Chemical Sensor Nanotechnology in Pharmaceutical Drug Research
Abstract
:1. Introduction
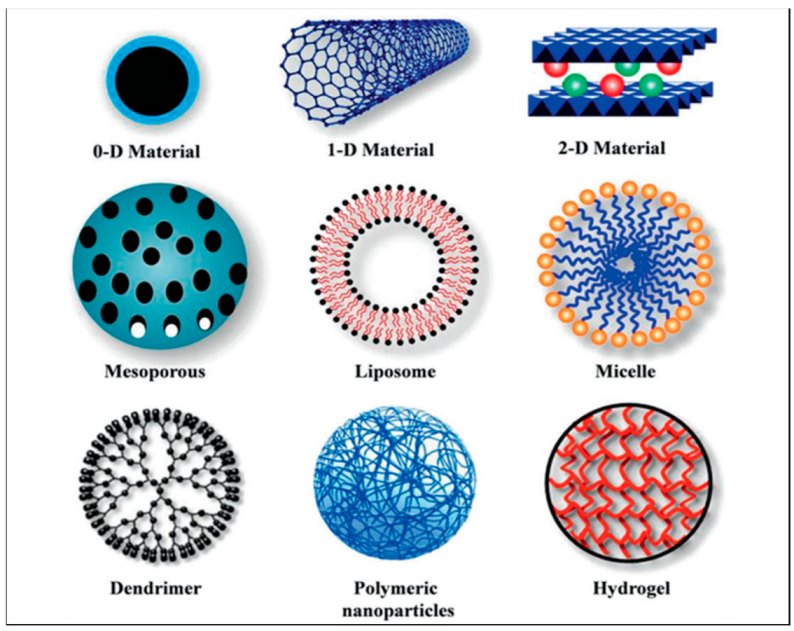
2. Nanomaterial Scaffolds and Therapeutic Drug Applications
2.1. Polymeric Nanomaterials and Their Applications
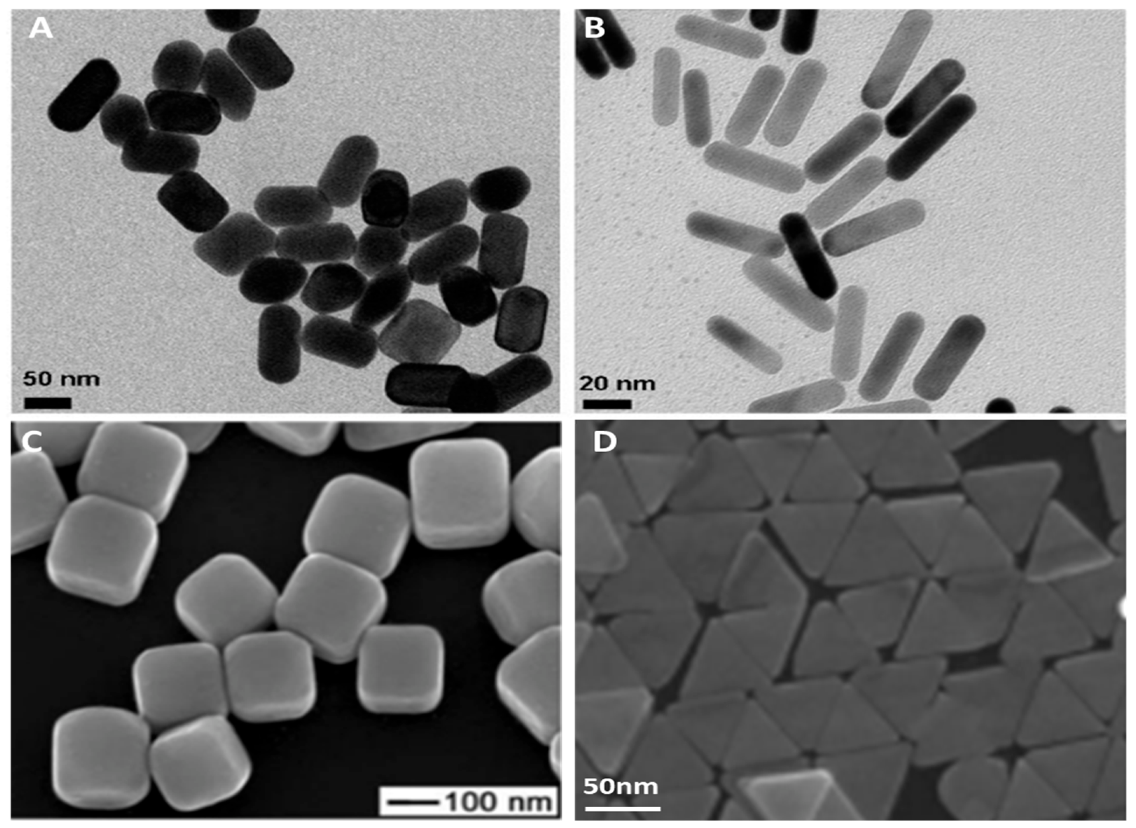
2.2. Metallic Nanomaterials and Their Applications
2.3. Graphene Based Nano-Sensors and Applications
3. Detection Methods and Sensing Techniques
3.1. Electrical Detection Methods
3.1.1. Field Effect Transistor (FET) Based Detection Method
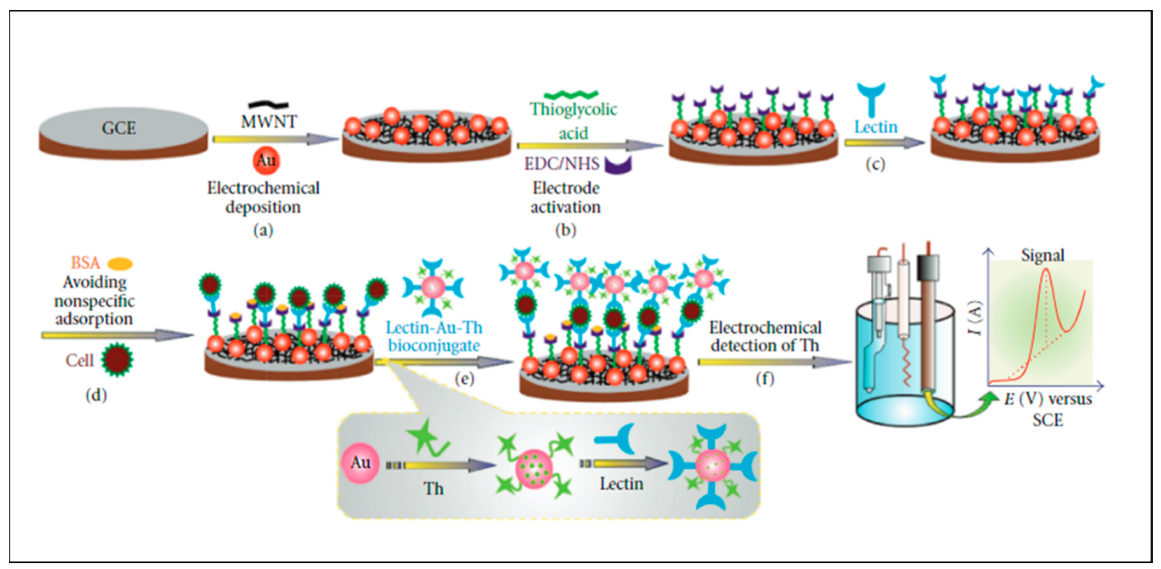
3.1.2. Electrochemical Detection Methods
3.2. Optical Detection Methods
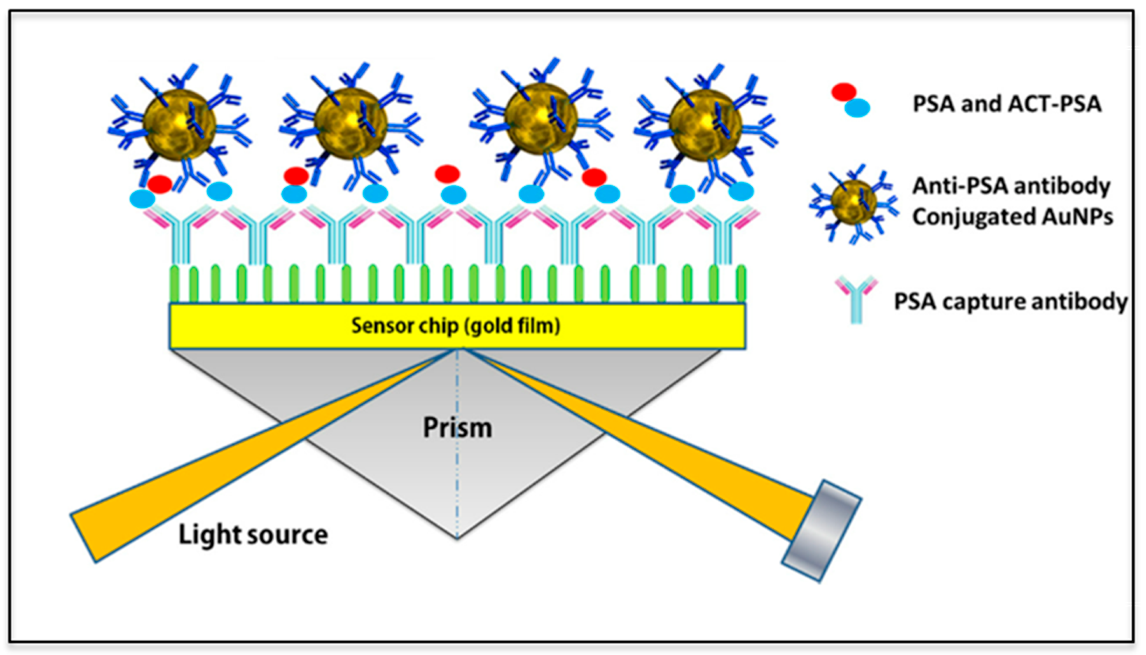
3.2.1. Surface Plasmon Resonance Spectroscopy
3.2.2. Surface Enhanced Raman Spectroscopy (SERS)
4. Prospects and Shortcomings
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Wu, F.; White, J.C.; Xing, B. 100 Nanometers: A Potentially Inappropriate Threshold for Environmental and Ecological Effects of Nanoparticles. Environ. Sci. Technol. 2014, 48, 3098–3099. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.K. Nanoparticles in modern medicine: State of the art and future challenges. Int. J. Nanomed. 2007, 2, 129–141. [Google Scholar]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical Applications of Graphene. Theranostics 2012, 2, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Shi, X.; Ji, A.; Shi, L.; Zhou, C.; Cui, Y. Fabrication and Characteristics of Reduced Graphene Oxide Produced with Different Green Reductants. PLoS ONE 2015, 10, e0144842. [Google Scholar]
- Yadav, S.; Sharma, A.K.; Kumar, P. Nanoscale Self-Assembly for Therapeutic Delivery. Front. Bioeng. Biotechnol. 2020, 8, 127. [Google Scholar] [CrossRef]
- Bissantz, C.; Kuhn, B.; Stahl, M. A Medicinal Chemist’s Guide to Molecular Interactions. J. Med. Chem. 2010, 53, 5061–5084. [Google Scholar] [CrossRef]
- Shovsky, A.; Varga, I.; Makuška, R.; Claesson, P.M. Formation and Stability of Water-Soluble, Molecular Polyelectrolyte Complexes: Effects of Charge Density, Mixing Ratio, and Polyelectrolyte Concentration. Langmuir 2009, 25, 6113–6121. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Magazù, S.; Calandra, P. Amphiphiles Self-Assembly: Basic Concepts and Future Perspectives of Supramolecular Approaches. Adv. Condens. Matter Phys. 2015, 2015, 151683. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Carney, R.P.; Stellacci, F.; Lau, B.L.T. Protein-nanoparticle interactions: The effects of surface compositional and structural heterogeneity are scale dependent. Nanoscale 2013, 5, 6928–6935. [Google Scholar] [CrossRef] [Green Version]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [Green Version]
- Amina, S.J.; Guo, B. A Review on the Synthesis and Functionalization of Gold Nanoparticles as a Drug Delivery Vehicle. Int. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, R.; Suni, I.I.; Bever, C.S.; Hammock, B.D. Impedance Biosensors: Applications to Sustainability and Remaining Technical Challenges. ACS Sustain. Chem. Eng. 2014, 2, 1649–1655. [Google Scholar] [CrossRef]
- Bharti, K.; Sadhu, K.K. Syntheses of metal oxide-gold nanocomposites for biological applications. Results Chem. 2022, 4, 100288. [Google Scholar] [CrossRef]
