Synergistic Interaction of Clusters of Iron Oxide Nanoparticles and Reduced Graphene Oxide for High Supercapacitor Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials Synthesis
2.3. Materials Characterization
2.4. Electrochemical Tests
3. Results and Discussion
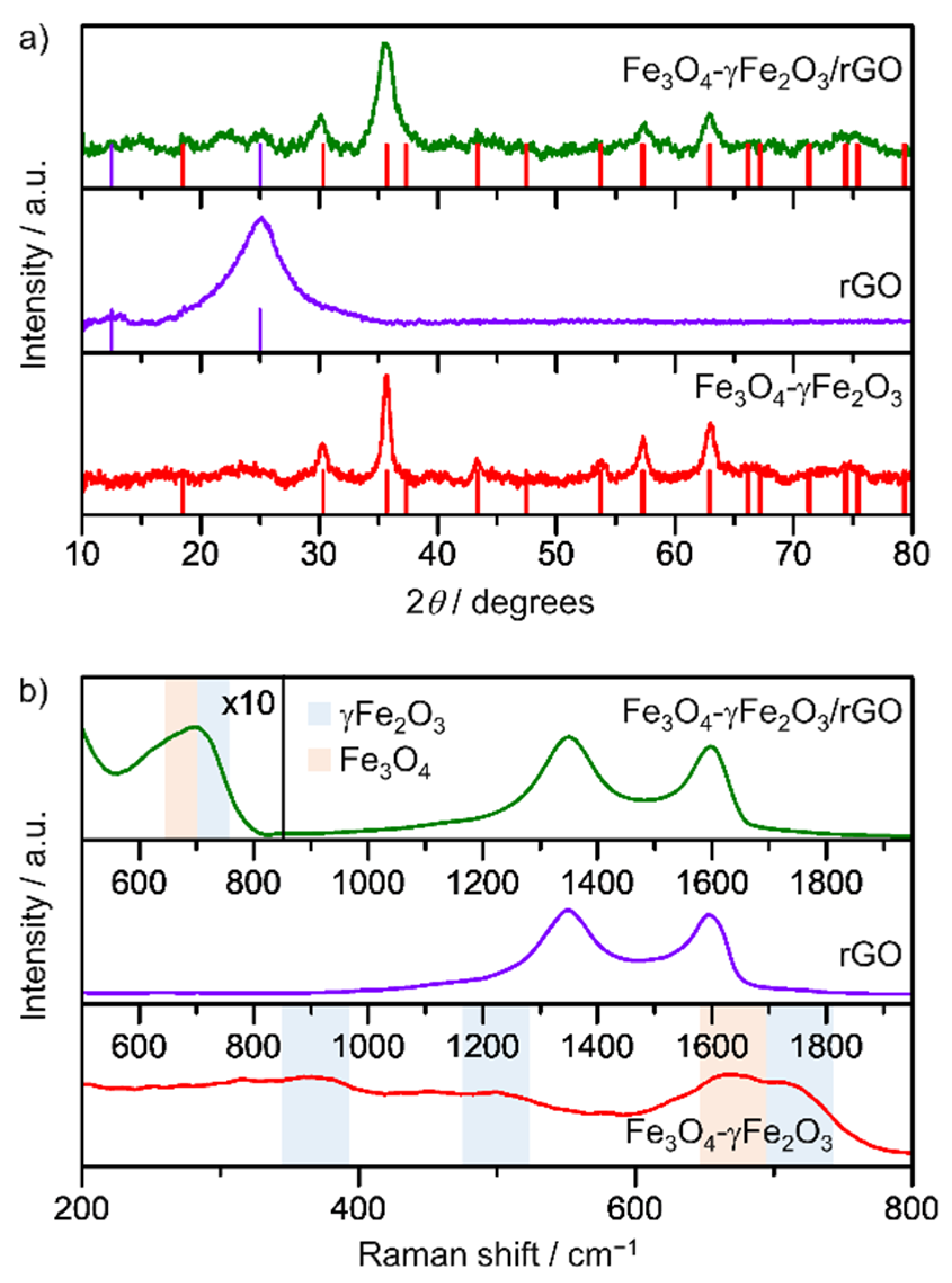
3.1. Sample Characterization
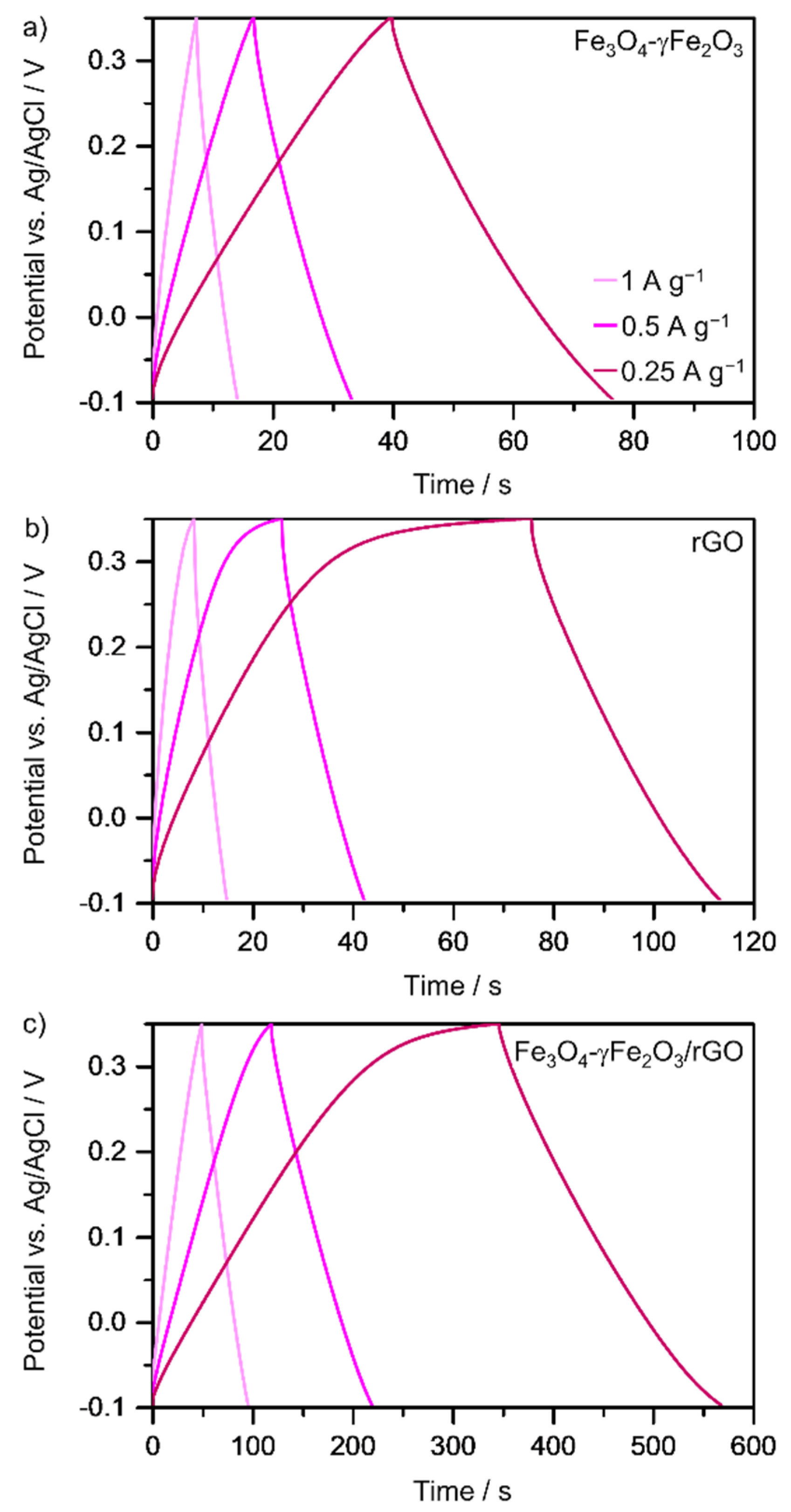
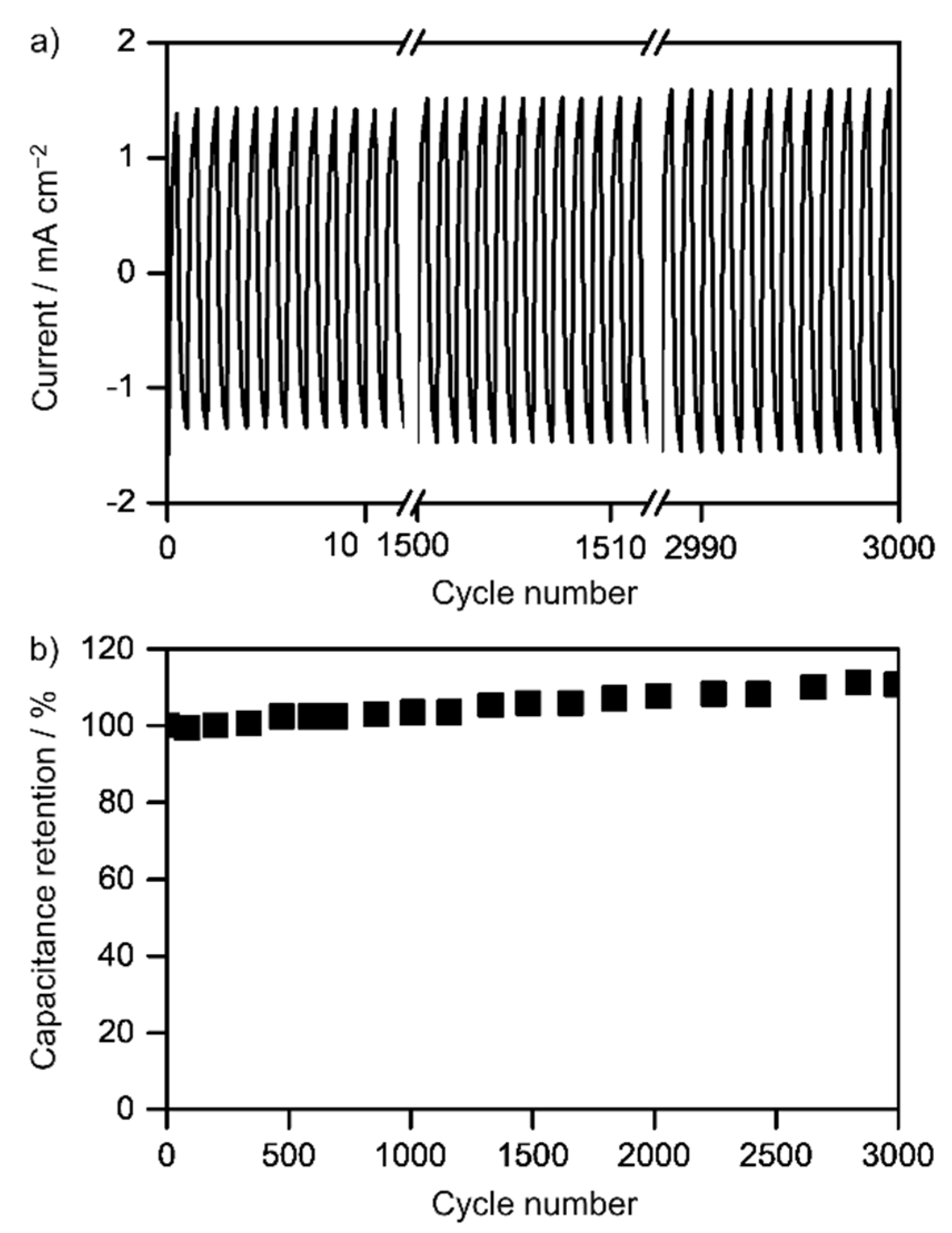
3.2. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khiew, P.; Ho, M.; Chiu, W.; Shamsudin, R.; Abd-Hamid, M.A.; Chia, C. Synthesis and electrochemical characterization of iron oxide/activated carbon composite electrode for symmetrical supercapacitor. Int. J. Mater. Metall. Eng. 2013, 7, 615–619. [Google Scholar]
- Rantho, M.N.; Madito, M.; Oyedotun, K.O.; Tarimo, D.J.; Manyala, N. Hybrid electrochemical supercapacitor based on birnessite-type MnO2/carbon composite as the positive electrode and carbonized iron-polyaniline/nickel graphene foam as a negative electrode. AIP Adv. 2020, 10, 065113. [Google Scholar] [CrossRef]
- Liu, L.; Niu, Z.; Chen, J. Unconventional supercapacitors from nanocarbon-based electrode materials to device configurations. Chem. Soc. Rev. 2016, 45, 4340–4363. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Pei, J.; Yan, C.; Chen, D.; Hu, Y.; Chen, G. Layered nickel metal–organic framework for high performance alkaline battery-supercapacitor hybrid devices. J. Mater. Chem. A 2016, 4, 13344–13351. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Cheng, H.-M. Hierarchical porous nickel oxide and carbon as electrode materials for asymmetric supercapacitor. J. Power Sources 2008, 185, 1563–1568. [Google Scholar] [CrossRef]
