Palladium Nanoparticles Synthesized by Laser Ablation in Liquids for Antimicrobial Applications
Abstract
:1. Introduction
2. Materials and Methods
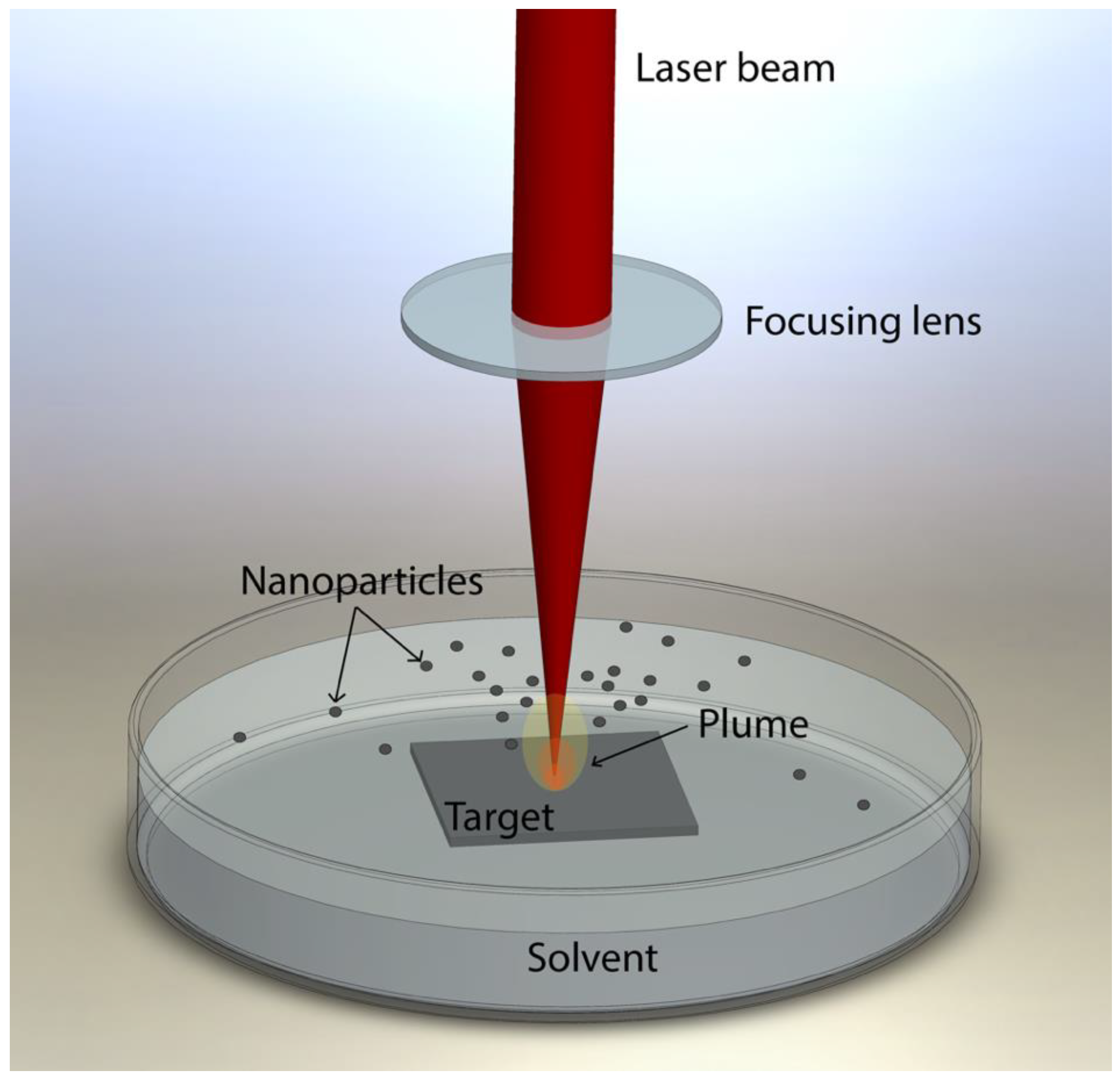
2.1. Laser Ablation
2.2. Colloidal Nanoparticles
Physicochemical Characterization
2.3. Immobilized Nanoparticles
2.3.1. Ion Release
2.3.2. Bacterial Growth Inhibition
2.3.3. Cytotoxicity
2.3.4. Statistical Analysis
3. Results and Discussion
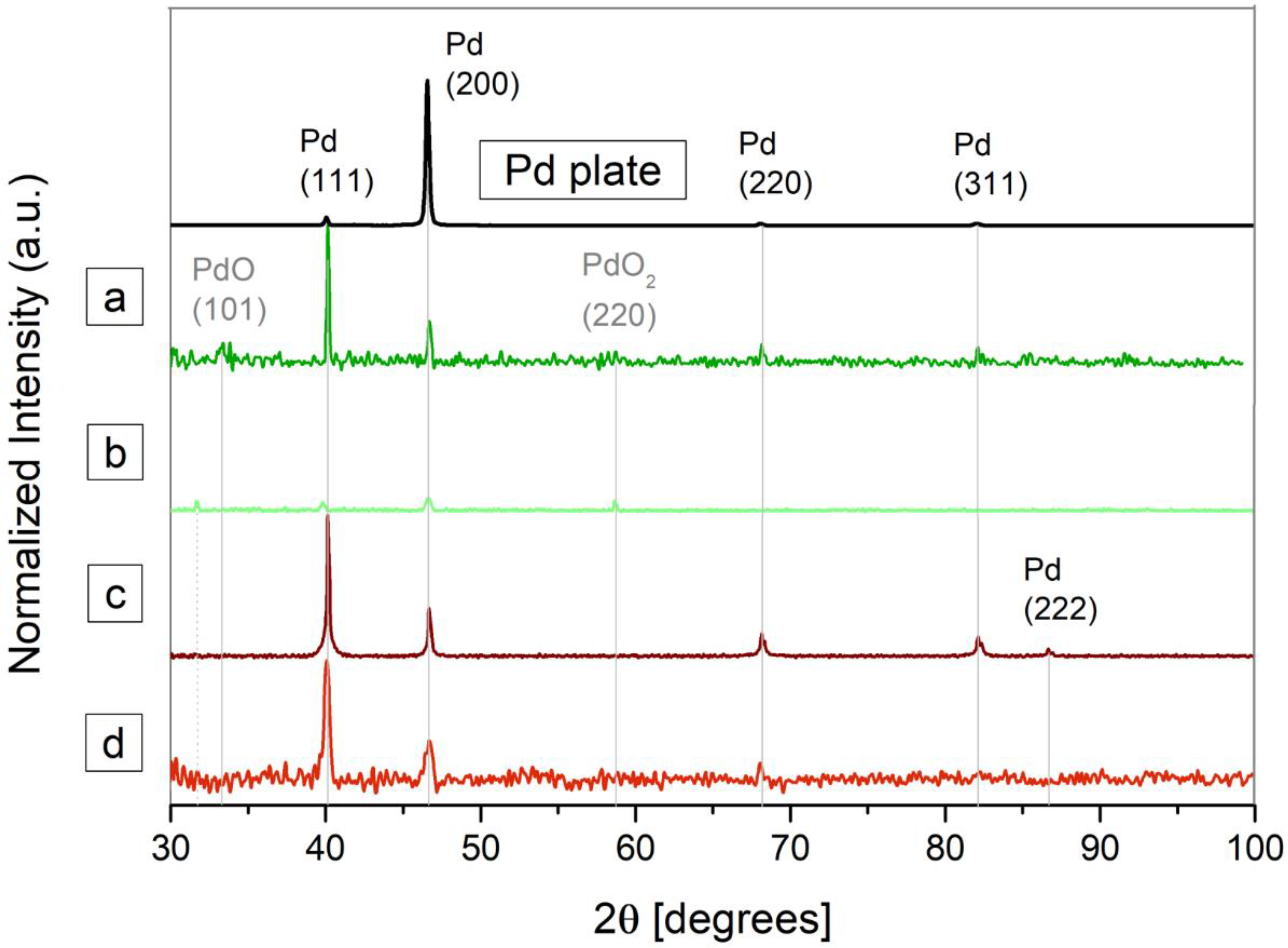
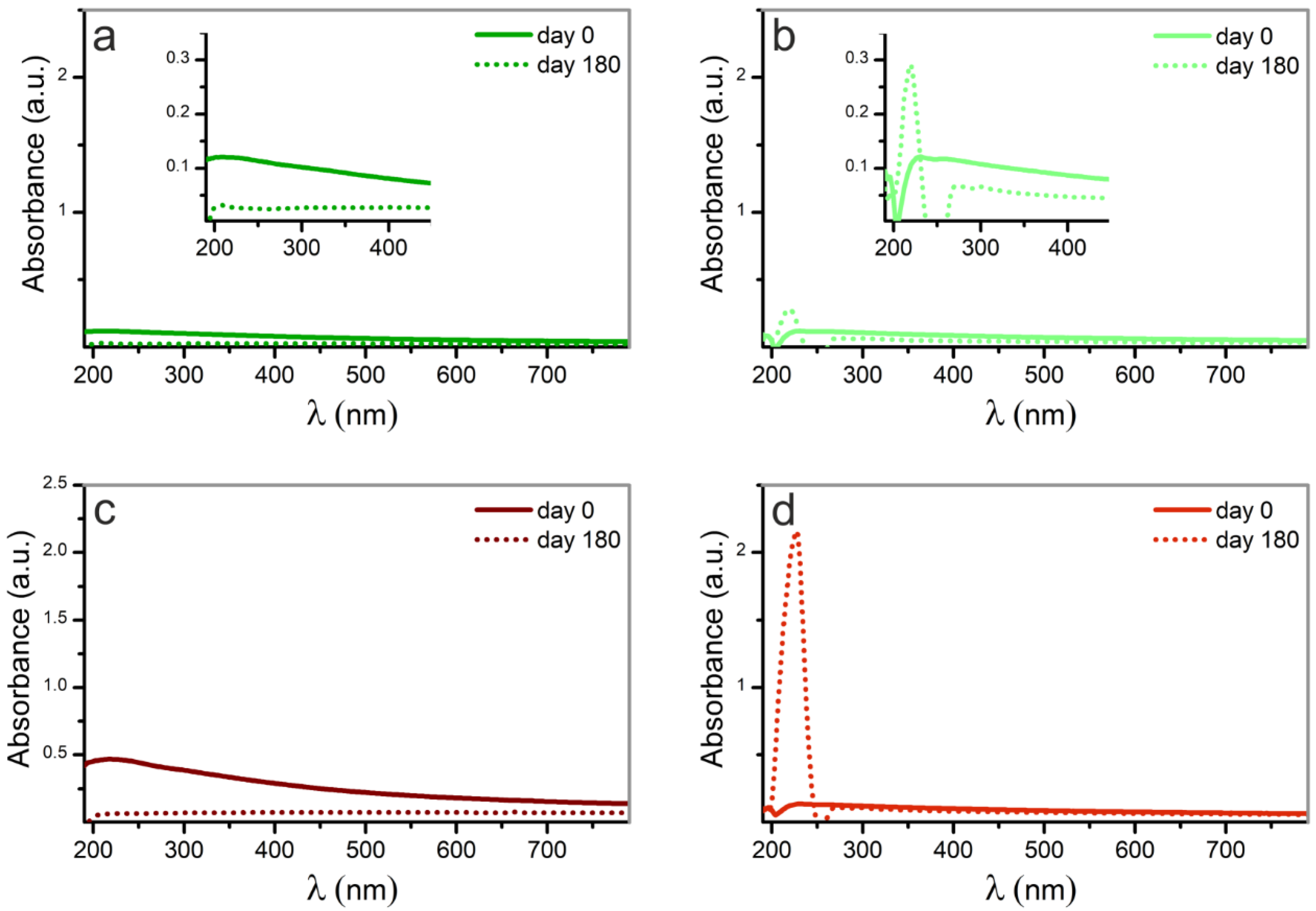
3.1. Characterization of the Nanoparticles in Solution
3.2. Ions Release Rate from the Nanoparticles Coating
3.3. Antimicrobial Activity
3.4. Cell Viability Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Munir, M.U.; Ahmad, M.M. Nanomaterials Aiming to Tackle Antibiotic-Resistant Bacteria. Pharmaceutics 2022, 14, 582. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis. Part 1: Causes and Threats. P&T 2015, 40, 277–283. [Google Scholar]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Reardon, S. WHO warns against ’post-antibiotic’ era. Nature 2014. [Google Scholar] [CrossRef]
- Ondusko, D.S.; Nolt, D. Staphylococcus aureus. Pediatr. Rev. 2018, 39, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Martens, E.; Demain, A.L. The antibiotic resistance crisis, with a focus on the United States. J. Antibiot. 2017, 70, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Kotigadde, S.; Anusha, G.R.; Sandya, V.; Subbannayya, Y.; Subbannayya, T.; Nayak, S. Effect of water storage in silver container on the viability of enteric bacterial pathogens. J. Commun. Dis. 2012, 44, 239–243. [Google Scholar]
