Engineering Gold Shelled Nanomagnets for Pre-Setting the Operating Temperature for Magnetic Hyperthermia
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
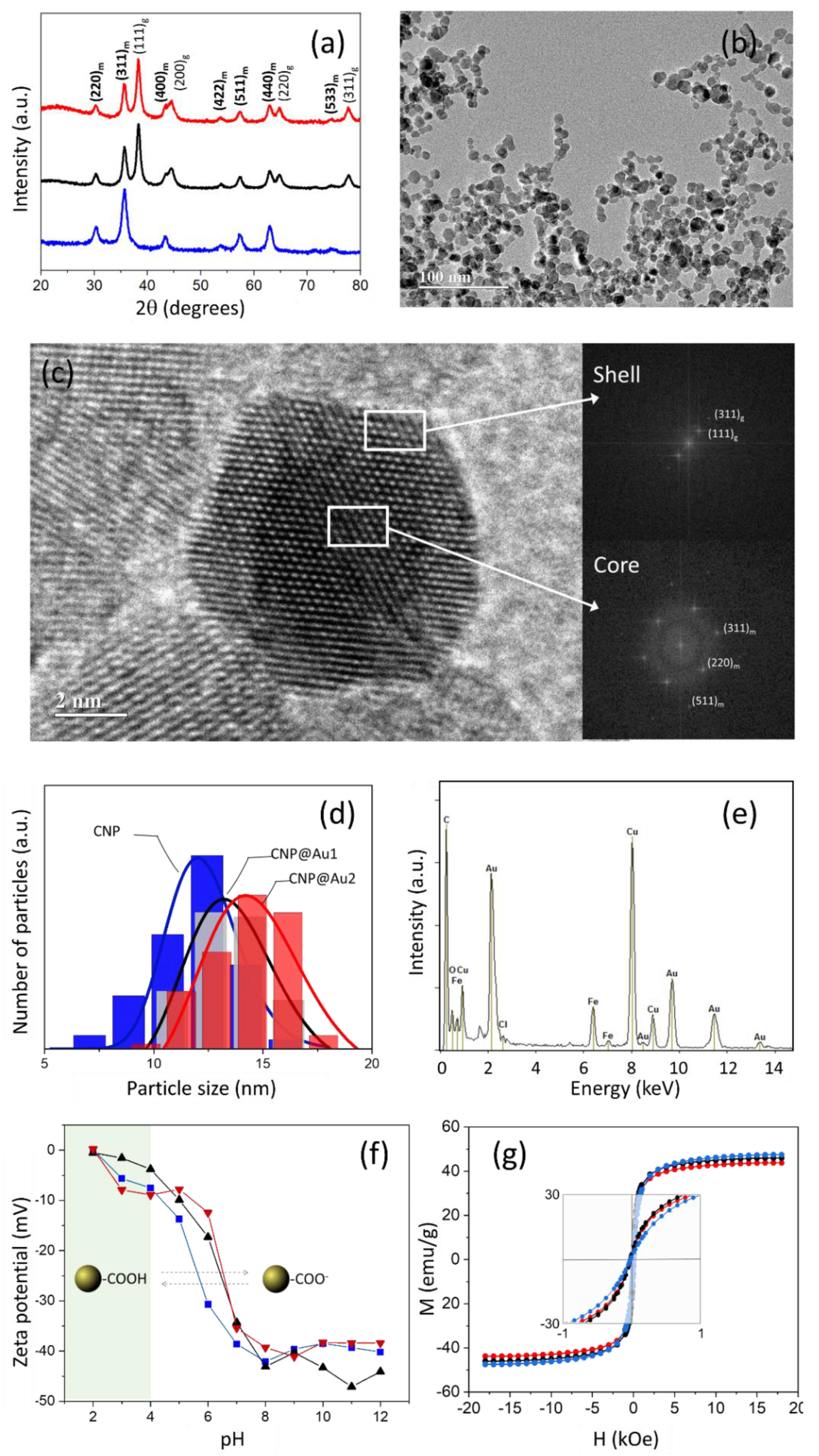
3.1. Sample Characterization
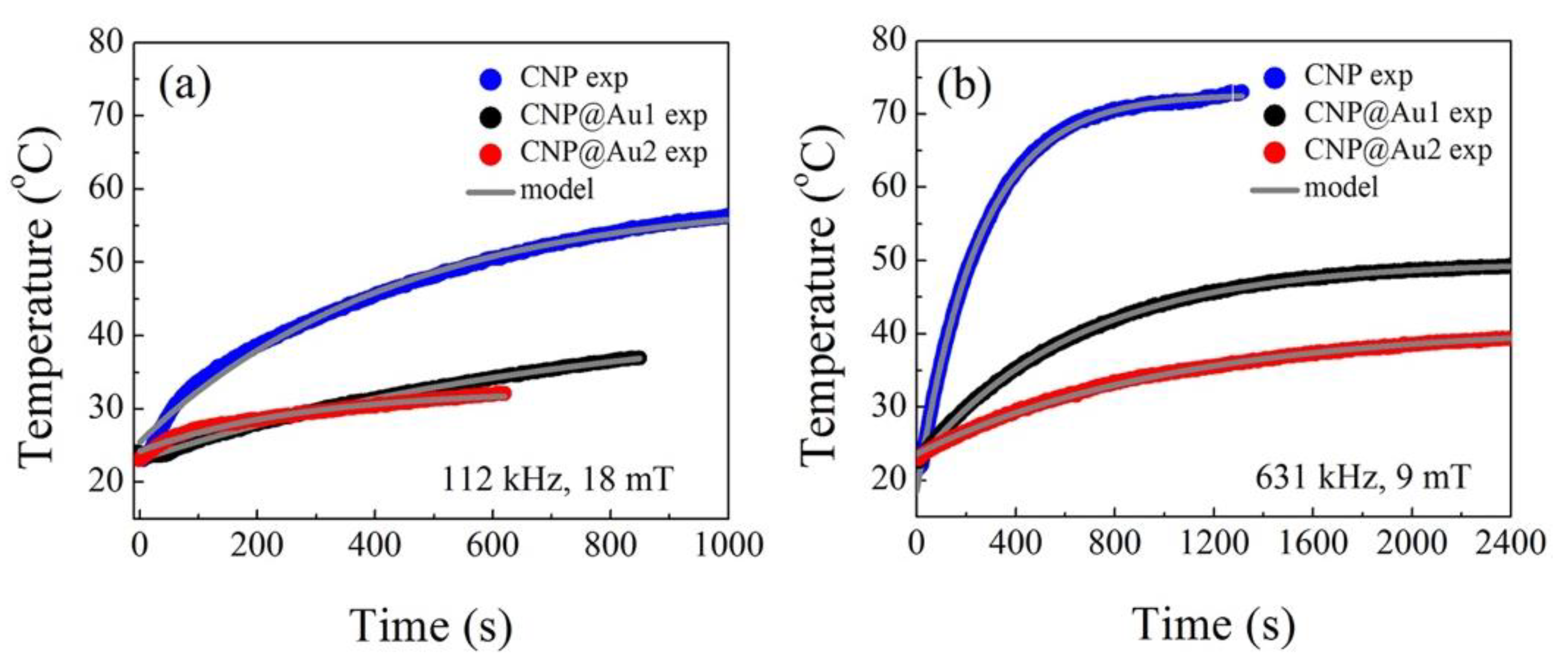
3.2. Magnetic Hyperthermia Data Analysis
3.3. Fitting Procedure for Versus Data
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fatima, H.; Charinpanitkul, T.; Kim, K.S. Fundamentals to Apply Magnetic Nanoparticles for Hyperthermia Therapy. Nanomaterials 2021, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Branquinho, L.C.; Carrião, M.S.; Costa, A.S.; Zufelato, N.; Sousa, M.H.; Miotto, R.; Ivkov, R.; Bakuzis, A.F. Effect of Magnetic Dipolar Interactions on Nanoparticle Heating Efficiency: Implications for Cancer Hyperthermia. Sci. Rep. 2013, 3, 2887. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef]
- Aquino, V.R.R.; Vinícius-Araújo, M.; Shrivastava, N.; Sousa, M.H.; Coaquira, J.A.H.; Bakuzis, A.F. Role of the Fraction of Blocked Nanoparticles on the Hyperthermia Efficiency of Mn-Based Ferrites at Clinically Relevant Conditions. J. Phys. Chem. C 2019, 123, 27725–27734. [Google Scholar] [CrossRef]
- Moise, B.; Byrne, J.M.; El Haj, A.J.; Telling, N.D. The Potential of Magnetic Hyperthermia for Triggering the Differentiation of Cancer Cells. Nanoscale 2018, 10, 20519–20525. [Google Scholar] [CrossRef]
- Myrovali, E.; Maniotis, N.; Samaras, T.; Angelakeris, M. Spatial Focusing of Magnetic Particle Hyperthermia. Nanoscale Adv. 2020, 2, 408–416. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, W.; Kong, L.; Morais, P.C. A New Approach for Highly Accurate, Remote Temperature Probing Using Magnetic Nanoparticles. Sci. Rep. 2014, 4, 6338. [Google Scholar] [CrossRef]
- Rodrigues, H.F.; Capistrano, G.; Bakuzis, A.F. In Vivo Magnetic Nanoparticle Hyperthermia: A Review on Preclinical Studies, Low-Field Nano-Heaters, Noninvasive Thermometry and Computer Simulations for Treatment Planning. Int. J. Hyperth. 2020, 37, 76–99. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, D. Evolution of Magnetic Hyperthermia for Glioblastoma Multiforme Therapy. ACS Chem. Neurosci. 2019, 10, 1157–1172. [Google Scholar] [CrossRef]