- Faraji, S.; Ani, F.N. Microwave-assisted synthesis of metal oxide/hydroxide composite electrodes for high power supercapacitors–a review. J. Power Sources 2014, 263, 338–360. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xiong, Y.; Liu, X.; Bo, X.; Zhang, Y.; Han, C.; Guo, L. Facile synthesis of electrospun MFe2O4 (M = Co, Ni, Cu, Mn) spinel nanofibers with excellent electrocatalytic properties for oxygen evolution and hydrogen peroxide reduction. Nanoscale 2015, 7, 8920–8930. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, M.; Meng, Y.; Fang, P.; Lu, X.; Tong, Y. Iron-based supercapacitor electrodes: Advances and challenges. Adv. Energy Mater. 2016, 6, 1601053. [Google Scholar] [CrossRef]
- Mu, J.; Chen, B.; Guo, Z.; Zhang, M.; Zhang, Z.; Zhang, P.; Shao, C.; Liu, Y. Highly dispersed Fe3O4 nanosheets on one-dimensional carbon nanofibers: Synthesis, formation mechanism, and electrochemical performance as supercapacitor electrode materials. Nanoscale 2011, 3, 5034–5040. [Google Scholar] [CrossRef]
- Nawwar, M.; Poon, R.; Chen, R.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. High areal capacitance of Fe3O4-decorated carbon nanotubes for supercapacitor electrodes. Carbon Energy 2019, 1, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Guan, C.; Liu, J.; Wang, Y.; Mao, L.; Fan, Z.; Shen, Z.; Zhang, H.; Wang, J. Iron oxide-decorated carbon for supercapacitor anodes with ultrahigh energy density and outstanding cycling stability. ACS Nano 2015, 9, 5198–5207. [Google Scholar] [CrossRef]
- Lu, X.; Zeng, Y.; Yu, M.; Zhai, T.; Liang, C.; Xie, S.; Balogun, M.S.; Tong, Y. Oxygen-deficient hematite nanorods as high-performance and novel negative electrodes for flexible asymmetric supercapacitors. Adv. Mater. 2014, 26, 3148–3155. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, J.; Xing, W.; Wang, G.; Cui, H.; Zhuo, S.; Xue, Q.; Yan, Z.; Qiao, S.Z. High-rate capacitive performance of graphene aerogel with a superhigh C/O molar ratio. J. Mater. Chem. 2012, 22, 23186–23193. [Google Scholar] [CrossRef]
