green, transition-metal-free aerobic oxidation of alcohols using a highly durable supported organocatalyst. Angew. Chem. Int. Ed. Engl. 2007, 46, 7210–7213. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Kuroboshi, M.; Goto, K. Electrooxidation of Alcohols in N-Oxyl-Immobilized Silica Gel/Water Disperse System: Approach to Totally Closed System. Synthesis 2009, 2009, 903–908. [Google Scholar]
- Ciriminna, R.; Pagliaro, M.; Blum, J.; Avnir, D. Dramatic ligand effect in copper(II) complex promoted transesterification of a phosphate diester. Chem. Commun. 2000, 2000, 1441–1442. [Google Scholar] [CrossRef]
- Palmisano, G.; Ciriminna, R.; Pagliaro, M. Waste-free electrochemical oxidation of alcohols in water. Adv. Synth. Catal. 2006, 348, 2033–2037. [Google Scholar] [CrossRef]
- Karimi, B.; Rafiee, M.; Alizadeh, S.; Vali, H. Eco-Friendly Electrocatalytic Oxidation of Alcohols on a Novel Electro Generated TEMPO-Functionalized MCM-41 Modified Electrode. Green Chem. 2015, 17, 991–1000. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M. Industrial Oxidations with Organocatalyst TEMPO and Its Derivatives. Org. Process Res. Dev. 2009, 14, 245–251. [Google Scholar] [CrossRef]
- Ciriminna, R.; Palmisano, G.; Pagliaro, M. Electrodes Functionalized with the 2,2,6,6-Tetramethylpiperidinyloxy Radical for the Waste-Free Oxidation of Alcohols. ChemCatChem 2015, 7, 552–558. [Google Scholar] [CrossRef]
- Ciriminna, R.; Ghahremani, M.; Karimi, B.; Pagliaro, M. Electrochemical Alcohol Oxidation Mediated by TEMPO-like Nitroxyl Radicals. ChemistryOpen 2017, 6, 5–10. [Google Scholar] [CrossRef]
- Piotrowski, P.; Pawłowska, J.; Sadło, J.G.; Bilewicz, R.; Kaim, A. TEMPO functionalized C60 fullerene deposited on gold surface for catalytic oxidation of selected alcohols. J. Nanopart. Res. 2017, 19, 161. [Google Scholar] [CrossRef][Green Version]
- Beejapur, H.A.; Zhang, Q.; Hu, K.; Zhu, L.; Wang, J.; Ye, Z. TEMPO in Chemical Transformations: From Homogeneous to Heterogeneous. ACS Catal. 2019, 9, 2777–2830. [Google Scholar] [CrossRef]