- Yaqoob, S.B.; Adnan, R.; Rameez Khan, R.M.; Rashid, M. Gold, Silver, and Palladium Nanoparticles: A Chemical Tool for Biomedical Applications. Front. Chem. 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Yougbare, S.; Chang, T.; Tan, S.; Kuo, J.; Hsu, P.; Su, C.; Kuo, T. Antimicrobial Gold Nanoclusters: Recent Developments and Future Perspectives. Int. J. Mol. Sci. 2019, 20, 2924. [Google Scholar] [CrossRef] [Green Version]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolár, M.; Vecerová, R.; Pizúrová, N.; Sharma, V.K.; Nevecna, T.; Zboril, R. Silver Colloid Nanoparticles: Synthesis, Characterization, and Their Antibacterial Activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 1, 37–45. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Rozhin, A.; Batasheva, S.; Kruychkova, M.; Cherednichenko, Y.; Rozhina, E.; Fakhrullin, R. Biogenic Silver Nanoparticles: Synthesis and Application as Antibacterial and Antifungal Agents. Micromachines 2021, 12, 1480. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Tahir, K.; Nazir, S.; Ahmad, A.; Li, B.; Ali Shah, S.A.; Khan, A.U.; Khan, G.M.; Khan, Q.U.; Haq Khan, Z.U.; Khan, F.U. Biodirected synthesis of palladium nanoparticles using: Phoenix dactylifera leaves extract and their size dependent biomedical and catalytic applications. RSC Adv. 2016, 6, 85903–85916. [Google Scholar] [CrossRef]
- Adams, B.D.; Chen, A. The role of palladium in a hydrogen economy. Mater. Today. 2011, 14, 282–289. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X. Recent development on palladium enhanced photocatalytic activity: A review. J. Alloys Compd. 2020, 830, 154669. [Google Scholar] [CrossRef]
