Green and Efficient Preparation of Tailed Lignin Nanoparticles and UV Shielding Composite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lignin Solubilization and Purification
2.3. Preparation of Tailed Lignin Nanoparticles (T-LNP) by Solvent-Antisolvent Method
2.4. Preparation of T-LNP-Based Composite Films
2.5. Antiradical Activity of Migrating Substances
2.6. Characterization
3. Results and Discussion
3.1. Lignin Nanoparticles
3.1.1. Microstructure
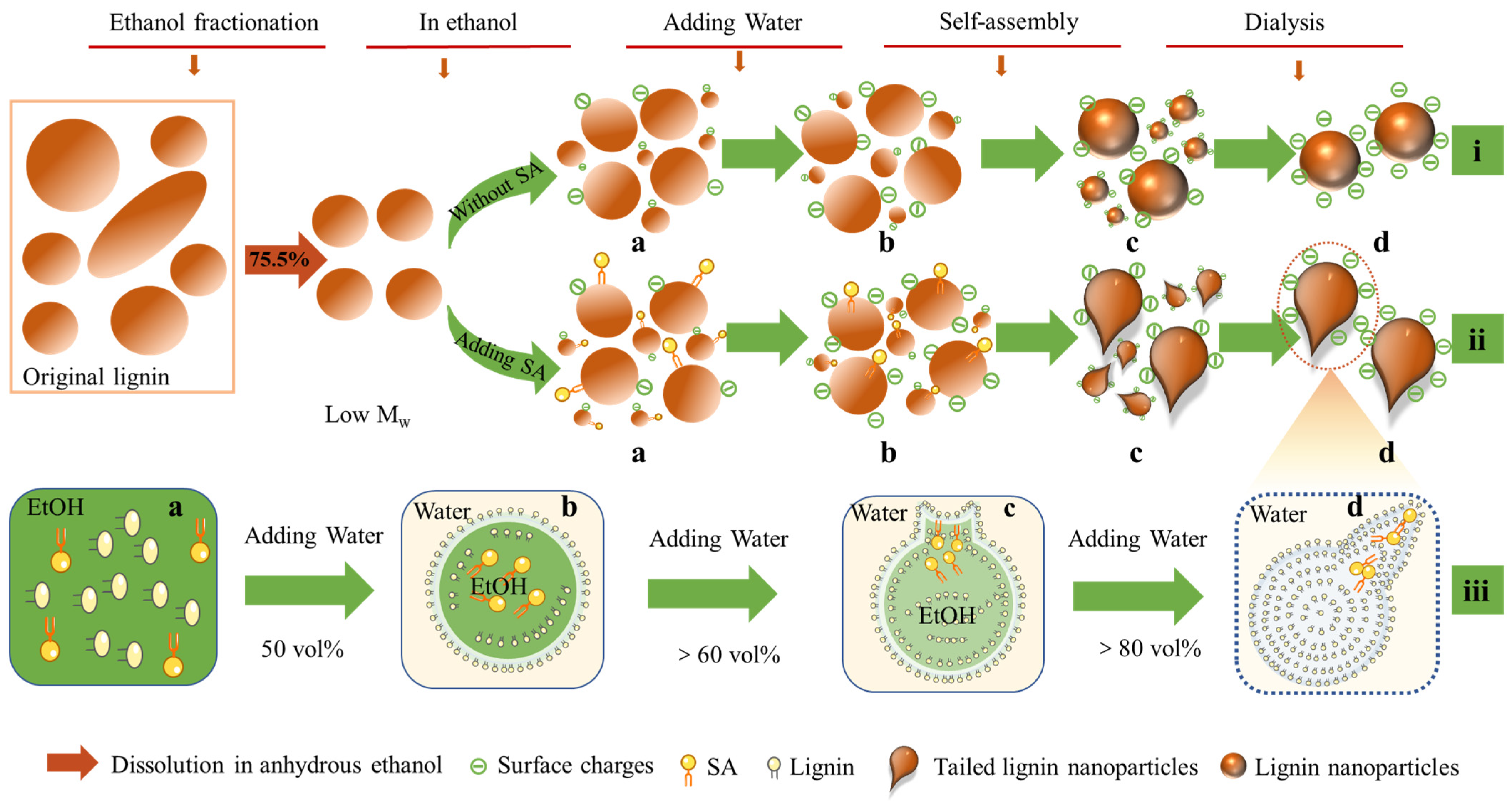
3.1.2. Proposed Formation Mechanism
3.1.3. Morphology Analysis
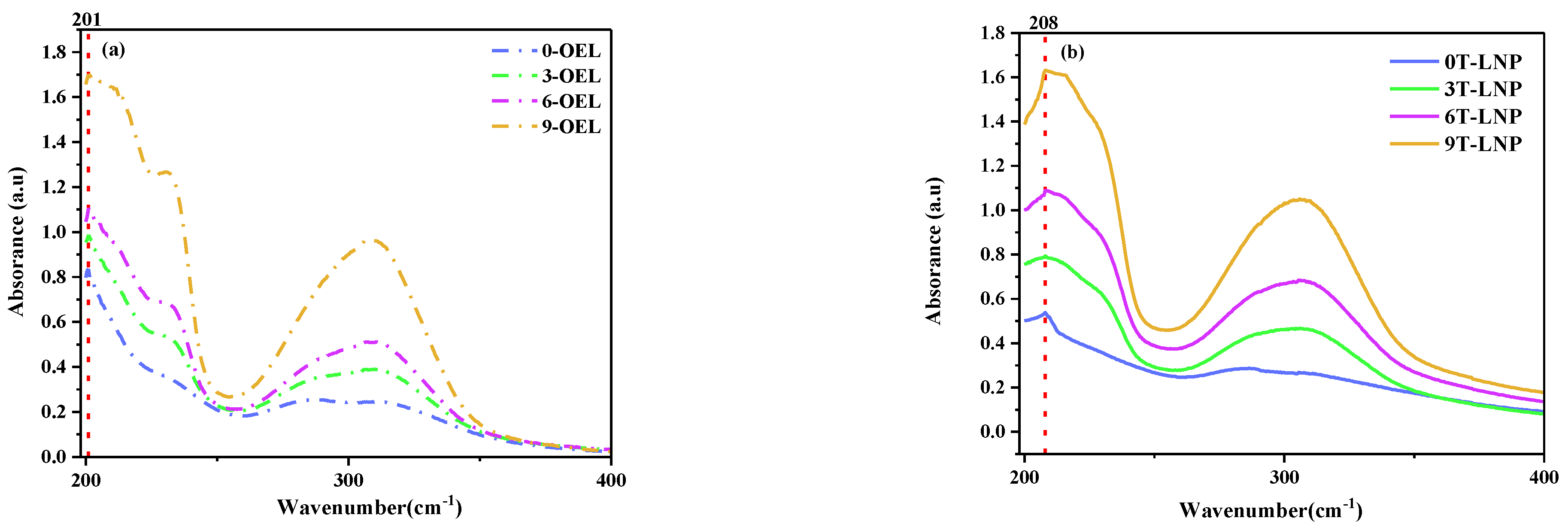
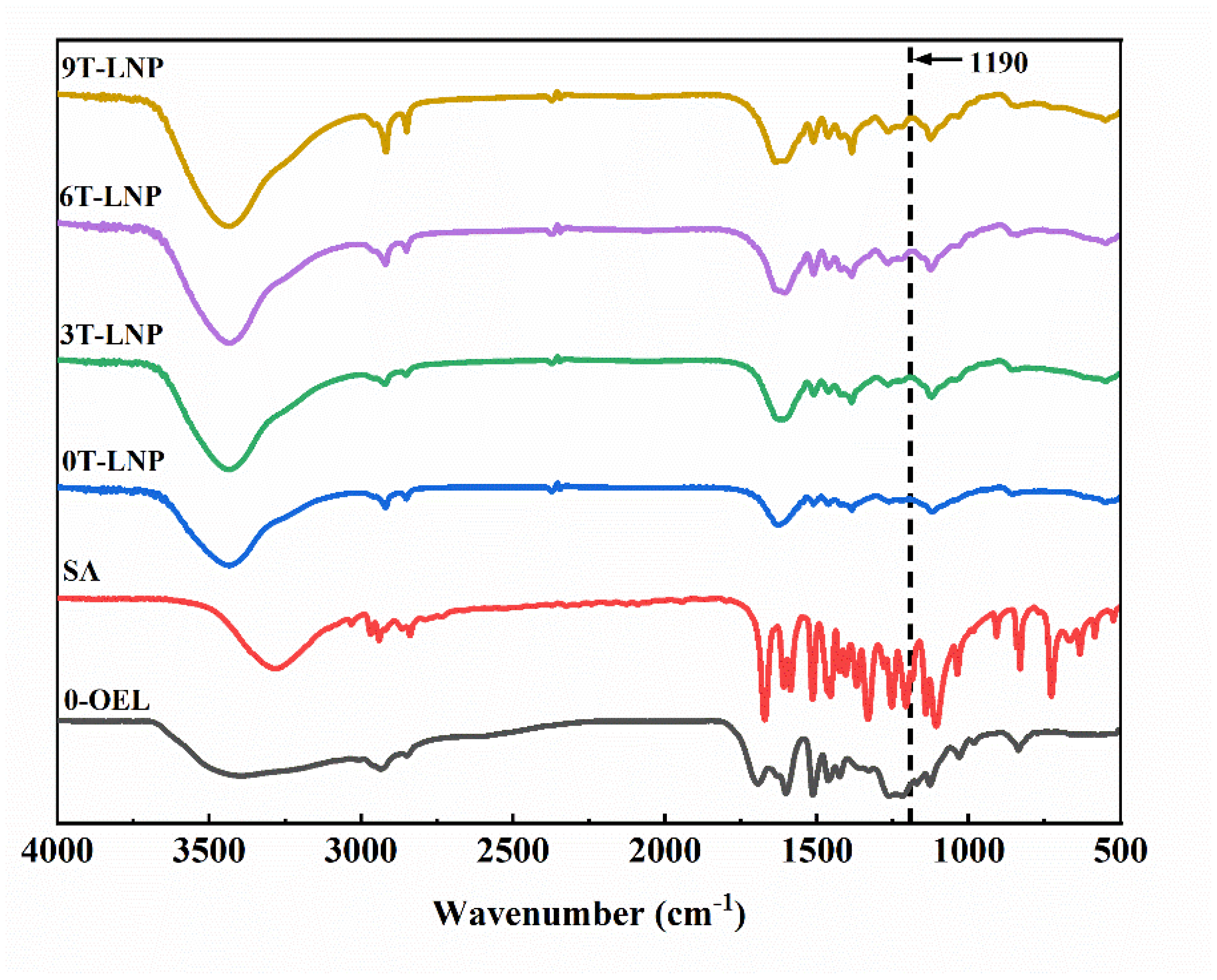
3.1.4. Chemical Characteristics
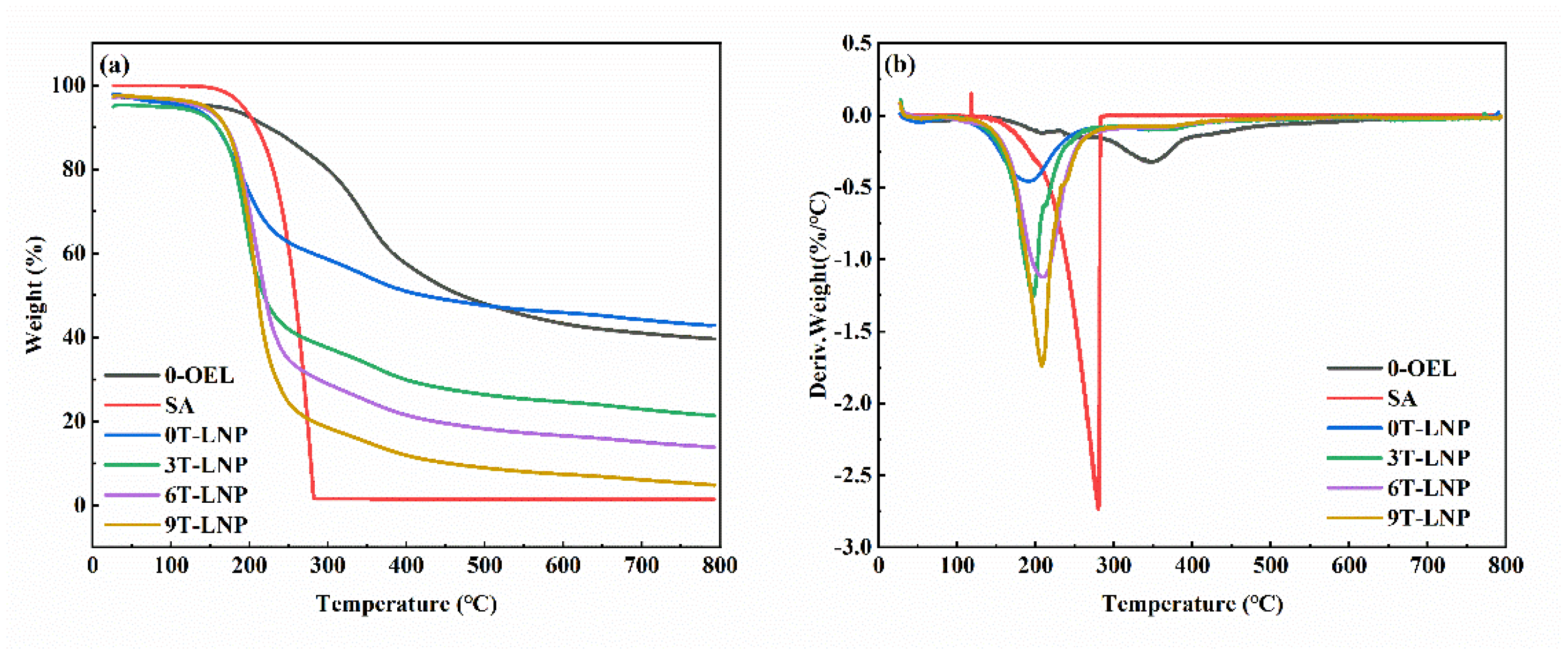
3.1.5. Thermal Properties
3.2. T-LNP Based Composite Films
3.2.1. UV Shielding Performance
3.2.2. Antiradical Activity of Migrating Substances
3.2.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, B.; Sun, D.; Wang, H.-M.; Yuan, T.-Q.; Sun, R.-C. Green and facile preparation of regular lignin nanoparticles with high yield and their natural broad-spectrum sunscreens. ACS Sustain. Chem. Eng. 2018, 7, 2658–2666. [Google Scholar] [CrossRef]
- Fakayode, O.A.; Aboagarib, E.A.A.; Zhou, C.; Ma, H. Co-pyrolysis of lignocellulosic and macroalgae biomasses for the production of biochar—A review. Bioresour. Technol. 2020, 297, 122408. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products—Strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef]
- Farooq, M.; Zou, T.; Riviere, G.; Sipponen, M.H.; Österberg, M. Strong, ductile, and waterproof cellulose nanofibril composite films with colloidal lignin particles. Biomacromolecules 2018, 20, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Österberg, M.; Sipponen, M.H.; Mattos, B.D.; Rojas, O.J. Spherical lignin particles: A review on their sustainability and applications. Green Chem. 2020, 22, 2712–2733. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Fortunati, E.; Gao, D.; Balestra, G.M.; Giovanale, G.; He, X.; Torre, L.; Kenny, J.M.; Puglia, D. Valorization of acid isolated high yield lignin nanoparticles as innovative antioxidant/antimicrobial organic materials. ACS Sustain. Chem. Eng. 2018, 6, 3502–3514. [Google Scholar] [CrossRef]
