Phosphorylated Poly(vinyl alcohol) Electrospun Mats for Protective Equipment Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Preparation of the Polymers PVA-OP (1-3)
2.4. Preparation of The Polymer Solution
2.5. Electrospinning Process of PVA-OP (1-3)
3. Results
3.1. FTIR Investigation
3.2. Thermal Stability
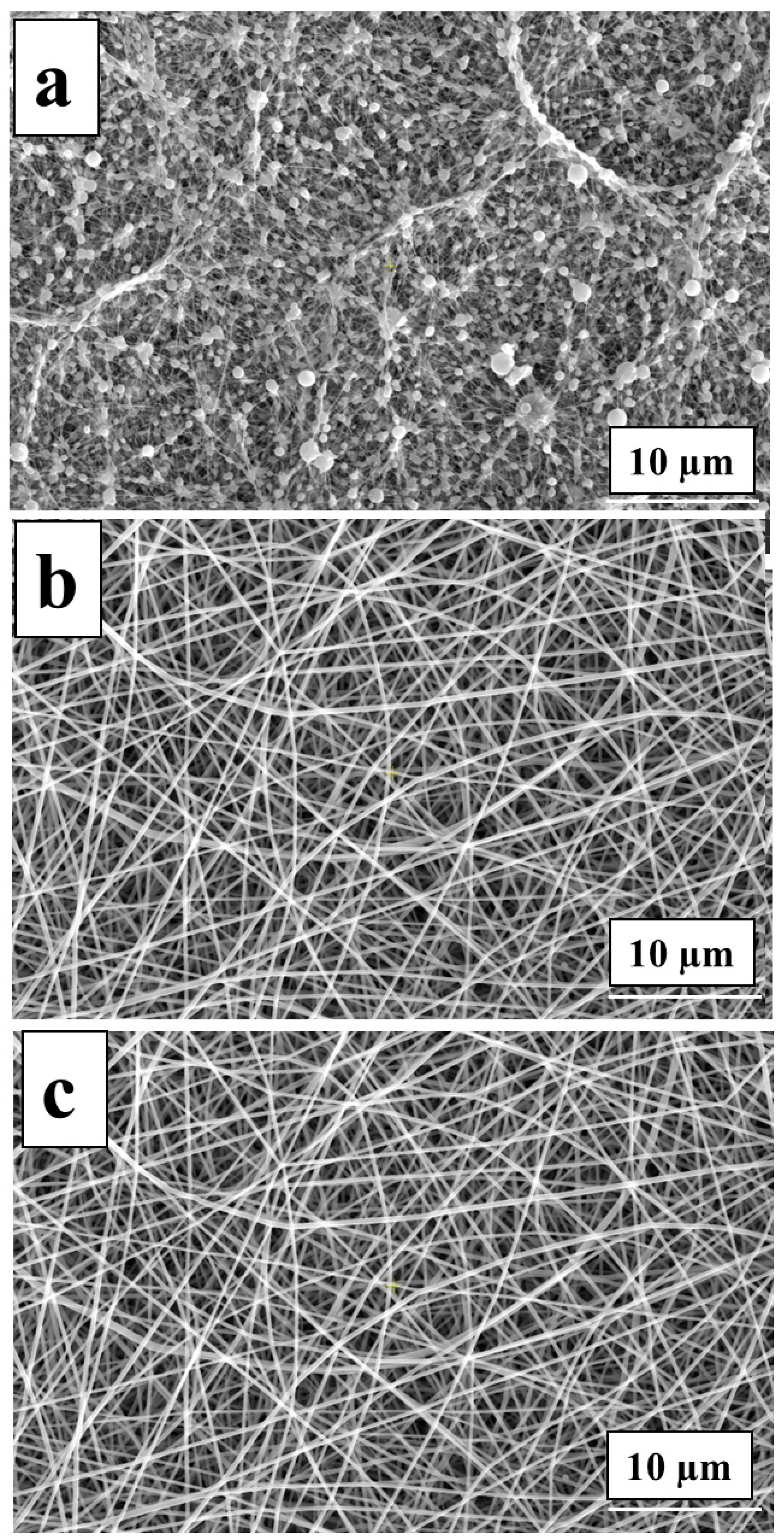
3.3. Morphological Investigation of tbeads only)he Char Residues of PVA-OP (1-3)
3.4. MCC Test
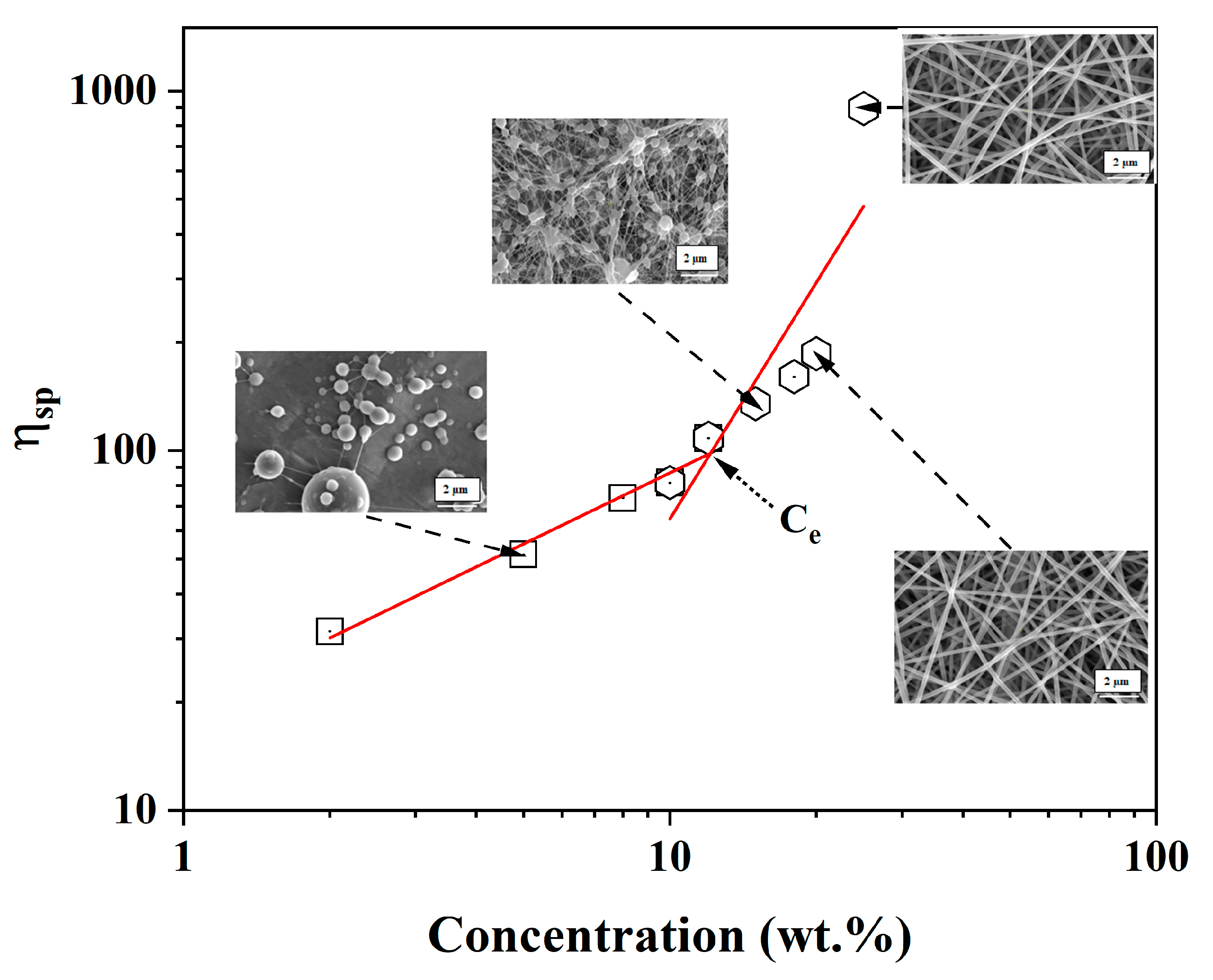
3.5. Rheological Study of PVA-OP (1-3)
3.6. Electrospinning of PVA-OP (1-3) Solutions
3.7. Air Permeability Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wotherspoon, S.; Conroy, S. COVID-19 personal protective equipment protocol compliance audit. Infect. Dis. Health 2021, 26, 273–275. [Google Scholar] [CrossRef]
- Song, G.; Mandal, S.; Rossi, R.M. (Eds.) 4—Development of high performance thermal protective clothing. In Thermal Protective Clothing for Firefighters; Woodhead Publishing: Cambridge, UK, 2017; pp. 27–55. [Google Scholar]
- Zhang, Q.; Li, Y.; Lin, Z.Y.; Wong, K.K.Y.; Lin, M.; Yildirimer, L.; Zhao, X. Electrospun polymeric micro/nanofibrous scaffolds for long-term drug release and their biomedical applications. Drug Discov. Today 2017, 22, 1351–1366. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Drioli, E. A review on membrane engineering for innovation in wearable fabrics and protective textiles. J. Membr. Sci. 2013, 446, 350–375. [Google Scholar] [CrossRef]