- Mohammad-Rezaei, R.; Golmohammadpour, M. Controlled Electrodeposition of Au-Copper Oxide Nanocomposite on a Renewable Carbon Ceramic Electrode for Sensitive Determination of NADH in Serum Samples. Electroanalysis 2020, 32, 606–612. [Google Scholar] [CrossRef]
- Gooding, J.J.; Chou, A.; Liu, J.; Losic, D.; Shapter, J.G.; Hibbert, D.B. The effects of the lengths and orientations of single-walled carbon nanotubes on the electrochemistry of nanotube-modified electrodes. Electrochem. Commun. 2007, 9, 1677–1683. [Google Scholar] [CrossRef]
- Heller, I.; Kong, J.; Heering, H.A.; Williams, K.A.; Lemay, A.S.G.; Dekker, C. Individual Single-Walled Carbon Nanotubes as Nanoelectrodes for Electrochemistry. Nano Lett. 2005, 5, 137–142. [Google Scholar] [CrossRef]
- Gao, X.P.A.; Zheng, G.; Lieber, C.M. Subthreshold Regime has the Optimal Sensitivity for Nanowire FET Biosensors. Nano Lett. 2010, 10, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Tu, Q.; Chang, C. Diagnostic applications of Raman spectroscopy. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 545–558. [Google Scholar] [CrossRef]
- Kazuma, E.; Tatsuma, T. Localized surface plasmon resonance sensors based on wavelength-tunable spectral dips †. Nanoscale 2014, 6, 2397–2405. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Ràfols, C.P.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. A Chemically-Bound Glutathione Sensor Bioinspired by the Defense of Organisms against Heavy Metal Contamination: Optimization of the Immobilization Conditions. Chemosensors 2017, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Cushing, S.K.; Wu, N.Q. Plasmon-enhanced optical sensors: A review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R.; Ray, K.; Chowdhury, M.; Szmacinski, H.; Fu, Y.; Zhang, J.; Nowaczyk, K. Plasmon-controlled fluorescence: A new paradigm in fluorescence spectroscopy. Analyst 2008, 133, 1308–1346. [Google Scholar] [CrossRef] [Green Version]
- Meyer, S.A.; Le Ru, E.C.; Etchegoin, P.G.; Champion, P.M.; Ziegler, L.D. Quantifying Resonant Raman Cross Sections with SERS. AIP Conf. Proc. 2010, 1267, 204–205. [Google Scholar]
- Cao, Y.C.; Jin, R.; Nam, J.-M.; Thaxton, C.S.; Mirkin, C.A. Raman Dye-Labeled Nanoparticle Probes for Proteins. J. Am. Chem. Soc. 2003, 125, 14676–14677. [Google Scholar] [CrossRef]
- Bertoldo Menezes, D.; Reyer, A.; Marietta, A.; Musso, M. Glass transition of polystyrene (PS) studied by Raman spectroscopic investigation of its phenyl functional groups. Mater. Res. Express 2017, 4, 015303. [Google Scholar] [CrossRef]
- Littleford, R.E.; Cunningham, D.; Matousek, P.; Towrie, M.; Parker, A.W.; Khan, I.; McComb, D.; Smith, W.E. Surface-enhanced resonance Raman scattering using pulsed and continuous-wave laser excitation. J. Raman Spectrosc. 2005, 36, 600–605. [Google Scholar] [CrossRef]
- Bumbrah, G.S.; Sharma, R.M. Raman spectroscopy–Basic principle, instrumentation and selected applications for the characterization of drugs of abuse. Egypt J. Forensic. Sci. 2016, 6, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Robinson, J.T.; Sun, X.M.; Dai, H.J. PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar] [CrossRef]