- Gordobil, O.; Herrera, R.; Yahyaoui, M.; Ilk, S.; Kaya, M.; Labidi, J. Potential use of kraft and organosolv lignins as a natural additive for healthcare products. RSC Adv. 2018, 8, 24525–24533. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Zhang, K.; Liu, P.; Han, H.; Zhao, S.; Kakade, A.; Khan, A.; Du, D.; Li, X. Lignin depolymerization and utilization by bacteria. Bioresour. Technol. 2018, 269, 557–566. [Google Scholar] [CrossRef]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; da Costa Lopes, A.M.; Łukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Thermochemical conversion of lignin to functional materials: A review and future directions. Green Chem. 2015, 17, 4888–4907. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and application of lignosulfonates and sulfonated lignin. Chemsuschem 2017, 10, 1861–1877. [Google Scholar] [CrossRef] [Green Version]
- Cha, Y.-L.; Alam, A.-M.; Park, S.-M.; Moon, Y.-H.; Kim, K.-S.; Lee, J.-E.; Kwon, D.-E.; Kang, Y.-G. Hydrothermal-process-based direct extraction of polydisperse lignin microspheres from black liquor and their physicochemical characterization. Bioresour. Technol. 2020, 297, 122399. [Google Scholar] [CrossRef]
- Richter, A.P.; Bharti, B.; Armstrong, H.B.; Brown, J.S.; Plemmons, D.A.; Paunov, V.; Stoyanov, S.; Velev, O. Synthesis and characterization of biodegradable lignin nanoparticles with tunable surface properties. Langmuir 2016, 32, 6468–6477. [Google Scholar] [CrossRef] [PubMed]
- Frangville, C.; Rutkevičius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of environmentally biodegradable lignin nanoparticles. Chemphyschem 2012, 13, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.-L.; Hult, E.-L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yang, M.; Yuan, Q.; Cheng, G. Controlled preparation of corncob lignin nanoparticles and their size-dependent antioxidant properties: Toward high value utilization of lignin. ACS Sustain. Chem. Eng. 2019, 7, 17166–17174. [Google Scholar] [CrossRef]
