Studies of the Structure and Optical Properties of BaSrMgWO6 Thin Films Deposited by a Spin-Coating Method
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Structural Investigation
3.2. Morphology and Elemental Chemical Composition
3.3. Chemical States
3.4. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galasso, F.S. Structure, Properties and Preparation of Perovskite-Type Compounds; Pergamon Press: Oxford, UK, 1969. [Google Scholar]
- Howard, C.J.; Stookes, H.T. Structures and phase transitions in perovskites—A group-theoretical approach. Acta Crystallogr. A Found. Crystallogr. 2005, 61, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.J.; Kennedy, B.J.; Woodward, P.M. Ordered double perovskites—A group-theoretical analysis. Acta Crystallogr. B 2003, 59 Pt 4, 463–471. [Google Scholar] [CrossRef]
- Doroftei, C.; Popa, P.D.; Iacomi, F.; Leontie, L. The influence of Zn2+ ions on the microstructure, electrical and gas sensing properties of La0.8Pb0.2FeO3 perovskite. Sens. Actuators B Chem. 2014, 191, 239–245. [Google Scholar] [CrossRef]
- Rezlescu, N.; Rezlescu, E.; Popa, P.D.; Doroftei, C.; Ignat, M. Partial substitution of manganese with cerium in SrMnO3 nano-perovskite catalyst. Effect of the modification on the catalytic combustion of dilute acetone. Mater. Chem. Phys. 2016, 182, 332–337. [Google Scholar] [CrossRef]
- Knapp, M.C.P.; Woodward, M. A-site cation ordering in AA’BB′O6 perovskites. J. Solid State Chem. 2006, 179, 1076–1085. [Google Scholar] [CrossRef]
- Davies, P.K. Cation ordering in complex oxides. Curr. Opin. Solid State Mater. Sci. 1999, 4, 467–471. [Google Scholar] [CrossRef]
- Karen, P.; Kjekshus, A.; Huang, Q.; Lynn, V.L.W.; Rosov, N.; Natali Sora, I.; Santoro, A. Neutron powder diffraction study of nuclear and magnetic structures of oxidized and reduced YBa2Fe3O8+w. J. Solid State Chem. 2003, 174, 87–95. [Google Scholar] [CrossRef]
- Barnes, P.W.; Lufaso, M.W.; Woodward, P.M. Structure prediction of ordered and disordered multiple octahedral cation perovskites using SPuDS. Acta Crystallogr. B 2006, 62, 397–410. [Google Scholar] [CrossRef]
- Saha-Dasgupta, T. Double perovskites with 3d and 4d/5d transition metals: Compounds with promises. Mater. Res. Express 2020, 7, 014003. [Google Scholar] [CrossRef]
