Transition Metal Nitrides for Electrocatalytic Application: Progress and Rational Design
Abstract
:1. Introduction
2. Synthetic Methods and Structural Properties of TMNs
2.1. Physical Synthetic Methods
2.2. Chemical Synthetic Methods
2.2.1. Transition Metal Oxide Method
2.2.2. Transition Metal Chloride Method
2.2.3. Metal-Organic Frameworks Method
2.2.4. Other Chemical Methods
3. Transition Metal Nitrides as Electrocatalysts
3.1. Transition Metal Nitrides as ORR Electrocatalysts
3.1.1. Mono-Metallic TMNs
3.1.2. Multi-Metallic TMNs
3.2. Transition Metal Nitrides as OER Electrocatalysts
3.2.1. Mono-Metallic TMNs
3.2.2. Multi-Metallic TMNs
3.3. Transition Metal Nitrides as Bifunctional ORR&OER Electrocatalysts
3.4. Design Principles of TMNs Electrocatalysts for Oriented Applications
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, X.; He, B.; Zhao, L.; Gong, Y.; Wang, R.; Wang, H. FeS2–CoS2 incorporated into nitrogen-doped carbon nanofibers to boost oxygen electrocatalysis for durable rechargeable Zn-air batteries. J. Power Source 2021, 482, 228955. [Google Scholar] [CrossRef]
- Qiao, M.; Wang, Y.; Wang, Q.; Hu, G.; Mamat, X.; Zhang, S.; Wang, S. Hierarchically ordered porous carbon with atomically dispersed FeN4 for ultraefficient oxygen reduction reaction in proton-exchange membrane fuel cells. Angew. Chem. Int. Ed. 2020, 59, 2688–2694. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, D.-D.; Chen, B.; Zhang, K.; Zou, R.; Wu, X.-T.; Zhu, Q.-L. Metal–organic framework-derived mesoporous carbon nanoframes embedded with atomically dispersed Fe–N active sites for efficient bifunctional oxygen and carbon dioxide electroreduction. Appl. Catal. B-Environ. 2020, 267, 118720. [Google Scholar] [CrossRef]
- Jeon, J.; Park, R.K.; Kim, K.M.; Ko, D.; Han, H.; Oh, N.; Yeo, S.; Ahn, C.; Mhin, S. CoFeS2@CoS2 nanocubes entangled with CT for efficient bifunctional performance for oxygen evolution and oxygen reduction reactions. Nanomaterials 2022, 12, 983. [Google Scholar] [CrossRef]
- Sathiskumar, C.; Ramakrishnan, S.; Vinothkannan, M.; Karthikeyan, S.; Yoo, D.J.; Rhan Kim, A. Nitrogen-doped porous carbon derived from biomass used as trifunctional electrocatalyst toward oxygen reduction, oxygen evolution and hydrogen evolution reactions. Nanomaterials 2019, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Cheng, Y.; Meng, Z.; Li, G.; Wu, J.; Kan, E.; Ouyang, B.; Zhang, H.; Tang, H. The design of single iron atoms dispersed with nitrogen coordination environment electrocatalyst for zinc -air battery. J. Power Source 2022, 529, 231174. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Jiang, M.; Wei, J.; Ding, X.; Zhu, C.; He, H.; Lai, H.; Shi, J. Engineering the coordination environment in atomic Fe/Ni dual-sites for efficient oxygen electrocatalysis in Zn-air and Mg-air batteries. Chem. Eng. J. 2021, 426, 130758. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, L.; Liu, S.; Xiang, Z. Binary ligand strategy toward interweaved encapsulation-nanotubes structured electrocatalyst for proton exchange membrane fuel cell. J. Energy Chem. 2022, 64, 129–135. [Google Scholar] [CrossRef]
- Logeshwaran, N.; Ramakrishnan, S.; Chandrasekaran, S.S.; Vinothkannan, M.; Kim, A.R.; Sengodan, S.; Velusamy, D.B.; Varadhan, P.; He, J.-H.; Yoo, D.J. An efficient and durable trifunctional electrocatalyst for zinc–air batteries driven overall water splitting. Appl. Catal. B-Environ. 2021, 297, 120405. [Google Scholar] [CrossRef]
- Peugeot, A.; Creissen, C.E.; Karapinar, D.; Tran, H.N.; Schreiber, M.; Fontecave, M. Benchmarking of oxygen evolution catalysts on porous nickel supports. Joule 2021, 5, 1281–1300. [Google Scholar] [CrossRef]
- Meng, Z.; Chen, N.; Cai, S.; Wang, R.; Wu, J.; Tang, H. Recent advances of hierarchically porous bifunctional oxygen electrocatalysts derived from metal–organic frameworks for Zn–air batteries. Mat. Chem. Front. 2021, 5, 2649–2667. [Google Scholar] [CrossRef]