- Shee, M.; Singh, N.D.P. Cooperative photoredox and palladium catalysis: Recent advances in various functionalization reactions. Catal. Sci. Technol. 2021, 11, 742–767. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Huynh, T.C.; Manivasagan, P.; Mondal, S.; Oh, J. An up-to-date review on biomedical applications of palladium nanoparticles. Nanomaterials 2020, 10, 66. [Google Scholar] [CrossRef] [Green Version]
- Adams, C.P.; Walker, K.A.; Obare, S.O.; Docherty, K.M. Size-dependent antimicrobial effects of novel palladium nanoparticles. PLoS ONE 2014, 9, e85981. [Google Scholar] [CrossRef] [Green Version]
- Fang, G.; Li, W.; Shen, X.; Perez-Aguilar, J.M.; Chong, Y.; Gao, X.; Chai, Z.; Chen, C.; Ge, C.; Zhou, R. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against Gram-positive and Gram-negative bacteria. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- MubarakAli, D.; Kim, H.; Venkatesh, P.S.; Kim, J.-W.; Lee, S.-Y. A systemic review on the synthesis, characterization, and applications of palladium nanoparticles in biomedicine. Appl. Biochem. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Simakin, A.V.; Voronov, V.V.; Kirichenko, N.A.; Shafeev, G.A. Nanoparticles produced by laser ablation of solids in liquid environment. Appl. Phys. A 2004, 79, 1127–1132. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. C 2009, 11, 3805–3821. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Rodriguez-Gonzalez, B.; del Val, J.; Comesaña, R.; Lusquiños, F.; Pou, J. Laser-assisted production of spherical TiO2 nanoparticles in water. Nanotechnology 2011, 22, 195606. [Google Scholar] [CrossRef]