- Car, A.; Baumann, P.; Duskey, J.T.; Chami, M.; Bruns, N.; Meier, W. PH-responsive PDMS-b-PDMAEMA micelles for intracellular anticancer drug delivery. Biomacromolecules 2014, 15, 3235–4325. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Taylor, J.; Huefner, A.; Li, L.; Wingfield, J.; Mahajan, S. Nanoparticles and intracellular applications of surface-enhanced Raman spectroscopy. Analyst 2016, 141, 5037–5055. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Beatty, A.; Lu, L.; Abdalrahman, A.; Makris, T.M.; Wang, G.; Wang, Q. Microfluidic-assisted polymer-protein assembly to fabricate homogeneous functionalnanoparticles. Mater. Sci. Eng. C 2020, 111, 110768. [Google Scholar] [CrossRef]
- Alyaudtin, R.N.; Reichel, A.; Löbenberg, R.; Ramge, P.; Kreuter, J.; Begley, D.J. Interaction ofpoly(butylcyanoacrylate) nano-particles with the blood-brain barrier in vivo and in vitro. J. Drug Target. 2001, 9, 209–221. [Google Scholar] [CrossRef]
- Yavuz, B.; Bozdag Pehlivan, S.; Sumer Bolu, B.; Nomak Sanyal, R.; Vural, I.; Unlu, N. Dexamethasone-PAMAM dendri-mer conjugates for retinal delivery: Preparation, characterization and in vivo evaluation. J. Pharm. Pharmacol. 2016, 68, 1010–1020. [Google Scholar] [CrossRef]
- Lima, T.; Bernfur, K.; Vilanova, M.; Cedervall, T. Understanding the Lipid and Protein Corona Formation on Different Sized Polymeric Nanoparticles. Sci. Rep. 2020, 10, 1129. [Google Scholar] [CrossRef]
- Osorno, L.L.; Brandley, A.N.; Maldonado, D.E.; Yiantsos, A.; Mosley, R.J.; Byrne, M.E. Review of Contemporary Self-Assembled Systems for the Controlled Delivery of Therapeutics in Medicine. Nanomaterials 2021, 11, 278. [Google Scholar] [CrossRef]
- Scarpa, E.; Bailey, J.L.; Janeczek, A.A.; Stumpf, P.S.; Johnston, A.H.; Oreffo, R.O.C.; Woo, Y.L.; Cheong, Y.C.; Evans, N.D.; Newman, T.A. Quantification of intracellular payload release from polymersome nanoparticles. Sci. Rep. 2016, 6, 29460. [Google Scholar] [CrossRef] [Green Version]
- Braun, A.C.; Gutmann, M.; Mueller, T.D.; Lühmann, T.; Meinel, L. Bioresponsive release of insulin-like growth factor-I from its PEGylated conjugate. J. Control. Release 2018, 279, 17–28. [Google Scholar] [CrossRef]
- Song, J.; Xu, B.; Yao, H.; Lu, X.; Tan, Y.; Wang, B.; Wang, X.; Yang, Z. Schiff-Linked PEGylated Doxorubicin Prodrug Forming pH-Responsive Nanoparticles with High Drug Loading and Effective Anticancer Therapy. Front. Oncol. 2021, 11, 656717. [Google Scholar] [CrossRef]
- Shi, H.; Xu, M.; Zhu, J.; Li, Y.; He, Z.; Zhang, Y.; Xu, Q.; Niu, Y.; Liu, Y. Programmed co-delivery of platinum nanodrugs and gemcitabine by a clustered nanocarrier for precision chemotherapy for NSCLC tumors. J. Mater. Chem. B 2020, 8, 332–342. [Google Scholar] [CrossRef]
- Thakur, S.; Tekade, R.K.; Kesharwani, P.; Jain, N.K. The effect of polyethylene glycol spacer chain length on the tumor-targeting potential of folate-modified PPI dendrimers. J. Nanopart. Res. 2013, 15, 1625. [Google Scholar] [CrossRef]
- Dutta, T.; Agashe, H.B.; Garg, M.; Balakrishnan, P.; Kabra, M.; Jain, N.K. Poly (propyleneimine) dendrimer based nanocontainers for targeting of efavirenz to human monocytes/macrophages in vitro. J. Drug Target. 2007, 15, 89–98. [Google Scholar] [CrossRef]
