Fabrication of Nylon-6 and Nylon-11 Nanoplastics and Evaluation in Mammalian Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication and Purification of NPs
2.1.1. Fabrication of Nylon-11 NPs
2.1.2. Fabrication of Nylon-6 NPs
2.2. Characterization of Particles
2.2.1. Quantification of Fluorescence of NPs
2.2.2. Fluorescence Leaching
2.2.3. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.2.4. Dynamic Light Scattering (DLS) and Zeta Potential
2.2.5. Formic Acid Protocol
2.2.6. Field Emission Scanning Electron Microscopy (FE-SEM)
2.3. Studies with Mammalian Cells
2.3.1. In Vitro Sedimentation, Diffusion, and Dosimetry (ISDD) Modeling
2.3.2. Cell Culture
2.3.3. Cytotoxicity Assays
2.3.4. Fluorescence Microscopy
2.3.5. Data Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Nylon Particle Formulations
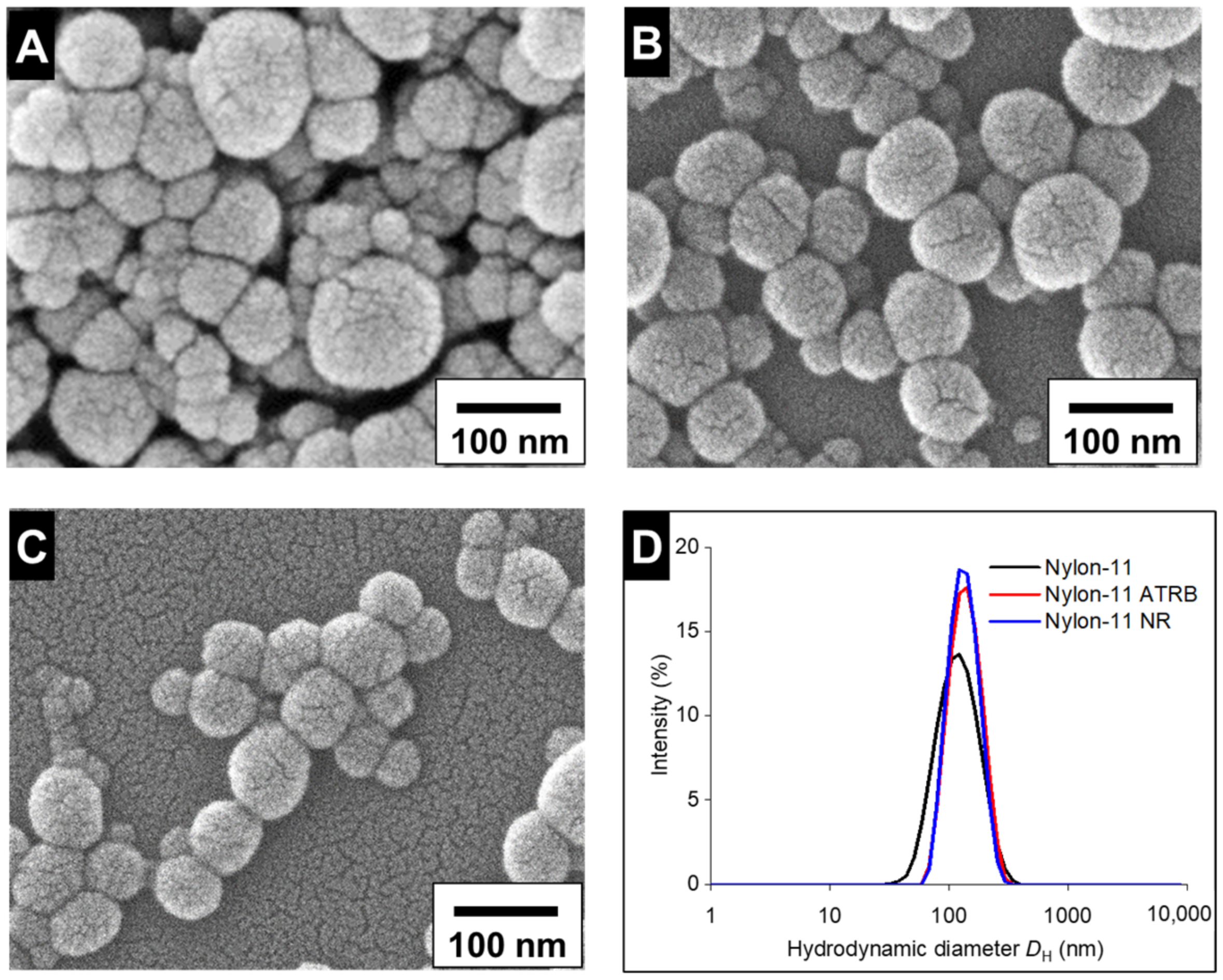
3.1.1. Fabrication and Characterization of Nylon-11 NPs
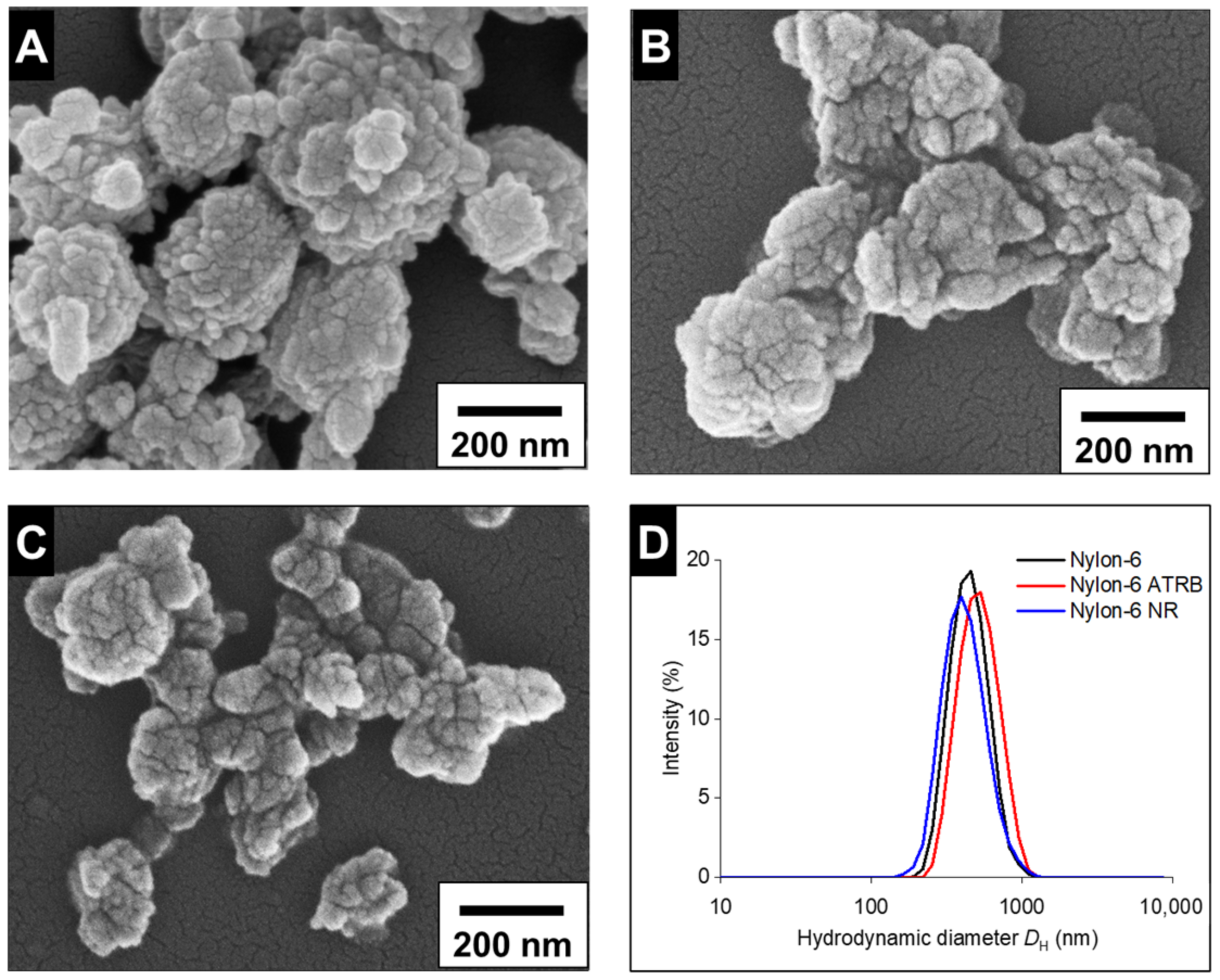
3.1.2. Fabrication and Characterization of Nylon-6 NPs
3.1.3. Fluorescence Leaching
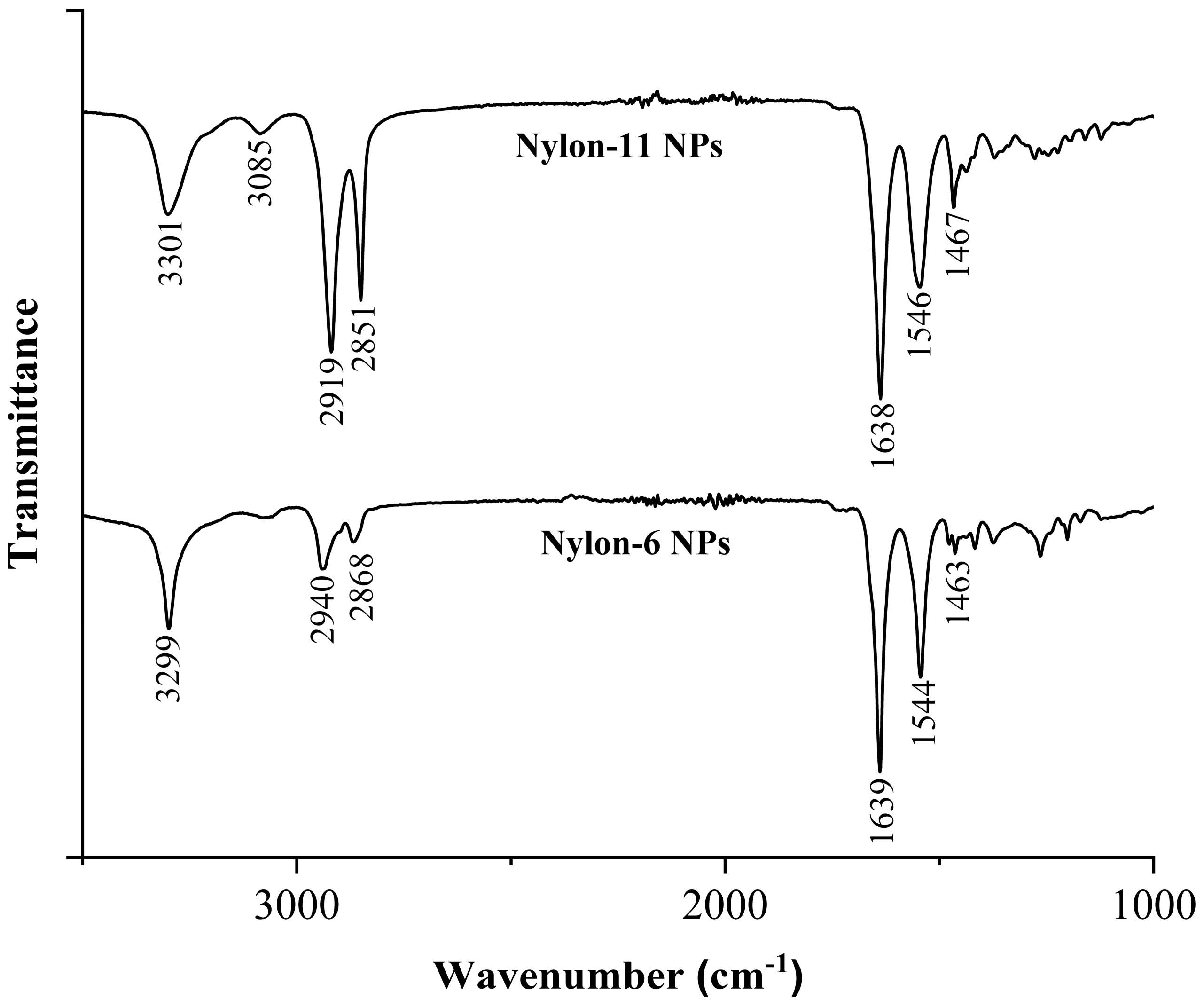
3.1.4. FT-IR Characterization of Nylon-11 and Nylon-6 NPs
3.2. Exposure of Mammalian Cells to Nylon NPs
3.2.1. Particle Characterization and Stability
3.2.2. In Vitro Sedimentation, Diffusion, and Dosimetry (ISDD) Modeling
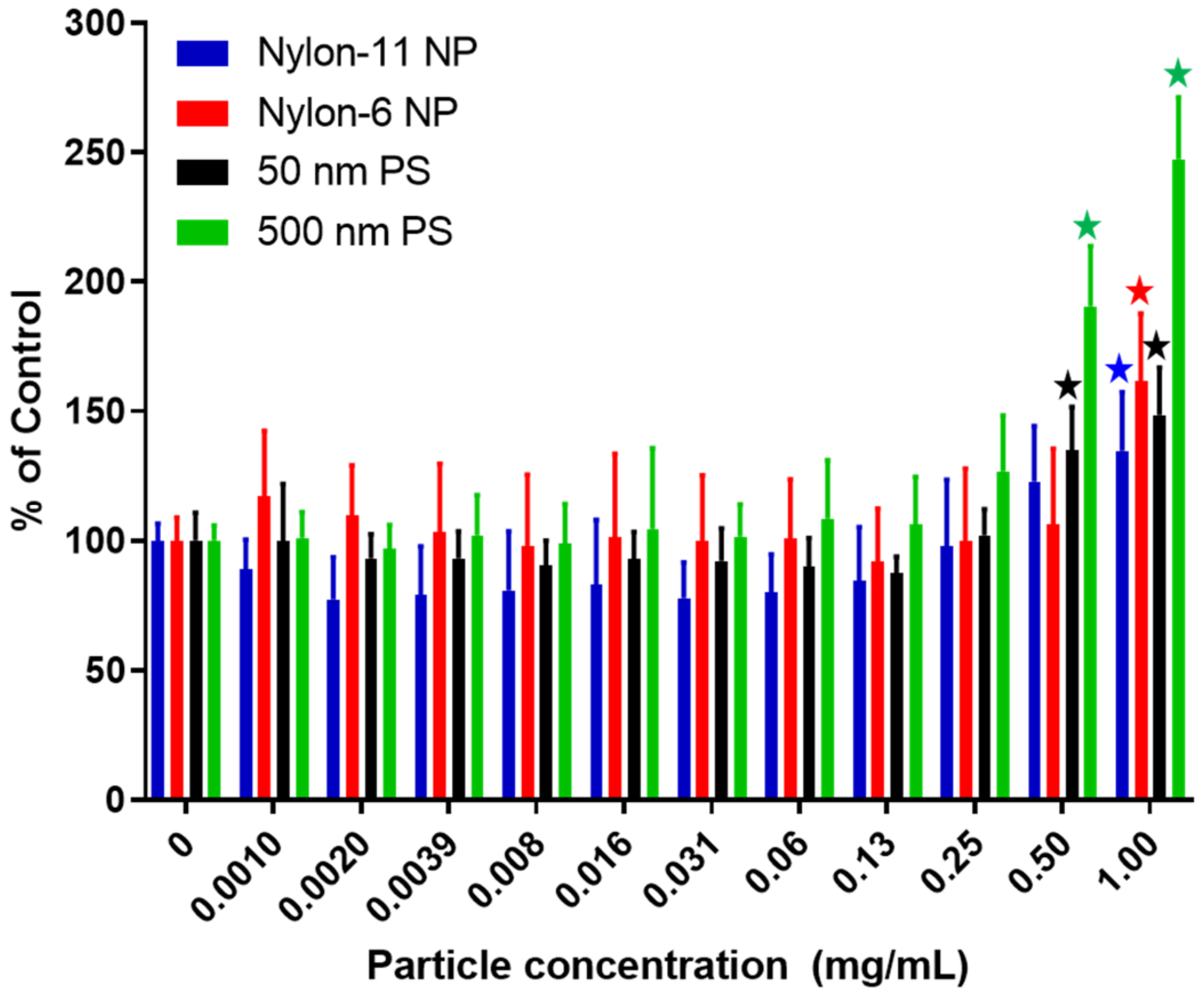
3.2.3. Cytotoxicity Studies with NPs