- Beejapur, H.A.; Campisciano, V.; Giacalone, F.; Gruttadauria, M. Catalytic Synergism in a C60IL10TEMPO2 Hybrid in the Efficient Oxidation of Alcohols. Adv. Synth. Catal. 2015, 357, 51–58. [Google Scholar] [CrossRef]
- Beejapur, H.A.; Campisciano, V.; Franchi, P.; Lucarini, M.; Giacalone, F.; Gruttadauria, M. Synthesis of Bis (Cyclic Dithiocarbonates) Bridged by a Polyoxyethylene Chain. ChemCatChem 2014, 6, 2419–2424. [Google Scholar] [CrossRef]
- Shakir, A.J.; Culita, D.C.; Calderon-Moreno, J.; Musuc, A.; Carp, O.; Ionita, G.; Ionita, P. Covalently grafted TEMPO on graphene oxide: A composite material for selective oxidations of alcohols. Carbon 2016, 105, 607–614. [Google Scholar] [CrossRef]
- Yuan, B.; Xu, C.; Zhang, D.; Zhang, R.; Su, H.; Guan, P.; Nie, J.; Fernandez, C. Electrografting of amino-TEMPO on graphene oxide and electrochemically reduced graphene oxide for electrocatalytic applications. Electrochem. Commun. 2017, 81, 18–23. [Google Scholar] [CrossRef]
- Singh, S.B.; Tandon, P.K. Catalysis: A brief review on Nano-Catalyst. J. Energy Chem. Eng. 2014, 2, 106–115. [Google Scholar]
- Narayan, N.; Meiyazhagan, A.; Vajtai, R. Metal Nanoparticles as Green Catalysts. Materials 2019, 12, 3602. [Google Scholar] [CrossRef]
- Swiech, O.; Bilewicz, R.; Megiel, E. TEMPO coated Au nanoparticles: Synthesis and tethering to gold surfaces. RSC Adv. 2013, 3, 5979. [Google Scholar] [CrossRef]
- Krzywicka, A.; Megiel, E. Silver-Polystyrene (Ag/PS) Nanocomposites Doped with Polyvinyl Alcohol (PVA)—Fabrication and Bactericidal Activity. Nanomaterials 2020, 10, 2245. [Google Scholar] [CrossRef]
- Krystosiak, P.; Tomaszewski, W.; Megiel, E. High-density polystyrene-grafted silver nanoparticles and their use in the preparation of nanocomposites with antibacterial properties. J. Colloid Interface Sci. 2017, 498, 9–21. [Google Scholar] [CrossRef]
- Gozdziewska, M.; Cichowicz, G.; Markowska, K.; Zawada, K.; Megiel, E. Nitroxide-coated silver nanoparticles: Synthesis, surface physicochemistry and antibacterial activity. RSC Adv. 2015, 5, 58403–58415. [Google Scholar] [CrossRef]