- Barcikowski, S.; Compagnini, G. Advanced nanoparticle generation and excitation by lasers in liquids. Phys. Chem. Chem. Phys. 2013, 15, 3022. [Google Scholar] [CrossRef]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser synthesis and processing of colloids: Fundamentals and applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef]
- Zeng, H.; Du, X.W.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via laser ablation/irradiation in liquid: A review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Fazio, E.; Gökce, B.; De Giacomo, A.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nanoparticles Engineering by Pulsed Laser Ablation in Liquids: Concepts and Applications. Nanomaterials 2020, 10, 2317. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Takeichi, A.; Azuma, H.; Suzuki, N.; Hioki, T.; Motohiro, T. Fabrication of Palladium Nanoparticles by Laser Ablation in Liquid. J. Laser Micro/Nanoeng. 2010, 5, 192–196. [Google Scholar] [CrossRef]
- Cristoforetti, G.; Pitzalis, E.; Spiniello, R.; Ishak, R.; Muniz-Miranda, M. Production of palladium nanoparticles by pulsed laser ablation in water and their characterization. J. Phys. Chem. C. 2011, 115, 5073–5083. [Google Scholar] [CrossRef]
- Mortazavi, S.Z.; Parvin, P.; Reyhani, A.; Golikand, A.N.; Mirershadi, S. Effect of laser wavelength at IR (1064 nm) and UV (193 nm) on the structural formation of palladium nanoparticles in deionized water. J. Phys. Chem. C. 2011, 115, 5049–5057. [Google Scholar] [CrossRef]
- Cristoforetti, G.; Pitzalis, E.; Spiniello, R.; Ishak, R.; Giammanco, F.; Muniz-Miranda, M.; Caporali, S. Physico-chemical properties of Pd nanoparticles produced by Pulsed Laser Ablation in different organic solvents. Appl. Surf. Sci. 2012, 258, 3289–3297. [Google Scholar] [CrossRef]