- Dutta, T.; Jain, N.K. Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochim. Biophys. Acta 2007, 1770, 681–686. [Google Scholar] [CrossRef]
- Lim, W.Q.; Zeng, S.; Phua, F.; Zhao, Y. Redox-Responsive Polymeric Nanocomplex for Delivery of Cytotoxic Protein and Chemotherapeutics. ACS Appl. Mater. Interfaces 2019, 11, 31638–31648. [Google Scholar] [CrossRef]
- Lee, K.S.; Chung, H.C.; Im, S.A.; Park, Y.H.; Kim, C.S.; Kim, S.-B.; Rha, S.Y.; Lee, M.Y.; Ro, J. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008, 108, 241–250. [Google Scholar] [CrossRef]
- Koopaei, M.N.; Khoshayand, M.R.; Mostafavi, S.H.; Amini, M.; Khorramizadeh, M.R.; Tehrani, M.J.; Atyabi, F.; Dinarvand, R. Docetaxel loaded PEG-PLGA nanoparticles: Optimized drug loading, in vitro cytotoxicity and in vivo antitumor effect. Iran. J. Pharm. Res. 2014, 13, 819–833. [Google Scholar]
- Taghipour-Sabzevar, V.; Sharifi, T.; Moghaddam, M.M. Polymeric nanoparticles as carrier for targeted and controlled delivery of anticancer agents. Ther. Deliv. 2019, 10, 527–550. [Google Scholar] [CrossRef]
- Kahraman, M.; Mullen, E.R.; Korkmaz, A.; Wachsmann-Hogiu, S. Fundamentals and applications of SERS-based bioanalytical sensing. Nanophotonics 2017, 6, 831–852. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Robertson, C.S.; Nguyen, A.H.; Kahraman, M.; Wachsmann-Hogiu, S. Thickness of a metallic film, in addition to its roughness, plays a significant role in SERS activity. Sci. Rep. 2015, 5, 11644. [Google Scholar] [CrossRef] [Green Version]
- Justino, C.I.L.; Freitas, A.C.; Pereira, R.; Duarte, A.C.; Rocha-Santos, T.A.P. Recent developments in recognition elements for chemical sensors and biosensors. TrAC Trends Anal. Chem. 2015, 68, 2–17. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Kim, J.; Tran, V.T.; Suzuki, T.; Neethirajan, S.; Lee, J.; Park, E.Y. In situ self-assembly of gold nanoparticles on hydrophilic and hydrophobic substrates for influenza virus-sensing platform. Sci. Rep. 2017, 7, 44495. [Google Scholar] [CrossRef] [Green Version]
- Bao, L.-L.; Mahurin, S.M.; Liang, C.-D.; Dai, S. Study of silver films over silica beads as a surface-enhanced Raman scattering (SERS) substrate for detection of benzoic acid. J. Raman Spectrosc. 2003, 34, 394–398. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.J.G.; Porteiro, J.; Míguez, J.L.; Pinto, G. Sputtering Physical Vapour Deposition (PVD) Coatings: A Critical Review on Process Improvement and Market Trend Demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef] [Green Version]
- Mattevi, C.; Kim, H.; Chhowalla, M. AReviewof Chemical Vapour Deposition of Graphene on Copper. J. Mater. Chem. 2011, 21, 3324–3334. [Google Scholar] [CrossRef]
- Keeney, M.; Jiang, X.Y.; Yamane, M.; Lee, M.; Goodman, S.; Yang, F. Nanocoating for biomolecule delivery using layer-by-layer self-assembly. J. Mater. Chem. B 2015, 3, 8757–8770. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Song, Y.; Liu, Y.; Jiang, W.; Peng, J.; Shi, L.; Jia, R.; Muhammad, Y.; Huang, L. Controlled fabrication of Au@MnO2 core/shell assembled nanosheets by localized surface plasmon resonance. Appl. Surf. Sci. 2021, 537, 147912. [Google Scholar] [CrossRef]
