Biocompatible Casein Electrolyte-Based Electric-Double-Layer for Artificial Synaptic Transistors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
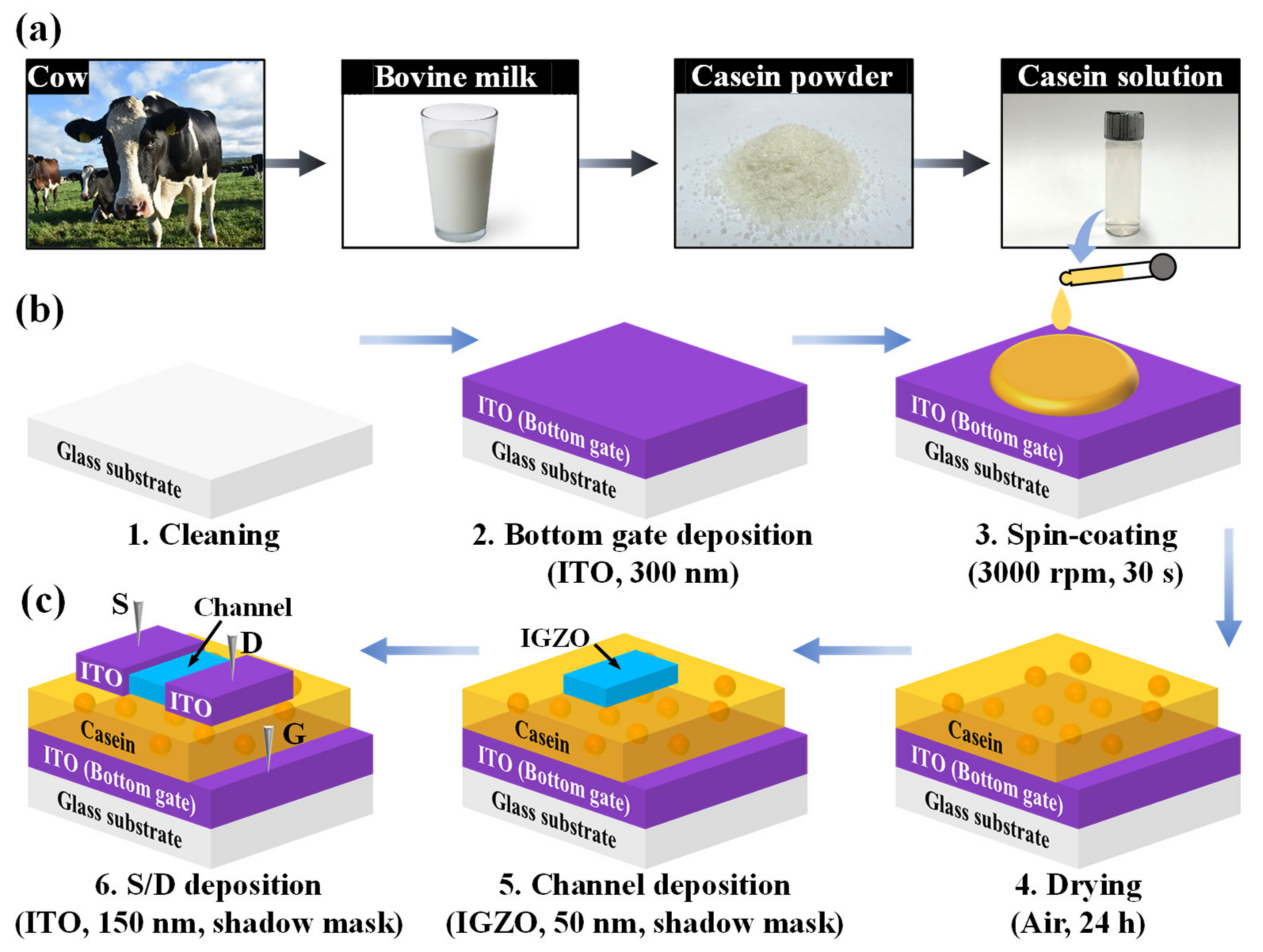
2.2. Preparation of Casein Solution
2.3. Fabrication of Casein Electrolyte-Based EDL Synaptic Transistors
2.4. Characterizations
3. Results
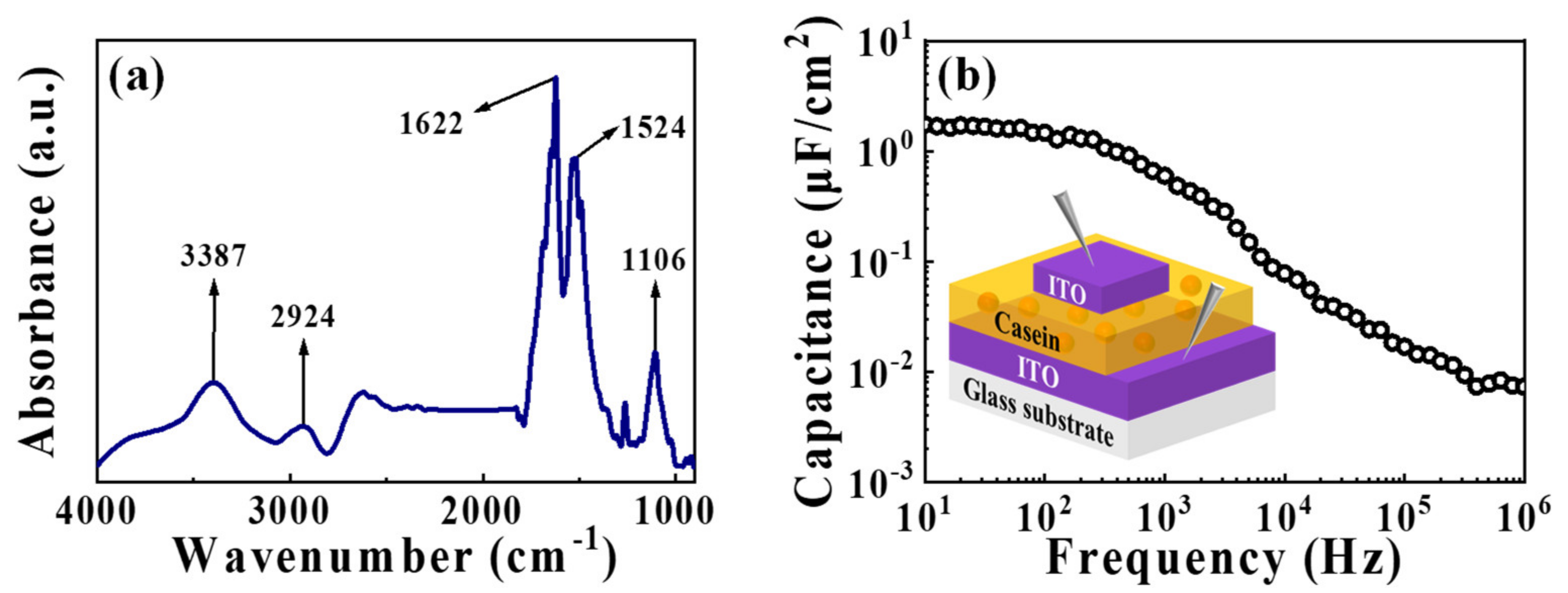
3.1. Verification of Casein Electrolyte Properties for EDL
3.2. Electrical Characteristics of Casein Electrolyte-Based EDL Synaptic Transistors
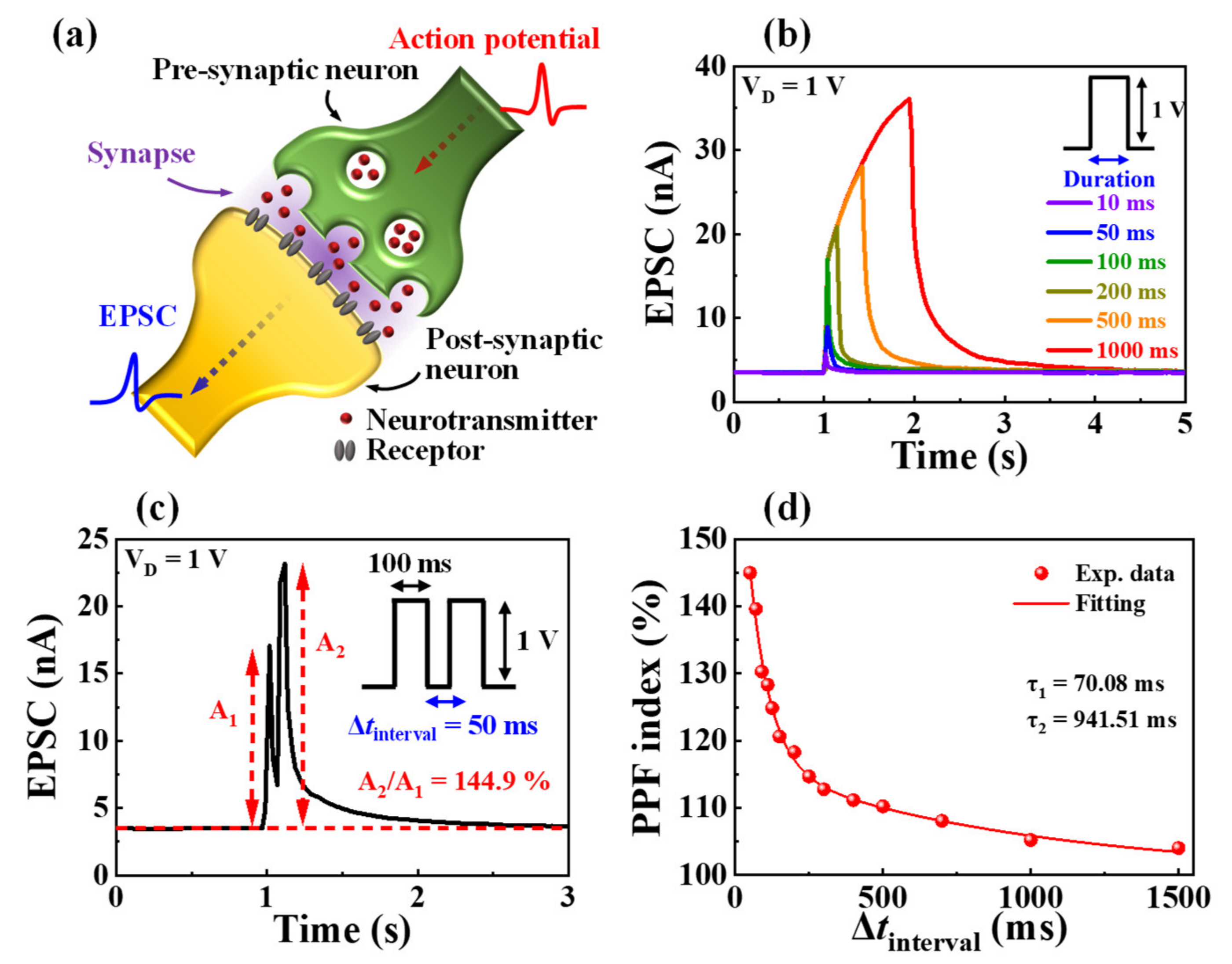
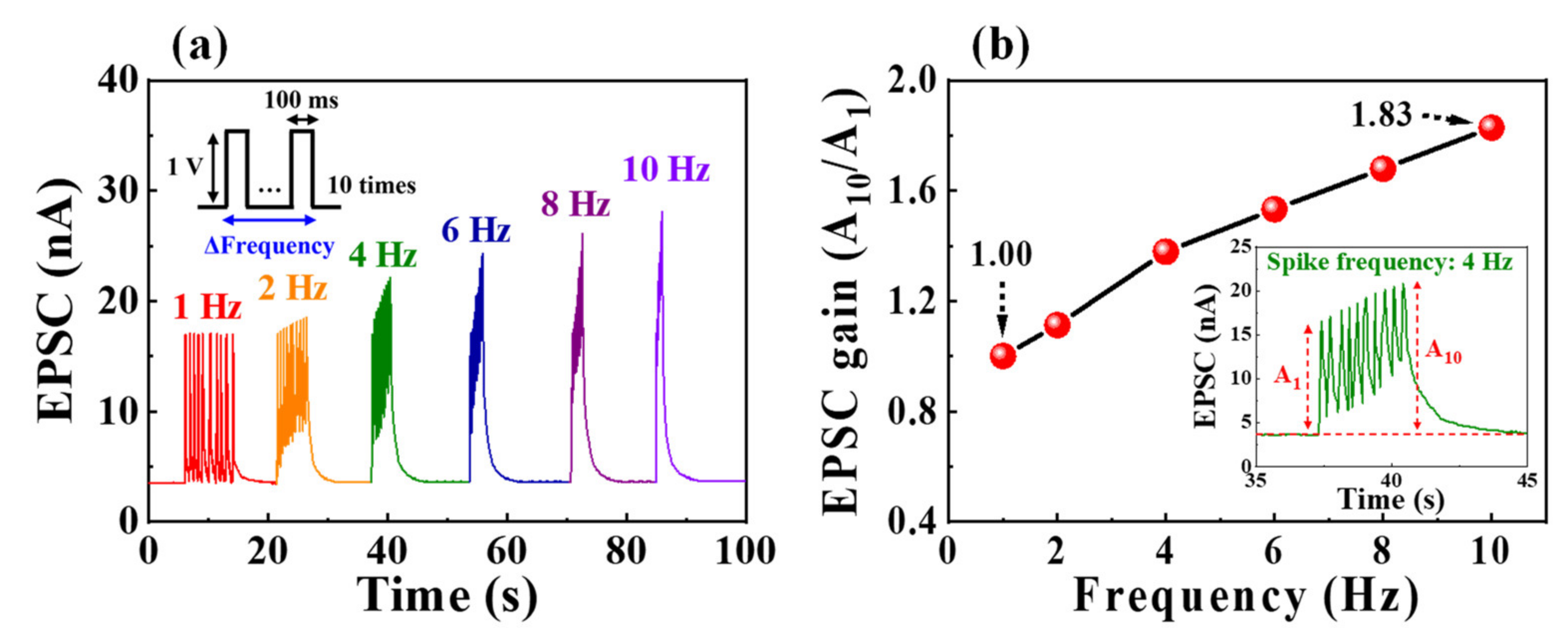
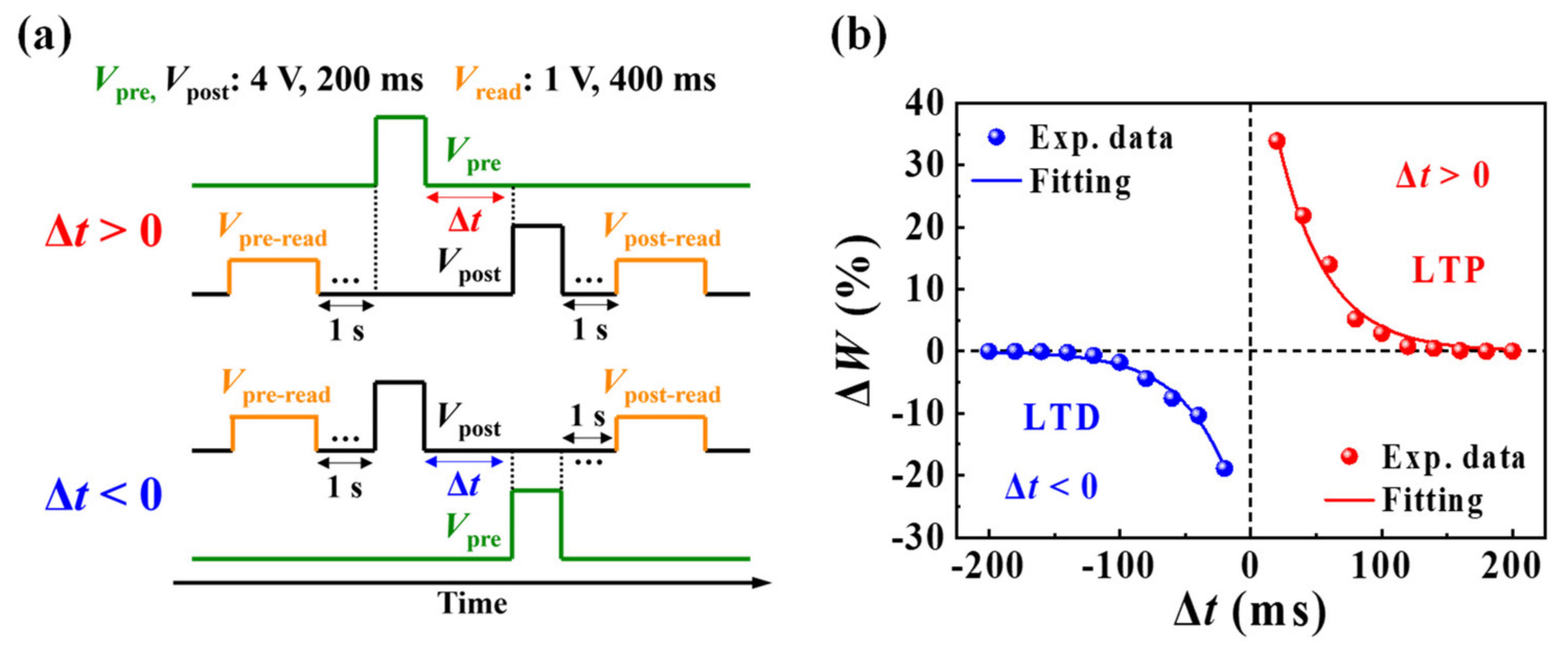
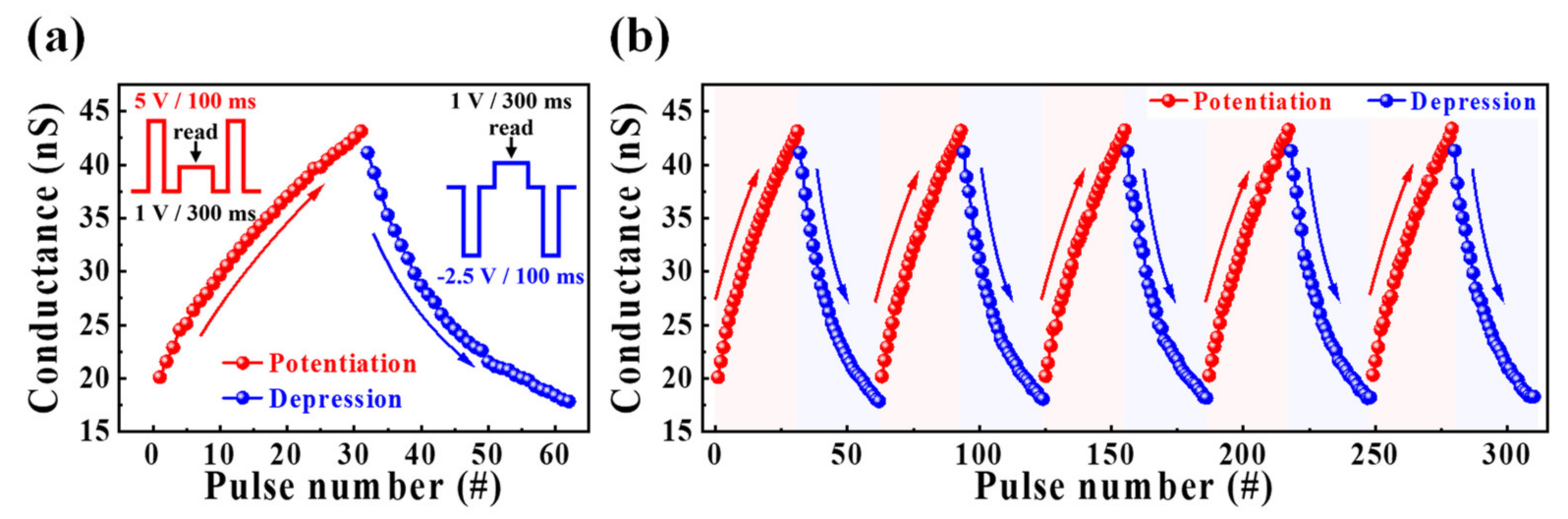
3.3. Synaptic Properties of Casein Electrolyte-Based EDL Synaptic Transistors
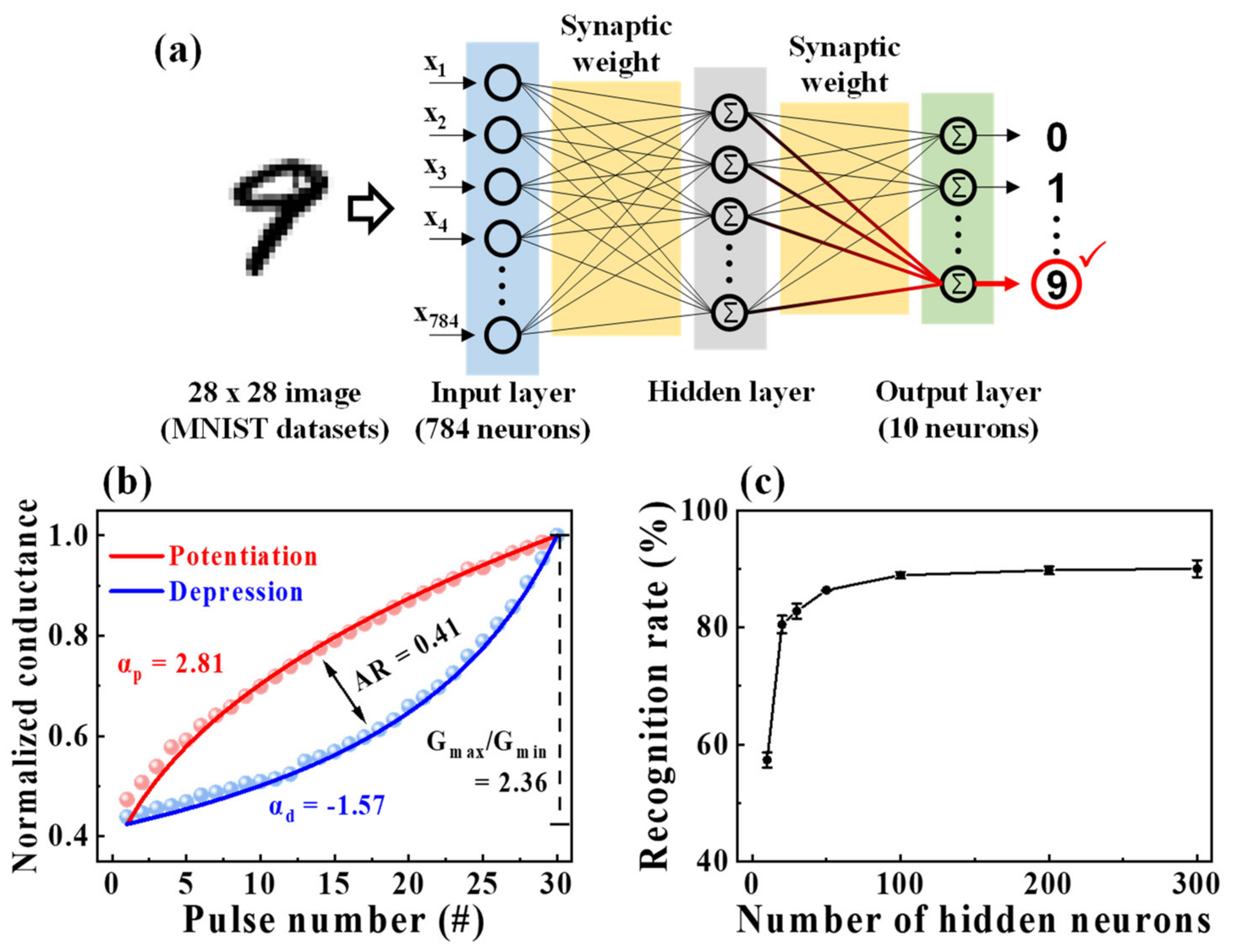
3.4. MNIST ANN Simulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, Q.; Sharbati, M.T.; Erickson, J.R.; Du, Y.; Xiong, F. Emerging artificial synaptic devices for neuromorphic computing. Adv. Mater. Technol. 2019, 4, 1900037. [Google Scholar] [CrossRef] [Green Version]
- Slavakis, K.; Giannakis, G.B.; Mateos, G. Modeling and optimization for big data analytics:(statistical) learning tools for our era of data deluge. IEEE Signal Process. Mag. 2014, 31, 18–31. [Google Scholar] [CrossRef]
- Pershin, Y.V.; Di Ventra, M. Neuromorphic, digital, and quantum computation with memory circuit elements. Proc. IEEE 2011, 100, 2071–2080. [Google Scholar] [CrossRef] [Green Version]