- Lintinen, K.; Xiao, Y.; Ashok, R.B.; Leskinen, T.; Sakarinen, E.; Sipponen, M.; Muhammad, F.; Oinas, P.; Österberg, M.; Kostiainen, M. Closed cycle production of concentrated and dry redispersible colloidal lignin particles with a three solvent polarity exchange method. Green Chem. 2018, 20, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and formation mechanism of size-controlled lignin nanospheres by self-assembly. Ind. Crops Prod. 2017, 100, 146–152. [Google Scholar] [CrossRef]
- Jiang, P.; Li, Q.; Gao, C.; Lu, J.; Cheng, Y.; Zhai, S.; An, Q.; Wang, H. Fractionation of alkali lignin by organic solvents for biodegradable microsphere through self-assembly. Bioresour. Technol. 2019, 289, 121640. [Google Scholar] [CrossRef]
- Li, H.; Deng, Y.; Wu, H.; Ren, Y.; Qiu, X.; Zheng, D.; Li, C. Self-assembly of kraft lignin into nanospheres in dioxane-water mixtures. Holzforschung 2016, 70, 725–731. [Google Scholar] [CrossRef]
- Dai, L.; Liu, R.; Hu, L.-Q.; Zou, Z.-F.; Si, C.-L. Lignin nanoparticle as a novel green carrier for the efficient delivery of resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 8241–8249. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Lange, H.; Ago, M.; Crestini, C. Understanding lignin aggregation processes. A case study: Budesonide entrapment and stimuli controlled release from lignin nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 9342–9351. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-Y.; Peng, X.-P.; Sun, S.; Wen, J.-L.; Yuan, T.-Q. Short-time deep eutectic solvents pretreatment enhanced production of fermentable sugars and tailored lignin nanoparticles from abaca. Int. J. Biol. Macromol. 2021, 192, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Yearla, S.R.; Padmasree, K. Preparation and characterisation of lignin nanoparticles: Evaluation of their potential as antioxidants and UV protectants. J. Exp. Nanosci. 2016, 11, 289–302. [Google Scholar] [CrossRef]
- Luo, T.; Wang, C.; Ji, X.; Yang, G.; Chen, J.; Yoo, C.G.; Janaswamy, S.; Lyu, G. Innovative production of lignin nanoparticles using deep eutectic solvents for multifunctional nanocomposites. Int. J. Biol. Macromol. 2021, 183, 781–789. [Google Scholar] [CrossRef]
- Trevisan, H.; Rezende, C.A. Pure, stable and highly antioxidant lignin nanoparticles from elephant grass. Ind. Crops Prod. 2020, 145, 112105. [Google Scholar] [CrossRef]