- Elayappan, V.; Shanmugam, R.; Chinnusamy, S.; Yoo, D.J.; Mayakrishnan, G.; Kim, K.; Noh, H.S.; Kim, M.K.; Lee, H. Three-dimensional bimetal tmo supported carbon based electrocatalyst developed via dry synthesis for hydrogen and oxygen evolution. Appl. Surf. Sci. 2020, 505, 144642. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Huang, C.; Ye, P.; Luo, X.; Ning, J.; Zhong, Y.; Hu, Y. Trifunctional electrocatalyst of N-doped graphitic carbon nanosheets encapsulated with CoFe alloy nanocrystals: The key roles of bimetal components and high-content graphitic-N. Appl. Catal. B-Environ. 2021, 298, 120512. [Google Scholar] [CrossRef]
- She, Y.Y.; Liu, J.; Wang, H.K.; Li, L.; Zhou, J.S.; Leung, M.K.H. Bubble-like Fe-encapsulated N,S-codoped carbon nanofibers as efficient bifunctional oxygen electrocatalysts for robust Zn-air batteries. Nano Res. 2020, 13, 2175–2182. [Google Scholar] [CrossRef]
- Meng, Z.; Zhu, G.; Wu, J.; Wang, R.; Tian, T.; Tang, H.; Luo, R.; Ye, D.; Zhang, R.; Kwofie, F.; et al. Gradient Co/Zn bimetallic coordinated polymer-derived hierarchically porous carbon for boosted oxygen electrocatalysts of rechargeable Zn–air batteries. Mater. Today Energy 2022, 24, 100935. [Google Scholar] [CrossRef]
- Zhu, Z.; Yin, H.; Wang, Y.; Chuang, H.C.; Xing, L.; Dong, M.; Lu, Y.R.; Casillas-Garcia, G.; Zheng, Y.; Chen, S.; et al. Coexisting single-atomic Fe and Ni sites on hierarchically ordered porous carbon as a highly efficient ORR electrocatalyst. Adv. Mater. 2020, 32, 2004670. [Google Scholar] [CrossRef]
- Rao, P.; Liu, Y.; Su, Y.-Q.; Zhong, M.; Zhang, K.; Luo, J.; Li, J.; Jia, C.; Shen, Y.; Shen, C.; et al. S, N co-doped carbon nanotube encased Co NPs as efficient bifunctional oxygen electrocatalysts for zinc-air batteries. Chem. Eng. J. 2021, 422, 130135. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Velusamy, B.D.; Sengodan, S.; Nagaraju, G.; Kim, D.H.; Kim, A.R.; Yoo, D.J. Rational design of multifunctional electrocatalyst: An approach towards efficient overall water splitting and rechargeable flexible solid-state zinc–air battery. Appl. Catal. B-Environ. 2022, 300, 120752. [Google Scholar] [CrossRef]
- Zeng, R.; Yang, Y.; Feng, X.; Li, H.; Gibbs, L.M.; DiSalvo, F.J.; Abruna, H.D. Nonprecious transition metal nitrides as efficient oxygen reduction electrocatalysts for alkaline fuel cells. Sci. Adv. 2022, 8, eabj1584. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Xu, Y.; Tang, L.; Xu, S.; Li, D.; Zhu, J.; Jiang, D. Bimetallic co-mo nitride nanosheet arrays as high-performance bifunctional electrocatalysts for overall water splitting. Chem. Eng. J. 2021, 411, 128433. [Google Scholar] [CrossRef]
- Wu, S.; Deng, D.; Zhang, E.; Li, H.; Xu, L. Con nanoparticles anchored on ultra-thin N-doped graphene as the oxygen reduction electrocatalyst for highly stable zinc-air batteries. Carbon 2022, 196, 347–353. [Google Scholar] [CrossRef]
- Park, S.H.; Jo, T.H.; Lee, M.H.; Kawashima, K.; Mullins, C.B.; Lim, H.-K.; Youn, D.H. Highly active and stable nickel–molybdenum nitride (Ni2Mo3N) electrocatalyst for hydrogen evolution. J. Mater. Chem. A 2021, 9, 4945–4951. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, W.; Tian, W.; Wan, J.; Zhang, H.; Wang, Y. Distorted quantum dots enhance the efficiency of alkaline oxygen electrocatalysis. J. Mater. Chem. A 2020, 8, 21173–21180. [Google Scholar] [CrossRef]
- Chen, Q.; Gong, N.; Zhu, T.; Yang, C.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Surface phase engineering modulated iron-nickel nitrides/alloy nanospheres with tailored d-band center for efficient oxygen evolution reaction. Small 2022, 18, 2105696. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yan, H.; Jin, Z.; Jia, J.C. Facile synthesis of stable Mo2N nanobelts with high catalytic activity for ammonia decomposition. Chin. J. Chem. 2019, 37, 364–372. [Google Scholar] [CrossRef]