- Ghosal, K.; Agatemor, C.; Špitálsky, Z.; Thomas, S.; Kny, E. Electrospinning tissue engineering and wound dressing scaffolds from polymer-titanium dioxide nanocomposites. Chem. Eng. J. 2019, 358, 1262–1278. [Google Scholar] [CrossRef]
- Serbezeanu, D.; Vlad-Bubulac, T.; Rusu, D.; Grădișteanu Pircalabioru, G.; Samoilă, I.; Dinescu, S.; Aflori, M. Functional Polyimide-Based Electrospun Fibers for Biomedical Application. Materials 2019, 12, 3201. [Google Scholar]
- Pampal, E.S.; Stojanovska, E.; Simon, B.; Kilic, A. A review of nanofibrous structures in lithium ion batteries. J. Power Sources 2015, 300, 199–215. [Google Scholar] [CrossRef]
- Aflori, M.; Serbezeanu, D.; Carja, I.-D.; Fortunato, G. Gold Nanoparticles Incorporated into Electrospun Polyimide Fibers. Chem. Lett. 2015, 44, 1440–1442. [Google Scholar] [CrossRef]
- Serbezeanu, D.; Popa, A.M.; Sava, I.; Carja, I.-D.; Amberg, M.; Rossi, R.M.; Fortunato, G. Design and synthesis of polyimide—Gold nanofibers with tunable optical properties. Eur. Polym. J. 2015, 64, 10–20. [Google Scholar] [CrossRef]
- Orudzhev, F.; Ramazanov, S.; Sobola, D.; Kaspar, P.; Trčka, T.; Částková, K.; Kastyl, J.; Zvereva, I.; Wang, C.; Selimov, D.; et al. Ultrasound and water flow driven piezophototronic effect in self-polarized flexible α-Fe2O3 containing PVDF nanofibers film for enhanced catalytic oxidation. Nano Energy 2021, 90, 106586. [Google Scholar] [CrossRef]
- Chatterjee, K.; Tabor, J.; Ghosh, T.K. Electrically Conductive Coatings for Fiber-Based E-Textiles. Fibers 2019, 7, 51. [Google Scholar]