- Qian, Y.; Zhong, X.; Li, Y.; Qiu, X. Fabrication of uniform lignin colloidal spheres for developing natural broad-spectrum sunscreens with high sun protection factor. Ind. Crops Prod. 2017, 101, 54–60. [Google Scholar] [CrossRef]
- Hararak, B.; Winotapun, C.; Inyai, J.; Wannid, P.; Prahsarn, C. Production of UV-shielded spherical lignin particles as multifunctional bio-additives for polyvinyl alcohol composite films. J. Nanopart. Res. 2021, 23, 193. [Google Scholar] [CrossRef]
- Ju, T.; Zhang, Z.; Li, Y.; Miao, X.; Ji, J. Continuous production of lignin nanoparticles using a microchannel reactor and its application in UV-shielding films. RSC Adv. 2019, 9, 24915–24921. [Google Scholar] [CrossRef] [Green Version]
- Baumberger, S.; Abaecherli, A.; Fasching, M.; Gellerstedt, G.; Gosselink, R.; Hortling, B.; Li, J.; Saake, B.; de Jong, E. Molar mass determination of lignins by size-exclusion chromatography: Towards standardisation of the method. Holzforschung 2007, 61, 459–468. [Google Scholar] [CrossRef]
- Li, J.; Zhao, H.; Zhang, X.; Liang, C. Organosolv Fractionation of Waste Biomass for Lignin Production Enhanced by Oxygen. Bioresources 2020, 15, 2774–2783. [Google Scholar] [CrossRef]
- Domenek, S.; Louaifi, A.; Guinault, A.; Baumberger, S. Potential of lignins as antioxidant additive in active biodegradable packaging materials. J. Polym. Environ. 2013, 21, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Xiong, F.; Wang, H.; Xu, H.; Qing, Y.; Wu, Z.; Wu, Y. Self-assembled lignin nanospheres with solid and hollow tunable structures. Ind. Crops Prod. 2020, 144, 112063. [Google Scholar] [CrossRef]
- Ma, M.; Dai, L.; Xu, J.; Liu, Z.; Ni, Y. A simple and effective approach to fabricate lignin nanoparticles with tunable sizes based on lignin fractionation. Green Chem. 2020, 22, 2011–2017. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Tian, D.; Shen, F.; Wan, X.; Xu, L.; Chen, Y.; Zhang, H.; Hu, J.; Shen, F. Fabrication and characterization of lignin–xylan hybrid nanospheres as pesticide carriers with enzyme-mediated release property. Soft Matter 2020, 16, 9083–9093. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, S.; Wu, C.; Liu, Y.; Yang, G.; Ni, Y. Super-stable, solvent-resistant and uniform lignin nanorods and nanospheres with a high yield in a mild and facile process. Green Chem. 2020, 22, 8734–8744. [Google Scholar] [CrossRef]