- Indiveri, G.; Liu, S.C. Memory and information processing in neuromorphic systems. Proc. IEEE 2015, 103, 1379–1397. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, D.W.; Zhou, P. Two-dimensional materials for synaptic electronics and neuromorphic systems. Sci. Bull. 2019, 64, 1056–1066. [Google Scholar] [CrossRef] [Green Version]
- Kuzum, D.; Yu, S.; Wong, H.P. Synaptic electronics: Materials, devices and applications. Nanotechnology 2013, 24, 382001. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, G.; Xin, Z.; Fu, C.; Wan, Q.; Shan, F. Solution-processed, electrolyte-gated In2O3 flexible synaptic transistors for brain-inspired neuromorphic applications. ACS Appl. Mater. Interfaces 2019, 12, 1061–1068. [Google Scholar] [CrossRef]
- Furber, S. Large-scale neuromorphic computing systems. J. Neural Eng. 2016, 13, 051001. [Google Scholar] [CrossRef]
- Grollier, J.; Querlioz, D.; Stiles, M.D. Spintronic nanodevices for bioinspired computing. Proc. IEEE 2016, 104, 2024–2039. [Google Scholar] [CrossRef] [Green Version]
- Royer, S.; Paré, D. Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature 2003, 422, 518–522. [Google Scholar] [CrossRef]
- Jo, S.H.; Chang, T.; Ebong, I.; Bhadviya, B.B.; Mazumder, P.; Lu, W. Nanoscale memristor device as synapse in neuromorphic systems. Nano Lett. 2010, 10, 1297–1301. [Google Scholar] [CrossRef]