- Gautam, L.; Warkar, S.G.; Ahmad, S.I.; Kant, R.; Jain, M. A review on carboxylic acid cross-linked polyvinyl alcohol: Properties and applications. Polym. Eng. Sci. 2022, 62, 225–246. [Google Scholar]
- Kamoun, E.A.; Loutfy, S.A.; Hussein, Y.; Kenawy, E.-R.S. Recent advances in PVA-polysaccharide based hydrogels and electrospun nanofibers in biomedical applications: A review. Int. J. Biol. Macromol. 2021, 187, 755–768. [Google Scholar] [CrossRef]
- Rynkowska, E.; Fatyeyeva, K.; Marais, S.; Kujawa, J.; Kujawski, W. Chemically and Thermally Crosslinked PVA-Based Membranes: Effect on Swelling and Transport Behavior. Polymers 2019, 11, 1799. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sood, A.; Han, S.S. Poly (vinyl alcohol)-alginate as potential matrix for various applications: A focused review. Carbohydr. Polym. 2022, 277, 118881. [Google Scholar] [CrossRef]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Shebbrin Moonshi, S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar]
- Wang, Y.C.; Yuan, Y.Y.; Du, J.Z.; Yang, X.Z.; Wang, J.J. Recent progress in polyphosphoesters: From controlled synthesis to biomedical applications. Macromol. Biosci. 2009, 9, 1154–1164. [Google Scholar]
- Montembault, V.; Fontaine, L.J.P.-B.P. Polyphosphoesters. Phosphorus-Based Polym. 2014, 97–124. [Google Scholar]
- Olmedo-Martínez, J.L.; Meabe, L.; Riva, R.; Guzmán-González, G.; Porcarelli, L.; Forsyth, M.; Mugica, A.; Calafel, I.; Müller, A.J.; Lecomte, P.J. Flame retardant polyphosphoester copolymers as solid polymer electrolyte for lithium batteries. Polym. Chem. 2021, 12, 3441–3450. [Google Scholar]