- Xia, H.; Hong, C.; Li, B.; Zhao, B.; Lin, Z.; Zheng, M.; Savilov, S.V.; Aldoshin, S.M. Facile synthesis of hematite quantum-dot/functionalized graphene-sheet composites as advanced anode materials for asymmetric supercapacitors. Adv. Funct. Mater. 2015, 25, 627–635. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Zhou, C.; Wang, C.; Ba, X.; Li, Y.; Huang, X.; Liu, J. Carbon-stabilized high-capacity ferroferric oxide nanorod array for flexible solid-state alkaline battery–supercapacitor hybrid device with high environmental suitability. Adv. Funct. Mater. 2015, 25, 5384–5394. [Google Scholar] [CrossRef]
- Tang, X.; Jia, R.; Zhai, T.; Xia, H. Hierarchical Fe3O4@Fe2O3 core–shell nanorod arrays as high-performance anodes for asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 27518–27525. [Google Scholar] [CrossRef]
- Yang, Q.; Lu, Z.; Li, T.; Sun, X.; Liu, J. Hierarchical construction of core–shell metal oxide nanoarrays with ultrahigh areal capacitance. Nano Energy 2014, 7, 170–178. [Google Scholar] [CrossRef]
- Chen, D.; Li, S.; Xu, B.; Zheng, F.; Zhou, H.; Yu, H.; Lin, F.; Zhu, X. Polycrystalline iron oxide nanoparticles prepared by C-dot-mediated aggregation and reduction for supercapacitor application. RSC Adv. 2016, 6, 45023–45030. [Google Scholar] [CrossRef]
- Mitchell, E.; Gupta, R.K.; Mensah-Darkwa, K.; Kumar, D.; Ramasamy, K.; Gupta, B.K.; Kahol, P. Facile synthesis and morphogenesis of superparamagnetic iron oxide nanoparticles for high-performance supercapacitor applications. New J. Chem. 2014, 38, 4344–4350. [Google Scholar] [CrossRef]
- Chen, J.; Huang, K.; Liu, S. Hydrothermal preparation of octadecahedron Fe3O4 thin film for use in an electrochemical supercapacitor. Electrochim. Acta 2009, 55, 1–5. [Google Scholar] [CrossRef]
- Du, X.; Wang, C.; Chen, M.; Jiao, Y.; Wang, J. Electrochemical performances of nanoparticle Fe3O4/activated carbon supercapacitor using KOH electrolyte solution. J. Phys. Chem. C 2009, 113, 2643–2646. [Google Scholar] [CrossRef]