- Usov, N.A.; Liubimov, B.Y. Dynamics of magnetic nanoparticle in a viscous liquid: Application to magnetic nanoparticle hyperthermia. J. Appl. Phys. 2012, 112, 023901. [Google Scholar] [CrossRef]
- Jeon, S.; Park, B.C.; Lim, S.; Yoon, H.Y.; Jeon, Y.S.; Kim, B.S.; Kim, Y.K.; Kim, K. Heat-Generating Iron Oxide Multigranule Nanoclusters for Enhancing Hyperthermic Efficacy in Tumor Treatment. ACS Appl. Mater. Interfaces 2020, 12, 33483–33491. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.T.; Lee, J.; Seon, J.; Ju, E.; Kim, M.; Kim, Y.I.; Kim, M.G.; Takemura, Y.; Arbab, A.S.; Kang, K.W.; et al. Giant Magnetic Heat Induction of Magnesium-Doped γ–Fe2O3 Superparamagnetic Nanoparticles for Completely Killing Tumors. Adv. Mater. 2018, 30, 1704362. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Shu, Y.; Wu, Q.; Hu, P.; Shi, J. Mild Magnetic Hyperthermia-Activated Innate Immunity for Liver Cancer Therapy. J. Am. Chem. Soc. 2021, 143, 8116–8128. [Google Scholar] [CrossRef]
- Ortega, D.; Pankhurst, Q.A. Magnetic Hyperthermia. Nanoscience 2013, 1, 60–88. [Google Scholar] [CrossRef]
- Périgo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and Advances in Magnetic Hyperthermia. Appl. Phys. Rev. 2015, 2, 041302. [Google Scholar] [CrossRef]
- León Felix, L.; Sanz, B.; Sebastián, V.; Torres, T.E.; Sousa, M.H.; Coaquira, J.A.H.; Ibarra, M.R.; Goya, G.F. Gold-Decorated Magnetic Nanoparticles Design for Hyperthermia Applications and as a Potential Platform for Their Surface-Functionalization. Sci. Rep. 2019, 9, 4185. [Google Scholar] [CrossRef]
- Mohammad, F.; Balaji, G.; Weber, A.; Uppu, R.M.; Kumar, C.S.S.R. Influence of Gold Nanoshell on Hyperthermia of Superparamagnetic Iron Oxide Nanoparticles. J. Phys. Chem. C 2010, 114, 19194–19201. [Google Scholar] [CrossRef]
- Arsianti, M.; Lim, M.; Lou, S.N.; Goon, I.Y.; Marquis, C.P.; Amal, R. Bi-Functional Gold-Coated Magnetite Composites with Improved Biocompatibility. J. Colloid Interface Sci. 2011, 354, 536–545. [Google Scholar] [CrossRef]
- Coelho, B.C.P.; Siqueira, E.R.; Ombredane, A.S.; Joanitti, G.A.; Chaves, S.B.; da Silva, S.W.; Chaker, J.A.; Longo, J.P.F.; Azevedo, R.B.; Morais, P.C.; et al. Maghemite–Gold Core–Shell Nanostructures (γ-Fe2O3@Au) Surface-Functionalized with Aluminium Phthalocyanine for Multi-Task Imaging and Therapy. RSC Adv. 2017, 7, 11223–11232. [Google Scholar] [CrossRef]
