Template-Free Synthesis of g-C3N4 Nanoball/BiOCl Nanotube Heterojunction with Enhanced Photocatalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
2.1.1. Raw Materials
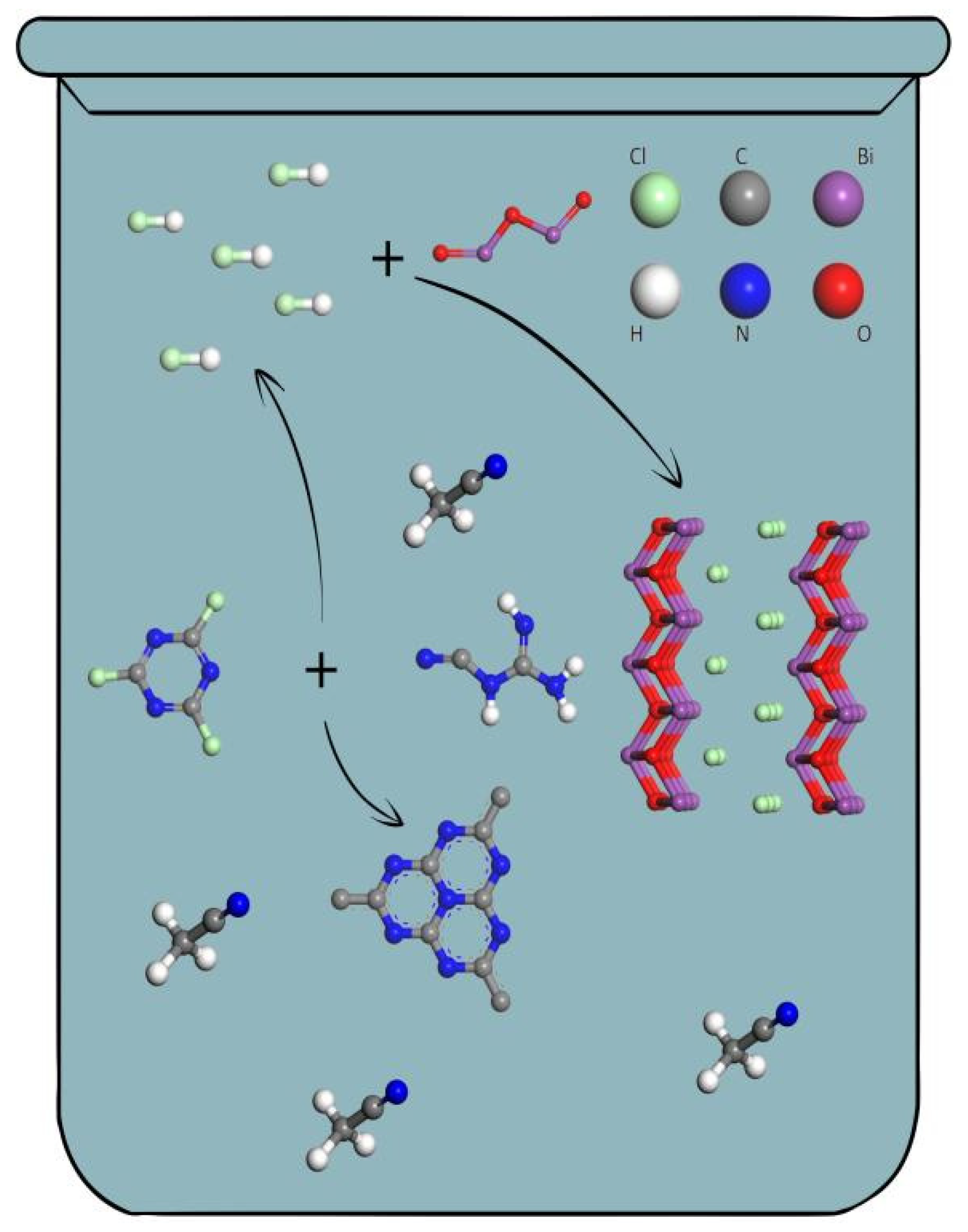
2.1.2. Synthesize of CB
2.1.3. Synthesize of BiOCl
2.2. Characterization
2.3. Photocatalytic Activity Experiment
3. Results
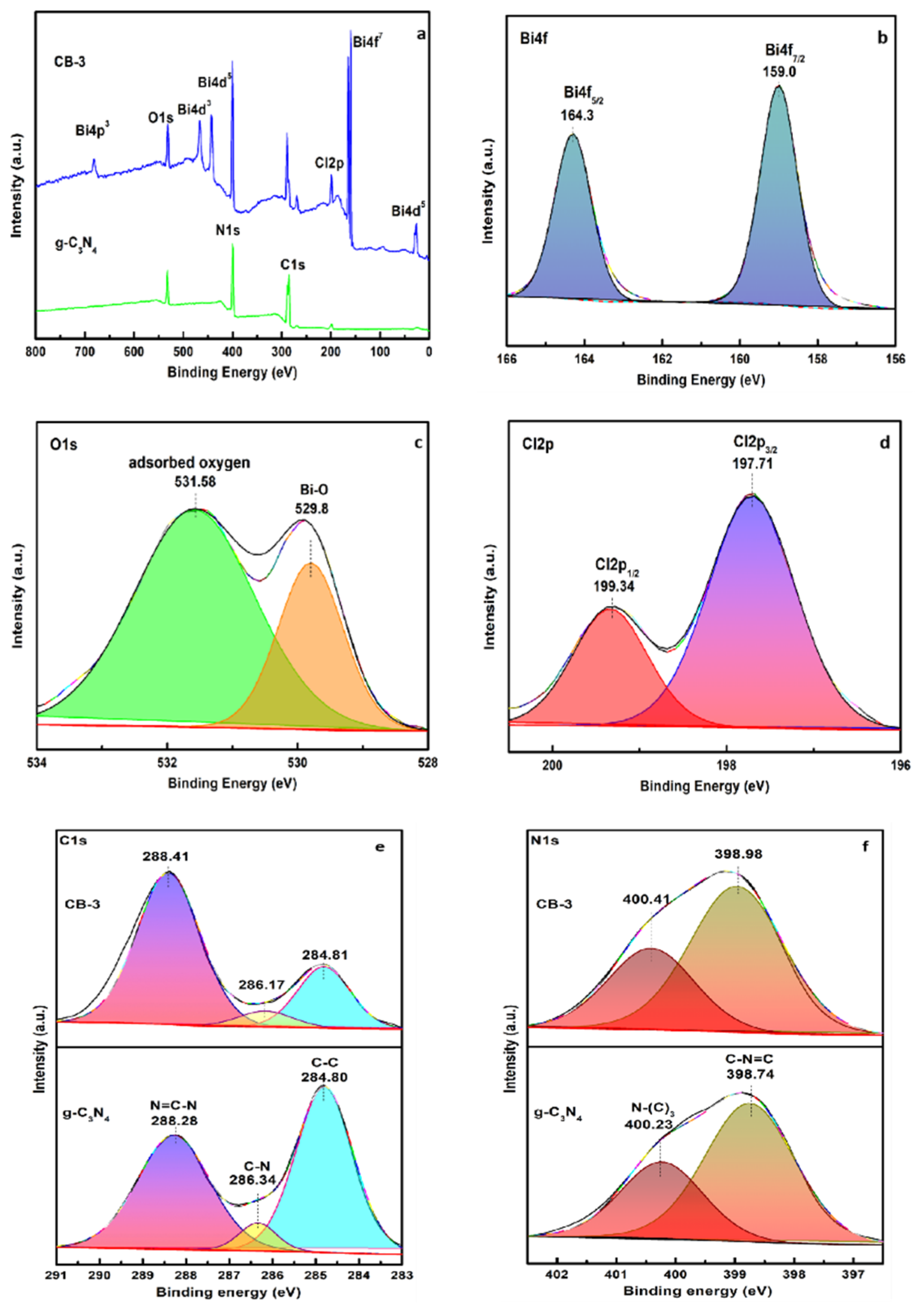
3.1. Structure and Composition
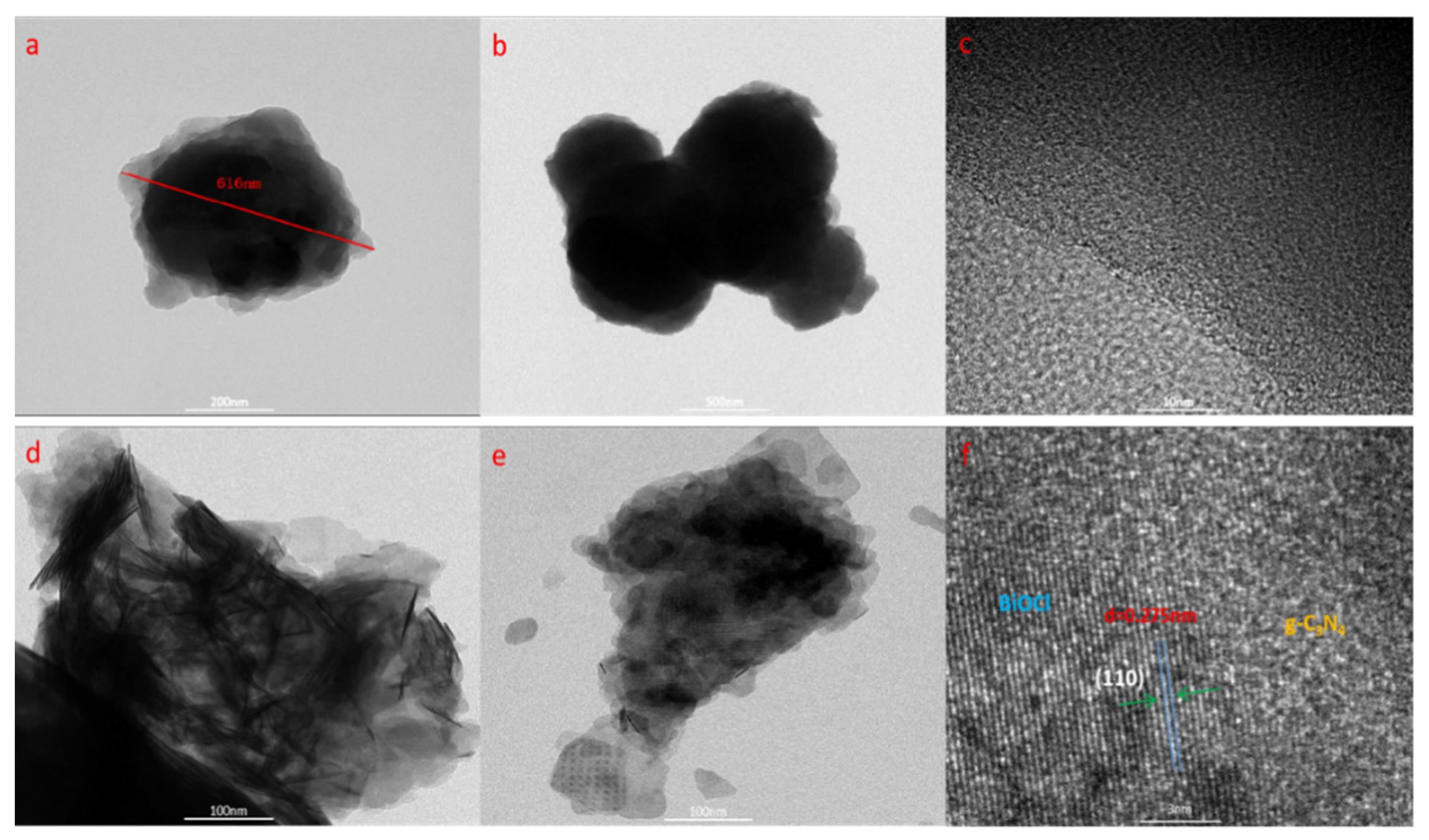
3.2. Morphology
3.3. Optical Properties
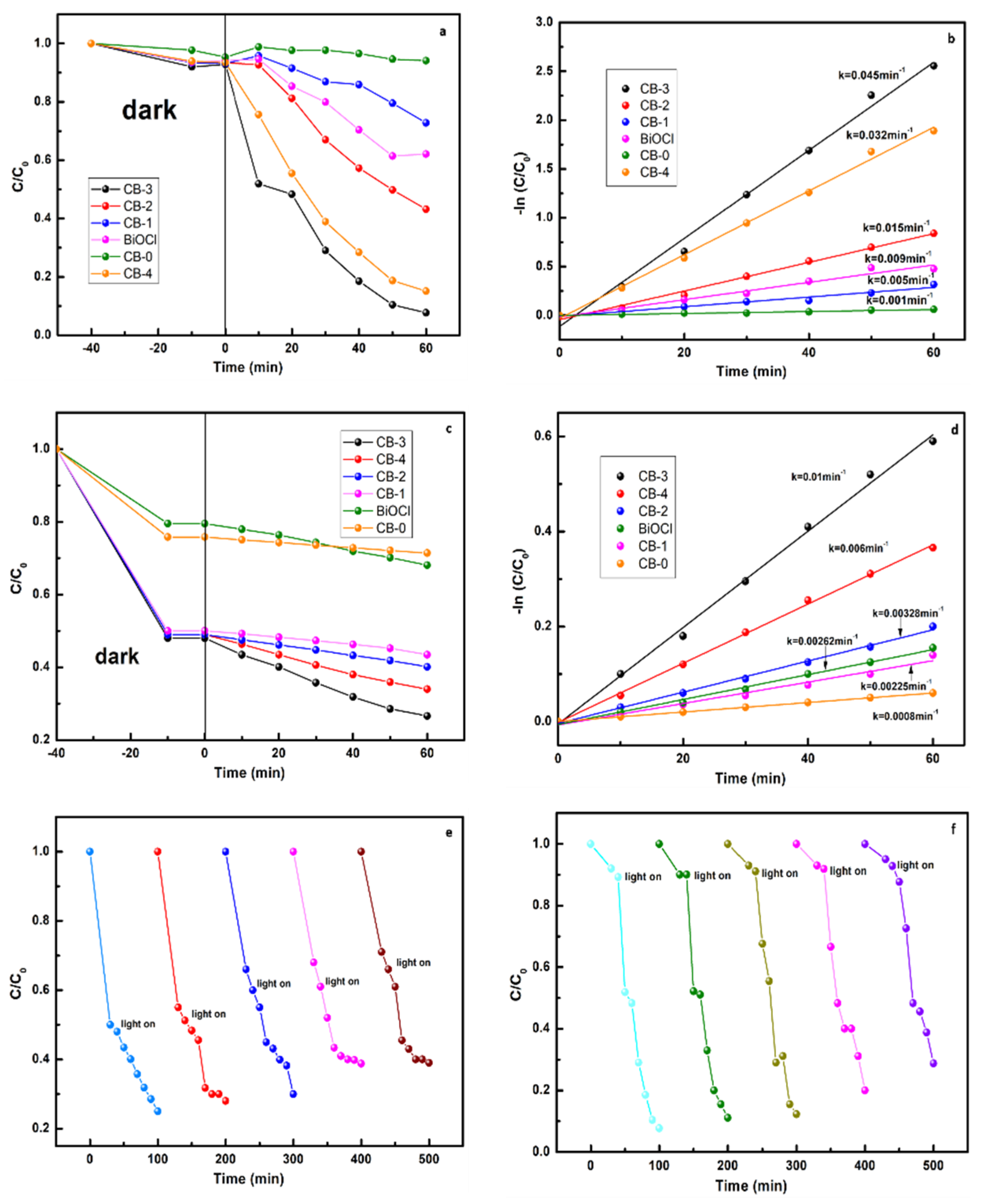
3.4. Photocatalytic Activity
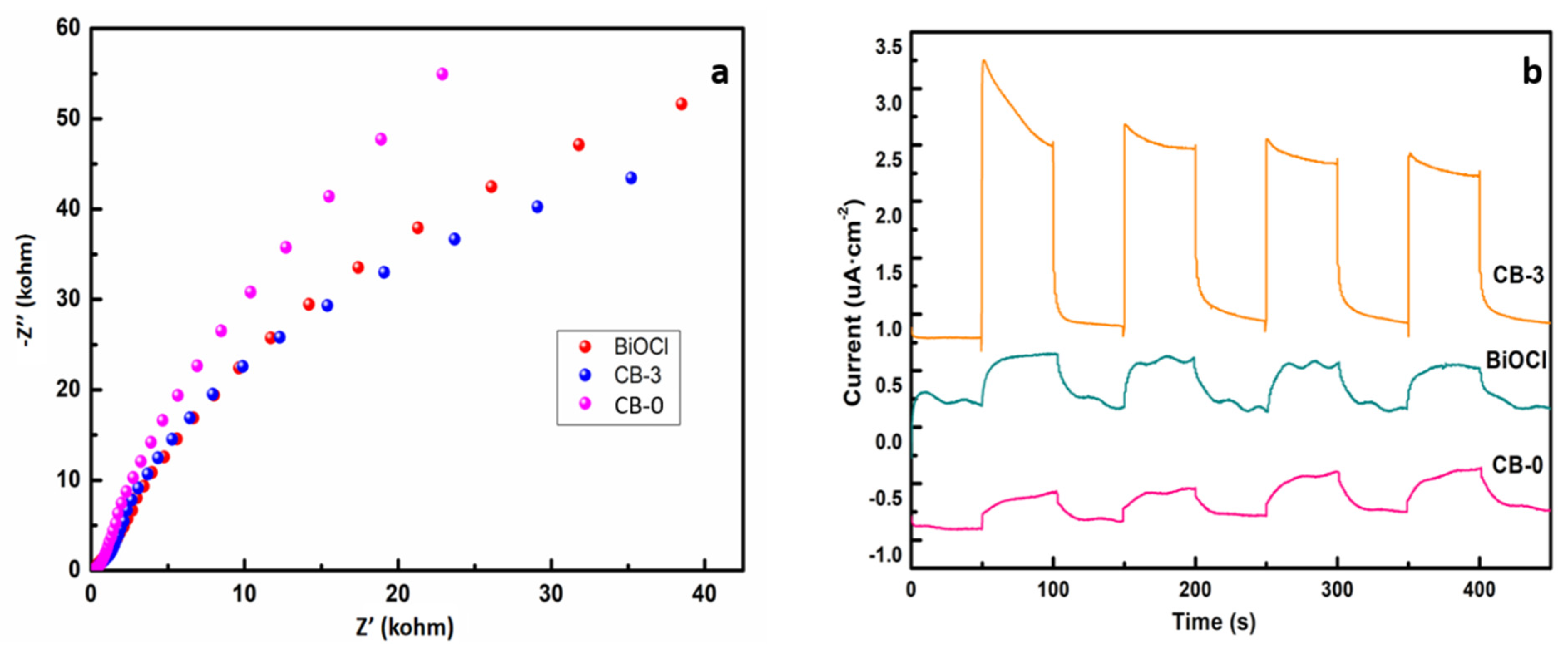
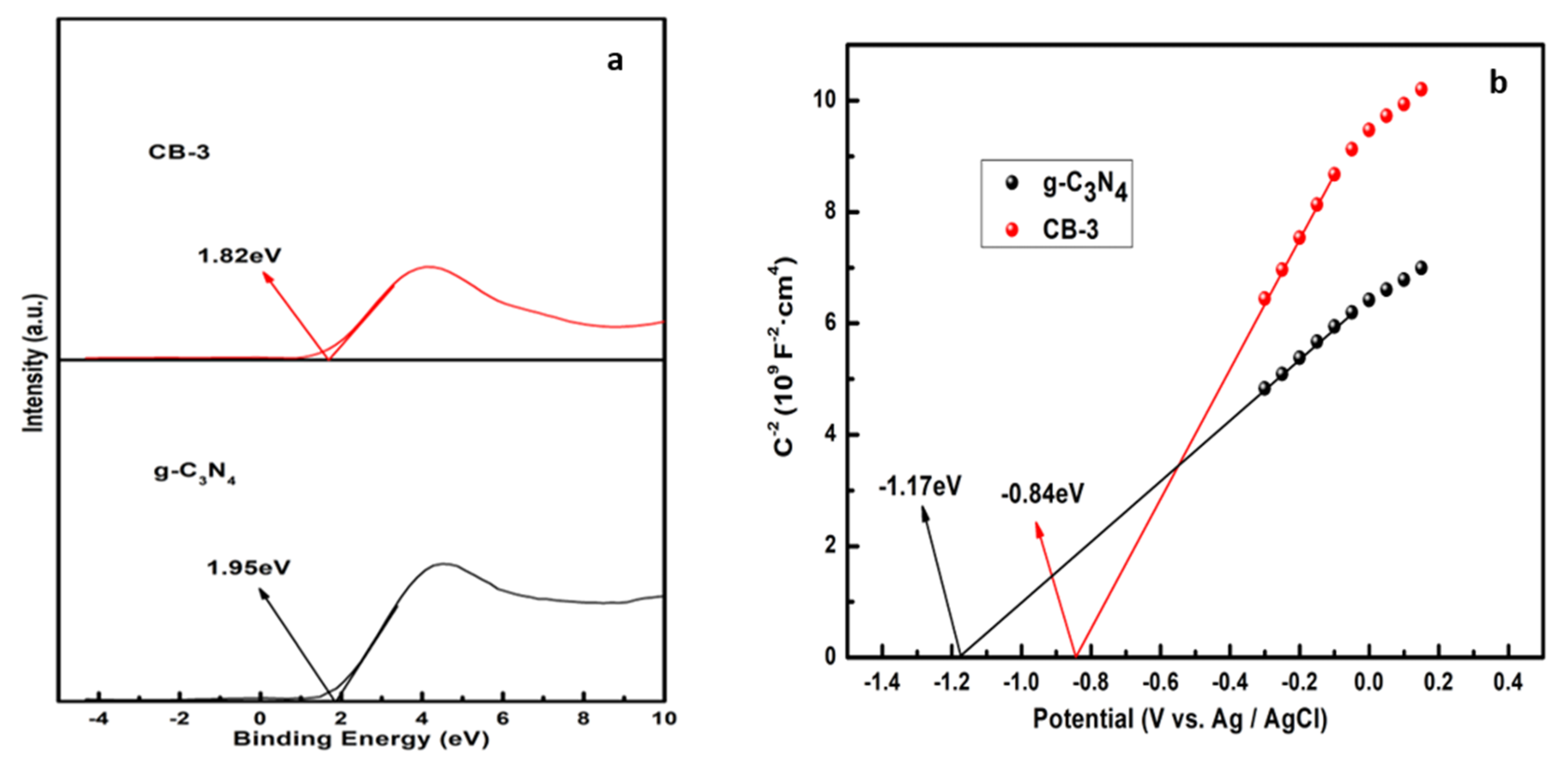
3.5. Photoelectric Properties
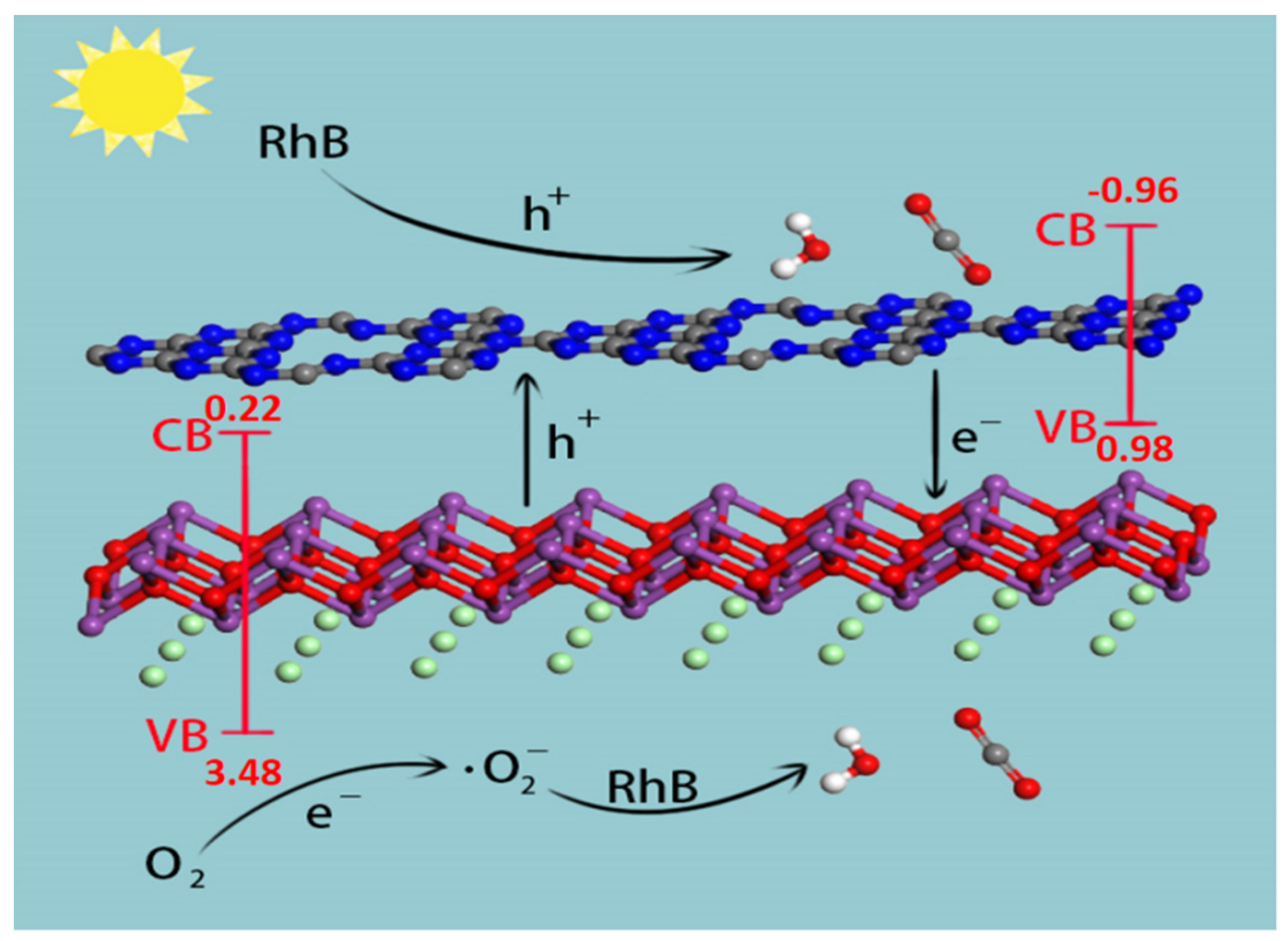
3.6. Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mclaren, A.; Valdes-Solis, T.; Li, G.; Tsang, S.C. Shape and size effects of ZnO nanocrystals on photocatalytic activity. J. Am. Chem. Soc. 2009, 131, 12540–12541. [Google Scholar] [CrossRef]
- Wu, N.; Wang, J.; Tafen, D.N.; Wang, H.; Zheng, J.-G.; Lewis, J.P.; Liu, X.; Leonard, S.S.; Manivannan, A. Shape-enhanced photocatalytic activity of single-crystalline anatase TiO2 (101) nanobelts. J. Am. Chem. Soc. 2010, 132, 6679–6685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.; Zheng, Z.; Yuan, Y.; Zhao, J.; Waclawik, E.R.; Ke, X.; Zhu, H. An efficient photocatalyst structure: TiO2(B) nanofibers with a shell of anatase nanocrystals. J. Am. Chem. Soc. 2009, 131, 17885–17893. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Bi, Q.-L.; Li, Q.; Su, Z.-P.; Chen, R.; Shi, C.-X.; Chen, T.-H. Room temperature synthesis of ultrathin iodine-doped BiOCl nanosheets. Colloid Surf. A-Physicochem. Eng. Asp. 2019, 582, 123899. [Google Scholar] [CrossRef]