- Huang, Y.-K.; Tian, H.-R.; Zhang, M.-Z.; He, J.-L.; Liu, J.; Ni, P.-H. Monoclonal Antibody-conjugated Polyphosphoester-hyd-DOX Prodrug Nanoparticles for Targeted Chemotherapy of Liver Cancer Cells. Chin. J. Polym. Sci. 2021, 39, 1392–1402. [Google Scholar]
- Yuan, Y.; Liu, J.; Li, C.; Fang, J.; Wang, S.; Guan, R. Preparation and properties of phosphorylated and crosslinked poly(vinyl alcohol)/bisphenol A–epoxy resin membranes for fuel cell applications. J. Appl. Polym. Sci. 2011, 122, 3071–3079. [Google Scholar] [CrossRef]
- You, H.; Song, G.; Liu, Q.; Yang, C.; Qiu, J.; Zang, L.; Liu, H.; Chen, J. A facile route for the fabrication of a superhydrophilic and underwater superoleophobic phosphorylated PVA-coated mesh for both oil/water immiscible mixture and emulsion separation. Appl. Surf. Sci. 2021, 537, 147986. [Google Scholar] [CrossRef]
- Vlad-Bubulac, T.; Oprea, A.-M.; Serbezeanu, D.; Carja, I.-D.; Hamciuc, C.J. In vitro Release of Metoprolol Tartrate from Poly (vinyl Alcohol)/Phosphoester–chondroitin Sulfate Semi-IPNs. Rev. Chim. 2013, 64, 663–666. [Google Scholar]
- Moon, S.; Ku, B.-C.; Emrick, T.; Coughlin, B.E.; Farris, R.J. Flame resistant electrospun polymer nanofibers from deoxybenzoin-based polymers. J. Appl. Polym. Sci. 2009, 111, 301–307. [Google Scholar] [CrossRef]
- Serbezeanu, D.; Butnaru, I.; Varganici, C.-D.; Bruma, M.; Fortunato, G.; Gaan, S. Phosphorus-containing polyimide fibers and their thermal properties. RSC Adv. 2016, 6, 38371–38379. [Google Scholar] [CrossRef] [Green Version]