- Yang, Y.-T.; Wu, H.-W.; Zou, Y.; Fang, X.-Y.; Li, S.; Song, Y.-F.; Wang, Z.-H.; Zhang, B. Facile synthesis of monodispersed titanium nitride quantum dots for harmonic mode-locking generation in an ultrafast fiber laser. Nanomaterials 2022, 12, 2280. [Google Scholar] [CrossRef]
- Li, P.; Chen, R.; Zhao, S.; Li, W.; Lin, Y.; Yu, Y. Architecture control and electronic structure engineering over Ni-based nitride nanocomposite for boosting ammonia borane dehydrogenation. Appl. Catal. B-Environ. 2021, 298, 120523. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Qi, Y.; Wang, C. Vanadium-doping and interface engineering for synergistically enhanced electrochemical overall water splitting and urea electrolysis. ACS Appl. Mater. Interfaces 2021, 13, 57392–57402. [Google Scholar] [CrossRef] [PubMed]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, J.; Ma, Y.; Yu, J.; Zhou, J.; Fan, Z. Recent advances in the controlled synthesis and catalytic applications of two-dimensional rhodium nanomaterials. ACS Mater. Lett. 2020, 3, 121–133. [Google Scholar] [CrossRef]
- Guy, K.; Tessier, F.; Kaper, H.; Grasset, F.; Dumait, N.; Demange, V.; Nishio, M.; Matsushita, Y.; Matsui, Y.; Takei, T.; et al. Original synthesis of molybdenum nitrides using metal cluster compounds as precursors: Applications in heterogeneous catalysis. Chem. Mater. 2020, 32, 6026–6034. [Google Scholar] [CrossRef]
- Choi, H.W.; Jeong, D.I.; Kwon, S.B.; Woo, S.; Kim, J.; Kim, J.H.; Yang, W.S.; Lim, B.; Kang, B.K.; Yoon, D.H. Nickel-iron nitrides and alloy heterojunction with amorphous n-doped carbon shell: High-efficiency synergistic electrocatalysts for oxygen evolution reaction. Appl. Surf. Sci. 2021, 566, 150706. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Z.; Song, H. Preparation and application of Fe-N co-doped GNR@CNT cathode oxygen reduction reaction catalyst in microbial fuel cells. Nanomaterials 2021, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Chen, N.; Cai, S.; Wu, J.; Wang, R.; Tian, T.; Tang, H. Rational design of hierarchically porous Fe-N-doped carbon as efficient electrocatalyst for oxygen reduction reaction and Zn-air batteries. Nano Res. 2021, 14, 4768–4775. [Google Scholar] [CrossRef]
- Zhu, P.W.; He, Z.; Zhao, Y.N.; Li, D.M.; Liu, H.W.; Zou, G.T. Preparation of high-pressure phase boron nitride films by physical vapor deposition. J. Vac. Sci. Technol. A 2002, 20, 622–624. [Google Scholar] [CrossRef]
- Soo Kang, J.; Park, M.A.; Kim, J.Y.; Ha Park, S.; Young Chung, D.; Yu, S.H.; Kim, J.; Park, J.; Choi, J.W.; Jae Lee, K.; et al. Reactively sputtered nickel nitride as electrocatalytic counter electrode for dye- and quantum dot-sensitized solar cells. Sci. Rep. 2015, 5, 10450. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Tang, G.; Liang, H.; Qi, Z.; Zhang, D.; Hu, W.; Shen, H.; Wang, Z. Bimetallic vanadium-molybdenum nitrides using magnetron co-sputtering as alkaline hydrogen evolution catalyst. Electrochem. Commun. 2018, 93, 166–170. [Google Scholar] [CrossRef]
- Murthy, A.P.; Govindarajan, D.; Theerthagiri, J.; Madhavan, J.; Parasuraman, K. Metal-doped molybdenum nitride films for enhanced hydrogen evolution in near-neutral strongly buffered aerobic media. Electrochim. Acta 2018, 283, 1525–1533. [Google Scholar] [CrossRef]
- Uchida, H.; Yamashita, M.; Hanaki, S.; Ueta, T. Characterization of (Ti, Al)n films prepared by ion mixing and vapor deposition. Mater. Sci. Eng. A 2004, 387, 758–762. [Google Scholar] [CrossRef]