- Huang, H.Y.; Ge, C.; Zhang, Q.H.; Liu, C.X.; Du, J.Y.; Li, J.K.; Wang, C.; Gu, L.; Yang, G.Z.; Jin, K.J. Electrolyte-gated synaptic transistor with oxygen ions. Adv. Funct. Mater. 2019, 29, 1902702. [Google Scholar] [CrossRef]
- Zhang, S.R.; Zhou, L.; Mao, J.Y.; Ren, Y.; Yang, J.Q.; Yang, G.H.; Zhu, X.; Han, S.T.; Roy, V.A.; Zhou, Y. Artificial synapse emulated by charge trapping-based resistive switching device. Adv. Mater. Technol. 2019, 4, 1800342. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, S.; Simanjuntak, F.M.; Saminathan, R.; Panda, D.; Tseng, T.Y. Improving linearity by introducing Al in HfO2 as a memristor synapse device. Nanotechnology 2019, 30, 445205. [Google Scholar] [CrossRef]
- Kuzum, D.; Jeyasingh, R.G.; Lee, B.; Wong, H.S.P. Nanoelectronic programmable synapses based on phase change materials for brain-inspired computing. Nano Lett. 2012, 12, 2179–2186. [Google Scholar] [CrossRef]
- Boyn, S.; Grollier, J.; Lecerf, G.; Xu, B.; Locatelli, N.; Fusil, S.; Girod, S.; Carrétéro, C.; Garcia, K.; Xavier, S.; et al. Learning through ferroelectric domain dynamics in solid-state synapses. Nat. Commun. 2017, 8, 14736. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Yang, Y.; Ren, T.L. Top-gate electric-double-layer IZO-based synaptic transistors for neuron networks. IEEE Electron Device Lett. 2017, 38, 588–591. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, K.; Xie, W.; Lee, K.H.; Zhang, S.; Lodge, T.P.; Frisbie, C.D. Electrolyte-gated transistors for organic and printed electronics. Adv. Mater. 2013, 25, 1822–1846. [Google Scholar] [CrossRef]