- Park, S.; Han, U.; Choi, D.; Hong, J. Layer-by-layer assembled polymeric thin films as prospective drug delivery carriers: Design and applications. Biomater. Res. 2018, 22, 29. [Google Scholar] [CrossRef]
- Mocan, T.; Matea, C.; Tabaran, F.; Iancu, C.; Orasan, R.; Mocan, L. In Vitro Administration of Gold Nanoparticles Functionalized with MUC-1 Protein Fragment Generates Anticancer Vaccine Response via Macrophage Activation and Polarization Mechanism. J. Cancer 2015, 6, 583–592. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Prajapati, S.; Padhan, B.; Amulyasai, B.; Sarkar, A. Nanotechnology-based sensors. In Biopolymer-Based Formulations Biomedical and Food Applications. In Biopolymer-Based Formulations Biomedical and Food Applications; Catalent: Somerset, NJ, USA, 2020; pp. 237–262. [Google Scholar]
- Vo, Q.K.; Nguyen, A.T.; Ho, H.T.; Huynh, L.T.N.; Nguyen, T.P.P.; Nguyen, T.H.-T. Environmentally Friendly Controlled Synthesis of Gold Nanostars with Collagen by One-Step Reduction Method. J. Nanomater. 2022, 2022, 4046389. [Google Scholar] [CrossRef]
- Sallum, L.F.; Soares, F.L.F.; Ardila, J.A.; Carneiro, R.L. Determination of acetylsalicylic acid in commercial tablets by SERS using silver nanoparticle-coated filter paper. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 107–111. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, W.; Xue, J.; Liu, Y.; Liu, Y.; Yan, P.; Liu, J.; Tang, J. Recent advances in synthetic methods and applications of silver nanostructures. Nanoscale Res. Lett. 2018, 13, 54. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Ferhan, A.R.; Gao, Y.; Dandapat, A.; Kim, D.-H. High-yield synthesis of triangular gold nanoplates with improved shape uniformity, tunable edge length and thickness. Nanoscale 2014, 6, 6496–6500. [Google Scholar] [CrossRef]
- Xiao, T.; Huang, J.; Wang, D.; Meng, T.; Yang, X. Au and Au-Based nanomaterials: Synthesis and recent progress in electrochemical sensor applications. Talanta 2020, 206, 120210. [Google Scholar] [CrossRef]
- Huynh, K.-H.; Pham, X.-H.; Kim, J.; Lee, S.H.; Chang, H.; Rho, W.-Y.; Jun, B.-H. Synthesis, Properties, and Biological Applications of Metallic Alloy Nanoparticles. Int. J. Mol. Sci. 2020, 21, 5174. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.C.; Reis, D.P.; Pereira, M.C.; Coelho, M.A.N. Doxorubicin and Varlitinib Delivery by Functionalized Gold Nanoparticles against Human Pancreatic Adenocarcinoma. Pharmaceutics 2019, 11, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagheri, S.; Yasemi, M.; Safaie-Qamsari, E.; Rashidiani, J.; Abkar, M.; Hassani, M.; Mirhosseini, S.A.; Kooshki, H. Using gold nanoparticles in diagnosis and treatment of melanoma cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 462–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dykman, L.A. Gold nanoparticles for preparation of antibodies and vaccines against infectious diseases. Expert Rev. Vaccines 2020, 19, 465–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehit, E.; Drzazgowska, J.; Buchenau, D.; Yesildag, C.; Lensen, M.; Altintas, Z. Ultrasensitive nonenzymatic electrochemical glucose sensor based on gold nanoparticles and molecularly imprinted polymers. Biosens. Bioelectron. 2020, 165, 112432. [Google Scholar] [CrossRef] [PubMed]