- Butnaru, I.; Serbezeanu, D.; Bruma, M.; Sava, I.; Gaan, S.; Fortunato, G. Physical and thermal properties of poly(ethylene terephthalate) fabric coated with electrospun polyimide fibers. High Perform. Polym. 2015, 27, 616–624. [Google Scholar] [CrossRef]
- Bonakdar, S.; Emami, S.H.; Shokrgozar, M.A.; Farhadi, A.; Ahmadi, S.A.H.; Amanzadeh, A. Preparation and characterization of polyvinyl alcohol hydrogels crosslinked by biodegradable polyurethane for tissue engineering of cartilage. Mater. Sci. Eng. C 2010, 30, 636–643. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Awada, H.; Daneault, C. Chemical Modification of Poly(Vinyl Alcohol) in Water. Appl. Sci. 2015, 5, 840–850. [Google Scholar]
- Restrepo, I.; Medina, C.; Meruane, V.; Akbari-Fakhrabadi, A.; Flores, P.; Rodríguez-Llamazares, S. The effect of molecular weight and hydrolysis degree of poly (vinyl alcohol)(PVA) on the thermal and mechanical properties of poly (lactic acid)/PVA blends. Polímeros 2018, 28, 169–177. [Google Scholar]
- Ayoola, B.; Balachandran, R.; Frank, J.; Mastorakos, E.; Kaminski, C. Spatially resolved heat release rate measurements in turbulent premixed flames. Combust. Flame 2006, 144, 1–16. [Google Scholar]
- Peng, S.; Zhou, M.; Liu, F.; Zhang, C.; Liu, X.; Liu, J.; Zou, L.; Chen, J. Flame-retardant polyvinyl alcohol membrane with high transparency based on a reactive phosphorus-containing compound. R. Soc. Open Sci. 2017, 4, 170512. [Google Scholar]