- Fujimoto, T.; Awaga, K. Electric-double-layer field-effect transistors with ionic liquids. Phys. Chem. Chem. Phys. 2013, 15, 8983–9006. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y.; Shi, Y.; Wan, Q. Solution-processed chitosan-gated IZO-based transistors for mimicking synaptic plasticity. IEEE Electron Device Lett. 2014, 35, 280–282. [Google Scholar] [CrossRef]
- Dai, S.; Wang, Y.; Zhang, J.; Zhao, Y.; Xiao, F.; Liu, D.; Wang, T.; Huang, J. Wood-derived nanopaper dielectrics for organic synaptic transistors. ACS Appl. Mater. Interfaces 2018, 10, 39983–39991. [Google Scholar] [CrossRef]
- Gao, W.T.; Zhu, L.Q.; Tao, J.; Wan, D.Y.; Xiao, H.; Yu, F. Dendrite integration mimicked on starch-based electrolyte-gated oxide dendrite transistors. ACS Appl. Mater. Interfaces 2018, 10, 40008–40013. [Google Scholar] [CrossRef]
- Wu, G.; Feng, P.; Wan, X.; Zhu, L.; Shi, Y.; Wan, Q. Artificial synaptic devices based on natural chicken albumen coupled electric-double-layer transistors. Sci. Rep. 2016, 6, 23578. [Google Scholar] [CrossRef]
- Sun, J.; Fu, Y.; Wan, Q. Organic synaptic devices for neuromorphic systems. J. Phys. D Appl. Phys. 2018, 51, 314004. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, W.J. Artificial synapses based on bovine milk biopolymer electric-double-layer transistors. Polymers 2022, 14, 1372. [Google Scholar] [CrossRef]