- Nemčeková, K.; Svitková, V.; Sochr, J.; Gemeiner, P.; Labuda, J. Gallic acid-coated silver nanoparticles as perspective drug nanocarriers: Bioanalytical study. Anal. Bioanal. Chem. 2022, 414, 5493–5505. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Rahimpour, E. Silver nanoparticles plasmon resonance-based method for the determination of uric acid in human plasma and urine samples. Microchim. Acta 2012, 178, 373–379. [Google Scholar] [CrossRef]
- Dou, Y.; Yang, X.; Liu, Z.; Zhu, S. Homocysteine-functionalized silver nanoparticles for selective sensing of Cu2+ ions and Lidocaine hydrochloride. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 423, 20–26. [Google Scholar] [CrossRef]
- Rajamanikandan, R.; Ilanchelian, M. Simple and visual approach for highly selective biosensing of vitamin B1 based on glutathione coated silver nanoparticles as a colorimetric probe. Sensors Actuators B Chem. 2017, 244, 380–386. [Google Scholar] [CrossRef]
- Gao, M.; Li, L.; Lu, S.; Liu, Q.; He, H. Silver nanoparticles for the visual detection of lomefloxacin in the presence of cystine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 72–78. [Google Scholar] [CrossRef]
- Qu, J.C.; Chang, Y.P.; Ma, Y.H.; Zheng, J.M.; Li, H.H.; Ou, Q.Q.; Ren, C.; Chen, X.G. A simple and sensitive colorimetric method for the determination of propafenone by silver nanoprobe. Sensors Actuators B Chem. 2012, 174, 133–139. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Genix, A.-C.; Oberdisse, J. Nanoparticle self-assembly: From interactions in suspension to polymer nanocomposites. Soft Matter 2018, 14, 5161–5179. [Google Scholar] [CrossRef]
- Cheon, Y.A.; Bae, J.H.; Chung, B.G. Reduced Graphene Oxide Nanosheet for Chemo-photothermal Therapy. Langmuir 2016, 32, 2731–2736. [Google Scholar] [CrossRef]
- Peik-See, T.; Pandikumar, A.; Nay-Ming, H.; Hong-Ngee, L.; Sulaiman, Y. Simultaneous Electrochemical Detection of Dopamine and Ascorbic Acid Using an Iron Oxide/Reduced Graphene Oxide Modified Glassy Carbon Electrode. Sensors 2014, 14, 15227–15243. [Google Scholar] [CrossRef]
- Hoa, L.T.; Sun, K.G.; Hur, S.H. Highly sensitive non-enzymatic glucose sensor based on Pt nanoparticle decorated graphene oxide hydrogel. Sensors Actuators B Chem. 2015, 210, 618–623. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Wang, L.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 2013, 3, 1604. [Google Scholar] [CrossRef] [Green Version]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors-Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Vu, C.-A.; Chen, W.-Y. Field-Effect Transistor Biosensors for Biomedical Applications: Recent Advances and Future Prospects. Sensors 2019, 19, 4214. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Dong, X.; Shi, Y.; Li, C.M.; Li, L.J.; Chen, P. Nanoelectronic biosensors based on CVD grown graphene †. Nanoscale 2010, 2, 1485–1488. [Google Scholar] [CrossRef]
- Bhat, K.S.; Ahmad, R.; Yoo, J.-Y.; Hahn, Y.-B. Nozzle-Jet Printed Flexible Field-Effect Transistor Biosensor for High Performance Glucose Detection. J. Colloid Interface Sci. 2017, 506, 188–196. [Google Scholar] [CrossRef]