- Huo, Y.; Zhang, J.-F.; Wang, Z.-L.; Dai, K.; Pan, C.-S.; Liang, C.-H. Efficient interfacial charge transfer of 2D/2D porous carbon nitride/bismuth oxychloride step-scheme heterojunction for boosted solar-driven CO2 reduction. J. Colloid Interface Sci. 2021, 585, 684–693. [Google Scholar] [CrossRef]
- Vinoth, S.; Ong, W.E.; Pandikumar, A. Sulfur-doped graphitic carbon nitride incorporated bismuth oxychloride/Cobalt based type-II heterojunction as a highly stable material for photoelectrochemical water splitting. J. Colloid Interface Sci. 2021, 591, 85–95. [Google Scholar] [CrossRef]
- Bellamkonda, S.; Rao, G.R. Nanojunction-mediated visible light photocatalytic enhancement in heterostructured ternary BiOCl/ CdS/g-C3N4 nanocomposites. Catal. Today 2019, 321, 18–25. [Google Scholar] [CrossRef]
- Li, H.; Xia, Z.-B.; Chen, J.-Q.; Lei, L.; Xing, J.-H. Constructing ternary CdS/reduced graphene oxide/TiO2 nanotube arrays hybrids for enhanced visible-light-driven photoelectrochemical and photocatalytic activity. Appl. Catal. B Environ. 2015, 168, 105–113. [Google Scholar]
- Zhu, Y.-Z.; Xu, Z.-X.; Lang, Q.-Q.; Jiang, W.-Y.; Yin, Q.-Q.; Zhong, S.-X.; Bai, S. Grain boundary engineered metal nanowire cocatalysts for enhanced photocatalytic reduction of carbon dioxide. Appl. Catal. B Environ. 2017, 206, 282–292. [Google Scholar] [CrossRef]
- Fu, J.-W.; Yu, J.-G.; Jiang, C.-J.; Cheng, B. g-C3N4-based heterostructured photocatalysts. Adv. Energy. Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Sridevi, A.; Ramji, B.R.; Venkatesan, G.K.D.P.; Sugumaran, V.; Selvakumar, P. A facile synthesis of TiO2/BiOCl and TiO2/BiOCl/La2O3 heterostructure photocatalyst for enhanced charge separation efficiency with improved UV-light catalytic activity towards Rhodamine B and Reactive Yellow 86. Inorg. Chem. Commun. 2021, 130, 108715. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.-Z.; Fang, F.; E, Y.-F.; Zhao, G.-Z. Novel visible-light-induced BiOCl/g-C3N4 photocatalyst for efficient degradation of metronidazole. Inorg. Chem. Commun. 2021, 132, 108820. [Google Scholar] [CrossRef]