- Hossain, A.; Bandyopadhyay, P.; Roy, S. An overview of double perovskites A2B′B″O6 with small ions at A site: Synthesis, structure and magnetic properties. J. Alloys Compd. 2018, 740, 414–427. [Google Scholar] [CrossRef]
- Hoye, R.L.Z.; Hidalgo, J.; Jagt, R.A.; Correa-Baena, J.-P.; Fix, T.; MacManus-Driscoll, J.L. The Role of Dimensionality on the Optoelectronic Properties of Oxide and Halide Perovskites, and their Halide Derivatives. Adv. Energy Mater. 2022, 12, 2100499. [Google Scholar] [CrossRef]
- Manoun, B.; Ezzahi, A.; Benmokhtar, S.; Ider, A.; Lazor, P.; Bih, L.; Igartua, J.M.; Gemmill, W.R.; Smith, M.D.; Zur Loye, H.-C. X-ray diffraction and Raman spectroscopy studies of temperature and composition induced phase transitions in Ba2−xSrxZnWO6 (0 ≤ x ≤ 2) double perovskite oxides. J. Solid State Chem. 2004, 177, 3560. [Google Scholar] [CrossRef]
- Bugaris, D.E.; Hodges, J.P.; Huq, A.; Loye, H.-C. Crystal growth, structures, and optical properties of the cubic double perovskites Ba2MgWO6 and Ba2ZnWO6. J. Solid State Chem. 2011, 184, 2293–2298. [Google Scholar] [CrossRef]
- Khalyavin, D.D.; Han, J.; Senos, A.M.R.; Mantas, P.Q. Synthesis and dielectric properties of tungsten-based complex perovskites. J. Mater. Res. 2003, 18, 2600–2607. [Google Scholar] [CrossRef]
- Fu, W.T.; Akerboom, S.; IJdo, D.J.W. Crystal structures of the double perovskites Ba2Sr1-xCaxWO6. J. Solid State Chem. 2007, 180, 1547–1552. [Google Scholar] [CrossRef]
- Ezzahi, A.; Manoun, B.; Ider, A.; Bih, L.; Benmokhtar, S.; Azrour, M.; Azdou, M.; Igartua, J.M.; Lazor, P. X-ray diffraction and Raman spectroscopy studies of BaSrMWO6 (M=Ni, Co, Mg) double perovskite oxides. J. Mol. Struct. 2011, 985, 339–345. [Google Scholar] [CrossRef]
- Zhou, Q.; Kennedy, B.J.; Elcombe, M.M. Composition and temperature dependent phase transitions in Co–W double perovskites, a synchrotron X-ray and neutron powder diffraction study. J. Solid State Chem. 2007, 180, 541–548. [Google Scholar] [CrossRef]
- Khattak, C.P.; Hurst, J.J.; Cox, D.E. Crystal growth, and electrical and magnetic properties of Ba2CoWO6. Mater. Res. Bull. 1975, 10, 1343–1347. [Google Scholar] [CrossRef]
- Depianti, J.B.; Orlando, M.; Cavichini, A.; Correa, H.P.S.; Rodrigues, V.A.; Passamai, J.L.; Piedade, E.L.O.; Belich, H.; Medeiros, E.F.; De Melo, F.C.L. Structural and magnetic investigation of Ca2MnReO6 doped with Ce. Ceramica 2013, 59, 262–268. [Google Scholar] [CrossRef]