- Brennan, G.; Thorat, N.D.; Pescio, M.; Bergamino, S.; Bauer, J.; Liu, N.; Tofail, S.A.M.; Silien, C. Spectral Drifts in Surface Textured Fe3O4-Au, Core–Shell Nanoparticles Enhance Spectra-Selective Photothermal Heating and Scatter Imaging. Nanoscale 2020, 12, 12632–12638. [Google Scholar] [CrossRef]
- Woodard, L.E.; Dennis, C.L.; Bochers, J.A.; Attaluri, A.; Velarde, E.; Dawidczyk, C.; Searson, P.C.; Pomper, M.G.; Ivkov, R. Nanoparticle Architecture Preserves Magnetic Properties during Coating to Enable Robust Multi-Modal Functionality. Sci. Rep. 2018, 8, 12706. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, R.; Wang, S.; Ding, L.; Li, J.; Luo, Y.; Wang, X.; Shen, M.; Shi, X. Multifunctional Fe3O4@Au Core/Shell Nanostars: A Unique Platform for Multimode Imaging and Photothermal Therapy of Tumors. Sci. Rep. 2016, 6, 28325. [Google Scholar] [CrossRef] [PubMed]
- Brennan, G.; Bergamino, S.; Pescio, M.; Tofail, S.A.M.; Silien, C. The Effects of a Varied Gold Shell Thickness on Iron Oxide Nanoparticle Cores in Magnetic Manipulation, T1 and T2 MRI Contrasting, and Magnetic Hyperthermia. Nanomaterials 2020, 10, 2424. [Google Scholar] [CrossRef]
- Sugumaran, P.J.; Yang, Y.; Wang, Y.; Liu, X.; Ding, J. Influence of the Aspect Ratio of Iron Oxide Nanorods on Hysteresis–Loss–Mediated Magnetic Hyperthermia. ACS Appl. Bio Mater. 2021, 4, 4809–4820. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.K. Comment on Engineering Shape Anisotropy of Fe3O4–γ-Fe2O3 Hollow Nanoparticles for Magnetic Hyperthermia. ACS Appl. Nano Mater. 2021, 4, 3148–3158. [Google Scholar] [CrossRef]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the Reliable Measurement of Specific Absorption Rates and Intrinsic Loss Parameters in Magnetic Hyperthermia Materials. J. Phys. D Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Lahiri, B.B.; Ranoo, S.; Philip, J. Uncertainties in the Estimation of Specific Absorption Rate during Radiofrequency Alternating Magnetic Field Induced Non–Adiabatic Heating of Ferrofluids. J. Phys. D Appl. Phys. 2017, 50, 455005. [Google Scholar] [CrossRef]