- Li, H.; Deng, Y.; Liu, B.; Ren, Y.; Liang, J.; Qian, Y.; Qiu, X.; Li, C.; Zheng, D. Preparation of nanocapsules via the self-assembly of kraft lignin: A totally green process with renewable resources. Acs Sustain. Chem. Eng. 2016, 4, 1946–1953. [Google Scholar] [CrossRef]
- Pang, T.; Wang, G.; Sun, H.; Wang, L.; Liu, Q.; Sui, W.; Parvez, A.M.; Si, C. Lignin fractionation for reduced heterogeneity in self-assembly nanosizing: Toward targeted preparation of uniform lignin nanoparticles with small size. Acs Sustain. Chem. Eng. 2020, 8, 9174–9183. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Yang, D.; Fang, Z.; Liu, W.; Xiang, T.; Qiu, X. Monodispersed Lignin Colloidal Spheres with Tailorable Sizes for Bio-Photonic Materials. Small 2022, 18, 2200671. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Hao, N.; Shinde, S.; Pu, Y.; Kang, X.; Ragauskas, A.J.; Yuan, J.S. Defining lignin nanoparticle properties through tailored lignin reactivity by sequential organosolv fragmentation approach (SOFA). Green Chem. 2019, 21, 245–260. [Google Scholar] [CrossRef]
- Selvaraj, S.; Rajkumar, P.; Thirunavukkarasu, K.; Gunasekaran, S.; Kumaresan, S. Vibrational (FT-IR and FT-Raman), electronic (UV–vis) and quantum chemical investigations on pyrogallol: A study on benzenetriol dimers. Vib. Spectrosc. 2018, 95, 16–22. [Google Scholar]
- Charanya, C.; Sampathkrishnan, S.; Balamurugan, N. Quantum mechanical analysis, spectroscopic (FT-IR, FT-Raman, UV-Visible) study, and HOMO-LUMO analysis of (1S, 2R)-2-amino-1-phenylpropan-1-ol using Density Functional Theory. J. Mol. Liq. 2017, 231, 116–125. [Google Scholar] [CrossRef]
- Selvaraj, S.; Rajkumar, P.; Santhiya, A.; Gunasekaran, S.; Kumaresan, S. 4-Methoxysalicylaldehyde: Spectroscopic and computational investigations. J. Emerg. Technol. Innov. Res. 2018, 5, 222–229. [Google Scholar]
- Gunasekaran, S.; Kumaresan, S.; Arunbalaji, R.; Anand, G.; Srinivasan, S. Density functional theory study of vibrational spectra, and assignment of fundamental modes of dacarbazine. J. Chem. Sci. 2008, 120, 315–324. [Google Scholar] [CrossRef]