- Rocha, T.C.; Oestereich, A.; Demidov, D.V.; Hävecker, M.; Zafeiratos, S.; Weinberg, G.; Bukhtiyarov, V.I.; Knop-Gericke, A.; Schlögl, R. The silver–oxygen system in catalysis: New insights by near ambient pressure X-ray photoelectron spectroscopy. PCCP 2012, 14, 4554–4564. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Chen, M.; Wu, L. Novel Method to Fabricate SiO2/Ag Composite Spheres and Their Catalytic, Surface-Enhanced Raman Scattering Properties. J. Phys. Chem. C 2007, 111, 11692–11698. [Google Scholar] [CrossRef]
- Briggs, D. Analysis of polymer surfaces by sims.2. fingerprint spectra from simple polymer- films. Surf. Interface Anal. 1982, 4, 151–155. [Google Scholar] [CrossRef]
- Schönherr, H.; Ringsdorf, H. Self-Assembled Monolayers of Symmetrical and Mixed Alkyl Fluoroalkyl Disulfides on Gold. 1. Synthesis of Disulfides and Investigation of Monolayer Properties. Langmuir 1996, 12, 3891–3897. [Google Scholar] [CrossRef]
- Swiech, O.; Hrynkiewicz-Sudnik, N.; Palys, B.; Kaim, A.; Bilewicz, R. Gold Nanoparticles Tethered to Gold Surfaces Using Nitroxyl Radicals. J. Phys. Chem. C 2011, 115, 7347–7354. [Google Scholar] [CrossRef]
- Ling, X.; Schaeffer, N.; Roland, S.; Pileni, M.-P. Superior Oxygen Stability of N-Heterocyclic Carbene-Coated Au Nanocrystals: Comparison with Dodecanethiol. Langmuir 2015, 31, 12873–12882. [Google Scholar] [CrossRef]
- Kumpulainen, E.T.; Koskinen, A.M. Catalytic Activity Dependency on Catalyst Components in Aerobic Copper–TEMPO Oxidation. Chem. Eur. J. 2009, 15, 10901–10911. [Google Scholar] [CrossRef]


| Orbital | Position [eV] | FWHM [eV] | Concentration [atom%] |
|---|---|---|---|
| O 1s | 532.0 | 1.51 | 10.6 |
| 533.2 | 1.51 | 4.47 | |
| 534.2 | 1.51 | 2.60 | |
| 535.9 | 1.51 | 0.52 | |
| N 1s | 398.6 | 1.45 | 0.74 |
| 400.1 | 1.45 | 1.00 | |
| 402.0 | 1.45 | 0.60 | |
| Ag 3d5/2 | 367.8 | 1.03 | 6.38 |
| 368.6 | 1.84 | 1.46 | |
| Ag 3d3/2 | 373.8 | 1.03 | 4.25 |
| 374.6 | 1.84 | 0.97 | |
| C 1s | 284.6 | 1.43 | 31.0 |
| 285.8 | 1.43 | 12.6 | |
| 286.9 | 1.43 | 13.6 | |
| 288.6 | 1.43 | 3.50 | |
| S 2p3/2 | 161.1 | 1.60 | 3.09 |
| 162.8 | 1.60 | 0.42 | |
| S 2p1/2 | 162.3 | 1.60 | 1.55 |
| 164.0 | 1.60 | 0.21 |
| Entry | No Cycle | Yield b [%] | TON c | Yield b [%] | TON c |