- Sinan, N.; Unur, E. Fe3O4/carbon nanocomposite: Investigation of capacitive & magnetic properties for supercapacitor applications. Mater. Chem. Phys. 2016, 183, 571–579. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramaprabhu, S. Functionalized graphene-based nanocomposites for supercapacitor application. J. Phys. Chem. C 2011, 115, 14006–14013. [Google Scholar] [CrossRef]
- Sheng, S.; Liu, W.; Zhu, K.; Cheng, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. Fe3O4 nanospheres in situ decorated graphene as high-performance anode for asymmetric supercapacitor with impressive energy density. J. Colloid Interface Sci. 2019, 536, 235–244. [Google Scholar] [CrossRef]
- Sayahi, H.; Mohsenzadeh, F.; Darabi, H.R.; Aghapoor, K. Facile and economical fabrication of magnetite/graphite nanocomposites for supercapacitor electrodes with significantly extended potential window. J. Alloys Compd. 2019, 778, 633–642. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramaprabhu, S. Magnetite decorated multiwalled carbon nanotube based supercapacitor for arsenic removal and desalination of seawater. J. Phys. Chem. C 2010, 114, 2583–2590. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, L.; Du, H.; Wang, Y.; Yuan, H. Fe3O4 nanoparticles grown on graphene as advanced electrode materials for supercapacitors. J. Power Sources 2014, 245, 101–106. [Google Scholar] [CrossRef]
- Li, L.; Dou, Y.; Wang, L.; Luo, M.; Liang, J. One-step synthesis of high-quality N-doped graphene/Fe3O4 hybrid nanocomposite and its improved supercapacitor performances. RSC Adv. 2014, 4, 25658–25665. [Google Scholar] [CrossRef]
- Liu, M.; Sun, J. In situ growth of monodisperse Fe3O4 nanoparticles on graphene as flexible paper for supercapacitor. J. Mater. Chem. A 2014, 2, 12068–12074. [Google Scholar] [CrossRef]
- Ke, Q.; Tang, C.; Liu, Y.; Liu, H.; Wang, J. Intercalating graphene with clusters of Fe3O4 nanocrystals for electrochemical supercapacitors. Mater. Res. Express 2014, 1, 025015. [Google Scholar] [CrossRef]