- Alsabah, Y.A.; AlSalhi, M.S.; Mustafa, E.M.; Elbadawi, A.A.; Devanesan, S.; Siddig, M.A. Synthesis, Phase Transition, and Optical Studies of Ba2−xSrxZnWO6 (x = 1.00, 1.25, 1.50, 1.75, 2.00) Tungsten Double Perovskite Oxides. Crystals 2020, 10, 299. [Google Scholar] [CrossRef]
- Patwe, S.J.; Achary, S.N.; Mathews, M.D.; Tyag, A.K. Synthesis, phase transition and thermal expansion studies on M2MgWO6 (M = Ba 2+ and Sr 2+) double perovskites. J. Alloys Compd. 2005, 390, 100–105. [Google Scholar] [CrossRef]
- Chen, Y.C.; Wang, Y.N.; Syu, R.Y. Effect of sintering temperature on microstructures and microwave dielectric properties of Ba2MgWO6 ceramics. J. Mater. Sci. Mater. Electron. 2016, 27, 4259–4264. [Google Scholar] [CrossRef]
- Vu, Q.T.H.; Bondzior, B.; Stefanska, D.; Miniajluk-Gaweł, N.; Winiarski, M.J.; Deren, P.J. On how the mechanochemical and co-precipitation synthesis method changes the sensitivity and operating range of the Ba2Mg1-xEuxWO6 optical thermometer. Sci. Rep. 2021, 11, 22847. [Google Scholar] [CrossRef] [PubMed]
- Leng, K.; Tang, Q.; Wei, Y.; Yang, L.; Xie, Y.; Wu, Z.; Zhu, X. Recent advances in Re-based double perovskites: Synthesis, structural characterization, physical properties, advanced applications, and theoretical studies. AIP Adv. 2020, 10, 120701. [Google Scholar] [CrossRef]
- Abbassi, A. Opto-Electronic Properties of the Co-Doped ZnO, Perovskites BiMO3 and New Cubic Double Perovskite BaSrMgWO6 Oxides: Theory and Experiment. Ph.D. Thesis, Faculte des Sciences, Universite Mohammed V, Rabat, Morocco, 2016. [Google Scholar]
- Butt, M.A. Thin-Film Coating Methods: A Successful Marriage of High-Quality and Cost-Effectiveness—A Brief Exploration. Coatings 2022, 12, 1115. [Google Scholar] [CrossRef]
- Opitz, A.K.; Rameshan, C.; Kubicek, M.; Rupp, G.M.; Nenning, A.; Götsch, T.; Blume, R.; Hävecker, M.; Knop-Gericke, ·A.; Rupprechter, G.; et al. Knop-Gericke,·A.; Rupprechter, G.; et al. The Chemical Evolution of the La0.6Sr0.4CoO3−δ Surface Under SOFC Operating Conditions and Its Implications for Electrochemical Oxygen Exchange Activity. Top. Catal. 2018, 61, 2129–2141. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, D.; Jing, H.; Zhang, F.; Zhang, X.; Hu, S.; Zhang, L.; Wang, J.; Zhang, L.; Zhang, W.; et al. Oxygen activation on Ba-containing perovskite materials. Sci. Adv. 2022, 8, eabn4072. [Google Scholar] [CrossRef]