3.2.4. Fluorescence Microscopy of Macrophages Exposed to NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EPRO. Plastics—The Facts 2021; EPRO: Brussels, Belgium, 2021. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, J.; McKeown, P.; Jones, M.D. A circular economy approach to plastic waste. Polym. Degrad. Stab. 2019, 165, 170–181. [Google Scholar] [CrossRef]
- Meys, R.; Frick, F.; Westhues, S.; Sternberg, A.; Klankermayer, J.; Bardow, A. Towards a circular economy for plastic packaging wastes—The environmental potential of chemical recycling. Resour. Conserv. Recycl. 2020, 162, 105010. [Google Scholar] [CrossRef]
- Bucknall, D.G. Plastics as a materials system in a circular economy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378, 20190268. [Google Scholar] [CrossRef]
- Ter Halle, A.; Jeanneau, L.; Martignac, M.; Jardé, E.; Pedrono, B.; Brach, L.; Gigault, J. Nanoplastic in the North Atlantic Subtropical Gyre. Environ. Sci. Technol. 2017, 51, 13689–13697. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Microplastics in Spanish Table Salt. Sci. Rep. 2017, 7, 8620. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Ho, Y.B.; Larat, V.; Salamatinia, B. Microplastics in eviscerated flesh and excised organs of dried fish. Sci. Rep. 2017, 7, 5473. [Google Scholar] [CrossRef]
- Gündoğdu, S. Contamination of table salts from Turkey with microplastics. Food Addit. Contam. Part A 2018, 35, 1006–1014. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Synthetic particles as contaminants in German beers. Food Addit. Contam. Part A 2014, 31, 1574–1578. [Google Scholar] [CrossRef] [PubMed]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef] [PubMed]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Shruti, V.C. Branded milks—Are they immune from microplastics contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef] [PubMed]
- Boyle, K.; Örmeci, B. Microplastics and Nanoplastics in the Freshwater and Terrestrial Environment: A Review. Water 2020, 12, 2633. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A. Sources and Pathways of Microplastics to Habitats. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 229–244. [Google Scholar] [CrossRef] [Green Version]
- Shim, W.J.; Hong, S.H.; Eo, S. Chapter 1—Marine Microplastics: Abundance, Distribution, and Composition. In Microplastic Contamination in Aquatic Environments; Zeng, E.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–29. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Mortensen, N.P.; Fennell, T.R.; Johnson, L.M. Unintended human ingestion of nanoplastics and small microplastics through drinking water, beverages, and food sources. NanoImpact 2021, 21, 100302. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Mailänder, V.; Landfester, K. Interaction of Nanoparticles with Cells. Biomacromolecules 2009, 10, 2379–2400. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, W.-C.; Westerhoff, P.; Posner, J.D. Biological accumulation of engineered nanomaterials: A review of current knowledge. Environ. Sci. Processes Impacts 2013, 15, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Cho, H.