- Ebrahimisadr, S.; Aslibeiki, B.; Asadi, R. Magnetic Hyperthermia Properties of Iron Oxide Nanoparticles: The Effect of Concentration. Phys. C Supercond. Appl. 2018, 549, 119–121. [Google Scholar] [CrossRef]
- Lanier, O.L.; Korotych, O.I.; Monsalve, A.G.; Wable, D.; Savliwala, S.; Grooms, N.W.F.; Nacea, C.; Tuitt, O.R.; Dobson, J. Evaluation of Magnetic Nanoparticles for Magnetic Fluid Hyperthermia. Int. J. Hyperth. 2019, 36, 686–700. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Efthimiadou, E.K.; Pissas, M.; Fuentes, D.; Boukos, N.; Psycharis, V.; Kordas, G.; Loukopoulos, V.C.; Kagadis, G.C. Magnetic Fluid Hyperthermia Simulations in Evaluation of SAR Calculation Methods. Phys. Med. 2020, 71, 39–52. [Google Scholar] [CrossRef]
- Zhong, D.; Zhao, J.; Li, Y.; Qiao, Y.; Wei, Q.; He, J.; Xie, T.; Li, W.; Zhou, M. Laser-Triggered Aggregated Cubic α-Fe2O3@Au Nanocomposites for Magnetic Resonance Imaging and Photothermal/Enhanced Radiation Synergistic Therapy. Biomaterials 2019, 219, 119369. [Google Scholar] [CrossRef]
- Dai, C.; Wang, C.; Hu, R.; Lin, H.; Liu, Z.; Yu, L.; Chen, Y.; Zhang, B. Photonic/Magnetic Hyperthermia–Synergistic Nanocatalytic Cancer Therapy Enabled by Zero–Valence Iron Nanocatalysts. Biomaterials 2019, 219, 119374. [Google Scholar] [CrossRef]
- Motoyama, J.; Hakat, T.; Kato, R.; Yamashita, N.; Morino, T.; Kobayashi, T.; Honda, H. Size dependent heat generation of magnetite nanoparticles under AC magnetic field for cancer therapy. Biomagn. Res. Technol. 2008, 6, 4. [Google Scholar] [CrossRef]
- Kallumadil, M.; Tada, M.; Nakagawa, T.; Abe, M.; Southern, P.; Pankhurst, Q.A. Suitability of commercial colloids for magnetic hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1509. [Google Scholar] [CrossRef]
- Rodriguez-Luccioni, H.L.; Latorre-Esteves, M.; Méndez-Veja, J.; Rodríguez, A.R.; Rinaldi, C.; Torres-Lugo, M. Enhanced reduction in cell viability by hyperthermia induced by magnetic nanoparticles. Int. J. Nanomed. 2011, 6, 373. [Google Scholar] [CrossRef]
- de la Presa, P.; Luengo, Y.; Multigner, M.; Costo, R.; Morales, M.P.; Rivero, G.; Hernando, A. Study of Heating Efficiency as a Function of Concentration, Size, and Applied Field in γ-Fe2O3 Nanoparticles. J. Phys. Chem. C 2012, 116, 25602. [Google Scholar] [CrossRef]