- Chen, F.-M.; Leong, Z.-Y.; Yang, H.-Y. An aqueous rechargeable chloride ion battery. Energy Storage Mater. 2017, 7, 189–194. [Google Scholar]
- Cui, Y.; Tang, Y.; Wang, X. Template-free synthesis of graphitic carbon nitride hollow spheres for photocatalytic degradation of organic pollutants. Mater. Lett. 2015, 161, 197–200. [Google Scholar] [CrossRef]
- Wang, S.-J.; Zhang, J.-Q.; Li, B.; Sun, H.-Q.; Wang, S.-B.; Duan, X.-G. Morphology-dependent photocatalysis of graphitic carbon nitride for sustainable remediation of aqueous pollutants: A mini review. J. Environ. Chem. Eng. 2022, 10, 107438. [Google Scholar] [CrossRef]
- Liu, B.-Y.; Nie, X.-Q.; Tang, Y.; Yang, S.; Bian, L.; Dong, F.-Q.; He, H.-C.; Zhou, Y.; Liu, K. Objective findings on the K-Doped g-C3N4 photocatalysts: The presence and influence of organic byproducts on K-doped g-C3N4 photocatalysis. Langmuir 2021, 37, 4859–4868. [Google Scholar] [CrossRef]
- Xie, M.; Wei, W.; Xu, Y.-G.; Xie, J.-M. Carbon nitride nanowires/nanofibers: A novel template-free synthesis from a cyanuric chloride–melamine precursor towards enhanced adsorption and visible-light photocatalytic performance. Ceram. Int. 2016, 42, 4158–4170. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.-X.; Li, Y.-X.; Peng, S.-Q.; Liu, B. The nonmetal modulation of composition and morphology of g-C3N4-based photocatalysts. Appl. Catal. B Environ. 2020, 269, 118828. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.-H.; Yong, S.-T.; Chai, S.-P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hu, H.; Hu, Y.-H. Role of ZnO morphology in its reduction and photocatalysis. Appl. Surf. Sci. 2020, 502, 144202. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, R.-G.; Mu, L.-C.; Li, C. Significance of Crystal Morphology Controlling in Semiconductor-Based Photocatalysis: A Case Study on BiVO4 Photocatalyst. Cryst. Growth Des. 2017, 17, 2923–2928. [Google Scholar] [CrossRef]
- Yao, W.-Z.; Zhang, J.-H.; Wang, Y.X.; Ren, F.Z. Hybrid density functional study on the mechanism for the enhanced photocatalytic properties of the ultrathin hybrid layered nanocomposite g-C3N4/BiOCl. Appl. Surf. Sci. 2018, 435, 1351–1360. [Google Scholar] [CrossRef]
- Lv, J.; Dai, K.; Zhang, J.-F.; Geng, L.; Liang, C.-H.; Liu, Q.-C.; Zhu, G.-P.; Chen, C. Facile synthesis of Z-scheme graphitic-C3N4/Bi2MoO6 nanocomposite for enhanced visible photocatalytic properties. Appl. Surf. Sci. 2015, 358, 377–384. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, J.; Zhang, J.; Dai, K.; Liang, C. Facile synthesis of Z-scheme BiVO4/porous graphite carbon nitride heterojunction for enhanced visible-light-driven photocatalyst. Appl. Surf. Sci. 2018, 430, 595–602. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Li, G.; Cao, Y.; Shao, Y.; Li, D. Highly efficient Bi2O2CO3/BiOCl photocatalyst based on heterojunction with enhanced dye-sensitization under visible light. Appl. Catal. B Environ. 2016, 187, 301–309. [Google Scholar] [CrossRef]
- Wang, J.-C.; Yao, H.-C.; Fan, Z.-Y.; Zhang, L.; Wang, J.-S.; Zang, S.-Q.; Li, Z.-J. Indirect Z-Scheme BiOI/g-C3N4 photocatalysts with enhanced photoreduction CO2 activity under visible light irradiation. ACS Appl. Mater. Interfaces 2016, 8, 3765–3775. [Google Scholar] [CrossRef]
- Wang, W.-J.; Huang, B.-B.; Ma, X.-C.; Wang, Z.-Y.; Qin, X.-Y.; Zhang, X.-Y.; Dai, Y. Efficient separation of photogenerated electron-hole Pairs by the combination of a heterolayered structure and internal polar field in pyroelectric BiOIO3 nanoplates. Chem. Eur. J. 2013, 19, 14777–14780. [Google Scholar] [CrossRef]