-J.; Kim, E.; Huh, Y.H.; Kim, H.-J.; Kim, B.; Kang, T.; Lee, J.-S.; Jeong, J. Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos. Nanoscale 2019, 11, 3173–3185. [Google Scholar] [CrossRef]
- Pitt, J.A.; Trevisan, R.; Massarsky, A.; Kozal, J.S.; Levin, E.D.; Di Giulio, R.T. Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): A case study with nanopolystyrene. Sci. Total Environ. 2018, 643, 324–334. [Google Scholar] [CrossRef]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef] [Green Version]
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delacher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailänder, V.; Landfester, K.; et al. Differential Uptake of Functionalized Polystyrene Nanoparticles by Human Macrophages and a Monocytic Cell Line. ACS Nano 2011, 5, 1657–1669. [Google Scholar] [CrossRef]
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef]
- Loos, C.; Syrovets, T.; Musyanovych, A.; Mailänder, V.; Landfester, K.; Nienhaus, G.U.; Simmet, T. Functionalized polystyrene nanoparticles as a platform for studying bio–nano interactions. Beilstein J. Nanotechnol. 2014, 5, 2403–2412. [Google Scholar] [CrossRef] [Green Version]
- Walczak, A.P.; Kramer, E.; Hendriksen, P.J.M.; Tromp, P.; Helsper, J.P.F.G.; van der Zande, M.; Rietjens, I.M.C.M.; Bouwmeester, H. Translocation of differently sized and charged polystyrene nanoparticles in in vitro intestinal cell models of increasing complexity. Nanotoxicology 2015, 9, 453–461. [Google Scholar] [CrossRef]