- Kumar, A.R.; Nithya, V.; Rajeshkumar, S.; Gunasekaran, S.; Kumaresan, S.; Selvaraj, S.; Selvam, K.A.; Devanathan, J. An experimental and theoretical evidence for structural and spectroscopic properties of 2-hydroxy-5-methoxybenzaldehyde. Int. J. Anal. Exp. Modal Anal. 2019, 9, 1990–2003. [Google Scholar]
- Poletto, M.; Zattera, A.J. Materials produced from plant biomass: Part III: Degradation kinetics and hydrogen bonding in lignin. Mater. Res. 2013, 16, 1065–1070. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Luzi, F.; Hao, X.; Yang, W.; Torre, L.; Xiao, Z.; Xie, Y.; Puglia, D. Thermal, antioxidant and swelling behaviour of transparent polyvinyl (alcohol) films in presence of hydrophobic citric acid-modified lignin nanoparticles. Int. J. Biol. Macromol. 2019, 127, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Owczarek, J.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.; Kenny, J.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crops Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone, S.; Gigli, V.; Avitabile, D.; Saladino, R. Nano-structured lignin as green antioxidant and UV shielding ingredient for sunscreen applications. Antioxidants 2021, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Piccinino, D.; Capecchi, E.; Delfino, I.; Crucianelli, M.; Conte, N.; Avitabile, D.; Saladino, R. Green and Scalable Preparation of Colloidal Suspension of Lignin Nanoparticles and Its Application in Eco-friendly Sunscreen Formulations. ACS Omega 2021, 6, 21444–21456. [Google Scholar] [CrossRef]
- Enayat, S.; Banerjee, S. Comparative antioxidant activity of extracts from leaves, bark and catkins of Salix aegyptiaca sp. Food Chem. 2009, 116, 23–28. [Google Scholar] [CrossRef]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Bertoglio, F.; Owczarek, J.; Bruni, G.; Kozanecki, M.; Kenny, J.; Torre, L.; Visai, L.; Puglia, D. Polyvinyl alcohol/chitosan hydrogels with enhanced antioxidant and antibacterial properties induced by lignin nanoparticles. Carbohydr. Polym. 2018, 181, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Su, W.Y.; Yang, M.C. Evaluation of chitosan/PVA blended hydrogel membranes. J. Membr. Sci. 2004, 236, 39–51. [Google Scholar] [CrossRef]