- Elsaidy, A.; Vallejo, J.P.; Salgueiriño, V.; Lugo, L. Tuning the thermal properties of aqueous nanofluids by taking advantage of size-customized clusters of iron oxide nanoparticles. J. Mol. Liq. 2021, 344, 117727. [Google Scholar] [CrossRef]
- Sun, H.; Cao, L.; Lu, L. Magnetite/reduced graphene oxide nanocomposites: One step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Res. 2011, 4, 550–562. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Y.; Ren, W. Structure switch between α-Fe2O3, γ-Fe2O3 and Fe3O4 during the large scale and low temperature sol–gel synthesis of nearly monodispersed iron oxide nanoparticles. Adv. Powder Technol. 2013, 24, 93–97. [Google Scholar] [CrossRef]
- Haneef, M.; Saleem, H.; Habib, A. Use of graphene nanosheets and barium titanate as fillers in PMMA for dielectric applications. Synth. Met. 2017, 223, 101–106. [Google Scholar] [CrossRef]
- Aparna, M.; Grace, A.N.; Sathyanarayanan, P.; Sahu, N.K. A comparative study on the supercapacitive behaviour of solvothermally prepared metal ferrite (MFe2O4, M = Fe, Co, Ni, Mn, Cu, Zn) nanoassemblies. J. Alloys Compd. 2018, 745, 385–395. [Google Scholar] [CrossRef]
- Otero-Lorenzo, R.; Ramos-Docampo, M.A.; Rodriguez-Gonzalez, B.; Comesaña-Hermo, M.; Salgueiriño, V. Solvothermal clustering of magnetic spinel ferrite nanocrystals: A Raman perspective. Chem. Mater. 2017, 29, 8729–8736. [Google Scholar] [CrossRef]
- Scott, J. Soft-mode spectroscopy: Experimental studies of structural phase transitions. Rev. Mod. Phys. 1974, 46, 83. [Google Scholar] [CrossRef]
- Rivas-Murias, B.; Testa-Anta, M.; Torruella, P.; Estradé, S.; Peiró, F.; Rodríguez-González, B.; Comesaña-Hermo, M.; Salgueiriño, V. Structural and magnetic implications of transition metal migration within octahedral core-shell nanocrystals. Chem. Mater. 2020, 32, 10435–10446. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venâncio Silva, S.; De Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Jubb, A.M.; Allen, H.C. Vibrational spectroscopic characterization of hematite, maghemite, and magnetite thin films produced by vapor deposition. ACS Appl. Mater. Interfaces 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueiriño, V. Raman spectroscopy to unravel the magnetic properties of iron oxide nanocrystals for bio-related applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Shou, Q.; Wu, J.; Liu, F.; Dravid, V.P.; Zhang, X. Influence of component content on the capacitance of magnetite/reduced graphene oxide composite. J. Electroanal. Chem. 2013, 698, 1–8. [Google Scholar] [CrossRef]
- Qi, T.; Jiang, J.; Chen, H.; Wan, H.; Miao, L.; Zhang, L. Synergistic effect of Fe3O4/reduced graphene oxide nanocomposites for supercapacitors with good cycling life. Electrochim. Acta 2013, 114, 674–680. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, X.; Zhang, Y.; Qian, X. Fe2O3/reduced graphene oxide/Fe3O4 composite in situ grown on Fe foil for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 30133–30142. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.E. Practical Aspects of Preparation and Evaluation of Electrochemical Capacitors. In Electrochemical Supercapacitors; Springer: Boston, MA, USA, 1999; pp. 597–607. [Google Scholar] [CrossRef]
- Lasia, A. Definition of Impedance and Impedance of Electrical Circuits. In Electrochemical Impedance Spectroscopy and Its Applications; Springer: New York, NY, USA, 2014; pp. 7–66. [Google Scholar] [CrossRef]
- Barcia, O.E.; D'Elia, E.; Frateur, I.; Mattos, O.R.; Pébère, N.; Tribollet, B. Application of the impedance model of de Levie for the characterization of porous electrodes. Electrochim. Acta 2002, 47, 2109–2116. [Google Scholar] [CrossRef]
- Park, J.; Macdonald, D. Impedance studies of the growth of porous magnetite films on carbon steel in high temperature aqueous systems. Corros. Sci. 1983, 23, 295–315. [Google Scholar] [CrossRef]