- Anguissola, S.; Garry, D.; Salvati, A.; O’Brien, P.J.; Dawson, K.A. High Content Analysis Provides Mechanistic Insights on the Pathways of Toxicity Induced by Amine-Modified Polystyrene Nanoparticles. PLoS ONE 2014, 9, e108025. [Google Scholar] [CrossRef] [PubMed]
- Murali, K.; Kenesei, K.; Li, Y.; Demeter, K.; Környei, Z.; Madarász, E. Uptake and bio-reactivity of polystyrene nanoparticles is affected by surface modifications, ageing and LPS adsorption: In vitro studies on neural tissue cells. Nanoscale 2015, 7, 4199–4210. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Mecham, J.; Krovi, A.; Caffaro, M.; Aravamudhan, S.; Kovach, A.; Fennell, T.; Pollas Mortensen, N. Fabrication of polyethylene terephthalate (PET) nanoparticles with fluorescent tracers for studies in mammalian cells. Nanoscale Adv. 2021, 3, 339–346. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, A.G.; Muñoz-Tabares, J.A.; Aguilar-Guzmán, J.C.; Vazquez-Duhalt, R. A novel and simple method for polyethylene terephthalate (PET) nanoparticle production. Environ. Sci. Nano 2019, 6, 2031–2036. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, H.; Sim, Y.; Kang, T.; Jeong, J. Fluorescent Polypropylene Nanoplastics for Studying Uptake, Biodistribution, and Excretion in Zebrafish Embryos. ACS Omega 2022, 7, 2467–2473. [Google Scholar] [CrossRef]
- Nylon—Global Market Trajectory & Analytics; Research and Markets: Dublin, Ireland, 2021; Volume 1227800.