- Das, P.; Colombo, M.; Prosperi, D. Recent advances in magnetic fluid hyperthermia for cancer therapy. Colloids Surf. B Biointerfaces 2019, 174, 42. [Google Scholar] [CrossRef]
- Meffre, A.; Mehdaoui, B.; Connord, V.; Carrey, J.; Fazzini, P.F.; Lachaize, S.; Respaud, M.; Chaudret, B. Complex nano-objects displaying both magnetic and catalytic properties: A proof of concept for magnetically induced heterogeneous catalysis. Nano Lett. 2015, 15, 3241. [Google Scholar] [CrossRef]
- Nica, V.; Caro, C.; Páez-Muñoz, J.M.; Leal, M.P.; Garcia-Martin, M.L. Bi-Magnetic Core-Shell CoFe2O4@MnFe2O4 Nanoparticles for In Vivo Theranostics. Nanomaterials 2020, 10, 907. [Google Scholar] [CrossRef]
- Tajabadi, M.; Rahmani, I.; Mirkazemi, S.M.; Orimi, H.G. Insights into the synthesis optimization of FE@SiO2 Core-Shell nanostructure as a highly efficient nano-heater for magnetic hyperthermia treatment. Adv. Powder Technol. 2022, 33, 103366. [Google Scholar] [CrossRef]
- Sebale, S.; Jadhav, V.; Mane-Gavade, S.; Yu, X.Y. Superparamagnetic CoFe2O4@Au with High Specific Absorption Rate and Intrinsic Loss Power for Magnetic Fluid Hyperthermia Applications. Acta Metall. Sin. (Engl. Lett.) 2018, 32, 719. [Google Scholar] [CrossRef]
- Cho, N.H.; Kim, Y.B.; Lee, Y.Y.; Im, S.W.; Kim, R.M.; Kim, J.W.; Namgung, S.D.; Lee, H.E.; Kim, H.; Han, J.H.; et al. Adenine oligomer directed synthesis of chiral gold nanoparticles. Nat. Commun. 2022, 13, 3831. [Google Scholar] [CrossRef] [PubMed]
- Exner, K.S.; Ivanova, A. A doxorubicin-peptide-gold nanoparticle conjugate as a functionalized drug delivery system: Exploring the limits. Phys. Chem. Chem. Phys. 2022, 24, 14985. [Google Scholar] [CrossRef] [PubMed]
- Leroy, P.; Devau, N.; Revil, A.; Bizi, M. Influence of surface conductivity on the apparent zeta potential of amorphous silica nanoparticles. J. Colloid Interface Sci. 2013, 410, 81–93. [Google Scholar] [CrossRef]
- Godoy, N.V.; Galazzi, R.M.; Chacon-Madrid, K.; Arruda, M.A.Z.; Mazali, I.O. Evaluating the total gold concentration in metallic nanoparticles with a high content of organic matter through microwave–assisted decomposition platform and plasma–based spectrometric techniques (ICP–MS and ICP OES). Talanta 2021, 224, 121808. [Google Scholar] [CrossRef]