- Qiu, M.; Wang, D.; Zhang, L.; Li, M.; Liu, M.; Fu, S. Electrochemical exfoliation of water-dispersible graphene from graphite towards reinforcing the mechanical and flame-retardant properties of poly (vinyl alcohol) composites. Mater. Chem. Phys. 2020, 254, 123430. [Google Scholar] [CrossRef]
- Yu, S.-j.; Lu, S.-y.; Tan, D.-f.; Zhu, Y.-f. Nitrogen and phosphorus co-doped carbon dots for developing highly flame retardant poly (vinyl alcohol) composite films. Eur. Polym. J. 2022, 164, 110970. [Google Scholar] [CrossRef]
- Ma, B.; Lii, J.-H.; Schaefer, H.F.; Allinger, N.L. Systematic comparison of experimental, quantum mechanical, and molecular mechanical bond lengths for organic molecules. J. Phys. Chem. 1996, 100, 8763–8769. [Google Scholar]
- McKee, M.G.; Wilkes, G.L.; Colby, R.H.; Long, T.E. Correlations of Solution Rheology with Electrospun Fiber Formation of Linear and Branched Polyesters. Macromolecules 2004, 37, 1760–1767. [Google Scholar] [CrossRef]
- McKee, M.G.; Elkins, C.L.; Long, T.E. Influence of self-complementary hydrogen bonding on solution rheology/electrospinning relationships. Polymer 2004, 45, 8705–8715. [Google Scholar] [CrossRef]

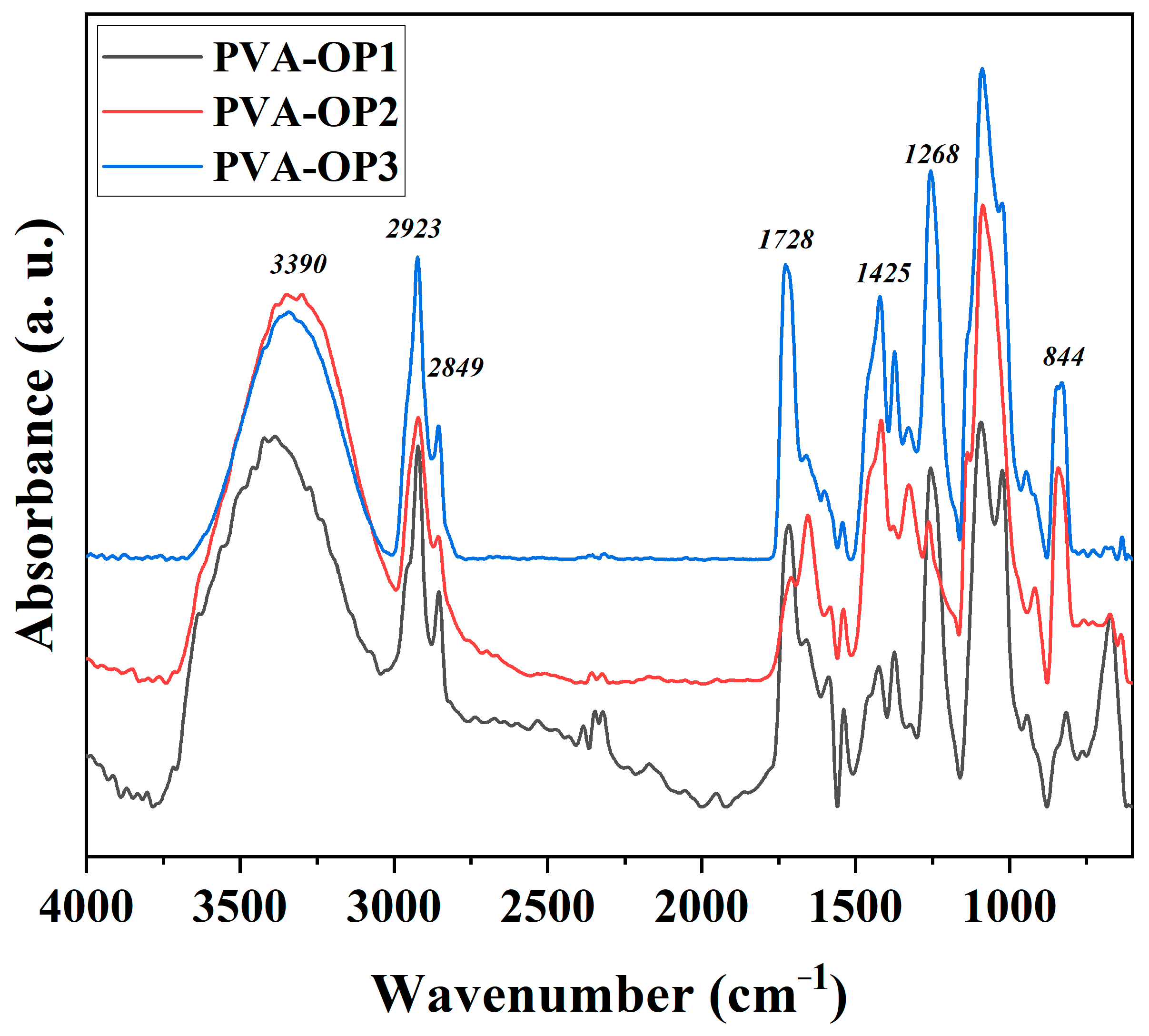
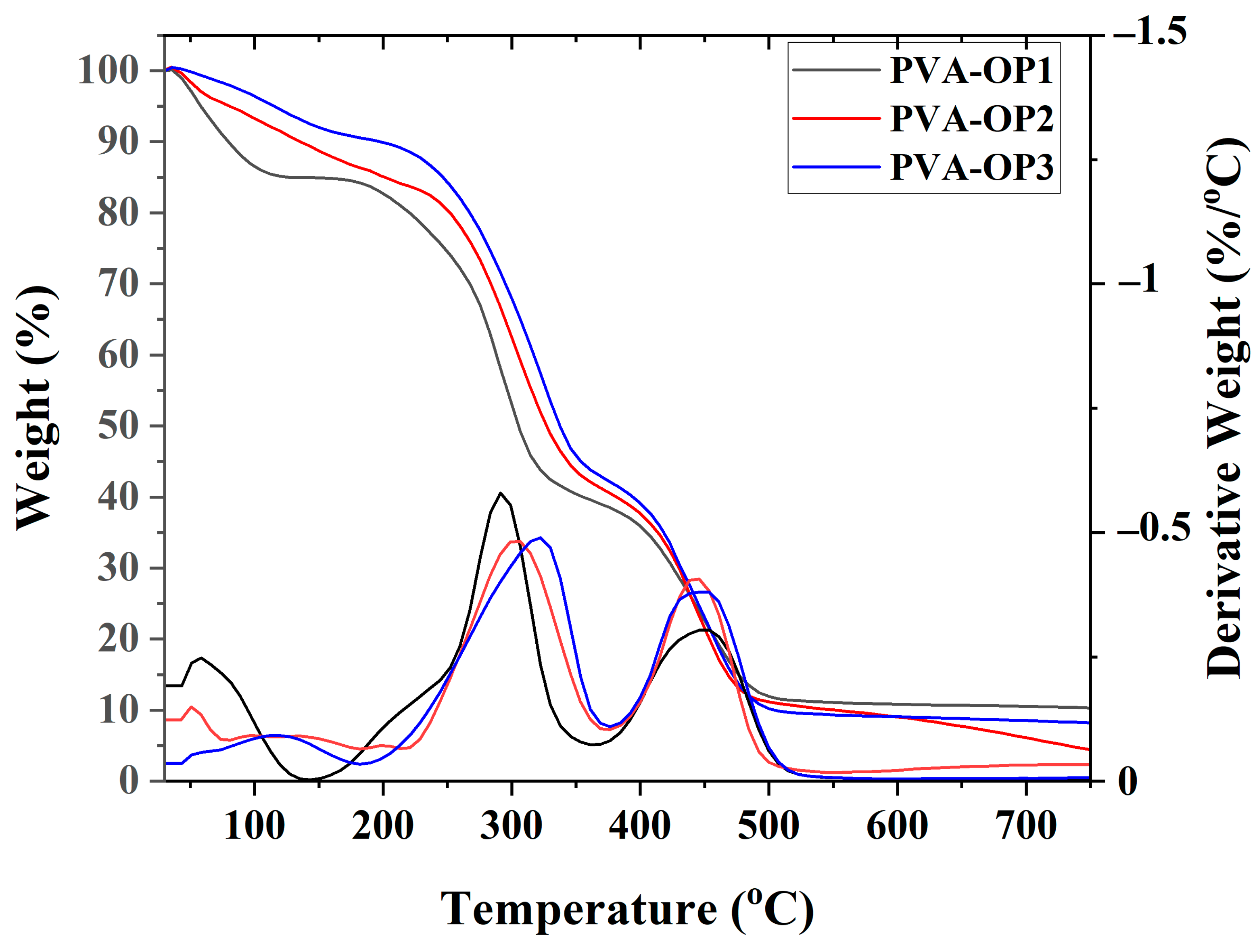
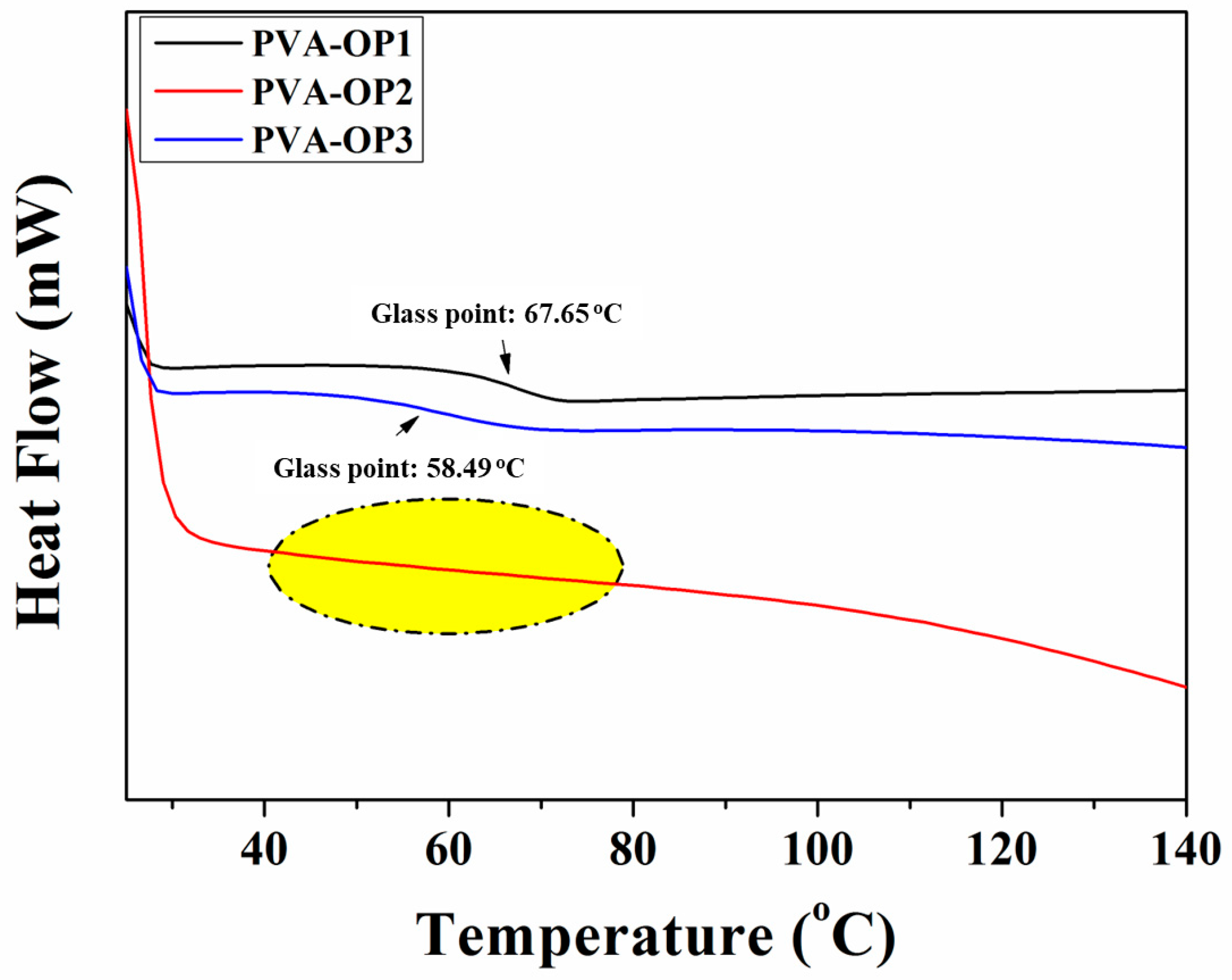

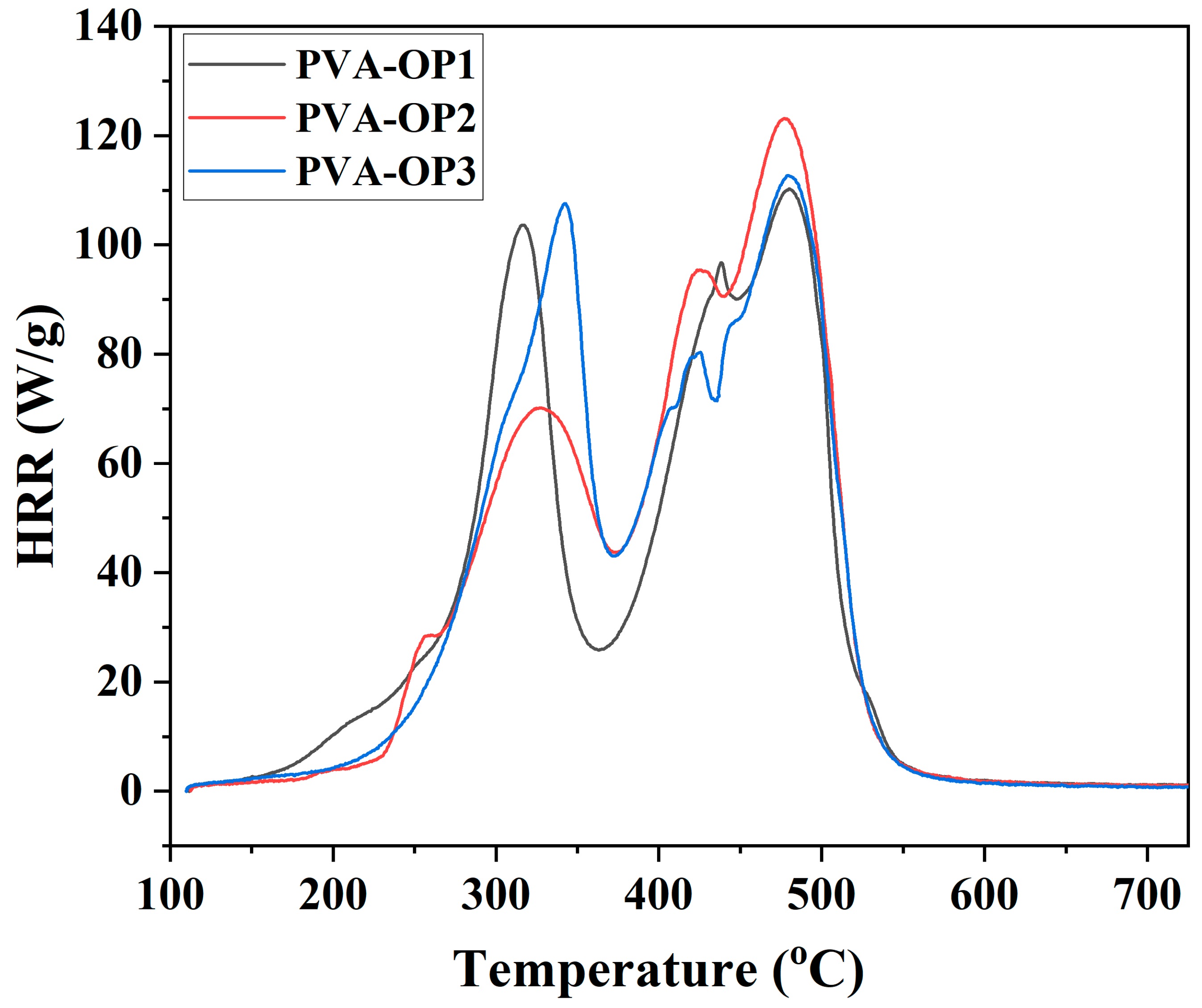

| Sample | TGA | DSC | |||
|---|---|---|---|---|---|
| Tonset (°C) 1 | Tmax (°C) 2 | Tendset (°C) 3 | Char Yield (%) 4 | Tg (°C) 5 | |
| PVA-OP1 | 46.62 255.65 404.47 | 56.15 292.41 451.15 | 101.61 315.20 486.16 | 10.34 | 67.65 |
| PVA-OP2 | 42.10 250.17 416.13 | 136.36 303.47 442.61 | 250.17 342.12 475.61 | 4.64 | - |