| T-LNP (wt.%) | CH (wt.%) | PVA (wt.%) | |
|---|---|---|---|
| PVA | - | - | 100 |
| PVA/1T-LNP | 1 | - | 99 |
| PVA/3T-LNP | 3 | - | 97 |
| CH | - | 100 | - |
| CH/1T-LNP | 1 | 99 | - |
| CH/3T-LNP | 3 | 97 | - |
| PVA/CH | - | 10 | 90 |
| PVA/CH/1T-LNP | 1 | 9.9 | 89.1 |
| PVA/CH/3T-LNP | 3 | 9.7 | 87.3 |
| T-LNP (wt.%) | CNF (wt.%) | ||
| CNF | - | 100 | |
| CNF/1T-LNP | 1 | 99 | |
| CNF/3T-LNP | 3 | 97 | |
| T-LNP (wt.%) | SAL (wt.%) | ||
| SAL | - | 100 | |
| SAL/1T-LNP | 1 | 99 | |
| SAL/3T-LNP | 3 | 97 | |
| T-LNP (wt.%) | CES (wt.%) | ||
| CES | - | 100 | |
| CES/1T-LNP | 1 | 99 | |
| CES/3T-LNP | 3 | 97 |
| Raw Materials | Methods and Main Chemicals | Morphology | Ref |
|---|---|---|---|
| Enzymatic hydrolysis lignin | Self-assembly, THF-water | Hollow, 419 nm | [18] |
| Alkali lignin | Self-assembly, THF-water | Sphere, 168 nm | [19] |
| Kraft lignin | Self-assembly, dioxane-water | Hollow, 200 nm | [20] |
| Alkali lignin | Self-assembly, methanol-water | Sphere, 100 nm | [21] |
| Soda lignin | Self-assembly, ethanol-water | Colloidal 160 nm | [22] |
| Enzymatic hydrolysis lignin | Self-assembly, acetone-water | Sphere, 100 nm | [39] |
| Organosolv lignin | Self-assembly, THF-water | Sphere, 132 nm | [40] |
| Ethanol lignin | Self-assembly, ethanol/SA -water | Tailed, 286 nm | This work |
| Sample | 0-OEL | SA | 0T-LNP | 3T-LNP | 6T-LNP | 9T-LNP |
|---|---|---|---|---|---|---|
| TGA T10% (°C) | 223.9 | 209.3 | 158.7 | 159.1 | 169.8 | 170.1 |
| DTG Tmax (°C) | 345.5 | 280.1 | 191.7 | 197.5 | 207.9 | 209.5 |
| Samples | Optical Property | Ref | |
|---|---|---|---|
| Transmittance (%, 320 nm) | Transmittance (%, 550 nm) | ||
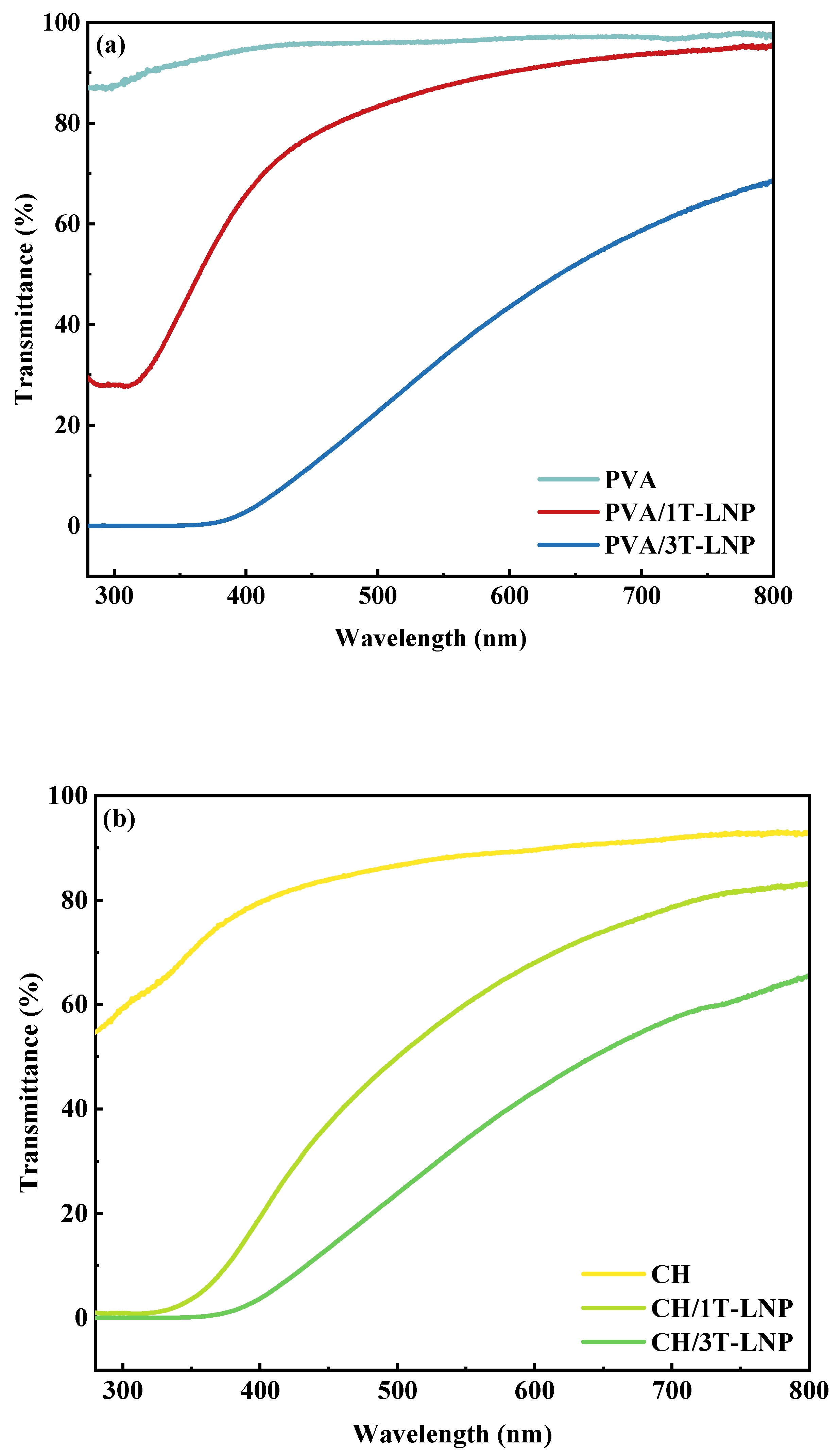
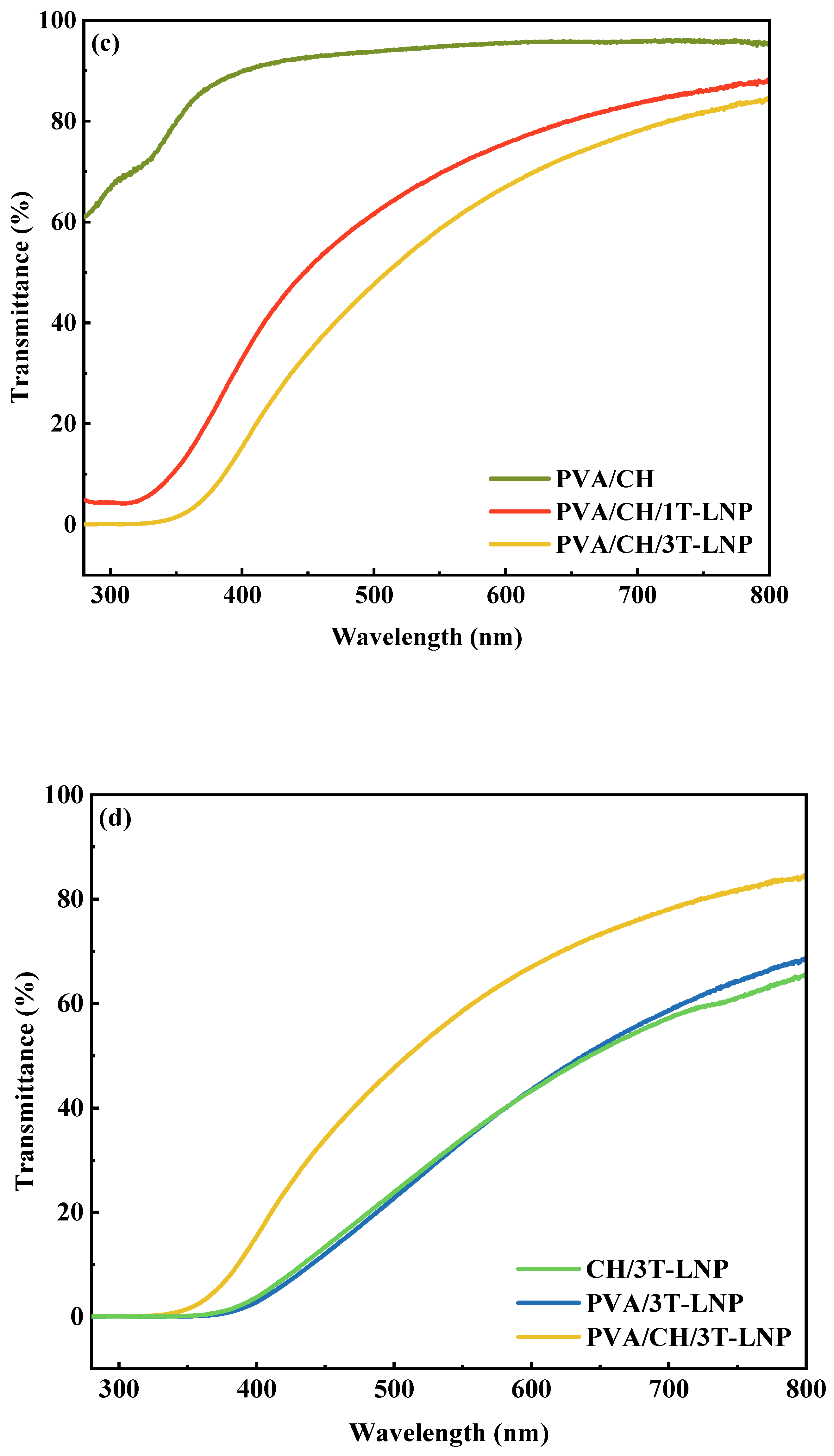
| PVA/3LNP | 0.39 | 38.40 | [48] |
| CH/3LNP | 1.52 | 44.02 | |
| PVA/CH/3LNP | 0.87 | 52.39 | |
| PVA/3T-LNP | 0.002 | 33.64 | This work |
| CH/3T-LNP | 0.01 | 34.11 | |
| PVA/CH/3T-LNP | 0.127 | 58.62 | |
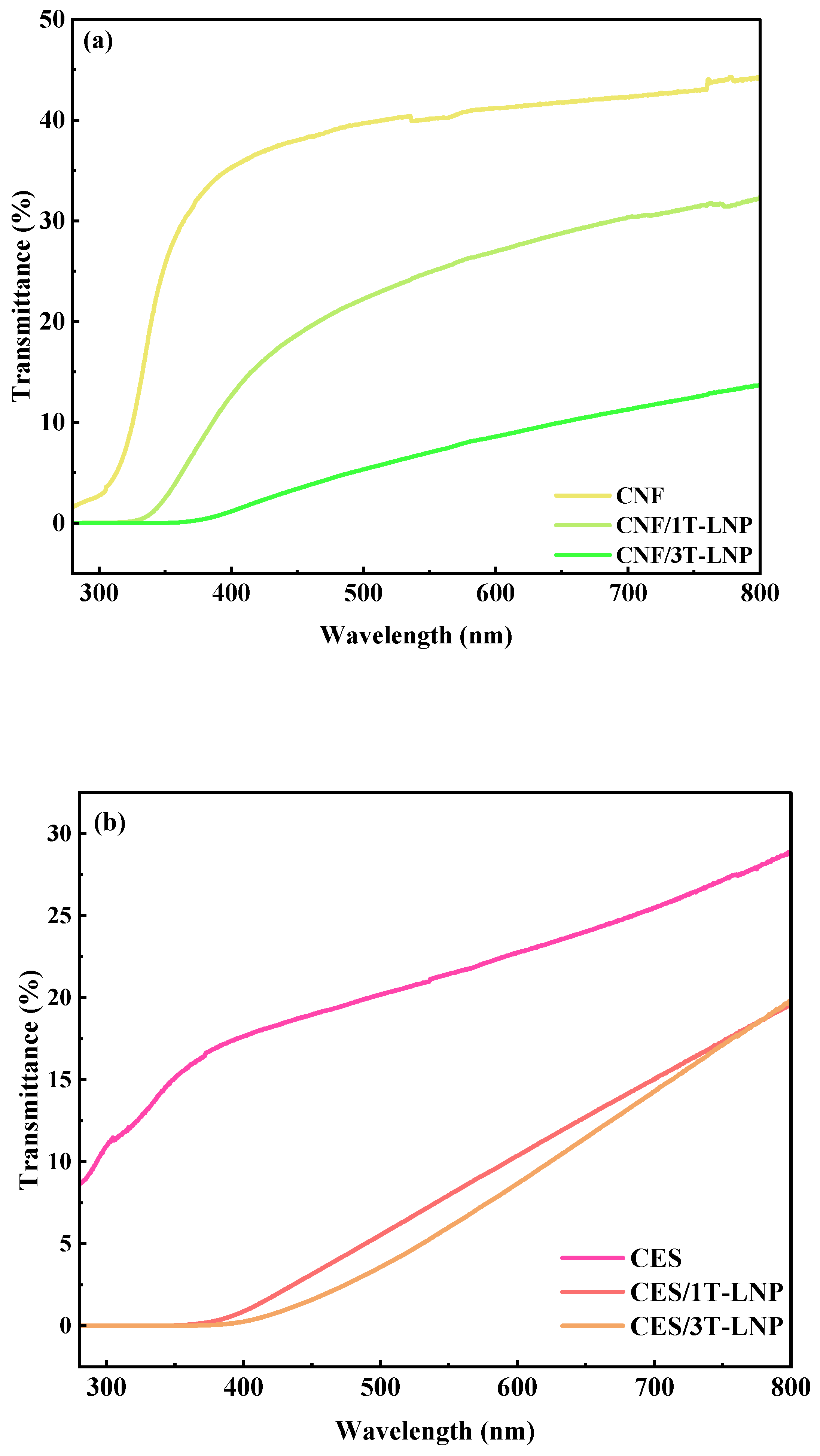
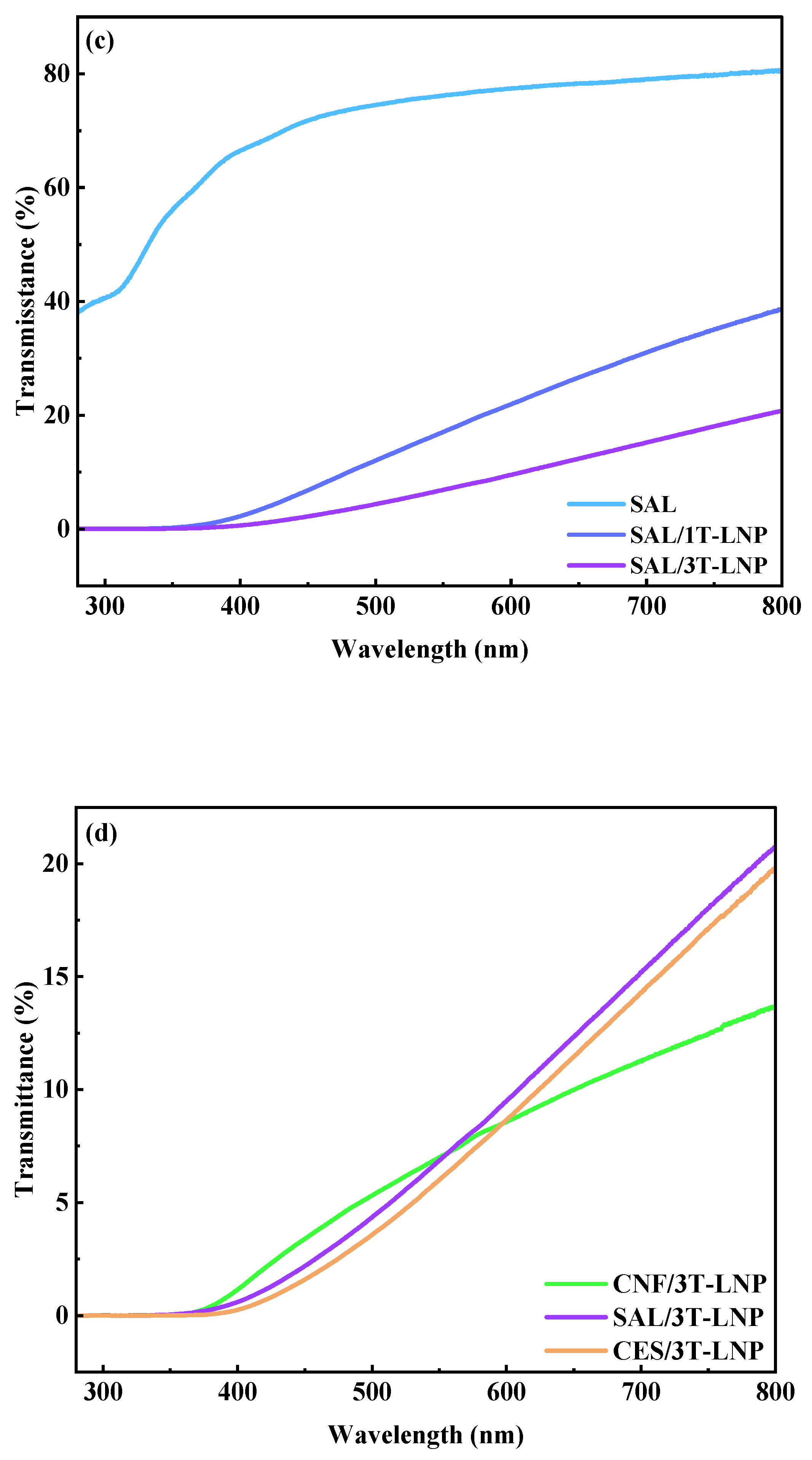
| Samples | Optical Property | |
|---|---|---|