- Wu, J.Y.; Bian, J.J. Structure stability and microwave dielectric properties of double perovskite ceramics—Ba2Mg1xCaxWO6 (0.0 ≤ x ≤ 0.15). Ceram. Int. 2012, 38, 3217–3225. [Google Scholar] [CrossRef]
- Morris, M.C.; McMurdie, H.F.; Evans, E.H.; Paretzkin, B.; Parker, H.S.; Wong-Ng, W.; Gladhill, D.M.; Hubbard, C.R. Standard X-ray Diffraction Powder Patterns. Natl. Bur. Stand. (U.S.) Monogr. 1982, 25, 21. Available online: https://nvlpubs.nist.gov/nistpubs/Legacy/MONO/nbsmonograph25-21.pdf (accessed on 23 July 2022).
- Swanson, H.E.; Gilfrich, N.T.; George, G.M. Standard X-ray Diffraction Powder Patterns. Natl. Bur. Stand. 1956, 539, 52. Available online: https://nvlpubs.nist.gov/nistpubs/Legacy/circ/nbscircular539v5.pdf (accessed on 23 July 2022).
- Hanawalt, J.D.; Rinn, H.W.; Frevel, L.K. Chemical Analysis by X-Ray Diffraction. Anal. Chem. 1938, 10, 475–512. [Google Scholar] [CrossRef]
- Yildiz, A.; Irimia, M.; Toma, M.; Spulber, I.; Zodieriu, G.; Dobromir, M.; Timpu, D.; Iacomi, F. Effect of the Substrate Nature on Electron Transport in Ga Doped ZnO Thin Films Grown by RF Sputtering. Mater. Today Proc. 2018, 5, 15888–15894. [Google Scholar] [CrossRef]
- Chong, C.; Liu, H.; Wang, S.; Yang, K. First-Principles Study on the Effect of Strain on Single-Layer Molybdenum Disulfide. Nanomaterials 2021, 11, 3127. [Google Scholar] [CrossRef]
- Ding-Jiang Xue, D.-J.; Hou, Y.; Liu, S.-C.; Wei, M.; Chen, B.; Huang, Z.; Li, Z.; Sun, B.; Proppe, A.H.; Dong, Y.; et al. Regulating strain in perovskite thin films through charge-transport layers. Nat. Commun. 2020, 11, 1514. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, S.K.; Knobel, M.; Choudhary, R.J.; Lee, C.G.; Koo, B.H.; Kumar, R. Structural and magnetic properties of bulk and thin films of Mg0.95Mn0.05Fe2O4. Curr. Appl. Phys. 2009, 9, 1009–1013. [Google Scholar] [CrossRef]
- Droubay, T.C.; Kong, L.; Chambers, S.A.; Hes, W.P. Work function reduction by BaO: Growth of crystalline barium oxide on Ag(001) and Ag(111) surfaces. Surf. Sci. 2015, 632, 201–206. [Google Scholar] [CrossRef]
- Wong-Ng, W.; Roth, R.S. Single-crystal structural investigation of BaO2. Phys. C Supercond. Appl. 1994, 233, 97–101. [Google Scholar] [CrossRef]