- Phelan, M.; Aherne, A.; FitzGerald, R.J.; O’Brien, N.M. Casein-derived bioactive peptides: Biological effects, industrial uses, safety aspects and regulatory status. Int. Dairy J. 2009, 19, 643–654. [Google Scholar] [CrossRef]
- Chen, H. Functional properties and applications of edible films made of milk proteins. J. Dairy Sci. 1995, 78, 2563–2583. [Google Scholar] [CrossRef]
- Brother, G.H. Casein plastics. Ind. Eng. Chem. 1940, 32, 31–33. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; El-Fotoh, W.S.; Elgindy, N.A. Casein-based formulations as promising controlled release drug delivery systems. J. Control. Release 2011, 153, 206–216. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Wang, R.; Chai, Y. Preparation and characterization of homogeneous and enhanced casein protein-based composite films via incorporating cellulose microgel. Sci. Rep. 2019, 9, 1221. [Google Scholar] [CrossRef] [Green Version]
- Prochoń, M.; Marzec, A.; Szadkowski, B. Preparation and characterization of new environmentally friendly starch-cellulose materials modified with casein or gelatin for agricultural applications. Materials 2019, 12, 1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curley, D.M.; Kumosinski, T.F.; Unruh, J.J.; Farrell Jr, H.M. Changes in the secondary structure of bovine casein by Fourier transform infrared spectroscopy: Effects of calcium and temperature. J. Dairy Sci. 1998, 81, 3154–3162. [Google Scholar] [CrossRef]