| PVA-OP3 | 52.57 246.92 410.33 | 140.00 325.51 445.16 | 150.37 349.30 486.12 | 8.24 | 58.49 |
| Sample | Weight (mg) | Char Yield (mg) | Char Yield (wt%) | Decomposition Rate (%) | HRC 1 (J/(g × K)) | THR 2 (kJ/g) | PHRR 3 (W/g) | TPHRR 4 (°C) | Time (s) |
|---|---|---|---|---|---|---|---|---|---|
| PVA-OP1 | 20.09 | 2.46 | 12.24 | 87.76 | 265.65 | 18.24 | 108.53 | 479.98 | 339.50 |
| PVA-OP2 | 20.07 | 1.92 | 9.57 | 90.43 | 257.70 | 19.53 | 121.83 | 477.26 | 336.50 |
| PVA-OP3 | 20.02 | 1.69 | 8.44 | 91.56 | 245.38 | 19.31 | 111.35 | 479.20 | 332.00 |
| Sample | Viscosity (Pa × s) | Electrospinning Conditions | Average Fiber Diameters (µm) |
|---|---|---|---|
| PVA-OP1 | 0.5677 | 30%, 20 cm, 50 μL/min, 22 kV, 20%, 25 °C | 0.111 ± 0.03 (fibers) |
| PVA-OP2 | 0.0961 | 15%, 20 cm, 50 μL/min, 22 kV, 20%, 25 °C | 0.056 ± 0.023 (fibers with beads) |
| 0.6154 | 25%, 20 cm, 50 μL/min, 22 kV, 20%, 25 °C | 0.217 ± 0.045 (uniform fibers) | |
| PVA-OP3 | 0.0289 | 2%, 20 cm, 50 μL/min, 22 kV, 20%, 25 °C | - (beads only) |
| 0.0467 | 5%, 20 cm, 50 μL/min, 22 kV, 20%, 25 °C | - (beads only) | |
| 0.0666 | 8%, 20 cm, 50 μL/min, 22 kV, 20%, 25 °C | - (beads with fibers) | |
| 0.0734 | 10%, 20 cm, 50 μL/min, 22 kV, 20%, 25 °C | - (fibers with beads) | |
| 0.0972 | 12%, 20 cm, 50 μL/min, 22 kV, 20%, 25 °C | 0.031 ± 0.019 (fibers with beads) | |
| 0.1211 | 15%, 20 cm, 50 μL/min, 22 kV, 20%, 25 °C | 0.048 ± 0.020 (fibers with beads) | |
| 0.1437 | 18%, 20 cm, 50 μL/min, 22 kV, 20%, 25 °C | 0.062 ± 0.032 (fibers with beads) | |