- Zimmerman, J.L.; Williams, R.; Knabashesku, V.N. Synthesis of spherical carbon nitride nanostructures. Nano Lett. 2001, 1, 731–734. [Google Scholar] [CrossRef]
- Fanchini, G.; Tagliaferro, A.; Ray, S.C. Electronic and vibrational structures of amorphous carbon nitrides. Diam. Relat. Mater. 2003, 12, 208–218. [Google Scholar] [CrossRef]
- Al Marzouqi, F.; Al Farsi, B.; Kuvarega, A.T.; Al Lawati, H.A.J.; Al Kindy, S.M.Z.; Kim, Y.; Selvaraj, R. Controlled Microwave-Assisted Synthesis of the 2D-BiOCl/2D-g-C3N4 Heterostructure for the Degradation of Amine-Based Pharmaceuticals under Solar Light Illumination. ACS Omega 2019, 4, 4671–4678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, K.; Xu, Z.; Wang, L.; Feng, H.; Pan, F.; Zhuang, J.; Hao, W. First-principles study on the electronic structures and diffusion behaviors of intrinsic defects in BiOCl. Comp. Mater. Sci. 2022, 203, 111088. [Google Scholar] [CrossRef]
- Hu, J.-L.; Wu, X.; Huang, C.-J.; Fan, W.-J.; Qiu, X.-Q. Visible light photocatalytic activity induced by Rh(III) modification on the surface of BiOCl. Appl. Surf. Sci. 2016, 387, 45–50. [Google Scholar] [CrossRef]
- She, X.; Wu, J.; Zhong, J.; Xu, H.; Yang, Y.; Vajtai, R.; Lou, J.; Liu, Y.; Du, D.; Li, H.; et al. Oxygenated monolayer carbon nitride for excellent photocatalytic hydrogen evolution and external quantum efficiency. Nano Energy 2016, 27, 138–146. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for selective photoreduction of CO2 into solar fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Abdelaziz, M.B.; Chouchene, B.; Balan, L.; Gries, T.; Medjahdi, G.; Ezzaouia, H.; Schneider, R. One pot synthesis of bismuth oxide/graphitic carbon nitride composites with high photocatalytic activity. Mol. Catal. 2019, 463, 110–118. [Google Scholar] [CrossRef]