- Abreu, C.; Izquierdo, M.; Merino, P.; Nóvoa, X.; Perez, C. A new approach to the determination of the cathodic protection period in zinc-rich paints. Corrosion 1999, 55, 1173–1181. [Google Scholar] [CrossRef]
- Guitián, B.; Nóvoa, X.; Pintos, A. Development of conversion coatings on iron via corrosion in LiPF6 solution. Electrochim. Acta 2019, 304, 428–436. [Google Scholar] [CrossRef]
- Díaz, B.; Guitián, B.; Nóvoa, X.; Pérez, M. The effect of long-term atmospheric aging and temperature on the electrochemical behaviour of steel rebars in mortar. Corros. Sci. 2018, 140, 143–150. [Google Scholar] [CrossRef]
- Godino, N.; Borrise, X.; Munoz, F.X.; Del Campo, F.J.; Compton, R.G. Mass transport to nanoelectrode arrays and limitations of the diffusion domain approach: Theory and experiment. J. Phys. Chem. C 2009, 113, 11119–11125. [Google Scholar] [CrossRef] [Green Version]
- Joiret, S.; Keddam, M.; Nóvoa, X.; Pérez, M.; Rangel, C.; Takenouti, H. Use of EIS, ring-disk electrode, EQCM and Raman spectroscopy to study the film of oxides formed on iron in 1 M NaOH. Cem. Concr. Compos. 2002, 24, 7–15. [Google Scholar] [CrossRef]
- Sankar, K.V.; Selvan, R.K.; Meyrick, D. Electrochemical performances of CoFe2O4 nanoparticles and a rGO based asymmetric supercapacitor. RSC Adv. 2015, 5, 99959–99967. [Google Scholar] [CrossRef]
- Kumaraguru, S.; Yesuraj, J.; Mohan, S. Reduced graphene oxide-wrapped micro-rod like Ni/Co organic-inorganic hybrid nanocomposite as an electrode material for high-performance supercapacitor. Compos. B Eng. 2020, 185, 107767. [Google Scholar] [CrossRef]
- Lee, J.W.; Hall, A.S.; Kim, J.-D.; Mallouk, T.E. A facile and template-free hydrothermal synthesis of Mn3O4 nanorods on graphene sheets for supercapacitor electrodes with long cycle stability. Chem. Mater. 2012, 24, 1158–1164. [Google Scholar] [CrossRef]
- Sethuraman, B.; Purushothaman, K.K.; Muralidharan, G. Synthesis of mesh-like Fe2O3/C nanocomposite via greener route for high performance supercapacitors. RSC Adv. 2014, 4, 4631–4637. [Google Scholar] [CrossRef]