- Zhang, J.Z. Optical Properties and Spectroscopy of Nanomaterials; World Scientific Publishing Co. Pte. Ltd.: Hackensack, NJ, USA, 2009; pp. 58–68. [Google Scholar]
- Evans, M.; Hastings, N.; Peacock, B. Statistical Distributions, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2000; pp. 129–133. [Google Scholar]
- Torrens-Serra, J.; Peral, I.; Rodriguez-Viejo, J.; Clavaguera-Mora, M.T. Microstructure evolution and grain size distribution in nanocrystalline FeNbBCu from synchroton XRD and TEM analysis. J. Non-Cryst. Solids 2012, 358, 107. [Google Scholar] [CrossRef]
- Aquino, J.C.R.; Aragón, F.H.; Coaquira, J.A.H.; Gratens, X.; Chitta, V.A.; Gonzales, I.; Macedo, W.A.A.; Morais, P.C. Evidence of Cr3+ and Cr4+ Coexitence in Chromium-Doped SnO2 Nanoparticles: A Structural and Magnetic Study. J. Phys. Chem. C 2017, 121, 21670. [Google Scholar] [CrossRef]
- Kahmei, R.D.R.; Seal, P.; Borah, J.P. Tunable heat generation in nickel-substituted zinc ferrite nanoparticles for magnetic hyperthermia. Nanoscale Adv. 2021, 3, 5339. [Google Scholar] [CrossRef]
- Subin, P.J.; Jacob, M.M. Determination of ferromagnetic and superparamagnetic components of magnetization and the effect of particle size on structural, magnetic and hyperfine properties of Mg0.5Zn0.5Fe2O4 nanoparticles. J. Alloys Compd. 2021, 869, 159242. [Google Scholar] [CrossRef]
- Qu, F.; Morais, P.C. Oxide semiconductor nanoparticle in aqueous medium: A surface charge density investigation. J. Phys. Chem. B 2000, 104, 5232–5236. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Tsopoe, S.P.; Borgohain, C.; Fopase, R.; Pandey, L.M.; Borah, J.P. A comparative investigation of normal and inverted exchange bias effect for magnetic fluid hyperthermia applications. Sci. Rep. 2020, 10, 18666. [Google Scholar] [CrossRef] [PubMed]



| Sample | CNP | CNP@Au1 | CNP@Au2 |
|---|---|---|---|
| (nm) | 11.8 | 12.3 | 12.1 |
| (nm) | 11.9 | 13.1 | 13.5 |
| (nm) | 14 ± 3 | 15 ± 3 | 15 ± 3 |
| 0.19 ± 0.02 | 0.21 ± 0.02 | 0.23 ± 0.02 | |
| (emu/g) | 48.5 | 46.0 | 43.8 |
| Sample | (s) | (kHz) | (mT) | |||
|---|---|---|---|---|---|---|