| 0.1663 | 20%, 20 cm, 50 μL/min, 22 kV, 20%, 25 °C | 0.304 ± 0.087 (uniform fibers) | |
| 0.7967 | 25%, 20 cm, 50 μL/min, 22 kV, 20%, 25 °C | 0.214 ± 0.048 (uniform fibers) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serbezeanu, D.; Vlad-Bubulac, T.; Onofrei, M.D.; Doroftei, F.; Hamciuc, C.; Ipate, A.-M.; Anisiei, A.; Lisa, G.; Anghel, I.; Şofran, I.-E.; et al. Phosphorylated Poly(vinyl alcohol) Electrospun Mats for Protective Equipment Applications. Nanomaterials 2022, 12, 2685. https://doi.org/10.3390/nano12152685
Serbezeanu D, Vlad-Bubulac T, Onofrei MD, Doroftei F, Hamciuc C, Ipate A-M, Anisiei A, Lisa G, Anghel I, Şofran I-E, et al. Phosphorylated Poly(vinyl alcohol) Electrospun Mats for Protective Equipment Applications. Nanomaterials. 2022; 12(15):2685. https://doi.org/10.3390/nano12152685
Chicago/Turabian StyleSerbezeanu, Diana, Tăchiță Vlad-Bubulac, Mihaela Dorina Onofrei, Florica Doroftei, Corneliu Hamciuc, Alina-Mirela Ipate, Alexandru Anisiei, Gabriela Lisa, Ion Anghel, Ioana-Emilia Şofran, and et al. 2022. "Phosphorylated Poly(vinyl alcohol) Electrospun Mats for Protective Equipment Applications" Nanomaterials 12, no. 15: 2685. https://doi.org/10.3390/nano12152685
APA StyleSerbezeanu, D., Vlad-Bubulac, T., Onofrei, M. D., Doroftei, F., Hamciuc, C., Ipate, A.-M., Anisiei, A., Lisa, G., Anghel, I., Şofran, I.-E., & Popescu, V. (2022). Phosphorylated Poly(vinyl alcohol) Electrospun Mats for Protective Equipment Applications. Nanomaterials, 12(15), 2685. https://doi.org/10.3390/nano12152685