- Hao, Z.; Zhu, Y.; Wang, X.; Rotti, P.G.; DiMarco, C.; Tyler, S.R.; Zhao, X.; Engelhardt, J.F.; Hone, J.; Lin, Q. Real-Time Monitoring of Insulin Using a Graphene Field-Effect Transistor Aptameric Nanosensor. ACS Appl. Mater. Interfaces 2017, 9, 27504–27511. [Google Scholar] [CrossRef]
- Martinkova, P.; Kostelnik, A.; Valek, T.; Pohanka, M. Main streams in the construction of biosensors and their applications. Int. J. Electrochem. Sci. 2017, 12, 7386–7403. [Google Scholar] [CrossRef]
- Alhadrami, H.A. Biosensors: Classifications, medical applications, and future prospective. Biotechnol. Appl. Biochem. 2018, 65, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pomales, G.; Zangmeister, R.A. Recent Advances in Electrochemical Glycobiosensing. Int. J. Electrochem. 2011, 2011, 825790. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhu, X.; Huo, Z.; He, X.; Liang, Y.; Xu, M. Electrochemical detection of dopamine in the presence of ascorbic acid using PVP/graphene modified electrodes. Talanta 2012, 97, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Anota, E.C.; Soto, A.T.; Cocoletzi, G.H. Studies of graphene–chitosan interactions and analysis of the bioadsorption of glucose and cholesterol. Appl. Nanosci. 2013, 4, 911–918. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, F.; Raoof, J.B.; Ojani, R.; Baghayeri, M.; Lakouraj, M.M.; Tashakkorian, H. Synthesis of Ag nanoparticles for the electrochemical detection of anticancer drug flutamide. Chin. J. Catal. 2015, 36, 439–445. [Google Scholar] [CrossRef]
- Radhapyari, K.; Khan, R. Biosensor for Detection of Selective Anticancer Drug Gemcitabine Based On Polyaniline-gold Nanocomposite. Adv. Mater. Lett. 2015, 6, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Monzó, J.; Insua, I.; Fernandez-Trillo, F.; Rodriguez, P. Fundamentals, achievements and challenges in the electrochemical sensing of pathogens. Analyst 2015, 140, 7116–7128. [Google Scholar] [CrossRef]
- Miyazaki, C.M.; Shimizu, F.M.; Ferreira, M. Surface Plasmon Resonance (SPR) for Sensors and Biosensors. In Nanocharacterization Techniques: A volume in Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 183–200. [Google Scholar]
- Canovi, M.; Lucchetti, J.; Stravalaci, M.; Re, F.; Moscatelli, D.; Bigini, P.; Salmona, M.; Gobbi, M. Applications of Surface Plasmon Resonance (SPR) for the Characterization of Nanoparticles Developed for Biomedical Purposes. Sensors 2012, 12, 16420–16432. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface Plasmon Resonance: A Versatile Technique for Biosensor Applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef] [Green Version]
- Firdous, S.; Anwar, S.; Rafya, R. Development of surface plasmon resonance (SPR) biosensors for use in the diagnostics of malignant and infectious diseases. Laser Phys. Lett. 2018, 15, 065602. [Google Scholar] [CrossRef]