- Yang, C.-L.; Wei, H.-G.; Guan, L.-T.; Guo, J.; Wang, Y.-R.; Yan, X.-R.; Zhang, X.; Wei, S.-Y.; Guo, Z.-H. Polymer nanocomposites for energy storage, energy saving, and anticorrosion. J. Mater. Chem. A 2015, 3, 14929–14941. [Google Scholar] [CrossRef]
- Hao, L.; Huang, H.-W.; Guo, Y.-X.; Du, X.; Zhang, Y.-H. Bismuth oxychloride homogeneous phasejunction BiOCl/Bi12O17Cl2 with unselectively efficient photocatalytic activity and mechanism insight. Appl. Surf. Sci. 2017, 420, 303–312. [Google Scholar] [CrossRef]
- Le, S.-L.; Jiang, T.-S.; Zhao, Q.; Liu, X.-F.; Li, Y.-Y.; Fang, B.-W.; Gong, M. Cu-doped mesoporous graphitic carbon nitride for enhanced visible-light driven photocatalysis. RSC Adv. 2016, 6, 38811–38819. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Shi, R.; Wang, B.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. Tuning oxygen vacancies in ultrathin TiO2 nanosheets to boost photocatalytic nitrogen fixation up to 700 nm. Adv. Mater. 2019, 31, 1806482. [Google Scholar] [CrossRef]
- Wang, S.; Ding, X.; Zhang, X.; Pang, H.; Hai, X.; Zhan, G.; Zhou, W.; Song, H.; Zhang, L.; Chen, H.; et al. In situ carbon homogeneous doping on ultrathin bismuth molybdate: A dual-purpose strategy for efficient molecular oxygen activation. Adv. Funct. Mater. 2017, 27, 1703923. [Google Scholar] [CrossRef]
- Tian, N.; Zhang, Y.; Li, X.; Xiao, K.; Du, X.; Dong, F.; Waterhouse, G.I.N.; Zhang, T.; Huang, H. Precursor-reforming protocol to 3D mesoporous g-C3N4 established by ultrathin self-doped nanosheets for superior hydrogen evolution. Nano Energy 2017, 38, 72–81. [Google Scholar] [CrossRef]