- Agrawal, A.K.; Jassal, M. 4—Manufacture of polyamide fibres. In Polyesters and Polyamides; Deopura, B.L., Alagirusamy, R., Joshi, M., Gupta, B., Eds.; Woodhead Publishing: Cambridge, UK, 2008; pp. 97–139. [Google Scholar] [CrossRef]
- Deopura, B.L. 2—Polyamide fibers. In Polyesters and Polyamides; Deopura, B.L., Alagirusamy, R., Joshi, M., Gupta, B., Eds.; Woodhead Publishing: Cambridge, UK, 2008; pp. 41–61. [Google Scholar] [CrossRef]
- Mei, B.Z.; Scheinbeim, J.I.; Newman, B.A. The ferroelectric behavior of odd-numbered nylons. Ferroelectrics 1993, 144, 51–60. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef] [Green Version]
- Seth, C.K.; Shriwastav, A. Contamination of Indian sea salts with microplastics and a potential prevention strategy. Environ. Sci. Pollut. Res. 2018, 25, 30122–30131. [Google Scholar] [CrossRef]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef]
- Li, J.; Yang, D.; Li, L.; Jabeen, K.; Shi, H. Microplastics in commercial bivalves from China. Environ. Pollut. 2015, 207, 190–195. [Google Scholar] [CrossRef]
- Naji, A.; Nuri, M.; Vethaak, A.D. Microplastics contamination in molluscs from the northern part of the Persian Gulf. Environ. Pollut. 2018, 235, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Wang, Q.; Ran, W.; Wu, D.; Liu, Y.; Sun, S.; Liu, H.; Cao, R.; Zhao, J. Microplastic in cultured oysters from different coastal areas of China. Sci. Total Environ. 2019, 653, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Crespy, D.; Landfester, K. Anionic Polymerization of ε-Caprolactam in Miniemulsion: Synthesis and Characterization of Polyamide-6 Nanoparticles. Macromolecules 2005, 38, 6882–6887. [Google Scholar] [CrossRef]
- Crespy, D.; Landfester, K. Preparation of Nylon 6 Nanoparticles and Nanocapsules by Two Novel Miniemulsion/Solvent Displacement Hybrid Techniques. Macromol. Chem. Phys. 2007, 208, 457–466. [Google Scholar] [CrossRef]
- Ma, B.; Liu, F.; Li, Z.; Duan, J.; Kong, Y.; Hao, M.; Ge, S.; Jiang, H.; Liu, H. Piezoelectric nylon-11 nanoparticles with ultrasound assistance for high-efficiency promotion of stem cell osteogenic differentiation. J. Mater. Chem. B 2019, 7, 1847–1854. [Google Scholar] [CrossRef]
- Polonskyi, O.; Kylián, O.; Solař, P.; Artemenko, A.; Kousal, J.; Slavínská, D.; Choukourov, A.; Biederman, H. Nylon-sputtered nanoparticles: Fabrication and basic properties. J. Phys. D Appl. Phys. 2012, 45, 495301. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Demokritou, P. Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat. Protoc. 2017, 12, 355–371. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L. Lipid-polymer hybrid nanoparticles: Synthesis, characterization and applications. Nano LIFE 2010, 01, 163–173. [Google Scholar] [CrossRef]
- Trombetta, D.; Understanding Nylon and Moisture. The Weekly Pellet. 2016. Available online: https://weeklypellet.com/2016/07/22/understanding-nylon-and-moisture/ (accessed on 2 April 2022).
- Pleasant, R. What is the difference between nylons? Pt. 2. 2016. Available online: https://inbound.teamppi.com/blog/what-is-the-difference-between-nylons-pt-2 (accessed on 2 April 2022).
- Long-Chain Polyamides: A Brief Introduction to Nylon 11 and Nylon 12; Nylon Corporation of America, Inc. (NYCOA): Manchester, NH, USA, 2020.
- Brown-Woodman, P.D.; Huq, F.; Hayes, L.; Herlihy, C.; Picker, K.; Webster, W.S. In vitro assessment of the effect of methanol and the metabolite, formic acid, on embryonic development of the rat. Teratology 1995, 52, 233–243. [Google Scholar] [CrossRef]
- Verstraete, A.G.; Vogelaers, D.P.; van den Bogaerde, J.F.; Colardyn, F.A.; Ackerman, C.M.; Buylaert, W.A. Formic acid poisoning: Case report and in vitro study of the hemolytic activity. Am. J. Emerg. Med. 1989, 7, 286–290. [Google Scholar] [CrossRef]
- Hernández-Giottonini, K.Y.; Rodríguez-Córdova, R.J.; Gutiérrez-Valenzuela, C.A.; Peñuñuri-Miranda, O.; Zavala-Rivera, P.; Guerrero-Germán, P.; Lucero-Acuña, A. PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: Effects of formulation parameters. RSC Adv. 2020, 10, 4218–4231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, S.K.; Panyam, J.; Prabha, S.; Labhasetwar, V. Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J. Control. Release 2002, 82, 105–114. [Google Scholar] [CrossRef]
- Sackett, D.L.; Wolff, J. Nile red as a polarity-sensitive fluorescent probe of hydrophobic protein surfaces. Anal. Biochem. 1987, 167, 228–234. [Google Scholar] [CrossRef]
- Chen, A.K.; Cheng, Z.; Behlke, M.A.; Tsourkas, A. Assessing the sensitivity of commercially available fluorophores to the intracellular environment. Anal. Chem. 2008, 80, 7437–7444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazares-Cortes, E.; Espinosa, A.; Guigner, J.-M.; Michel, A.; Griffete, N.; Wilhelm, C.; Ménager, C. Doxorubicin Intracellular Remote Release from Biocompatible Oligo(ethylene glycol) Methyl Ether Methacrylate-Based Magnetic Nanogels Triggered by Magnetic Hyperthermia. ACS Appl. Mater. Interfaces 2017, 9, 25775–25788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Texas Red (and Texas Red-X) Dye. Available online: https://www.thermofisher.com/us/en/home/life-science/cell-analysis/fluorophores/texas-red.html (accessed on 4 April 2022).