- Semaltianos, N.G.; Petkov, P.; Scholz, S.; Guetaz, L. Palladium or palladium hydride nanoparticles synthesized by laser ablation of a bulk palladium target in liquids. J. Colloid Interface Sci. 2013, 402, 307–311. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Comesaña, R.; Lusquiños, F.; Riveiro, A.; Del Val, J.; Pou, J. Palladium nanoparticles produced by CW and pulsed laser ablation in water. Appl. Surf. Sci. 2014, 302, 19–23. [Google Scholar] [CrossRef]
- De Bonis, A.; Sansone, M.; Galasso, A.; Santagata, A.; Teghil, R. The role of the solvent in the ultrashort laser ablation of palladium target in liquid. Appl. Phys. A 2014, 117, 211–216. [Google Scholar] [CrossRef]
- Kim, J.; Reddy, D.A.; Ma, R.; Kim, T.K. The influence of laser wavelength and fluence on palladium nanoparticles produced by pulsed laser ablation in deionized water. Solid State Sci. 2014, 37, 96–102. [Google Scholar] [CrossRef]
- Mendivil, M.I.; Krishnan, B.; Castillo, G.A.; Shaji, S. Synthesis and properties of palladium nanoparticles by pulsed laser ablation in liquid. Appl. Surf. Sci. 2015, 348, 45–53. [Google Scholar] [CrossRef]
- Marzun, G.; Nakamura, J.; Zhang, X.; Barcikowski, S.; Wagener, P. Size control and supporting of palladium nanoparticles made by laser ablation in saline solution as a facile route to heterogeneous catalysts. Appl. Surf. Sci. 2015, 348, 75–84. [Google Scholar] [CrossRef]
- Giorgetti, E.; Marsili, P.; Cicchi, S.; Lascialfari, L.; Albiani, M.; Severi, M.; Caporali, S.; Muniz-Miranda, M.; Pistone, A.; Giammanco, F. Preparation of small size palladium nanoparticles by picosecond laser ablation and control of metal concentration in the colloid. J. Colloid Interface Sci. 2015, 442, 89–96. [Google Scholar] [CrossRef]