- Van Der Ven, C.; Muresan, S.; Gruppen, H.; De Bont, D.B.; Merck, K.B.; Voragen, A.G. FTIR spectra of whey and casein hydrolysates in relation to their functional properties. J. Agric. Food Chem. 2002, 50, 6943–6950. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Shah, F.F.; Bajpai, M.; Jadaun, M.; Jyotish, P. Dynamic release of amoxicillin from orally dissolving film (ODF) composed of casein and sodium alginate. J. Drug Res. Dev. 2017, 3, 1–7. [Google Scholar]
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3, 17086. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Wan, C.J.; Guo, L.Q.; Shi, Y.; Wan, Q. Artificial synapse network on inorganic proton conductor for neuromorphic systems. Nat. Commun. 2014, 5, 3158. [Google Scholar] [CrossRef] [Green Version]
- Wee, G.; Larsson, O.; Srinivasan, M.; Berggren, M.; Crispin, X.; Mhaisalkar, S. Effect of the ionic conductivity on the performance of polyelectrolyte-based supercapacitors. Adv. Funct. Mater. 2010, 20, 4344–4350. [Google Scholar] [CrossRef]
- Liu, R.; He, Y.; Jiang, S.; Wang, L.; Wan, Q. Synaptic plasticity modulation and coincidence detection emulated in multi-terminal neuromorphic transistors. Org. Electron. 2021, 92, 106125. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, Y.; Wang, Y.; Zhang, J.; Fang, L.; Jin, S.; Shao, Y.; Huang, J. Recent advances in transistor-based artificial synapses. Adv. Funct. Mater. 2019, 29, 1903700. [Google Scholar] [CrossRef]