|---|---|---|---|---|---|
| Oxygen balloon d | Air balloon | ||||
| 1 | I | 99 | 236 | 90 | 214 |
| 2 | II | 22 | 52 | 36 | 86 |
| 3 | III | 16 | 38 | 40 | 95 |
| 4 | IV | 10 | 24 | 18 | 43 |
| 5 | V | 5 | 12 | 8 | 19 |
| Air balloon | Air atmospheree | ||||
| 6 | I | 96 | 114 | 96 | 114 |
| 7 | II | 27 | 32 | 85 | 101 |
| 8 | III | 35 f | 42 | 81 | 96 |
| 9 | IV | 28 | 33 | 73 | 87 |
| 10 | V | 75 f | 89 | 69 | 83 |
| 11 | VI | 48 | 57 | 65 | 78 |
| 12 | VII | 61 f | 73 | 55 | 66 |
| 13 | VIII | 48 | 57 | 50 | 60 |
| 14 | IX | 54 f | 64 | 51 f | 61 |
| 15 | X | 36 | 43 | 27 | 32 |
| 16 | XI | 6 | 7 | 24 | 29 |
| 17 | XII | 4 | 5 | 10 | 12 |
| Entry | No Cycle | N-AgNPs b [mg] | Time [h] | Yield c [%] | S d [%] | TON e | Entry | No Cycle | N-AgNPs b [mg] | Time [h] | Yield c [%] | S d [%] | TON e |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benzaldehyde (experiment 1) | Acetophenone (experiment 5) | ||||||||||||
| 1 | I | 9 | 2.5 | 96 | 96 | 114 | 16 | I | 9 | 2.5 | 16 | 85 | 19 |
| 2 | II | 9 | 4 | 96 | 96 | 114 | 17 | II | 9 | 2.5 | 10 | 80 | 12 |
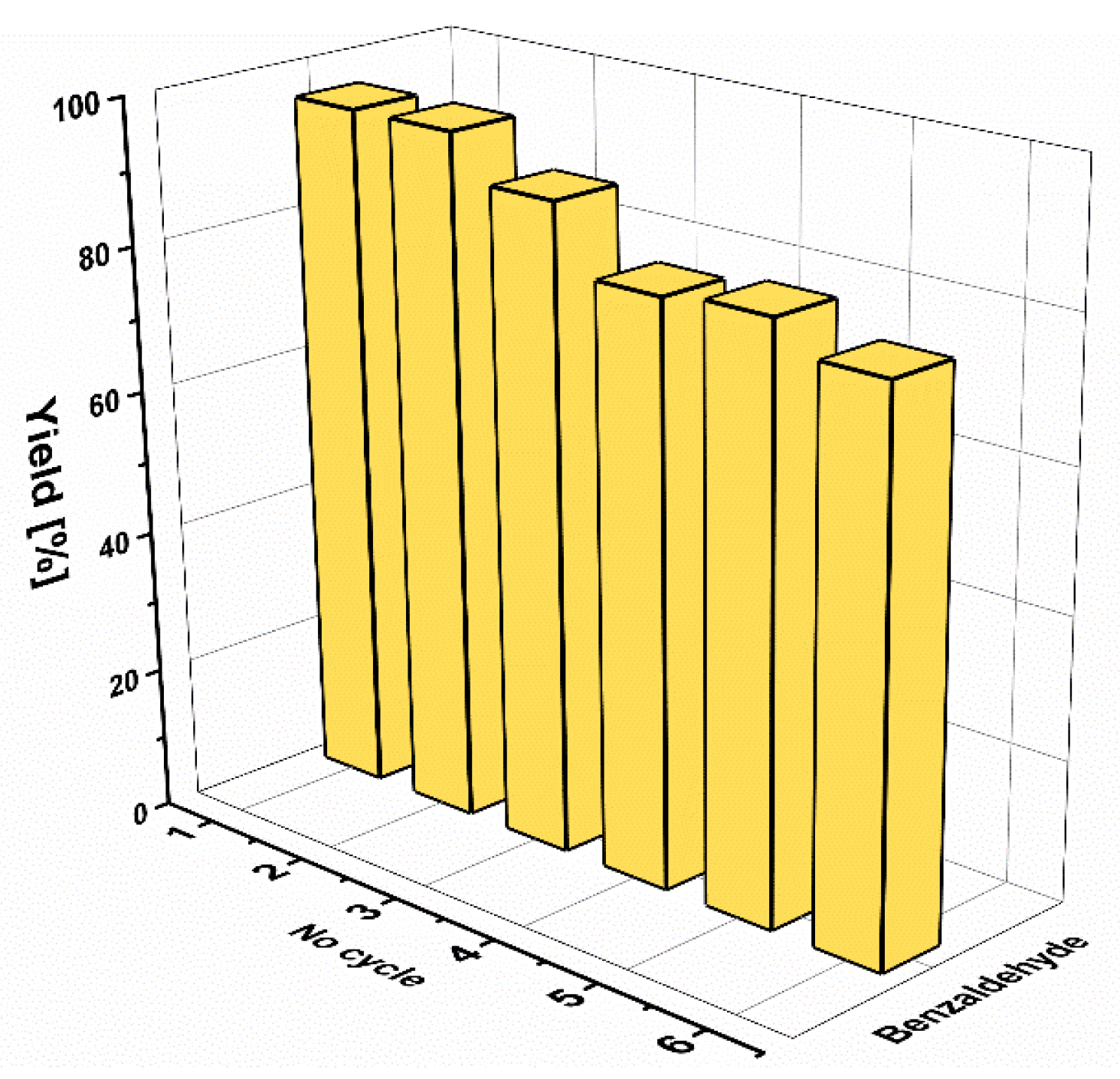
| 3 | III | 9 | 4 | 90 | 96 | 107 | Heptanal (experiment 6) | ||||||
| 4 | IV | 9 | 4 | 81 | 96 | 97 | 18 | I | 9 | 2.5 | 23 | 88 | 27 |
| 5 | V | 9 | 4 | 82 | 96 | 98 | 19 | II | 9 | 2.5 | 14 | 85 | 17 |
| 6 | VI | 9 | 4 | 78 | 97 | 93 | Allyl aldehyde (experiment 7) f | ||||||
| Benzaldehyde (experiment 2) | 20 | I | 9 | 5 | 24 | 100 | 29 | ||||||
| 7 | I | 4.5 | 5 | 92 | 100 | 219 | 21 | II | 9 | 24 | 22 | 100 | 26 |
| 8 | I | 4.5 | 6 | 96 | 100 | 229 | Furfural (experiment 8) g | ||||||
| 9 | II | 4.5 | 6 | 76 | 100 | 181 | 22 | I | 9 | 2.5 | 83 | 100 | 99 |
| 4-Pyridinecarboxaldehyde (experiment 3) | 23 | II | 9 | 2.5 | 68 | 100 | 81 | ||||||
| 10 | I | 4.5 | 5 | 79 | 100 | 188 | 24 | III | 9 | 2.5 | 61 | 100 | 73 |
| 11 | I | 4.5 | 6 | 90 | 100 | 214 | 25 | IV | 9 | 2.5 | 55 | 100 | 66 |
| 12 | II | 4.5 | 6 | 74 | 100 | 176 | 26 | V | 9 | 2.5 | 48 | 100 | 58 |
| Furfural (experiment 4) | 27 | VI | 9 | 2.5 | 43 | 100 | 51 | ||||||
| 13 | I | 9 | 2.5 | 94 | 100 | 112 | 28 | VII | 9 | 2.5 | 40 | 100 | 48 |