- Chen, X.-B.; Liu, L.; Yu, P.-Y.; Mao, S.-S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Sinhamahapatra, A.; Jeon, J.P.; Yu, J.-S. A new approach to prepare highly active and stable black titania for visible light-assisted hydrogen production. Energy Environ. Sci. 2015, 8, 3539–3544. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.-Y.; Yang, M.-Q.; Fu, X.-Z.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Kong, L.-N.; Jiang, Z.-Q.; Wang, C.-H.; Wan, F.-X.; Li, Y.-Y.; Wu, L.-Z.; Zhi, J.-F.; Zhang, X.-T.; Chen, S.-J.; Liu, Y.-C. Simple ethanol impregnation treatment can enhance photocatalytic activity of TiO2 nanoparticles under visible-light irradiation. ACS Appl. Mater. Interfaces 2015, 7, 7752–7758. [Google Scholar] [CrossRef]
- Chen, F.; Liu, H.-Q.; Bagwasi, S.; Shen, X.-X.; Zhang, J.-L. Photocatalytic study of BiOCl for degradation of organic pollutants under UV irradiation. J. Photoch. Photobiol. A 2010, 215, 76–80. [Google Scholar] [CrossRef]
- Tsukamoto, D.; Shiro, A.; Shiraishi, Y.; Sugano, Y.; Ichikawa, S.; Tanaka, S.; Hirai, T. Photocatalytic H2O2 production from ethanol/O2 system using TiO2 loaded with Au–Ag bimetallic alloy nanoparticles. ACS Catal. 2012, 2, 599–603. [Google Scholar] [CrossRef]
- Xu, J.; Gao, Q.-Z.; Bai, X.-J.; Wang, Z.-P.; Zhu, Y.-F. Enhanced visible-light-induced photocatalytic degradation and disinfection activities of oxidized porous g-C3N4 by loading Ag nanoparticles. Catal. Today 2019, 332, 227–235. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Huang, Y.; Chen, L.; Chen, M.-J.; Cao, J.-J.; Ho, W.K.; Lee, S.C. In situ g-C3N4 self-sacrificial synthesis of a g-C3N4/LaCO3OH heterostructure with strong interfacial charge transfer and separation for photocatalytic NO removal. J. Mater. Chem. A 2018, 6, 972–981. [Google Scholar] [CrossRef]




| C (%) | N (%) | O (%) | Cl (%) | Bi (%) | |
|---|---|---|---|---|---|
| EDS1 | 51.99 | 31.57 | 6.64 | 5.94 | 3.86 |