- Liu, W.; Li, A.; Wu, H.; Long, Y.; Huang, J.; Deng, X.; Wang, C.; Wang, Q.; Wu, S. Effects of gas pressure on microstructure and performance of (Ti, Al, Zr)n coatings produced by physical vapor deposition. Ceram. Int. 2016, 42, 17436–17441. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, B.; Li, C.; Yan, F.; Wang, D.; Yang, C.; Wan, J. Review of transition metal nitrides and transition metal nitrides/carbon nanocomposites for supercapacitor electrodes. Mater. Chem. Phys. 2020, 245, 122533. [Google Scholar] [CrossRef]
- Aslanzadeh, S.A. Transition metal-metal oxide hybrids as versatile materials for hydrogen storage. Chin. J. Phys. 2018, 56, 1917–1924. [Google Scholar] [CrossRef]
- Peng, X.; Huo, K.; Fu, J.; Gao, B.; Wang, L.; Hu, L.; Zhang, X.; Chu, K.P. Porous dual-layered mooxnanotube arrays with highly conductive tin cores for supercapacitors. ChemElectroChem 2015, 2, 512–517. [Google Scholar] [CrossRef]
- Nagai, M. Transition-metal nitrides for hydrotreating catalyst—Synthesis, surface properties, and reactivities. Appl. Catal. A-Gen. 2007, 322, 178–190. [Google Scholar] [CrossRef]
- Jiang, Y.; Dong, J.; Tan, S.; Wei, Q.; Xiong, F.; Yang, W.; Shen, Y.; Zhang, Q.; Liu, Z.A.; An, Q.; et al. Surface pseudocapacitance of mesoporous Mo3N2 nanowire anode toward reversible high-rate sodium-ion storage. J. Energy Chem. 2021, 55, 295–303. [Google Scholar] [CrossRef]
- Yang, M.; DiSalvo, F.J. Template-free synthesis of mesoporous transition metal nitride materials from ternary cadmium transition metal oxides. Chem. Mater. 2012, 24, 4406–4409. [Google Scholar] [CrossRef]
- Cheng, Z.; Saad, A.; Guo, H.; Wang, C.; Liu, S.; Thomas, T.; Yang, M. Ordered mesoporous transition metal nitrides prepared through hard template nanocasting and rapid nitridation process. J. Alloys Compd. 2020, 838, 155375. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Z.; Kong, J.; Wang, Z.; Zhang, M. Synthesis of transition metal nitride by nitridation of metastable oxide precursor. J. Solid State Chem. 2012, 194, 238–244. [Google Scholar] [CrossRef]
- Choi, D.; Kumta, P.N. Synthesis, structure, and electrochemical characterization of nanocrystalline tantalum and tungsten nitrides. J. Am. Ceram. Soc. 2007, 90, 3113–3120. [Google Scholar] [CrossRef]
- Afanasiev, P.; Laurenti, D. Ccl4-assisted preparation of highly dispersed molybdenum and tungsten nitrides. Top. Catal. 2012, 55, 940–949. [Google Scholar] [CrossRef]
- Cheng, F.; Kelly, S.M.; Young, N.A.; Clark, S.; Francesconi, M.G.; Lefebvre, F.; Bradley, J.S. General method of preparation of mesoporous m/Si3N4 nano-composites via a non-aqueous sol-gel route. Chem. Commun. 2005, 45, 5662–5664. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Gao, L. Metal-urea complex-a precursor to metal nitrides. J. Am. Ceram. Soc. 2004, 87, 352–357. [Google Scholar] [CrossRef]
- Weil, K.S.C.; Kumta, P.R.N. Synthesis of transition metal nitride powders and coatings using alkanolamine chelated precursors. Mater. Des. 2001, 22, 605–615. [Google Scholar] [CrossRef]
- Sheng, J.; Wang, L.; Deng, L.; Zhang, M.; He, H.; Zeng, K.; Tang, F.; Liu, Y.N. MOF-templated fabrication of hollow Co4N@n-doped carbon porous nanocages with superior catalytic activity. ACS Appl. Mater. Interfaces 2018, 10, 7191–7200. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, H.; Bo, X.; Guo, L. Bimetal-organic framework-derived porous rodlike cobalt/nickel nitride for all-pH value electrochemical hydrogen evolution. ACS Appl. Mater. Interfaces 2019, 11, 8018–8024. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.K.; Im, S.Y.; Lee, J.; Kwag, S.H.; Kwon, S.B.; Tiruneh, S.; Kim, M.-J.; Kim, J.H.; Yang, W.S.; Lim, B.; et al. In-situ formation of mof derived mesoporous Co3N/amorphous n-doped carbon nanocubes as an efficient electrocatalytic oxygen evolution reaction. Nano Res. 2019, 12, 1605–1611. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, C.; Sun, J.; Xing, Y.; Quan, B.; Li, D.; Jiang, D. Interfacial engineering of Co3FeN embedded n-doped carbon nanoarray derived from metal–organic frameworks for enhanced oxygen evolution reaction. Electrochim. Acta 2020, 354, 136629. [Google Scholar] [CrossRef]