| 14 | I | 9 | 6 | 100 | 100 | 119 | 29 | VIII | 9 | 2.5 | 35 | 100 | 42 |
| 15 | II | 9 | 6 | 75 | 100 | 89 | 30 | IX | 9 | 2.5 | 30 | 100 | 36 |
| Entry | No Cycle | Benzaldehyde | 4-Pyridine Carboxaldehyde | Furfural | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yield b [%] | S c [%] | TON d | Yield b [%] | S c [%] | TON d | Yield b [%] | S c [%] | TON d | ||
| 1 | I | 96 | 96 | 114 | 79 | 92 | 94 | 94 | 100 | 112 |
| 2 | II | 85 | 91 | 101 | 73 | 91 | 87 | 64 | 100 | 76 |
| 3 | III | 81 | 96 | 96 | 65 | 90 | 77 | 55 | 100 | 65 |
| 4 | IV | 73 | 95 | 87 | 58 | 91 | 69 | 52 | 100 | 62 |
| 5 | V | 69 | 95 | 83 | 56 | 94 | 67 | 44 | 100 | 52 |
| 6 | VI | 65 | 93 | 78 | 62 e | 93 | 73 | 44 | 100 | 53 |
| 7 | VII | 55 | 94 | 66 | 60 | 91 | 71 | 38 | 100 | 45 |
| 8 | VIII | 50 | 94 | 60 | 64 e | 93 | 76 | 34 | 100 | 41 |
| 9 | IX | 51 e | 95 | 61 | 59 | 93 | 70 | 31 | 100 | 37 |
| 10 | X | 27 | 92 | 32 | 54 | 92 | 64 | 28 | 100 | 33 |
| 11 | XI | 24 | 91 | 29 | 55 | 92 | 66 | 26 | 100 | 31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krogul-Sobczak, A.; Pisarek, N.; Cieciórski, P.; Megiel, E. Silver Nanoparticles Densely Grafted with Nitroxides as a Recyclable Green Catalyst in the Selective Oxidation of Alcohols. Nanomaterials 2022, 12, 2542. https://doi.org/10.3390/nano12152542
Krogul-Sobczak A, Pisarek N, Cieciórski P, Megiel E. Silver Nanoparticles Densely Grafted with Nitroxides as a Recyclable Green Catalyst in the Selective Oxidation of Alcohols. Nanomaterials. 2022; 12(15):2542. https://doi.org/10.3390/nano12152542
Chicago/Turabian StyleKrogul-Sobczak, Agnieszka, Natalia Pisarek, Piotr Cieciórski, and Elżbieta Megiel. 2022. "Silver Nanoparticles Densely Grafted with Nitroxides as a Recyclable Green Catalyst in the Selective Oxidation of Alcohols" Nanomaterials 12, no. 15: 2542. https://doi.org/10.3390/nano12152542
APA StyleKrogul-Sobczak, A., Pisarek, N., Cieciórski, P., & Megiel, E. (2022). Silver Nanoparticles Densely Grafted with Nitroxides as a Recyclable Green Catalyst in the Selective Oxidation of Alcohols. Nanomaterials, 12(15), 2542. https://doi.org/10.3390/nano12152542