- Navas, M.P.; Soni, R.K. Bromide (Br−) ion-mediated synthesis of anisotropic palladium nanocrystals by laser ablation. Appl. Surf. Sci. 2016, 390, 718–727. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Meixus, M.; Del Val, J.; Riveiro, A.; Comesaña, R.; Lusquiños, F.; Pou, J. Synthesis and characterization of Pd nanoparticles by laser ablation in water using nanosecond laser. Phys. Procedia 2016, 83, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Reddy, D.A.; Kim, Y.; Lee, S.; Ma, R.; Kim, T.K. Synthesis of ultra-small palladium nanoparticles deposited on CdS nanorods by pulsed laser ablation in liquid: Role of metal nanocrystal size in the photocatalytic hydrogen production. Chem. Eur. J. 2017, 23, 13112–13119. [Google Scholar] [CrossRef]
- Censabella, M.; Torrisi, V.; Boninelli, S.; Bongiorno, C.; Grimaldi, M.G.; Ruffino, F. Laser ablation synthesis of mono- and bimetallic Pt and Pd nanoparticles and fabrication of Pt-Pd/Graphene nanocomposites. Appl. Surf. Sci. 2019, 475, 494–503. [Google Scholar] [CrossRef]
- Oshida, Y. Bioscience and Bioengineering of Titanium Materials; Elsevier: Amsterdam, The Netherlands, 2007; pp. 217–256. [Google Scholar]
- Sarraf, M.; Rezvani Ghomi, E.; Alipour, S.; Ramakrishna, S.; Liana Sukiman, N. A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical application. Bio-Des. Manuf. 2021, 5, 371–395. [Google Scholar] [CrossRef]