| EDS2 | 39.48 | 41.77 | 8.21 | 5.62 | 4.92 |
| EDS3 | 42.44 | 44.27 | 6.55 | 5.38 | 1.36 |
| AVERAGE | 44.64 | 39.20 | 7.13 | 5.65 | 3.38 |
| XPS | 39.83 | 36.88 | 10.76 | 7.73 | 4.80 |
| Catalyst | Dyes | Removal Ratio (%) | Time (min) | Dye (mg/L) | Catalyst (g/L) | Refs |
|---|---|---|---|---|---|---|
| g-C3N4/CdS/BiOCl | RhB | ~90 | 30 | 20 | 1 | [9] |
| g-C3N4/BiOCl | RhB | ~90 | 150 | 20 | 1 | [24] |
| g-C3N4/Bi2O3 | RhB | ~90 | 210 | 10 | 0.25 | [37] |
| g-C3N4/BiOCl | RhB | ~90 | 50 | 10 | 0.46 | In this study |
| Ag/g-C3N4 | MO | ~95 | 300 | 20 | 2 | [38] |
| BiOCl/Bi12O17Cl2 | MO | ~70 | 300 | 10 | 0.6 | [39] |
| Cu/g-C3N4 | MO | ~90 | 70 | 10 | 0.5 | [40] |
| g-C3N4/BiOCl | MO | ~75 | 60 | 10 | 0.46 | In this study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Fan, Z.; Cao, X.; Fan, P.; Xie, Y.; Sun, Q.; Zhao, J. Template-Free Synthesis of g-C3N4 Nanoball/BiOCl Nanotube Heterojunction with Enhanced Photocatalytic Activity. Nanomaterials 2022, 12, 2569. https://doi.org/10.3390/nano12152569
Wang L, Fan Z, Cao X, Fan P, Xie Y, Sun Q, Zhao J. Template-Free Synthesis of g-C3N4 Nanoball/BiOCl Nanotube Heterojunction with Enhanced Photocatalytic Activity. Nanomaterials. 2022; 12(15):2569. https://doi.org/10.3390/nano12152569
Chicago/Turabian StyleWang, Longfei, Zheyuan Fan, Xixi Cao, Panfeng Fan, Yu Xie, Qing Sun, and Jinsheng Zhao. 2022. "Template-Free Synthesis of g-C3N4 Nanoball/BiOCl Nanotube Heterojunction with Enhanced Photocatalytic Activity" Nanomaterials 12, no. 15: 2569. https://doi.org/10.3390/nano12152569
APA StyleWang, L., Fan, Z., Cao, X., Fan, P., Xie, Y., Sun, Q., & Zhao, J. (2022). Template-Free Synthesis of g-C3N4 Nanoball/BiOCl Nanotube Heterojunction with Enhanced Photocatalytic Activity. Nanomaterials, 12(15), 2569. https://doi.org/10.3390/nano12152569