- Kim, K.; Chen, C.L.; Truong, Q.; Shen, A.M.; Chen, Y. A carbon nanotube synapse with dynamic logic and learning. Adv. Mater. 2013, 25, 1693–1698. [Google Scholar] [CrossRef]
- Hu, W.; Jiang, J.; Xie, D.; Liu, B.; Yang, J.; He, J. Proton–electron-coupled MoS2 synaptic transistors with a natural renewable biopolymer neurotransmitter for brain-inspired neuromorphic learning. J. Mater. Chem. C 2019, 7, 682–691. [Google Scholar] [CrossRef]
- Zucker, R.S.; Regehr, W.G. Short-term synaptic plasticity. Ann. Rev. Physiol. 2002, 64, 355–405. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Yang, Y.; Nie, S.; Liu, R.; Wan, Q. Electric-double-layer transistors for synaptic devices and neuromorphic systems. J. Mater. Chem. C 2018, 6, 5336–5352. [Google Scholar] [CrossRef]
- Bliss, T.V.; Lømo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Bi, G.Q.; Poo, M.M. Synaptic modifications in cultured hippocampal neurons: Dependence on spike timing, synaptic strength, and postsynaptic cell type. J. Neurosci. 1998, 18, 10464–10472. [Google Scholar] [CrossRef]
- Song, S.; Miller, K.D.; Abbott, L.F. Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat. Neurosci. 2000, 3, 919–926. [Google Scholar] [CrossRef]
- Feldman, D.E. The spike-timing dependence of plasticity. Neuron 2012, 75, 556–571. [Google Scholar] [CrossRef] [Green Version]