- Chalyan, T.; Potrich, C.; Schreuder, E.; Falke, F.; Pasquardini, L.; Pederzolli, C.; Heideman, R.; Pavesi, L. AFM1 Detection in Milk by Fab’ Functionalized Si3N4 Asymmetric Mach–Zehnder Interferometric Biosensors. Toxins 2019, 11, 409. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, Q.; Chen, S.; Cheng, F.; Wang, H.; Peng, W. Surface Plasmon Resonance Biosensor Based on Smart Phone Platforms. Sci. Rep. 2015, 5, 12864. [Google Scholar] [CrossRef] [Green Version]
- Blasi, D.; Sarcina, L.; Tricase, A.; Stefanachi, A.; Leonetti, F.; Alberga, D.; Mangiatordi, G.F.; Manoli, K.; Scamarcio, G.; Picca, R.A.; et al. Enhancing the Sensitivity of Biotinylated Surfaces by Tailoring the Design of the Mixed Self-Assembled Monolayer Synthesis. ACS Omega 2020, 5, 16762–16771. [Google Scholar] [CrossRef]
- Jabbari, S.; Dabirmanesh, B.; Arab, S.S.; Amanlou, M.; Daneshjou, S.; Gholami, S.; Khajeh, K. A novel enzyme based SPR-biosensor to detect bromocriptine as an ergoline derivative drug. Sensors Actuators B Chem. 2017, 240, 519–527. [Google Scholar] [CrossRef]
- Villena Gonzales, W.; Mobashsher, A.T.; Abbosh, A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef] [Green Version]
- Izo, J.; Jesus SDe Löbenberg, R.; Bou-chacra, N.A. Raman Spectroscopy for Quantitative Analysis in the Pharmaceutical In-dustry. J. Pharm. Pharm. Sci. 2020, 23, 24–46. [Google Scholar]
- Abraham, S.; König, M.; Srivastava, S.K.; Kumar, V.; Walkenfort, B.; Srivastava, A. Carbon nanostructure (0–3 dimensional) supported isolated gold nanoparticles as an effective SERS substrate. Sensors Actuators B Chem. 2018, 273, 455–465. [Google Scholar] [CrossRef]
- Hidi, I.J.; Jahn, M.; Pletz, M.W.; Weber, K.; Cialla-May, D.; Popp, J. Toward Levofloxacin Monitoring in Human Urine Samples by Employing the LoC-SERS Technique. J. Phys. Chem. C 2016, 120, 20613–20623. [Google Scholar] [CrossRef]
- Garrido, E.; Pla, L.; Lozano-Torres, B.; Sameh, E.S.; Martínez-Máñez, R.; Sancenón, F. Chromogenic and Fluorogenic Probes for the Detection of Illicit Drugs. ChemistryOpen 2018, 7, 401–428. [Google Scholar] [CrossRef] [PubMed]
- Long, S.-Y.; Chen, Z.-P.; Chen, Y.; Yu, R.-Q. Quantitative detection of captopril in tablet and blood plasma samples by the combination of surface-enhanced Raman spectroscopy with multiplicative effects model. J. Raman Spectrosc. 2015, 46, 605–609. [Google Scholar] [CrossRef]
- Ouyang, L.; Ren, W.; Zhu, L.; Irudayaraj, J. Prosperity to challenges: Recent approaches in SERS substrate fabrication. Rev. Anal. Chem. 2017, 36, 20160027. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thobakgale, L.; Ombinda-Lemboumba, S.; Mthunzi-Kufa, P. Chemical Sensor Nanotechnology in Pharmaceutical Drug Research. Nanomaterials 2022, 12, 2688. https://doi.org/10.3390/nano12152688
Thobakgale L, Ombinda-Lemboumba S, Mthunzi-Kufa P. Chemical Sensor Nanotechnology in Pharmaceutical Drug Research. Nanomaterials. 2022; 12(15):2688. https://doi.org/10.3390/nano12152688
Chicago/Turabian StyleThobakgale, Lebogang, Saturnin Ombinda-Lemboumba, and Patience Mthunzi-Kufa. 2022. "Chemical Sensor Nanotechnology in Pharmaceutical Drug Research" Nanomaterials 12, no. 15: 2688. https://doi.org/10.3390/nano12152688
APA StyleThobakgale, L., Ombinda-Lemboumba, S., & Mthunzi-Kufa, P. (2022). Chemical Sensor Nanotechnology in Pharmaceutical Drug Research. Nanomaterials, 12(15), 2688. https://doi.org/10.3390/nano12152688