| CNP | 428 ± 4 | 59.1 ± 0.1 | 25.5 ± 0.1 | 112 | 18 | 10 ± 2 |
| CNP@Au1 | 771 ± 6 | 43.8 ± 0.1 | 23.1 ± 01 | 112 | 18 | 2.0 ± 0.7 |
| CNP@Au2 | 276±6 | 32.6 ± 0.1 | 24.2 ± 0.1 | 112 | 18 | 4 ± 1 |
| CNP | 231 ± 2 | 72.3 ± 0.2 | 16.1 ± 0.1 | 112 | 25 | 99 ± 30 |
| CNP@Au1 | 628 ± 3 | 48.7 ± 0.1 | 22.5 ± 0.1 | 112 | 25 | 34 ± 2 |
| CNP@Au2 | 850 ± 3 | 43.6 ± 0.1 | 24.7 ± 0.1 | 112 | 25 | 24 ± 5 |
| CNP | 382 ± 3 | 64.7 ± 0.1 | 21.4 ± 0.1 | 167 | 17 | 95 ± 20 |
| CNP@Au1 | 606 ± 6 | 43.9 ± 0.1 | 23.0 ± 0.1 | 167 | 17 | 38 ± 5 |
| CNP@Au2 | 672 ± 7 | 42.4 ± 0.1 | 26.3 ± 0.1 | 167 | 17 | 47 ± 10 |
| CNP | 216 ± 3 | 76.5 ± 0.3 | 21.6 ± 0.3 | 334 | 17 | 177 ± 40 |
| CNP@Au1 | 559 ± 3 | 57.7 ± 0.1 | 27.2 ± 0.1 | 334 | 17 | 75 ± 10 |
| CNP@Au2 | 961 ± 4 | 51.1 ± 0.1 | 25.6 ± 0.1 | 334 | 17 | 36±4 |
| CNP | 238 ± 3 | 74.2 ± 0.2 | 28.7 ± 0.2 | 473 | 11 | 172 ± 40 |
| CNP@Au1 | 493 ± 1 | 57.4 ± 0.1 | 23.8 ± 0.1 | 473 | 11 | 53 ± 4 |
| CNP@Au2 | 607 ± 2 | 47.4 ± 0.1 | 26.4 ± 0.1 | 473 | 11 | 43 ± 4 |
| CNP | 250 ± 1 | 72.8 ± 0.1 | 18.5 ± 0.1 | 631 | 9 | 103 ± 20 |
| CNP@Au1 | 639 ± 1 | 49.9 ± 0.1 | 22.5 ± 0.1 | 631 | 9 | 32 ± 1 |
| CNP@Au2 | 1038 ± 1 | 41.2 ± 0.1 | 23.6 ± 0.1 | 631 | 9 | 24 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siqueira, E.R.L.; Pinheiro, W.O.; Aquino, V.R.R.; Coelho, B.C.P.; Bakuzis, A.F.; Azevedo, R.B.; Sousa, M.H.; Morais, P.C. Engineering Gold Shelled Nanomagnets for Pre-Setting the Operating Temperature for Magnetic Hyperthermia. Nanomaterials 2022, 12, 2760. https://doi.org/10.3390/nano12162760
Siqueira ERL, Pinheiro WO, Aquino VRR, Coelho BCP, Bakuzis AF, Azevedo RB, Sousa MH, Morais PC. Engineering Gold Shelled Nanomagnets for Pre-Setting the Operating Temperature for Magnetic Hyperthermia. Nanomaterials. 2022; 12(16):2760. https://doi.org/10.3390/nano12162760
Chicago/Turabian StyleSiqueira, Elis Regina Lima, Willie Oliveira Pinheiro, Victor Raul Romero Aquino, Breno Cunha Pinto Coelho, Andris Figueiroa Bakuzis, Ricardo Bentes Azevedo, Marcelo Henrique Sousa, and Paulo Cesar Morais. 2022. "Engineering Gold Shelled Nanomagnets for Pre-Setting the Operating Temperature for Magnetic Hyperthermia" Nanomaterials 12, no. 16: 2760. https://doi.org/10.3390/nano12162760