- De Angelis, B.A.; Schiavello, M. X-ray photoelectron spectroscopy study of nonstoichiometric tungsten oxides. J. Solid State Chem. 1977, 21, 67–72. [Google Scholar] [CrossRef]
- Wang, S.; Gao, H.; Chen, C.; Li, Q.; Li, C.; Wei, Y.; Fang, L. Effect of phase transition on optical and photoluminescence properties of nano-MgWO4 phosphor prepared by a gamma-ray irradiation assisted polyacrylamide gel method. J. Mater. Sci. Mater. Electron. 2019, 30, 15744–15753. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Soultati, A.; Georgiadou, D.G.; Stergiopoulos, T.; Palilis, L.C.; Kennou, S.; Stathopoulos, N.A.; Davazoglou, D.; Argitis, P. Hydrogenated under-stoichiometric tungsten oxide anode interlayers for efficient and stable organic photovoltaics. J. Mater. Chem. A 2014, 2, 1738–1749. [Google Scholar] [CrossRef]
- Hung, C.C.; Riman, R.E. An XPS Investigation of Hydrothermal and Commercial Barium Titanate Powders. KONA Powder Part. J. 1990, 8, 99–104. [Google Scholar] [CrossRef]
- Jha, D.; Himanshu, A.K.; Singh, B.K.; Shukla, D.; Kuma, U.; Kumar, R.; Bhattacharyya, K.; Shinde, A.B.; Krishna, P.S.R.; Dutta, A.; et al. The X-ray Photoelectron and Co K-Edge Absorption Spectra of Ba2CoWO6. Available online: https://arxiv.org/ftp/arxiv/papers/1907/1907.00342.pdf (accessed on 4 July 2019).
- Wlodarczyk, D.; Amilusik, M.; Kosyl, K.M.; Chrunik, M.; Lawniczak-Jablonska, K.; Strankowski, M.; Zajac, M.; Tsiumra, V.; Grochot, A.; Reszka, A.; et al. Synthesis Attempt and Structural Studies of Novel A2CeWO6 Double Perovskites (A2+ = Ba, Ca) in and outside of Ambient Conditions. ACS Omega 2022, 27, 18382–18408. [Google Scholar] [CrossRef]
- Cai, Z.; Kubicek, M.; Fleig, J.; Yildiz, B. Chemical heterogeneities on La0.6Sr0.4CoO3–δ thin films—correlations to cathode surface activity and stability. Chem. Mater. 2012, 24, 1116–1127. [Google Scholar] [CrossRef]
- Jantz, S.G.; Pielnhofer, F.; Dialer, M.; Höppe, H.A. On Tungstates of Divalent Cations (I)—Structural Investigation and Spectroscopic Properties of Sr2[WO5] and Ba2[WO5]. Z. Anorg. Allg. Chem. 2017, 643, 2024–2030. [Google Scholar] [CrossRef]
- Triyono, D.; Hannisa, A.; Laysandra, H. Structural, magnetic, and dielectric studies of cubically ordered Sr2FeMnO6. Appl. Phys. A 2022, 128, 232. [Google Scholar] [CrossRef]