- Hu, Y.; Li, C.; Xi, S.; Deng, Z.; Liu, X.; Cheetham, A.K.; Wang, J. Direct pyrolysis of a manganese-triazolate metal-organic framework into air-stable manganese nitride nanoparticles. Adv. Sci. 2021, 8, 2003212. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, H.; Ma, Y.; Yang, Y.; Li, B.; Cui, Y.; Guo, Z.; Wang, L. Core-shell-structured Co@Co4N nanoparticles encapsulated into MnO-modified porous n-doping carbon nanocubes as bifunctional catalysts for rechargeable Zn–air batteries. J. Energy Chem. 2020, 50, 52–62. [Google Scholar] [CrossRef]
- Cheng, Z.; Saad, A.; Adimi, S.; Guo, H.; Liu, S.; Thomas, T.; Yang, M. Metal organic framework-derived porous Fe2N nanocubes by rapid-nitridation for efficient photocatalytic hydrogen evolution. Mater. Adv. 2020, 1, 1161–1167. [Google Scholar] [CrossRef]
- Barker, M.G.; Grazia Francesconi, M.; O’Meara, P.M.; Baker, C.F. New synthetic routes to transition metal ternary nitrides and sulfides. J. Alloys Compd. 2001, 317, 186–189. [Google Scholar] [CrossRef]
- Xiao, X.; Urbankowski, P.; Hantanasirisakul, K.; Yang, Y.; Sasaki, S.; Yang, L.; Chen, C.; Wang, H.; Miao, L.; Tolbert, S.H.; et al. Scalable synthesis of ultrathin Mn3N2 exhibiting room-temperature antiferromagnetism. Adv. Funct. Mater. 2019, 29, 1809001. [Google Scholar] [CrossRef]
- Yao, W.; Makowski, P.; Giordano, C.; Goettmann, F. Synthesis of early-transition-metal carbide and nitride nanoparticles through the urea route and their use as alkylation catalysts. Chemistry 2009, 15, 11999–12004. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhao, Y.; Chen, G.; Shang, L.; Shi, R.; Kang, X.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Ni3FeN nanoparticles derived from ultrathin nife-layered double hydroxide nanosheets: An efficient overall water splitting electrocatalyst. Adv. Energy Mater. 2016, 6, 1502585. [Google Scholar] [CrossRef]
- Ghimbeu, C.M.; Raymundo-Piñero, E.; Fioux, P.; Béguin, F.; Vix-Guterl, C. Vanadium nitride/carbon nanotube nanocomposites as electrodes for supercapacitors. J. Mater. Chem. 2011, 21, 13268–13275. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.-Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel electronic and magnetic properties of two-dimensional transition metal carbides and nitrides. Adv. Funct. Mater. 2013, 23, 2185–2192. [Google Scholar] [CrossRef]
- Salamat, A.; Hector, A.L.; Kroll, P.; McMillan, P.F. Nitrogen-rich transition metal nitrides. Coord. Chem. Rev. 2013, 257, 2063–2072. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Qiao, X.; Jin, J.; Tian, X.; Fan, H.; Yu, D.; Wang, W.; Liao, S.; Yu, N.; Deng, Y. A strategy to unlock the potential of crn as a highly active oxygen reduction reaction catalyst. J. Mater. Chem. A 2020, 8, 8575–8585. [Google Scholar] [CrossRef]
- Kreider, M.E.; Gallo, A.; Back, S.; Liu, Y.; Siahrostami, S.; Nordlund, D.; Sinclair, R.; Norskov, J.K.; King, L.A.; Jaramillo, T.F. Precious metal-free nickel nitride catalyst for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2019, 11, 26863–26871. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, R. A new fuel cell cathode catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J.; Zhan, H.; Sun, D.; Dang, D.; Tian, L.X. Porous and three dimensional titanium nitride supported platinum as an electrocatalyst for oxygen reduction reaction. Electrochem. Commun. 2018, 91, 31–35. [Google Scholar] [CrossRef]
- Yang, M.; Guarecuco, R.; DiSalvo, F.J. Mesoporous chromium nitride as high performance catalyst support for methanol electrooxidation. Chem. Mater. 2013, 25, 1783–1787. [Google Scholar] [CrossRef]