- Roehm, N.W.; Rodgers, G.H.; Hatfield, S.M.; Glasebrook, A.L. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J. Immunol. Methods 1991, 142, 257–265. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Fernández-Arias, M.; Boutinguiza, M.; Del Val, J.; Covarrubias, C.; Bastias, F.; Gómez, L.; Maureira, M.; Arias-González, F.; Riveiro, A.; Pou, J. Copper nanoparticles obtained by laser ablation in liquids as bactericidal agent for dental applications. Appl. Surf. Sci. 2020, 507, 145032. [Google Scholar] [CrossRef]
- Favier, I.; Pla, D.; Gómez, M. Palladium Nanoparticles in Polyols: Synthesis, Catalytic Couplings, and Hydrogenations. Chem. Rev. 2020, 120, 1146–1183. [Google Scholar] [CrossRef]
- Markopoulos, A.P.; Koralli, P.; Kyriakakis, G.; Kompitsas, M.; Manolakos, D.E. Molecular dynamics simulation of material removal with the use of laser beam. In Materials Forming and Machining; Research and Development; Elsevier: Amsterdam, The Netherlands, 2016; pp. 117–153. [Google Scholar] [CrossRef]
- Wagener, P.; Jakobi, J.; Rehbock, C.; Chakravadhanula, V.S.K.; Thede, C.; Wiedwald, U.; Bartsch, M.; Kienle, L.; Barcikowski, S. Solvent-surface interactions control the phase structure in laser generated iron-gold core-shell nanoparticles. Sci. Rep. 2016, 6, 23352. [Google Scholar] [CrossRef] [Green Version]
- Kempasiddaiah, M.; Kandathil, V.; Dateer, R.B.; Sasidhar, B.S.; Patil, S.A.; Patil, S.A. Immobilizing biogenically synthesized palladium nanoparticles on cellulose support as a green and sustainable dip catalyst for cross-coupling reaction. Cellulose 2020, 27, 3335–3357. [Google Scholar] [CrossRef]
- Ingham, B. X-ray diffraction for characterizing metallic films. In Metallic Films for Electronic, Optical and Magnetic Applications; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef] [Green Version]