- Kim, D.; Bliem, R.; Hess, F.; Gallet, J.-J.; Yildiz, B. Electrochemical polarization dependence of the elastic and electrostatic driving forces to aliovalent dopant segregation on LaMnO3. J. Am. Chem. Soc. 2020, 142, 3548–3563. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Samundsett, C.; Bullock, J.; Hettick, M.; Allen, T.; Yan, D.; Peng, J.; Wu, Y.; Cui, J.; Javey, A.; et al. Conductive and Stable Magnesium Oxide Electron-Selective Contacts for Efficient Silicon Solar Cells. Adv. Energy Mater. 2016, 7, 1601863. [Google Scholar] [CrossRef]
- Yoshinaga, N.; Kumakura, S.; Kubota, K.; Horiba, T.; Komaba, S. Lithium Magnesium Tungstate Solid as an Additive into Li(Ni1/3 Mn1/3 Co1/3)O2 Electrodes for Li-Ion Batteries. J. Electrochem. Soc. 2019, 166, A5430–A5436. [Google Scholar] [CrossRef]
- Tang, Q.; Zhu, X. Structural Characterization and Physical Properties of Double Perovskite La2FeReO6+δ Powders. Nanomaterials 2022, 12, 244. [Google Scholar] [CrossRef] [PubMed]
- Nenning, A.; Opitz, A.K.; Rameshan, C.; Rameshan, R.; Blume, R.; Hävecker, M.; Knop-Gericke, A.; Rupprechter, G.; Klötzer, B.; Fleig, J. Ambient Pressure XPS Study of Mixed Conducting Perovskite-Type SOFC Cathode and Anode Materials under Well-Defined Electrochemical Polarization. J. Phys. Chem. 2016, 120, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Crumlin, E.J.; Mutoro, E.; Hong, W.T.; Biegalski, M.D.; Christen, H.M.; Liu, Z.; Bluhm, H.; Shao-Horn, Y. In Situ Ambient Pressure X-ray Photoelectron Spectroscopy of Cobalt Perovskite Surfaces under Cathodic Polarization at High Temperatures. J. Phys. Chem. C 2017, 2117, 16087–16094. [Google Scholar] [CrossRef]
- Zorn, G.; Dave, S.R.; Gao, X.; Castner, D.G. New Method for Determining the Elemental Composition and Distribution in Semiconductor Core-Shell Quantum Dots. Anal. Chem. 2011, 83, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Benguerine, O.; Nabi, Z.; Hachilif, A.; Bouabdallah, B.; Benichou, B. Bright future in optoelectronics, photovoltaics and thermoelectric using the double perovskites oxides BaSrMgB′O6 (B′=Te, W). Comput. Condens. Matter. 2022, 30, e00649. [Google Scholar] [CrossRef]
- Sharma, N.; Prabakar, K.; Ilango, S.; Dash, S.; Tyag, A.K. Optical band-gap and associated Urbach energy tails in defected AlN thin films grown by ion beam sputter deposition: Effect of assisted ion energy. Adv. Matter. Proc. 2017, 2, 342–346. [Google Scholar] [CrossRef]