- Grafmueller, S.; Manser, P.; Diener, L.; Maurizi, L.; Diener, P.-A.; Hofmann, H.; Jochum, W.; Krug, H.F.; Buerki-Thurnherr, T.; von Mandach, U.; et al. Transfer studies of polystyrene nanoparticles in the ex vivo human placenta perfusion model: Key sources of artifacts. Sci. Technol. Adv. Mater. 2015, 16, 044602. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.A.; Escobar, A.; Bastús, N.G.; Andreozzi, P.; Puntes, V.; Moya, S.E. Fluorescently labelled nanomaterials in nanosafety research: Practical advice to avoid artefacts and trace unbound dye. NanoImpact 2018, 9, 102–113. [Google Scholar] [CrossRef] [Green Version]
- Schür, C.; Rist, S.; Baun, A.; Mayer, P.; Hartmann, N.B.; Wagner, M. When Fluorescence Is not a Particle: The Tissue Translocation of Microplastics in Daphnia magna Seems an Artifact. Environ. Toxicol. Chem. 2019, 38, 1495–1503. [Google Scholar] [CrossRef]
- Malafaia, G.; da Luz, T.M.; Ahmed, M.A.I.; Karthi, S.; Araújo, A.P.d.C. When toxicity of plastic particles comes from their fluorescent dye: A preliminary study involving neotropical Physalaemus cuvieri tadpoles and polyethylene microplastics. J. Hazard. Mater. Adv. 2022, 6, 100054. [Google Scholar] [CrossRef]
- Catarino, A.I.; Frutos, A.; Henry, T.B. Use of fluorescent-labelled nanoplastics (NPs) to demonstrate NP absorption is inconclusive without adequate controls. Sci. Total Environ. 2019, 670, 915–920. [Google Scholar] [CrossRef]
- Snipstad, S.; Hak, S.; Baghirov, H.; Sulheim, E.; Mørch, Ý.; Lélu, S.; von Haartman, E.; Bäck, M.; Nilsson, K.P.R.; Klymchenko, A.S.; et al. Labeling nanoparticles: Dye leakage and altered cellular uptake. Cytometry. Part A J. Int. Soc. Anal. Cytol. 2017, 91, 760–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, T.; Ayoub, A.B.; Psaltis, D.; Klok, H.A. Fluorescence-Based and Fluorescent Label-Free Characterization of Polymer Nanoparticle Decorated T Cells. Biomacromolecules 2021, 22, 190–200. [Google Scholar] [CrossRef]
- Iwamoto, R.; Murase, H. Infrared spectroscopic study of the interactions of nylon-6 with water. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 1722–1729. [Google Scholar] [CrossRef]
- Bahrami, M.; Abenojar, J.; Martínez, M.A. Comparative Characterization of Hot-Pressed Polyamide 11 and 12: Mechanical, Thermal and Durability Properties. Polymers 2021, 13, 3553. [Google Scholar] [CrossRef] [PubMed]
- Halim, K.A.A.; Farrell, J.B.; Kennedy, J.E. Preparation and characterisation of polyamide 11/montmorillonite (MMT) nanocomposites for use in angioplasty balloon applications. Mater. Chem. Phys. 2013, 143, 336–348. [Google Scholar] [CrossRef]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent Advances in Understanding the Protein Corona of Nanoparticles and in the Formulation of “Stealthy” Nanomaterials. Front. Bioeng. Biotechnol. 2020, 8, 166. [Google Scholar] [CrossRef]
- Mortensen, N.P.; Hurst, G.B.; Wang, W.; Foster, C.M.; Nallathamby, P.D.; Retterer, S.T. Dynamic development of the protein corona on silica nanoparticles: Composition and role in toxicity. Nanoscale 2013, 5, 6372–6380. [Google Scholar] [CrossRef]
- Mortensen, N.P.; Moreno Caffaro, M.; Patel, P.R.; Uddin, M.J.; Aravamudhan, S.; Sumner, S.J.; Fennell, T.R. Investigation of twenty metal, metal oxide, and metal sulfide nanoparticles’ impact on differentiated Caco-2 monolayer integrity. NanoImpact 2020, 17, 100212. [Google Scholar] [CrossRef]