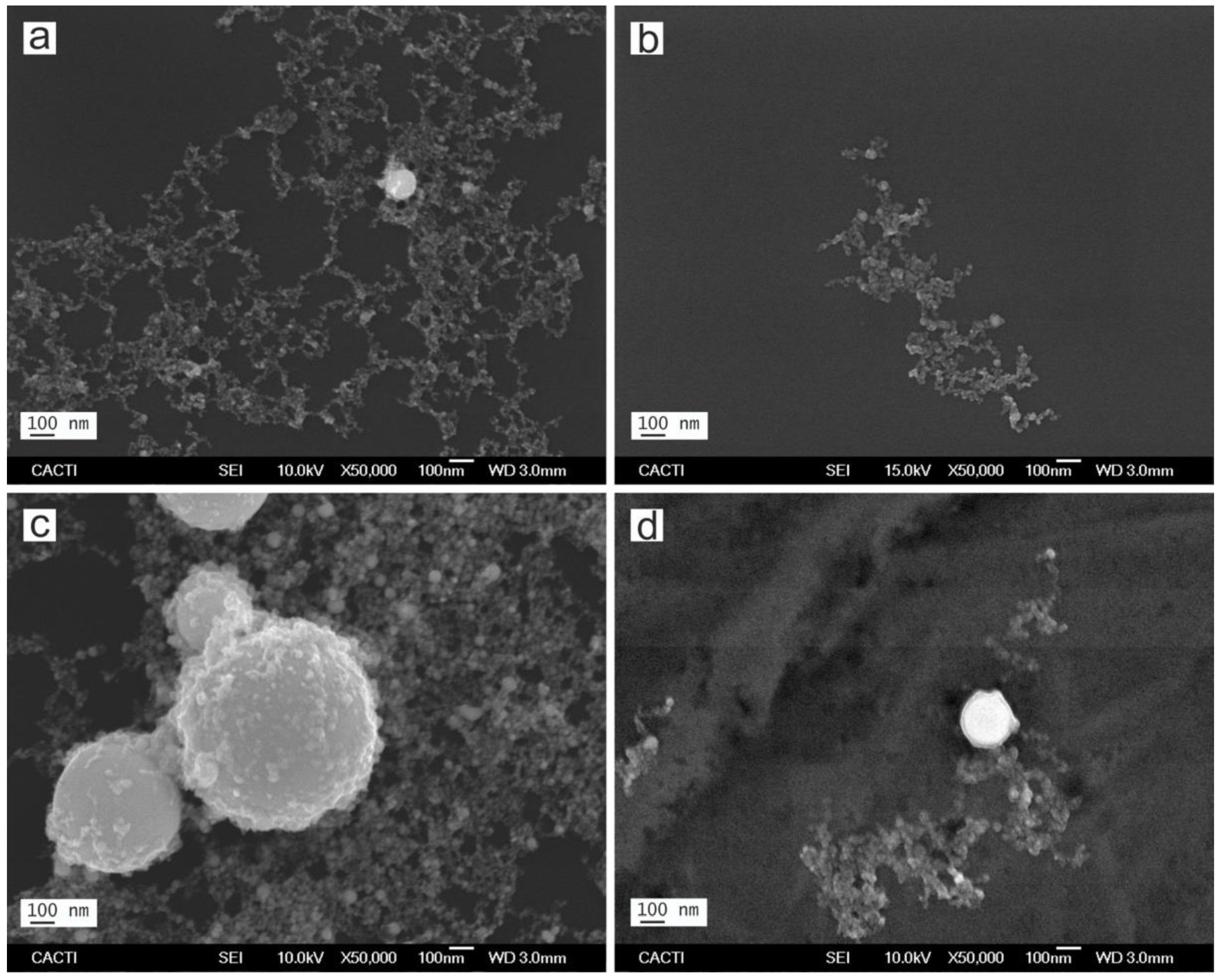

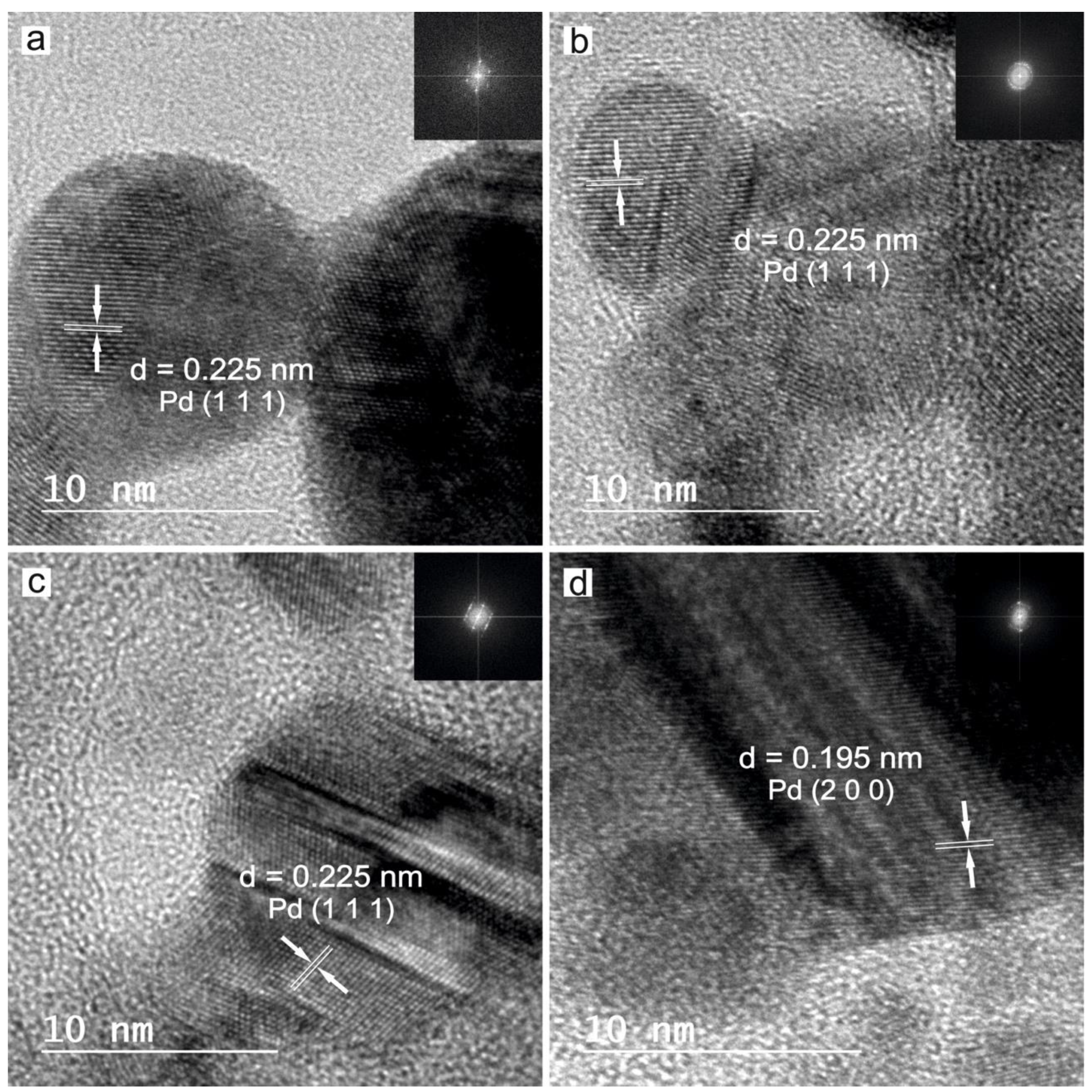





| Sample | Laser Source | Liquid Medium |
|---|---|---|
| a | 532 nm | Water |
| b | 532 nm | Methanol |
| c | 1064 nm | Water |
| d | 1064 nm | Methanol |
| Wavelength (nm) | Pulse Duration | Pulse Frequency (kHz) | Pulse Energy (mJ) |
|---|---|---|---|
| 532 | 14 | 20 | 0.26 |
| 1064 | 20 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Arias, M.; Vilas, A.M.; Boutinguiza, M.; Rodríguez, D.; Arias-González, F.; Pou-Álvarez, P.; Riveiro, A.; Gil, J.; Pou, J. Palladium Nanoparticles Synthesized by Laser Ablation in Liquids for Antimicrobial Applications. Nanomaterials 2022, 12, 2621. https://doi.org/10.3390/nano12152621
Fernández-Arias M, Vilas AM, Boutinguiza M, Rodríguez D, Arias-González F, Pou-Álvarez P, Riveiro A, Gil J, Pou J. Palladium Nanoparticles Synthesized by Laser Ablation in Liquids for Antimicrobial Applications. Nanomaterials. 2022; 12(15):2621. https://doi.org/10.3390/nano12152621
Chicago/Turabian StyleFernández-Arias, Mónica, Ana M. Vilas, Mohamed Boutinguiza, Daniel Rodríguez, Felipe Arias-González, Pablo Pou-Álvarez, Antonio Riveiro, Javier Gil, and Juan Pou. 2022. "Palladium Nanoparticles Synthesized by Laser Ablation in Liquids for Antimicrobial Applications" Nanomaterials 12, no. 15: 2621. https://doi.org/10.3390/nano12152621
APA StyleFernández-Arias, M., Vilas, A. M., Boutinguiza, M., Rodríguez, D., Arias-González, F., Pou-Álvarez, P., Riveiro, A., Gil, J., & Pou, J. (2022). Palladium Nanoparticles Synthesized by Laser Ablation in Liquids for Antimicrobial Applications. Nanomaterials, 12(15), 2621. https://doi.org/10.3390/nano12152621