| Transmittance (%, 320 nm) | Transmittance (%, 550 nm) | |
| CES/3T-LNP | 0.006 | 6.04 |
| CNF/3T-LNP | 0.001 | 7.02 |
| SAL/3T-LNP | 0.008 | 6.90 |
| Samples | Antioxidation Activity | RSA (%) |
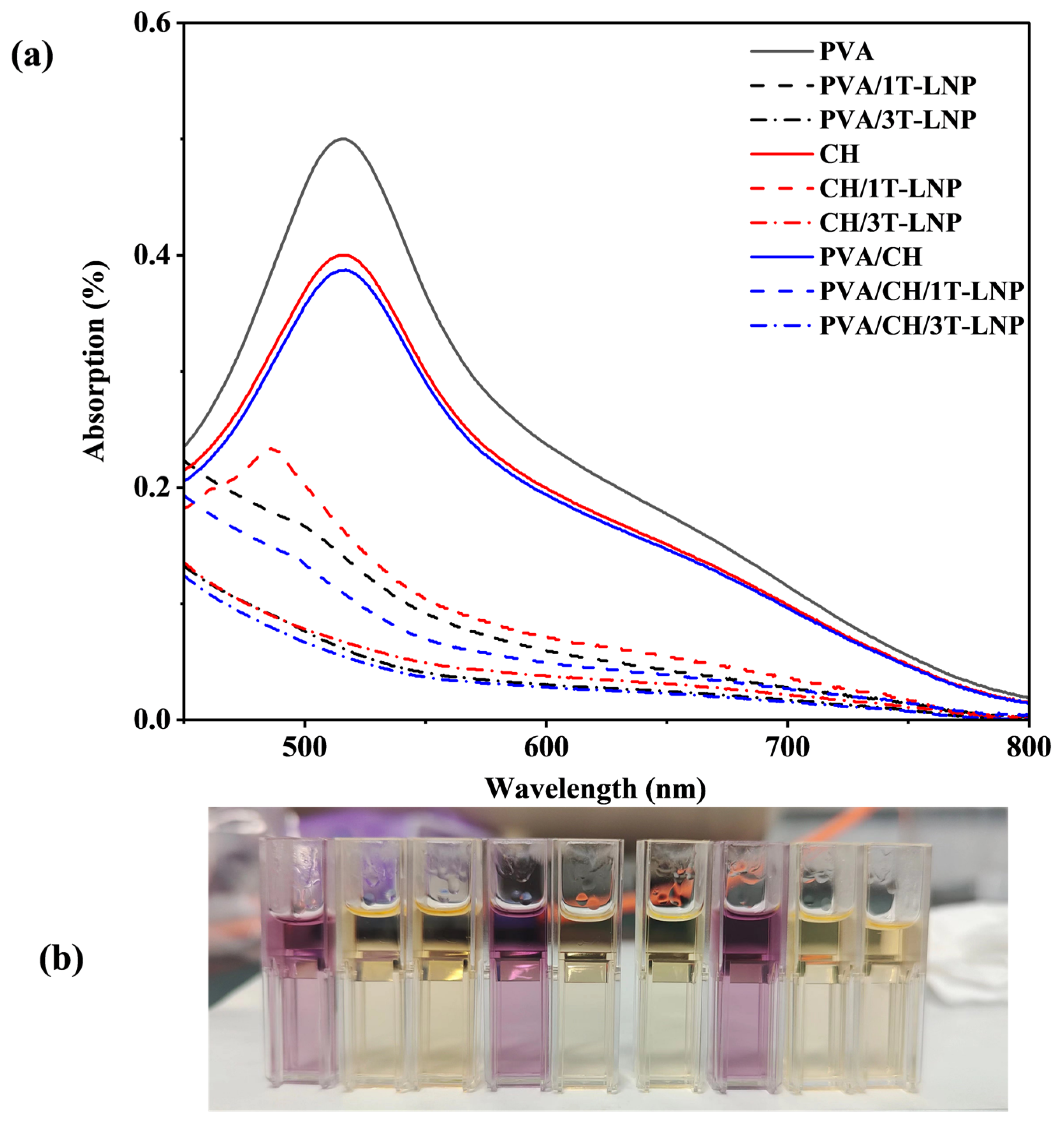
|---|---|---|
| Absorption (λ = 517 nm, %) | ||
| PVA | 0.499 | 0 |
| PVA/1T-LNP | 0.139 | 72.1 |
| PVA/3T-LNP | 0.067 | 86.6 |
| CH | 0.399 | 20.0 |
| CH/1T-LNP | 0.159 | 68.1 |
| CH/3T-LNP | 0.066 | 86.8 |
| PVA/CH | 0.388 | 22.2 |
| PVA/CH/1T-LNP | 0.108 | 78.4 |
| PVA/CH/3T-LNP | 0.055 | 89.0 |
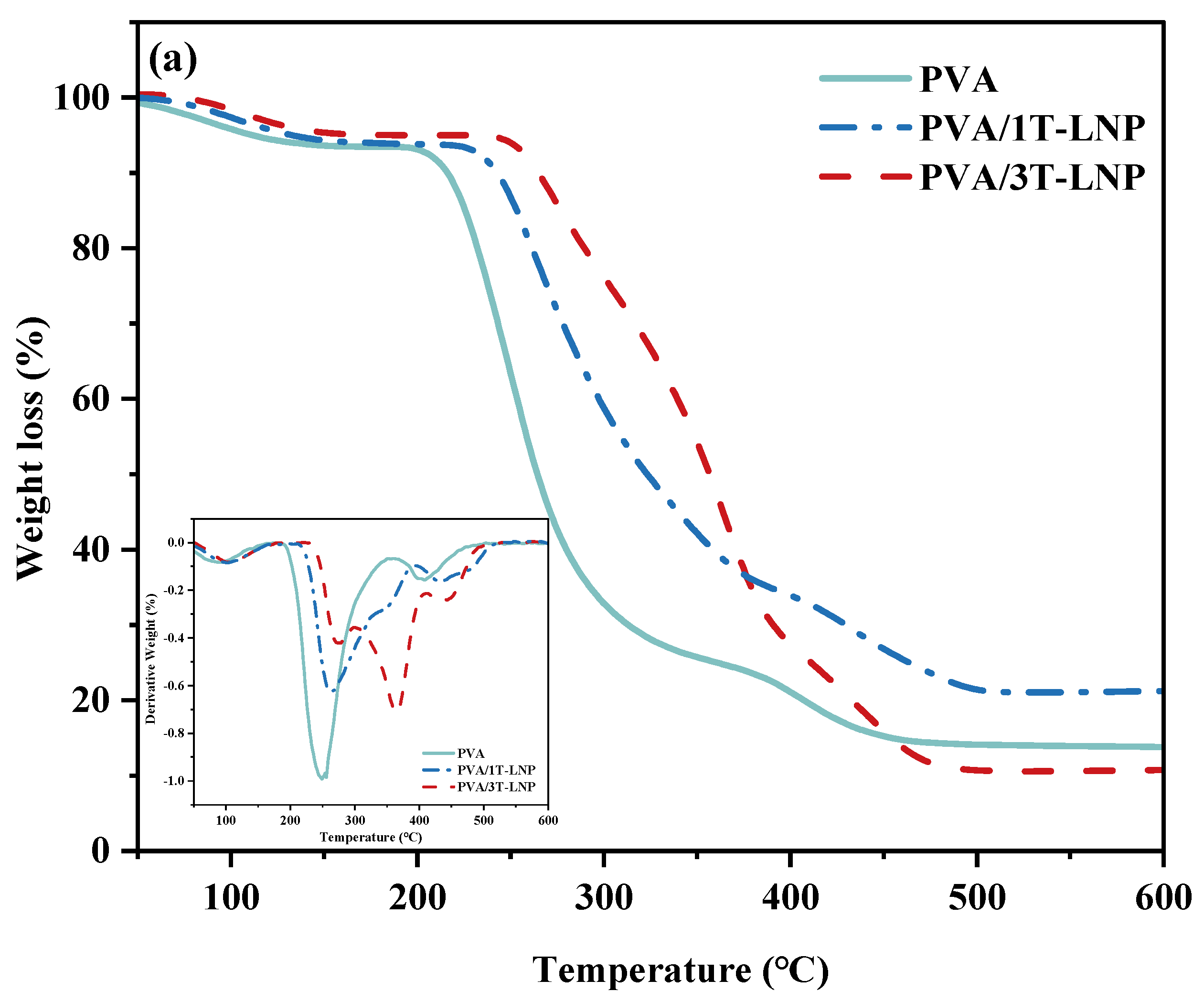
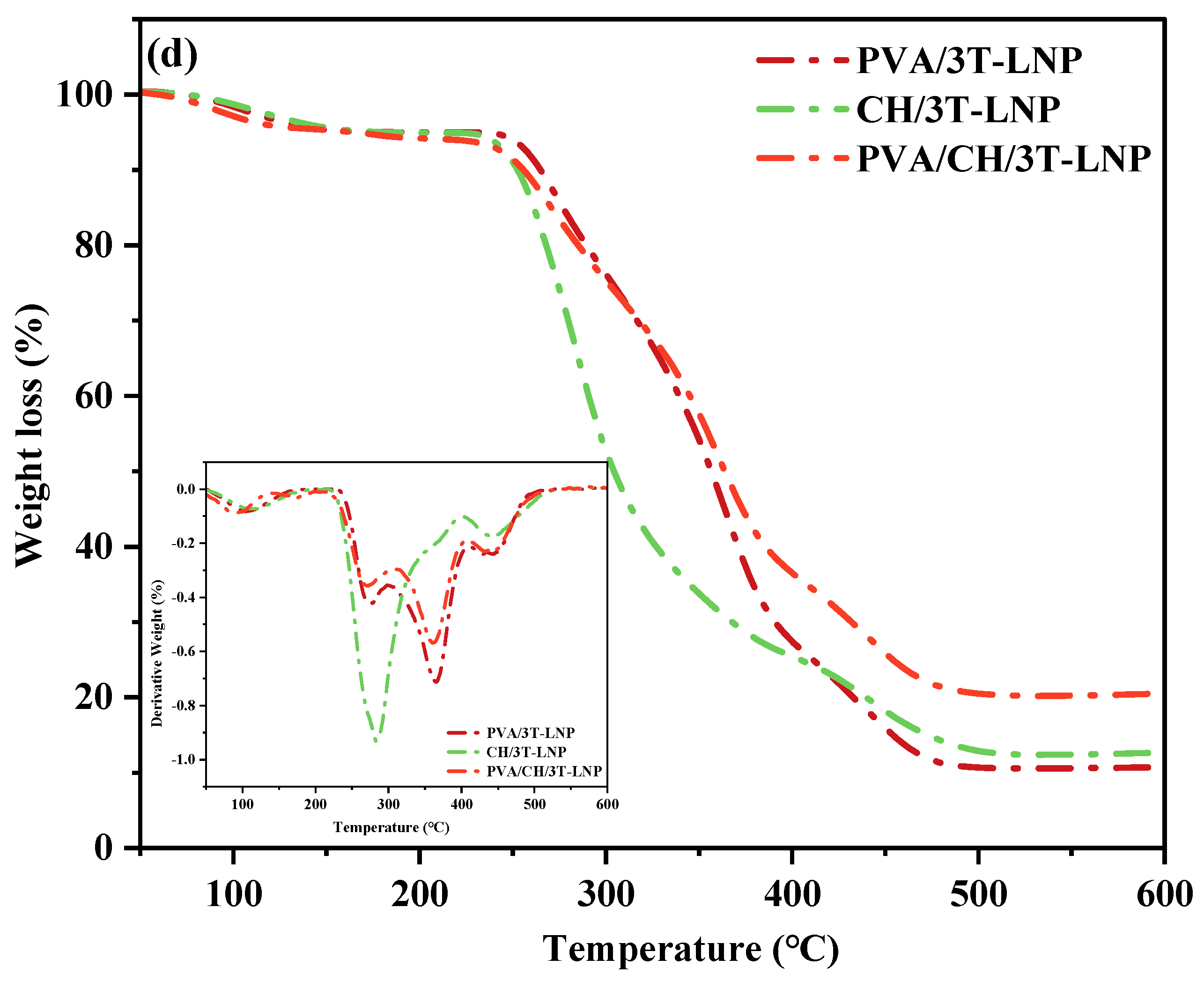
| Materials | T10% (°C) | T50% (°C) |
|---|---|---|
| PVA | 215.7 | 263.7 |
| PVA/1T-LNP | 242.4 | 322.7 |
| PVA/3T-LNP | 264.6 | 355.2 |
| CH | 137.7 | 366.1 |
| CH/1T-LNP | 242.8 | 285.9 |
| CH/3T-LNP | 251.6 | 303.4 |
| PVA/CH | 222.4 | 316.8 |
| PVA/CH/1T-LNP | 225.3 | 328.7 |
| PVA/CH/3T-LNP | 254.9 | 363.5 |
| Materials | Temperature at Peak (°C) | |||
|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 3 | Peak 4 | |
| PVA | 88.9 | 255.7 | - | 406.8 |
| PVA/1T-LNP | 97.3 | 265.1 | 432.8 | |
| PVA/3T-LNP | 100.7 | 272.5 | 364.2 | 428.9 |
| CH | 77.7 | 275.7 | - | - |
| CH/1T-LNP | 124.8 | 271.9 | - | 435.6 |
| CH/3T-LNP | 122.1 | 280.0 | - | 444.1 |
| PVA/CH | 90.7 | 254.7 | 347.7 | 429.3 |
| PVA/CH/1T-LNP | 96.9 | 272.8 | 353.2 | 430.7 |
| PVA/CH/3T-LNP | 93.8 | 271.0 | 360.8 | 434.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, S.; Zhang, S.; Liu, X.; Zhao, H.; Guo, D.; Tong, X.; Li, J. Green and Efficient Preparation of Tailed Lignin Nanoparticles and UV Shielding Composite Films. Nanomaterials 2022, 12, 2561. https://doi.org/10.3390/nano12152561
Zeng S, Zhang S, Liu X, Zhao H, Guo D, Tong X, Li J. Green and Efficient Preparation of Tailed Lignin Nanoparticles and UV Shielding Composite Films. Nanomaterials. 2022; 12(15):2561. https://doi.org/10.3390/nano12152561
Chicago/Turabian StyleZeng, Shiyi, Shenchong Zhang, Xiaogang Liu, Huifang Zhao, Daliang Guo, Xin Tong, and Jing Li. 2022. "Green and Efficient Preparation of Tailed Lignin Nanoparticles and UV Shielding Composite Films" Nanomaterials 12, no. 15: 2561. https://doi.org/10.3390/nano12152561