- Nasibi, M.; Golozar, M.A.; Rashed, G. Nano iron oxide (Fe2O3)/carbon black electrodes for electrochemical capacitors. Mater. Lett. 2012, 85, 40–43. [Google Scholar] [CrossRef]
- Chen, H.-C.; Wang, C.-C.; Lu, S.-Y. γ-Fe2O3/graphene nanocomposites as a stable high performance anode material for neutral aqueous supercapacitors. J. Mater. Chem. A 2014, 2, 16955–16962. [Google Scholar] [CrossRef]


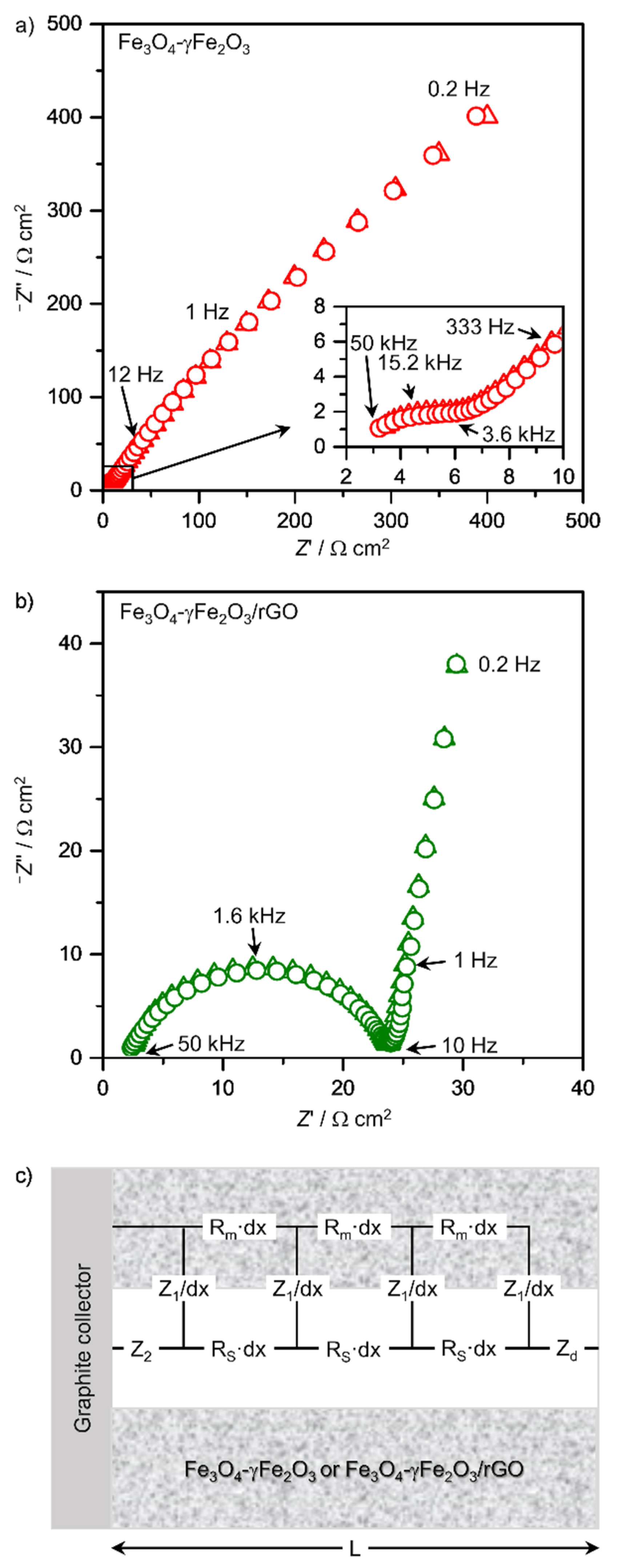
| R0/Ω cm2 | Rm/kΩ cm | Rs/kΩ cm | R1/Ω cm3 | C1/mF cm−3 | α2 | R2/kΩ cm2 | C2/mF cm2 | α2 | Rd/Ω cm2 | τd/s | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe3O4-Fe2O3 | 0.2 | 11.2 | 16 | 1.1 | 7.1 | - | 20.8 | 9.3 | 0.639 | 38.9 | 0.23 |
| Fe3O4-Fe2O3/rGO-C | 1.8 | 0.023 | 24 | 1.6 | 63.1 | - | 20.6 | 15.0 | 0.657 | 247.4 | 17.7 |
| Fe3O4-Fe2O3/rGO | 0.1 | 5.5 | 6.2 | 7 × 10−3 | 56 × 103 | 0.905 | 0.2 | 2 × 10−3 | 0.846 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsaidy, A.; Majcherkiewicz, J.N.; Puértolas, B.; Salgueiriño, V.; Nóvoa, X.R.; Correa-Duarte, M.A. Synergistic Interaction of Clusters of Iron Oxide Nanoparticles and Reduced Graphene Oxide for High Supercapacitor Performance. Nanomaterials 2022, 12, 2695. https://doi.org/10.3390/nano12152695
Elsaidy A, Majcherkiewicz JN, Puértolas B, Salgueiriño V, Nóvoa XR, Correa-Duarte MA. Synergistic Interaction of Clusters of Iron Oxide Nanoparticles and Reduced Graphene Oxide for High Supercapacitor Performance. Nanomaterials. 2022; 12(15):2695. https://doi.org/10.3390/nano12152695
Chicago/Turabian StyleElsaidy, Amir, Julia N. Majcherkiewicz, Begoña Puértolas, Verónica Salgueiriño, Xosé Ramón Nóvoa, and Miguel A. Correa-Duarte. 2022. "Synergistic Interaction of Clusters of Iron Oxide Nanoparticles and Reduced Graphene Oxide for High Supercapacitor Performance" Nanomaterials 12, no. 15: 2695. https://doi.org/10.3390/nano12152695