- Amironesei, A.; Airinei, A.; Timpu, D.; Cozan, V.; Rambu, A.P.; Irimia, M.; Iacomi, F.; Rusu, G.I. Electrical and optical properties of some polyazomethine thin films prepared by a spin-coating method. J. Optoelectron. Adv. Mater. 2011, 13, 802–806. [Google Scholar]
- Prepelita, P.; Medianu, R.; Garoi, F.; Stefan, N.; Iacomi, F. On the structural and electrical characteristics of zinc oxide thin films. Thin Solid Film. 2010, 518, 4615–4618. [Google Scholar] [CrossRef]
- John, G.M.; Mugo, S.W.; Ngaruiya, J.M.; Mugambi, N.; Riungu, G.G. Correlation of Bond Energy and Optical Band Energy of Annealed TiO2 Thin Films. Am. J. Energy Res. 2021, 9, 1–5. [Google Scholar] [CrossRef]

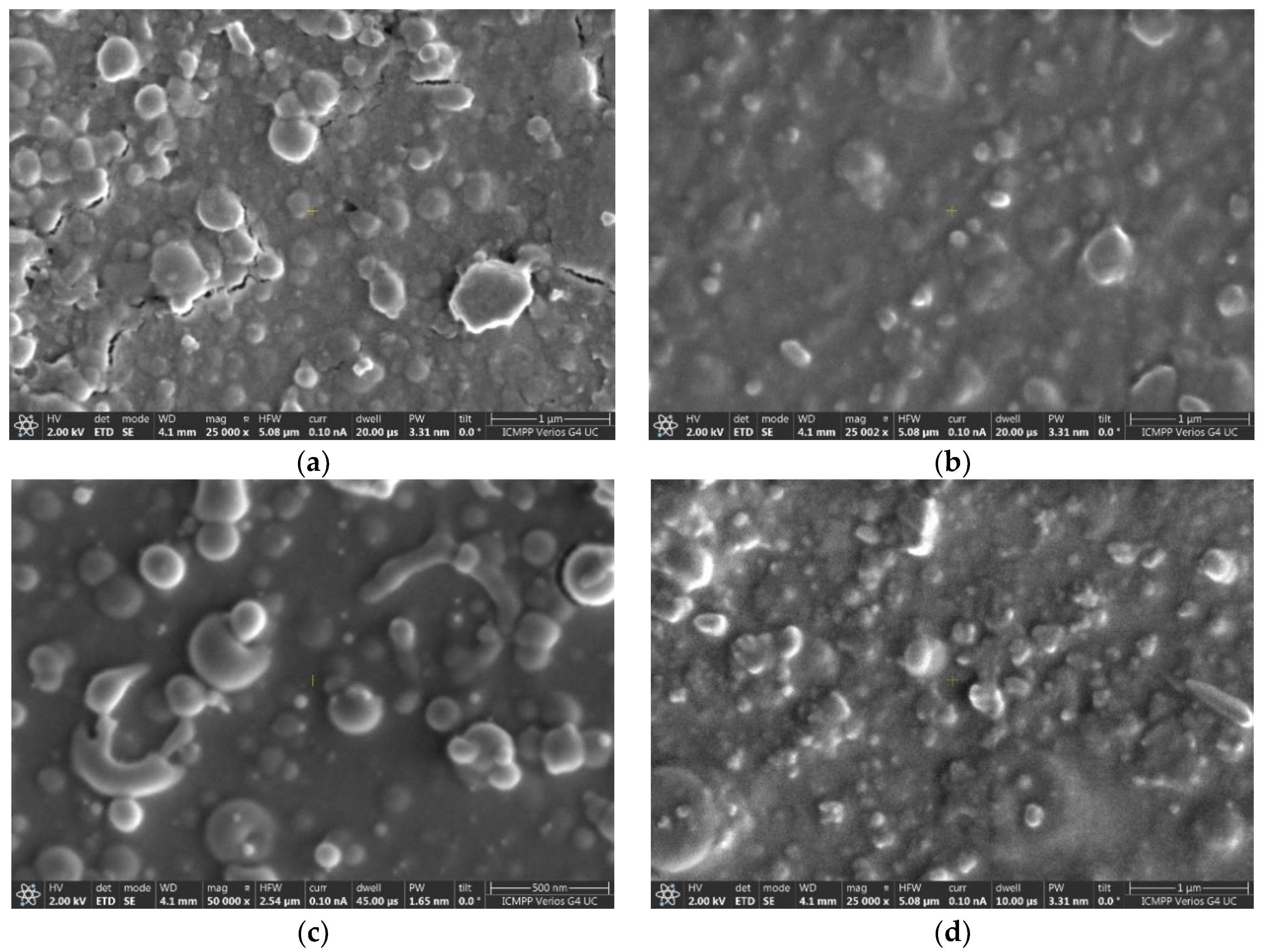
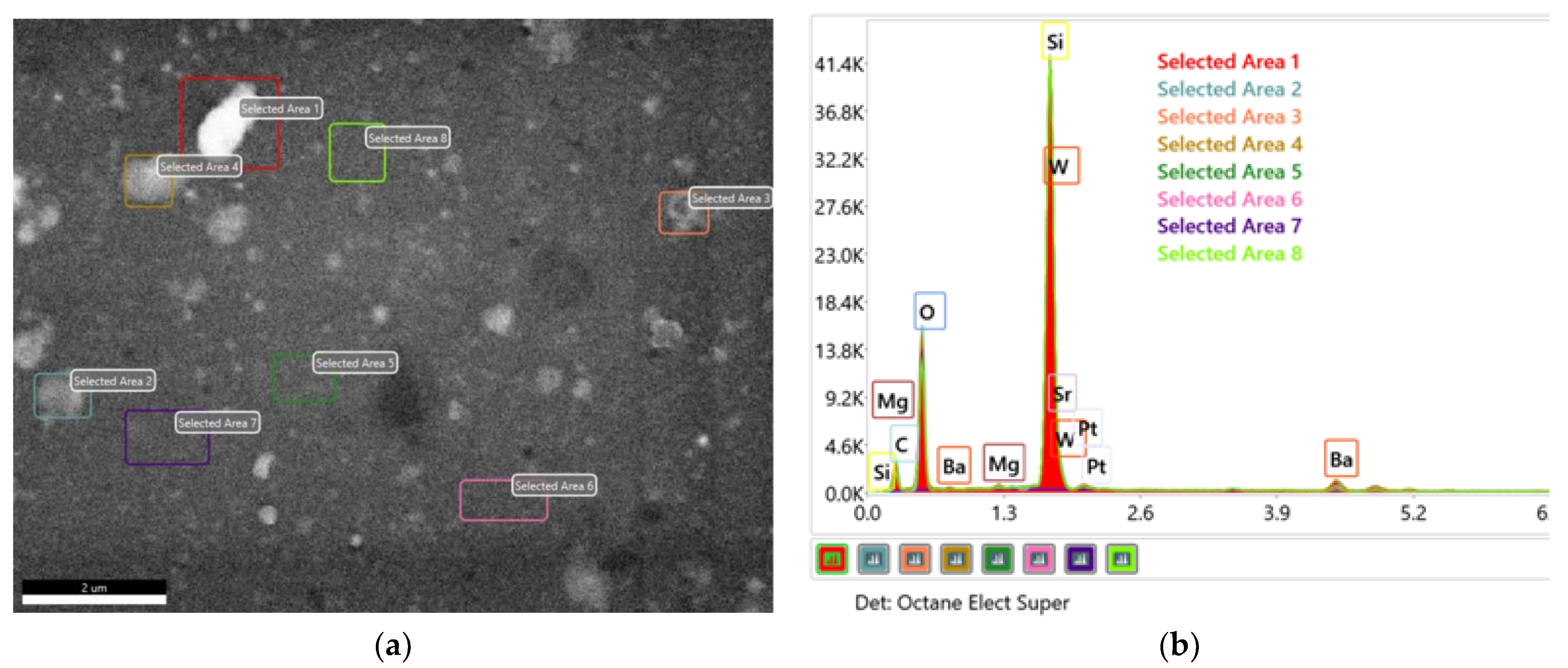



| Sample | Post Deposition Annealing | a Å | V Å3 | Dm nm | Symmetry | Tolerance Factor, t | ε % |
|---|---|---|---|---|---|---|---|
| BSMWO−I | 800 °C, 1 h | - | - | amorphous | |||
| BSMWO−II | 800 °C 1 h and 850 °C, 45 min | 8.1954 ± 0.0757 | 550.436 ± 15.257 | 42.70 ± 0.05 | Fm-3m | 1.012 ± 0.005 | 2.22 ± 0.05 |
| Elements | Area 1 Atomic % | Area 2 Atomic % | Area 3 Atomic % | Area 4 Atomic % | Area 5 Atomic % | Area 6 Atomic % | Area 7 Atomic % | Area 8 Atomic % |
|---|---|---|---|---|---|---|---|---|
| C K | 34.6 | 31.1 | 30.4 | 34.1 | 28.5 | 27.8 | 28.7 | 30 |
| O K | 44.1 | 46.6 | 47 | 44.7 | 47.6 | 47.3 | 47.5 | 46.3 |
| Si K | 19.6 | 20.9 | 21.2 | 19.3 | 22.7 | 22.8 | 22.6 | 22.5 |
| Mg K | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Sr L | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.4 | 0.4 | 0.4 |
| Ba L | 0.8 | 0.5 | 0.4 | 0.8 | 0.2 | 0.2 | 0.2 | 0.2 |
| W L | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Pt L | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 |
| Compound | BE (eV) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ba 3d | Sr 3d | Mg 1s | W 4f | O 1s | C1s | ||||
| 3d5/2 | 3d3/2 | 3d5/2 | 3d3/2 | 1s | 4f7/2 | 4f5/2 | 1s | 1s | |
| BaSrMgWO6 | 779.96 | 795.48 | 133.18 | 134.88 | 1304.27 | 37.60 | 39.90 | 530.11 | |
| Peak area % | 61.9 | 61.9 | 61.9 | 61.9 | 33.3 | ||||
| Ba2−(x+y) SrxMgyWO5 | 781.48 | 797.02 | 135.12 | 136.73 | 1305.33 | 35.93 | 38.73 | 531.63 | |