- Yue, R.; Xia, M.; Wang, M.; Chen, P.; Gong, W.; Liao, S.; Li, Z.; Gao, F.; Zhang, L.; Wang, J. Tin and tic as stable and promising supports for oxygen reduction reaction: Theoretical and experimental study. Appl. Surf. Sci. 2019, 495, 143620. [Google Scholar] [CrossRef]
- Qi, J.; Jiang, L.; Jiang, Q.; Wang, S.; Sun, G. Theoretical and experimental studies on the relationship between the structures of molybdenum nitrides and their catalytic activities toward the oxygen reduction reaction. J. Phys. Chem. C 2010, 114, 18159–18166. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Cheng, W.; Li, Y.; Zhou, W.; Su, H.; Zhao, X.; Yao, P.; Liu, Q. Metallic Ni3N quantum dots as a synergistic promoter for NiO nanosheet toward efficient oxygen reduction electrocatalysis. J. Phys. Chem. C 2019, 123, 8633–8639. [Google Scholar] [CrossRef]
- Luo, J.; Tian, X.; Zeng, J.; Li, Y.; Song, H.; Liao, S. Limitations and improvement strategies for early-transition-metal nitrides as competitive catalysts toward the oxygen reduction reaction. ACS Catal. 2016, 6, 6165–6174. [Google Scholar] [CrossRef]
- Tian, X.L.; Luo, J.M.; Nan, H.X.; Fu, Z.Y.; Zeng, J.H.; Liao, S.J. Binary transition metal nitrides with enhanced activity and durability for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 16801–16809. [Google Scholar] [CrossRef]
- Tian, X.L.; Wang, L.; Chi, B.; Xu, Y.; Zaman, S.; Qi, K.; Liu, H.; Liao, S.; Xia, B.Y. Formation of a tubular assembly by ultrathin Ti0.8Co0.2N nanosheets as efficient oxygen reduction electrocatalysts for hydrogen–/metal–air fuel cells. ACS Catal. 2018, 8, 8970–8975. [Google Scholar] [CrossRef]
- Li, W.; Pan, Z.; Huang, Z.; Zhou, Q.; Xu, Y.; Wu, S.; Chen, C.; Lin, Y.; Hu, G. Pt nanoparticles supported on titanium iron nitride nanotubes prepared as a superior electrocatalysts for methanol electrooxidation. Int. J. Hydrog. Energy 2018, 43, 9777–9786. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel-iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Xu, K.; Fang, Z.; Tong, Y.; Wu, J.; Lu, X.; Peng, X.; Ding, H.; Wu, C.; Xie, Y. Metallic Co4N porous nanowire arrays activated by surface oxidation as electrocatalysts for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2015, 54, 14710–14714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, B.; Xu, J.; Jia, G.; Chen, S.; Rawat, R.S.; Fan, H.J. Rapid synthesis of cobalt nitride nanowires: Highly efficient and low-cost catalysts for oxygen evolution. Angew. Chem. Int. Ed. 2016, 55, 8670–8674. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chen, P.; Li, X.; Tong, Y.; Ding, H.; Wu, X.; Chu, W.; Peng, Z.; Wu, C.; Xie, Y. Metallic nickel nitride nanosheets realizing enhanced electrochemical water oxidation. J. Am. Chem. Soc. 2015, 137, 4119–4125. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Choi, S.M.; Bai, Y.; Jang, M.J.; Yu, R.; Cho, H.-S.; Kim, C.-H.; Myung, N.V.; Yin, Y. Plasmon-enhanced oxygen evolution catalyzed by Fe2N-embedded TiOxNy nanoshells. ACS Appl. Energ. Mater. 2019, 3, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Yu, Y.; Liang, Q.; Pang, Y.; Miao, L.; Liu, X.; Kou, Z.; He, J.; Pennycook, S.J.; Mu, S.; et al. Surface nitridation of nickel-cobalt alloy nanocactoids raises the performance of water oxidation and splitting. Appl. Catal. B-Environ. 2020, 270, 118889. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Pan, W.; Ma, H.; Zhang, J. 3D porous nickel-cobalt nitrides supported on nickel foam as efficient electrocatalysts for overall water splitting. ChemSusChem 2017, 10, 4170–4177. [Google Scholar] [CrossRef]
- Li, D.; Xing, Y.; Yang, R.; Wen, T.; Jiang, D.; Shi, W.; Yuan, S. Holey cobalt-iron nitride nanosheet arrays as high-performance bifunctional electrocatalysts for overall water splitting. ACS Appl. Mater. Interfaces 2020, 12, 29253–29263. [Google Scholar] [CrossRef]