- Xi, F.; Han, Y.; Liu, M.; Bae, J.H.; Tiedemann, A.; Grützmacher, D.; Zhao, Q.T. Artificial synapses based on ferroelectric Schottky barrier field-effect transistors for neuromorphic applications. ACS Appl. Mater. Interfaces 2021, 13, 32005–32012. [Google Scholar] [CrossRef]
- Yang, C.S.; Shang, D.S.; Liu, N.; Fuller, E.J.; Agrawal, S.; Talin, A.A.; Li, Y.Q.; Shen, B.G.; Sun, Y. All-solid-state synaptic transistor with ultralow conductance for neuromorphic computing. Adv. Funct. Mater. 2018, 28, 1804170. [Google Scholar] [CrossRef]
- Jang, J.W.; Park, S.; Burr, G.W.; Hwang, H.; Jeong, Y.H. Optimization of conductance change in Pr1–xCaxMnO3-Based synaptic devices for neuromorphic systems. IEEE Electron Device Lett. 2015, 36, 457–459. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-S.; Park, H.; Cho, W.-J. Biocompatible Casein Electrolyte-Based Electric-Double-Layer for Artificial Synaptic Transistors. Nanomaterials 2022, 12, 2596. https://doi.org/10.3390/nano12152596
Kim H-S, Park H, Cho W-J. Biocompatible Casein Electrolyte-Based Electric-Double-Layer for Artificial Synaptic Transistors. Nanomaterials. 2022; 12(15):2596. https://doi.org/10.3390/nano12152596
Chicago/Turabian StyleKim, Hwi-Su, Hamin Park, and Won-Ju Cho. 2022. "Biocompatible Casein Electrolyte-Based Electric-Double-Layer for Artificial Synaptic Transistors" Nanomaterials 12, no. 15: 2596. https://doi.org/10.3390/nano12152596