| Peak area, % | 25.4 | 25.4 | 25.4 | 38.1 | 22.7 | ||||
| BaO2, SrO2 MgO | 782.32 | 797.71 | 136.33 | 137.33 | 1306.33 | 531.87 | |||
| Peak area, % | 12.7 | 12.7 | 12.7 | 11.4 | |||||
| C-C | 284.6 | ||||||||
| Peak area, % | 46.7 | ||||||||
| OH; C-OH | 532.68 | 286.27 | |||||||
| Peak area, % | 18.4 | 31.6 | |||||||
| H2O, O2 C=O | 534.00 | 287.41 | |||||||
| Peak area, % | 14.2 | 21.7 | |||||||
| Compound | Elements, Atomic % | ||||
|---|---|---|---|---|---|
| O | Ba | Sr | Mg | W | |
| BaS rMgWO6 | 40.39 | 5.38 | 5.38 | 5.38 | 5.38 |
| Ba2−(x+y) SrxMgyWO5 | 16.57 | 2.21 | 2.21 | 2.21 | 3.31 |
| BaO2, SrO2, MgO | 8.29 | 1.10 | 1.1 | 1.10 | 0.00 |
| Total | 65.25 | 8.69 | 8.69 | 8.69 | 8.69 |
| Sample | d nm | T (450 nm) % | α (2.75 eV) cm−1 | Egdir eV | Egind eV | EU eV | ET eV |
|---|---|---|---|---|---|---|---|
| BSMWO−I | 150 | 88.3 | 6734.6 | 5.21 | 3.90 | 0.756 | 1.433 |
| BSMWO−II | 150 | 83.3 | 9705.7 | 4.69 | 3.77 | 0.610 | 0.914 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punga, L.; Abbassi, A.; Toma, M.; Alupului, T.; Doroftei, C.; Dobromir, M.; Timpu, D.; Doroftei, F.; Hrostea, L.; Rusu, G.G.; et al. Studies of the Structure and Optical Properties of BaSrMgWO6 Thin Films Deposited by a Spin-Coating Method. Nanomaterials 2022, 12, 2756. https://doi.org/10.3390/nano12162756
Punga L, Abbassi A, Toma M, Alupului T, Doroftei C, Dobromir M, Timpu D, Doroftei F, Hrostea L, Rusu GG, et al. Studies of the Structure and Optical Properties of BaSrMgWO6 Thin Films Deposited by a Spin-Coating Method. Nanomaterials. 2022; 12(16):2756. https://doi.org/10.3390/nano12162756
Chicago/Turabian StylePunga, Luciana, Abderrahman Abbassi, Mihaela Toma, Teodor Alupului, Corneliu Doroftei, Marius Dobromir, Daniel Timpu, Florica Doroftei, Laura Hrostea, George G. Rusu, and et al. 2022. "Studies of the Structure and Optical Properties of BaSrMgWO6 Thin Films Deposited by a Spin-Coating Method" Nanomaterials 12, no. 16: 2756. https://doi.org/10.3390/nano12162756
APA StylePunga, L., Abbassi, A., Toma, M., Alupului, T., Doroftei, C., Dobromir, M., Timpu, D., Doroftei, F., Hrostea, L., Rusu, G. G., Razouk, A., & Iacomi, F. (2022). Studies of the Structure and Optical Properties of BaSrMgWO6 Thin Films Deposited by a Spin-Coating Method. Nanomaterials, 12(16), 2756. https://doi.org/10.3390/nano12162756