- Florance, I.; Ramasubbu, S.; Mukherjee, A.; Chandrasekaran, N. Polystyrene nanoplastics dysregulate lipid metabolism in murine macrophages in vitro. Toxicology 2021, 458, 152850. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, H.; He, C.; Jin, Y.; Fu, Z. Polystyrene nanoparticles trigger the activation of p38 MAPK and apoptosis via inducing oxidative stress in zebrafish and macrophage cells. Environ. Pollut. 2021, 269, 116075. [Google Scholar] [CrossRef]
- Aguilar-Guzmán, J.C.; Bejtka, K.; Fontana, M.; Valsami-Jones, E.; Villezcas, A.M.; Vazquez-Duhalt, R.; Rodríguez-Hernández, A.G. Polyethylene terephthalate nanoparticles effect on RAW 264.7 macrophage cells. Microplastics Nanoplastics 2022, 2, 9. [Google Scholar] [CrossRef]
- Mahadevan, G.; Valiyaveettil, S. Understanding the interactions of poly(methyl methacrylate) and poly(vinyl chloride) nanoparticles with BHK-21 cell line. Sci. Rep. 2021, 11, 2089. [Google Scholar] [CrossRef] [PubMed]


| Before Purification | Post Purification | |||||
|---|---|---|---|---|---|---|
| Average Hydrodynamic Diameter ± SD (nm) | Average PDI ± SD | Average Zeta Potential ± SD (mV) | Average Hydrodynamic Diameter ± SD (nm) | Average PDI ± SD | Average Zeta Potential ± SD (mV) | |
| Nylon-11 | 136 ± 45 | 0.16 ± 0.03 | 35.23 ± 1.35 | 127 ± 51 | 0.19 ± 0.00 | 33.07 ± 1.41 |
| Nylon-11-ATRB | 160 ± 49 | 0.08 ± 0.01 | 28.67 ± 0.63 | 142 ± 43 | 0.08 ± 0.01 | 34.31 ± 0.85 |
| Nylon-11-NR | 164 ± 56 | 0.09 ± 0.01 | 30.23 ± 0.49 | 137 ± 39 | 0.08 ± 0.01 | 30.67 ± 0.38 |
| Nylon-6 | 1686 ± 274 | 0.27 ± 0.11 | 5.09 ± 0.62 | 465 ± 132 | 0.10 ± 0.05 | 22.63 ± 0.05 |
| Nylon-6-ATRB | 755 ± 107 | 0.97 ± 0.04 | 7.39 ± 0.74 | 536 ± 160 | 0.08 ± 0.01 | 21.77 ± 0.26 |
| Nylon-6-NR | 2187 ± 261 | 0.41 ± 0.03 | 3.74 ± 0.38 | 436 ± 139 | 0.17 ± 0.05 | 22.73 ± 0.62 |
| Nylon-6-TR | 1398 ± 219 | 0.39 ± 0.10 | 5.71 ± 0.24 | 548 ± 248 | 0.23 ± 0.01 | 26.0 ± 0.78 |
| Deionized H2O | Cell Media | |||||
|---|---|---|---|---|---|---|
| Average Hydrodynamic Diameter ± SD (nm) | Average PDI ± SD | Average Zeta Potential ± SD (mV) | Average Hydrodynamic Diameter ± SD (nm) | Average PDI ± SD | Average Zeta Potential ± SD (mV) | |
| 50 nm PS | 61 ± 14 | 0.02 ± 0.02 | −68.80 ± 1.88 | 0 h = 242 ± 105 24 h = 171 ± 82 | 0 h = 0.50 ± 0.08 24 h = 0.47 ± 0.04 | 0 h = −12.30 ± 1.10 24 h = −12.93 ± 1.08 |
| 500 nm PS | 480 ± 97 | 0.04 ± 0.01 | −59.17 ± 0.26 | 0 h = 597 ± 168 24 h = 557 ± 155 | 0 h = 0.13 ± 0.06 24 h = 0.06 ± 0.02 | 0 h = −6.65 ± 1.95 24 h = −11.7 ± 1.36 |
| Nylon-11 NP | 109 ± 41 | 0.21 ± 0.03 | 47.87 ± 0.58 | 0 h = 751 ± 277 24 h = 620 ± 193 | 0 h = 0.61 ± 0.09 24 h = 0.52 ± 0.08 | 0 h = −10.04 ± 0.85 24 h = −12.10 ± 0.43 |
| Nylon-6 NP | 476 ± 134 | 0.11 ± 0.03 | 21.93 ± 0.12 | 0 h = 602 ± 192 24 h = 569 ± 312 | 0 h = 0.28 ± 0.02 24 h = 0.50 ± 0.01 | 0 h = −5.17 ± 0.74 24 h = −6.82 ± 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krovi, S.A.; Moreno Caffaro, M.M.; Aravamudhan, S.; Mortensen, N.P.; Johnson, L.M. Fabrication of Nylon-6 and Nylon-11 Nanoplastics and Evaluation in Mammalian Cells. Nanomaterials 2022, 12, 2699. https://doi.org/10.3390/nano12152699
Krovi SA, Moreno Caffaro MM, Aravamudhan S, Mortensen NP, Johnson LM. Fabrication of Nylon-6 and Nylon-11 Nanoplastics and Evaluation in Mammalian Cells. Nanomaterials. 2022; 12(15):2699. https://doi.org/10.3390/nano12152699
Chicago/Turabian StyleKrovi, Sai Archana, Maria M. Moreno Caffaro, Shyam Aravamudhan, Ninell P. Mortensen, and Leah M. Johnson. 2022. "Fabrication of Nylon-6 and Nylon-11 Nanoplastics and Evaluation in Mammalian Cells" Nanomaterials 12, no. 15: 2699. https://doi.org/10.3390/nano12152699