- Liu, T.; Li, M.; Bo, X.; Zhou, M. Comparison study toward the influence of the second metals doping on the oxygen evolution activity of cobalt nitrides. ACS Sustain. Chem. Eng. 2018, 6, 11457–11465. [Google Scholar] [CrossRef]
- Sun, H.; Tian, C.; Fan, G.; Qi, J.; Liu, Z.; Yan, Z.; Cheng, F.; Chen, J.; Li, C.P.; Du, M. Boosting activity on Co4N porous nanosheet by coupling CeO2 for efficient electrochemical overall water splitting at high current densities. Adv. Funct. Mater. 2020, 30, 1910596. [Google Scholar] [CrossRef]
- Kwag, S.H.; Lee, Y.S.; Lee, J.; Jeong, D.I.; Kwon, S.B.; Yoo, J.H.; Woo, S.; Lim, B.S.; Park, W.K.; Kim, M.-J.; et al. Design of 2d nanocrystalline Fe2Ni2N coated onto graphene nanohybrid sheets for efficient electrocatalytic oxygen evolution. ACS Appl. Energy Mater. 2019, 2, 8502–8510. [Google Scholar]
- Liang, S.; Jing, M.; Pervaiz, E.; Guo, H.; Thomas, T.; Song, W.; Xu, J.; Saad, A.; Wang, J.; Shen, H.; et al. Nickel-iron nitride-nickel sulfide composites for oxygen evolution electrocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 41464–41470. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xu, C.; Jiang, X.; Bando, Y.; Wang, X. Porous monolithic electrode of Ni3FeN on 3D graphene for efficient oxygen evolution. J. Nanosci. Nanotechnol. 2020, 20, 5175–5181. [Google Scholar] [CrossRef]
- Wang, J.; Cao, F.; Shen, C.; Li, G.; Li, X.; Yang, X.; Li, S.; Qin, G. Nanoscale nickel–iron nitride-derived efficient electrochemical oxygen evolution catalysts. Catal. Sci. Technol. 2020, 10, 4458–4466. [Google Scholar] [CrossRef]
- Jia, J.; Zhai, M.; Lv, J.; Zhao, B.; Du, H.; Zhu, J. Nickel molybdenum nitride nanorods grown on ni foam as efficient and stable bifunctional electrocatalysts for overall water splitting. ACS Appl. Mater. Interfaces 2018, 10, 30400–30408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, B.; Long, G.; Tan, H.; Wang, Z.; Zhang, Z.; Gao, W.; Rawat, R.S.; Fan, H.J. Enhancing bifunctionality of CoN nanowires by mn doping for long-lasting Zn-air batteries. Sci. China Chem. 2020, 63, 890–896. [Google Scholar] [CrossRef]
- He, X.; Tian, Y.; Deng, D.; Chen, F.; Wu, J.; Qian, J.; Li, H.; Xu, L. Engineering antiperovskite Ni4N/VN heterostructure with improved intrinsic interfacial charge transfer as a bifunctional catalyst for rechargeable zinc–air batteries. ACS Sustain. Chem. Eng. 2021, 9, 17007–17015. [Google Scholar] [CrossRef]
- Fan, Y.; Ida, S.; Staykov, A.; Akbay, T.; Hagiwara, H.; Matsuda, J.; Kaneko, K.; Ishihara, T. Ni-Fe nitride nanoplates on nitrogen-doped graphene as a synergistic catalyst for reversible oxygen evolution reaction and rechargeable Zn-air battery. Small 2017, 13, 1700099. [Google Scholar] [CrossRef]
- Wang, Q.; Shang, L.; Shi, R.; Zhang, X.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. 3D carbon nanoframe scaffold-immobilized Ni3FeN nanoparticle electrocatalysts for rechargeable zinc-air batteries’ cathodes. Nano Energy 2017, 40, 382–389. [Google Scholar] [CrossRef]
- Zhang, N.; Xie, S.; Wang, W.; Xie, D.; Zhu, D.; Cheng, F. Ultra-small Fe2N/N-CNTs as efficient bifunctional catalysts for rechargeable Zn-air batteries. J. Electrochem. Soc. 2020, 167, 020505. [Google Scholar] [CrossRef]
- Fu, G.; Cui, Z.; Chen, Y.; Xu, L.; Tang, Y.; Goodenough, J.B. Hierarchically mesoporous nickel-iron nitride as a cost-efficient and highly durable electrocatalyst for Zn-air battery. Nano Energy 2017, 39, 77–85. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; Xiong, P.; Li, G.; Qiu, T.; Gong, W.B.; Zhao, F.; Li, C.; Li, Q.; Wang, G.; et al. Molecularly thin nitride sheets stabilized by titanium carbide as efficient bifunctional electrocatalysts for fiber-shaped rechargeable zinc-air batteries. Nano Lett. 2020, 20, 2892–2898. [Google Scholar] [CrossRef]
- Cui, Z.; Fu, G.; Li, Y.; Goodenough, J.B. Ni3FeN-supported Fe3Pt intermetallic nanoalloy as a high-performance bifunctional catalyst for metal-air batteries. Angew. Chem. Int. Ed. 2017, 56, 9901–9905. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Liu, X.; Long, L.; Wang, S.; Xu, X.; Liu, M.; Yang, W.; Jia, J. Bifunctional oxygen electrodes of homogeneous Co4N nanocrystals@N-doped carbon hybrids for rechargeable Zn-air batteries. Carbon 2019, 151, 10–17. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, G.; Li, J.; Wang, Y.; Zhang, Z. Surface-engineered cobalt nitride composite as efficient bifunctional oxygen electrocatalyst. Nanotechnology 2019, 30, 495406. [Google Scholar] [CrossRef]
- Gao, T.; Jin, Z.; Zhang, Y.; Tan, G.; Yuan, H.; Xiao, D. Coupling cobalt-iron bimetallic nitrides and N-doped multi-walled carbon nanotubes as high-performance bifunctional catalysts for oxygen evolution and reduction reaction. Electrochim. Acta 2017, 258, 51–60. [Google Scholar] [CrossRef]

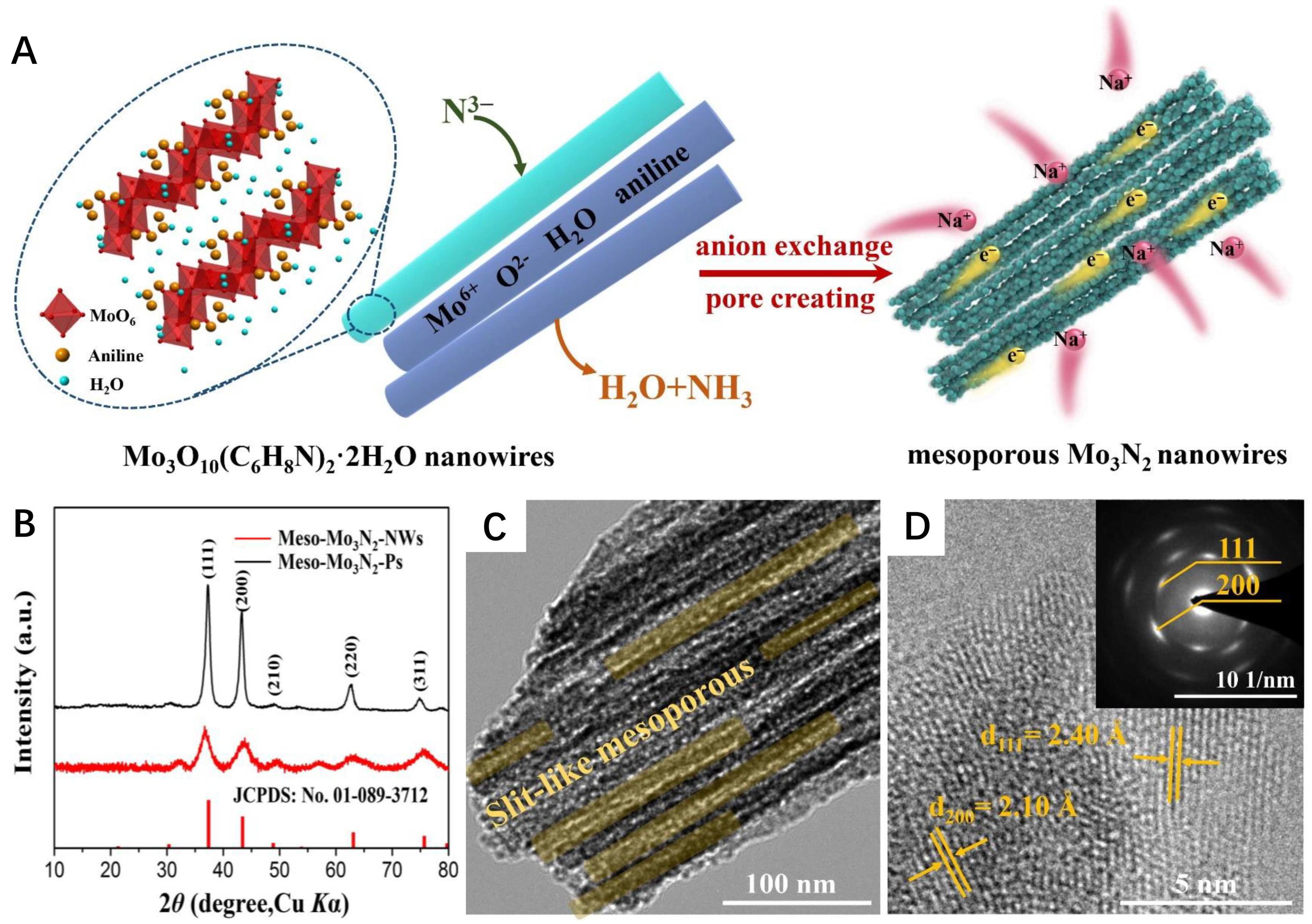
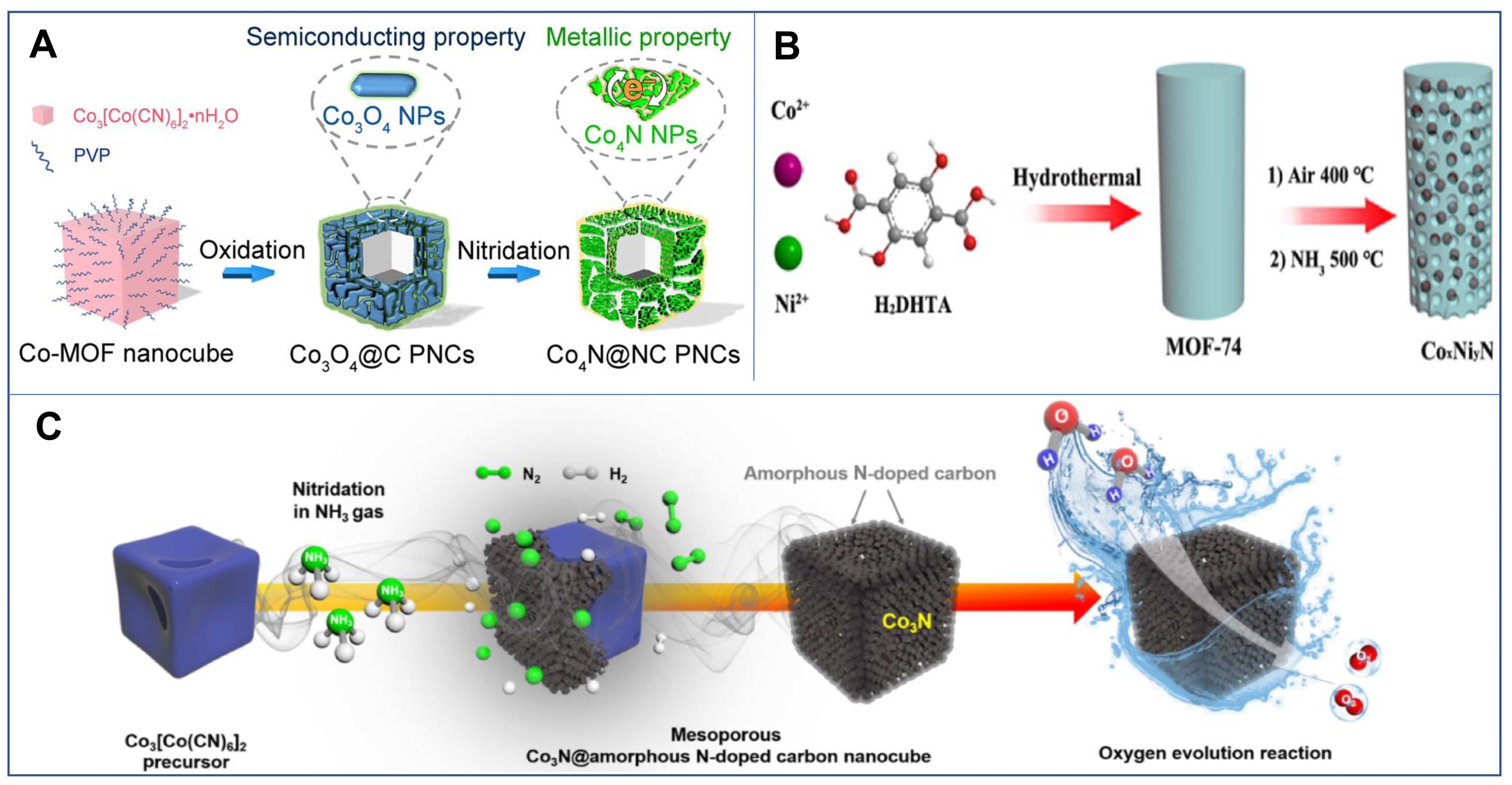
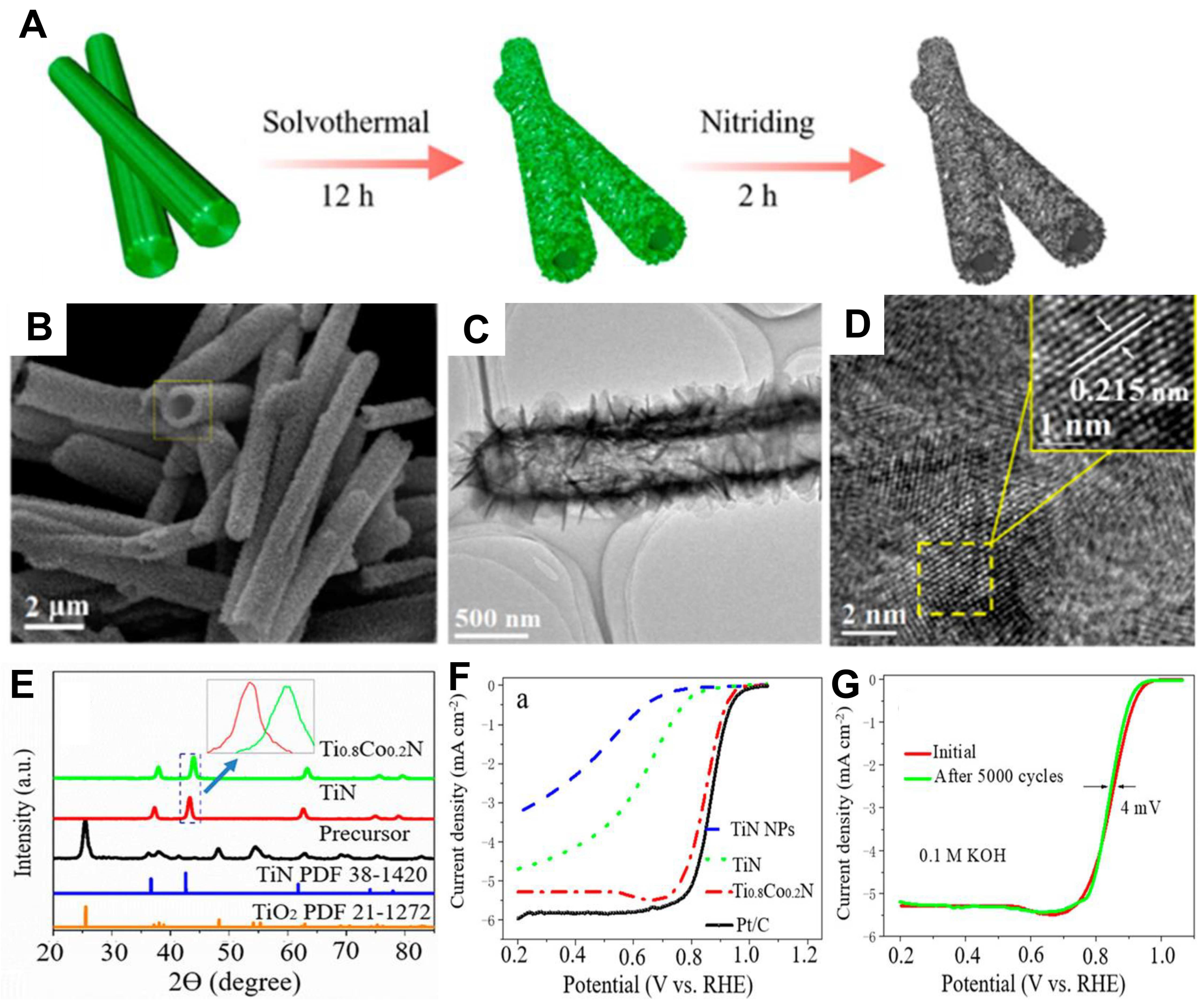

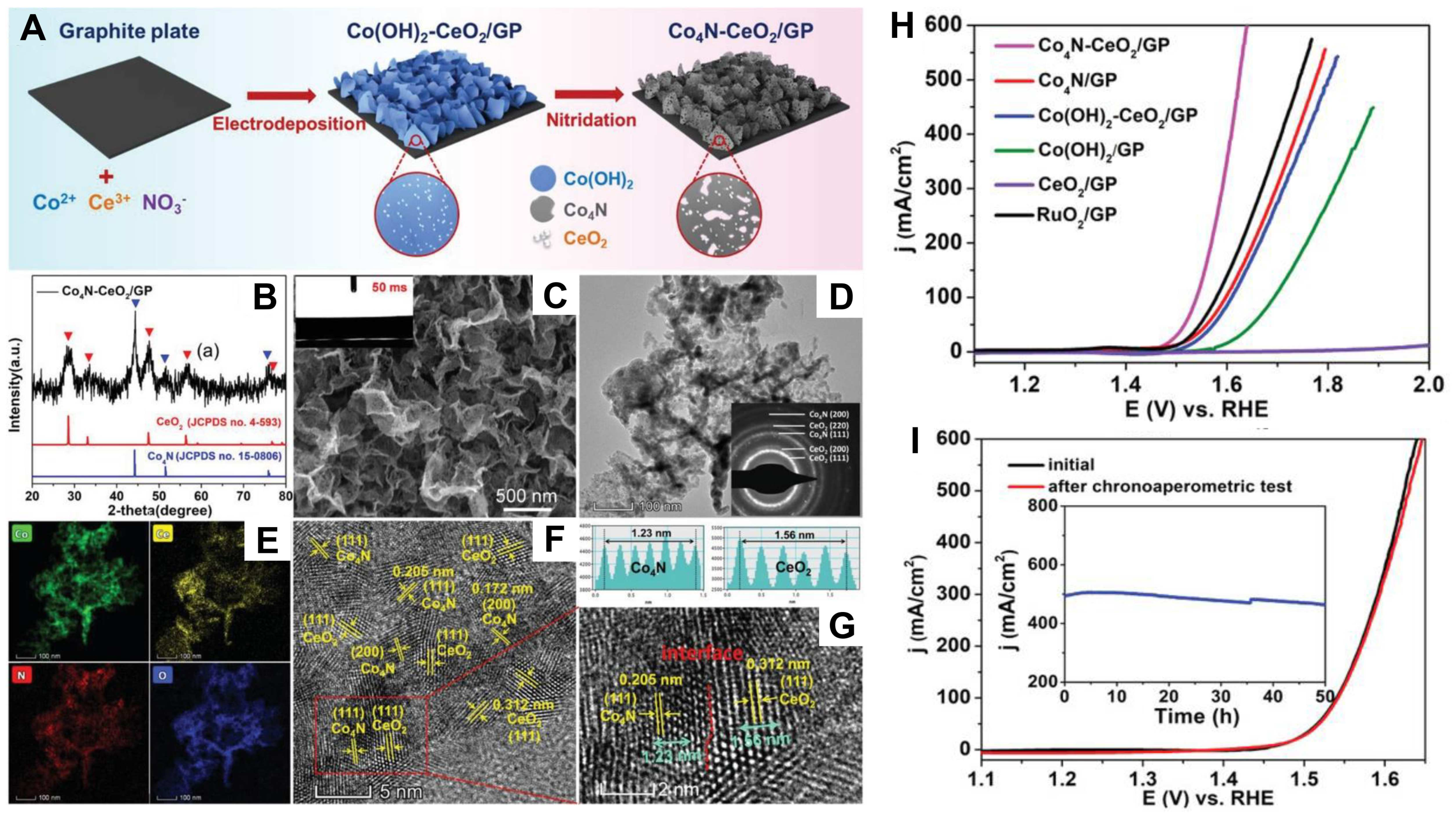
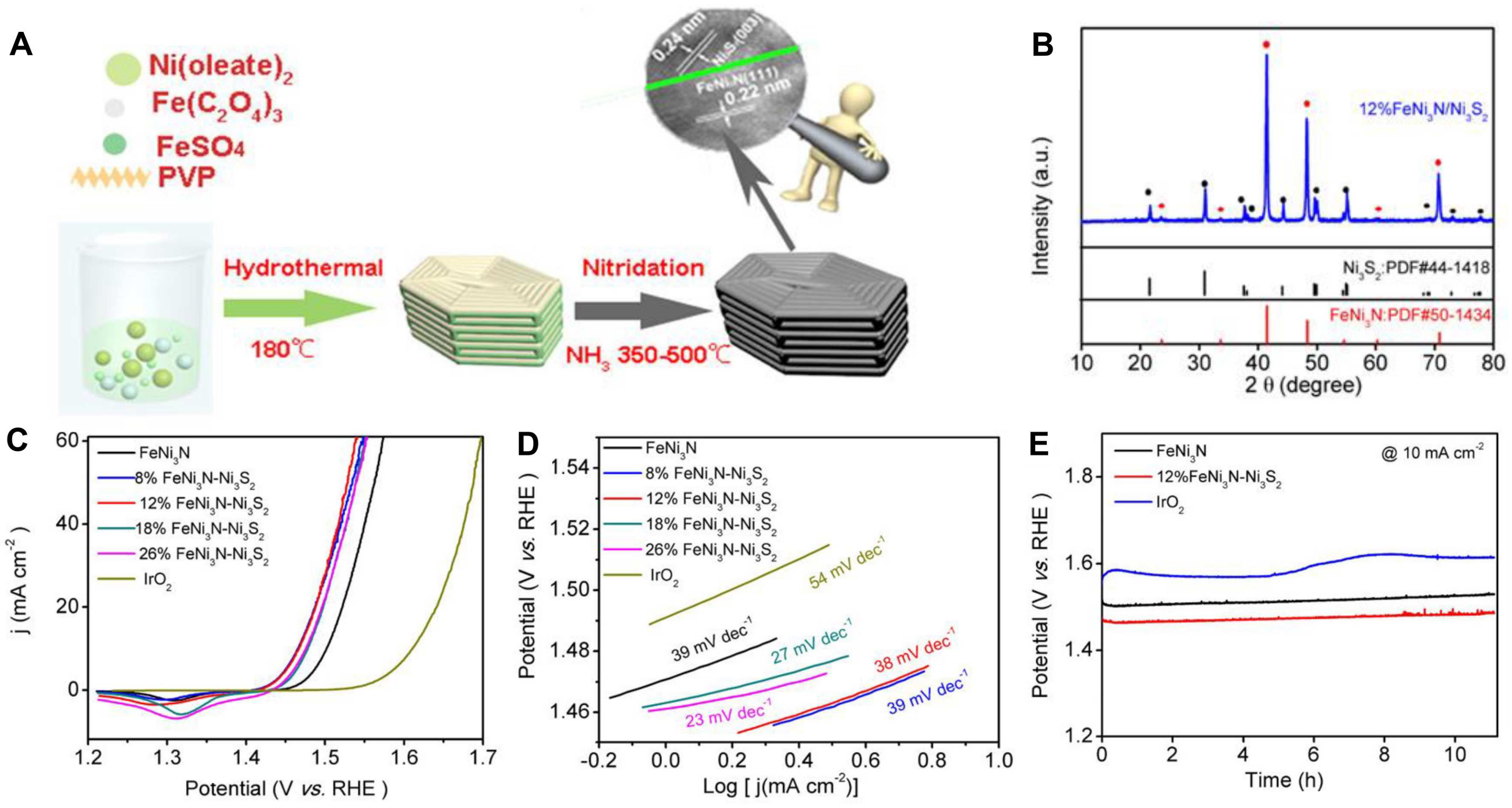
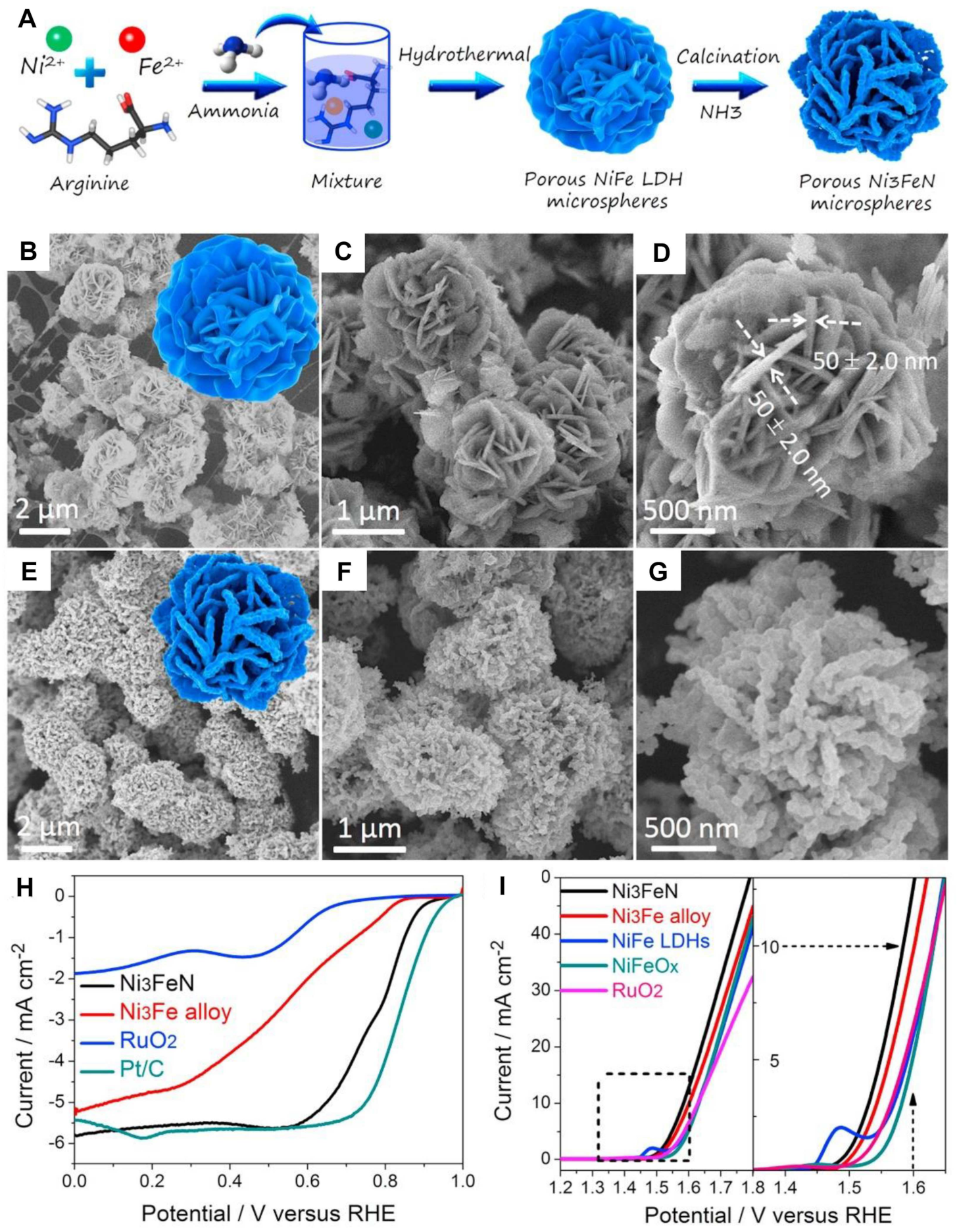

| Catalysts | Morphology | Electrolyte | Ej=10 [V vs. RHE] | Tafet Slope [mV dec−1] | Ref. |
|---|---|---|---|---|---|
| Co4N | 1D | 1 M KOH | 1.49 | 44 | [81] |
| CoN | 1D | 1 M KOH | 1.52 | 70 | [82] |
| Ni3N | 2D | 1 M KOH | 1.58 | 45 | [83] |
| TiOxNy-Fe2N | 3D | 1 M KOH | 1.54 | 59 | [84] |
| NiCo2N/NF | 1D | 1 M KOH | 1.52 | 65 | [85] |
| Ni2Co-N | 3D | 1 M KOH | 1.44 | 53 | [86] |
| CoFeNxHNAs /NF | 2D | 1 M KOH | 1.49 | 57 | [87] |
| CoFe(3:1)-N | 3D | 1 M KOH | 1.43 | 42 | [88] |
| Co4N-CeO2/GP | 2D | 1 M KOH | 1.47 | 46 | [89] |
| Fe2Ni2N/rGO | 2D | 1 M KOH | 1.52 | 49 | [90] |
| FeNi3N-Ni3S2 | 3D | 1 M KOH | 1.46 | 38 | [91] |
| Ni3FeN/SG | 2D | 1 M KOH | 1.46 | 43 | [92] |
| NiFeOOH /Ni3FeN/Ni | 3D | 1 M KOH | 1.43 | 36 | [93] |
| Nifoam@Ni- Ni0.2Mo0.8N | 1D | 1 M KOH | 1.45 | 39 | [94] |
| Catalysts | Morphology | Electrolyte | E1/2 for ORR [V] | Ei = 10 for OER [V] | ΔE [V] | Ref. |
|---|---|---|---|---|---|---|
| Ni3FeN/NRGO | 2D | 0.1 M KOH | 0.72 | 1.38 | 0.77 | [97] |
| Ni3FeN/Co,N-CNF | 0D | 0.1 M KOH | 0.81 | 1.50 | 0.69 | [98] |
| Fe2N/N-CNTs | 0D | 0.1 M KOH | 0.71 | 1.66 | 0.95 | [99] |
| Ni3FeN | 3D | 0.1 M KOH | 0.78 | 1.58 | 0.70 | [100] |
| NiFeMnN | 2D | 0.1 M KOH | 0.84 | 1.52 | 0.68 | [101] |
| Fe3Pt/Ni3FeN | 3D | 0.1 M KOH | 0.93 | 1.60 | 0.72 | [102] |
| Co4N@NC-m | 0D | 0.1 M KOH | 0.87 | 1.63 | 0.81 | [103] |
| Co5.47N | 0D | 0.1 M KOH | 0.82 | 1.61 | 0.80 | [104] |
| Co-Fe-N@MWCNT | 0D | 0.1 M KOH | 0.92 | 1.52 | 0.72 | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Z.; Zheng, S.; Luo, R.; Tang, H.; Wang, R.; Zhang, R.; Tian, T.; Tang, H. Transition Metal Nitrides for Electrocatalytic Application: Progress and Rational Design. Nanomaterials 2022, 12, 2660. https://doi.org/10.3390/nano12152660
Meng Z, Zheng S, Luo R, Tang H, Wang R, Zhang R, Tian T, Tang H. Transition Metal Nitrides for Electrocatalytic Application: Progress and Rational Design. Nanomaterials. 2022; 12(15):2660. https://doi.org/10.3390/nano12152660
Chicago/Turabian StyleMeng, Zihan, Shuhong Zheng, Ren Luo, Haibo Tang, Rui Wang, Ruiming Zhang, Tian Tian, and Haolin Tang. 2022. "Transition Metal Nitrides for Electrocatalytic Application: Progress and Rational Design" Nanomaterials 12, no. 15: 2660. https://doi.org/10.3390/nano12152660
APA StyleMeng, Z., Zheng, S., Luo, R., Tang, H., Wang, R., Zhang, R., Tian, T., & Tang, H. (2022). Transition Metal Nitrides for Electrocatalytic Application: Progress and Rational Design. Nanomaterials, 12(15), 2660. https://doi.org/10.3390/nano12152660







