Constructing a Superior Interfacial Microstructure on Carbon Fiber for High Interfacial and Mechanical Properties of Epoxy Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Amination of Carbon Fibers
2.2.2. Synthesis of HP–COOH
2.2.3. Chemical Grafting of HP–COOH onto Carbon Fibers
2.3. Preparation of CF/EP Composites
2.4. Characterization
3. Results and Discussions
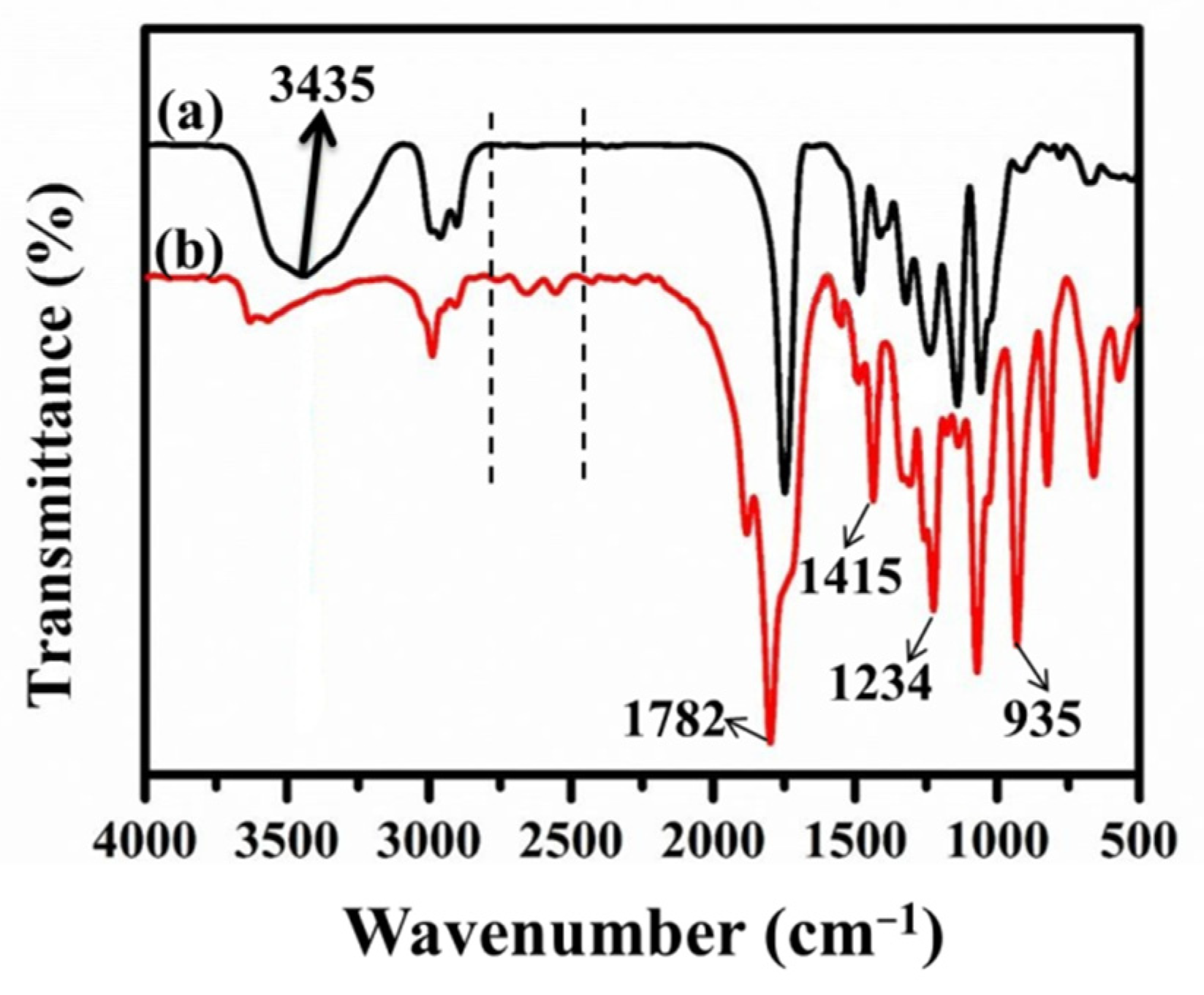
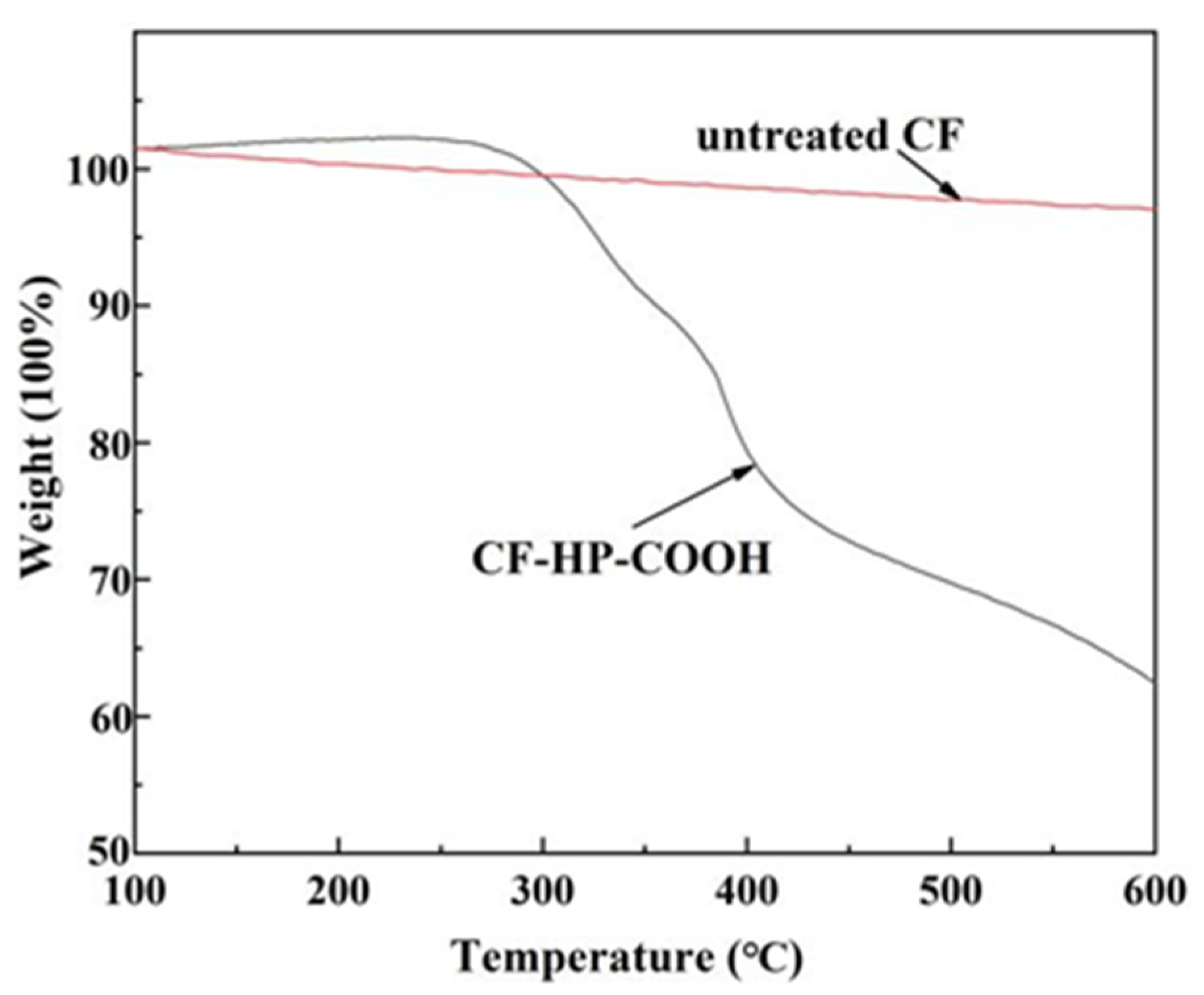
3.1. Chemical Compositions of the Carbon Fibers
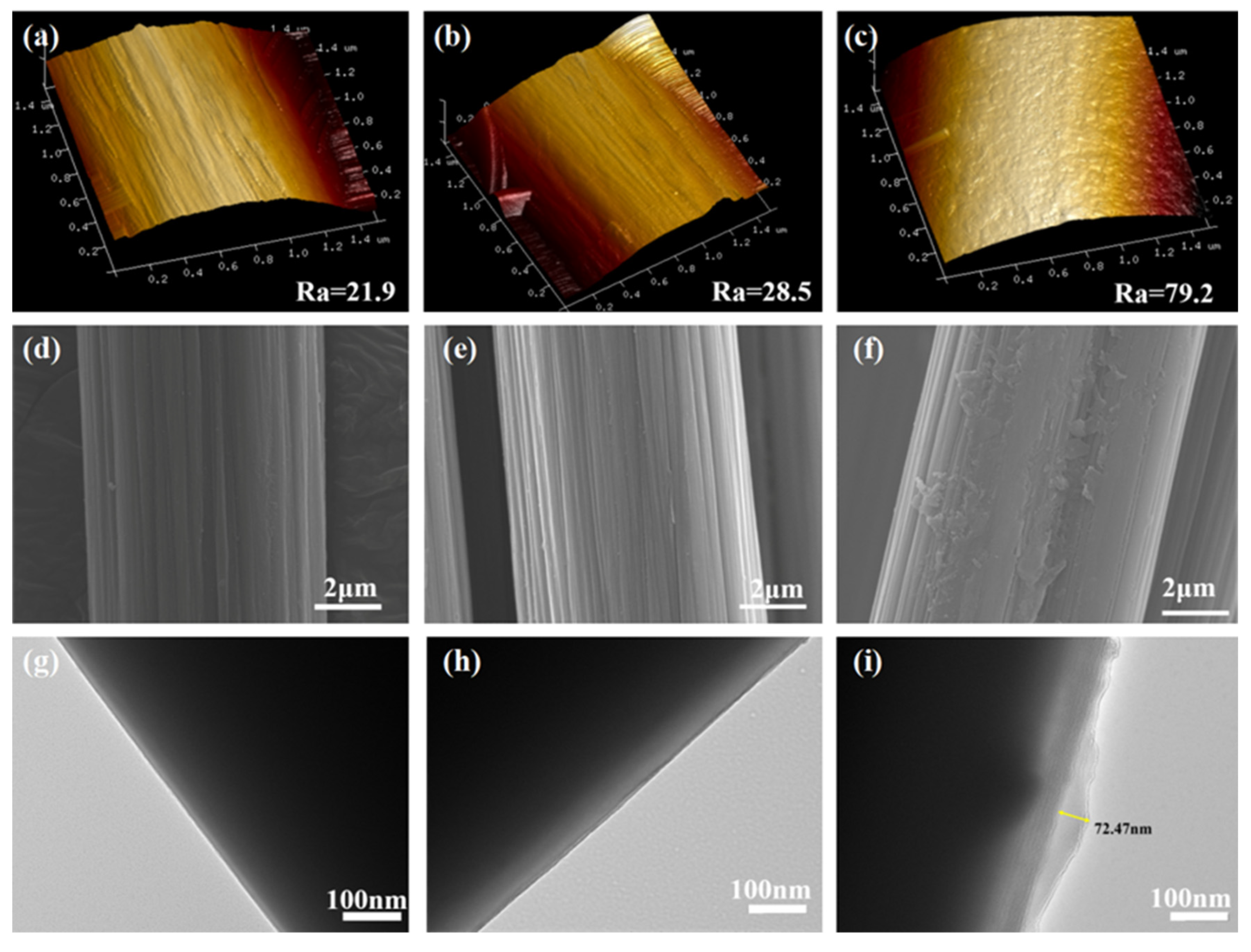
3.2. Surface Morphology of Carbon Fibers
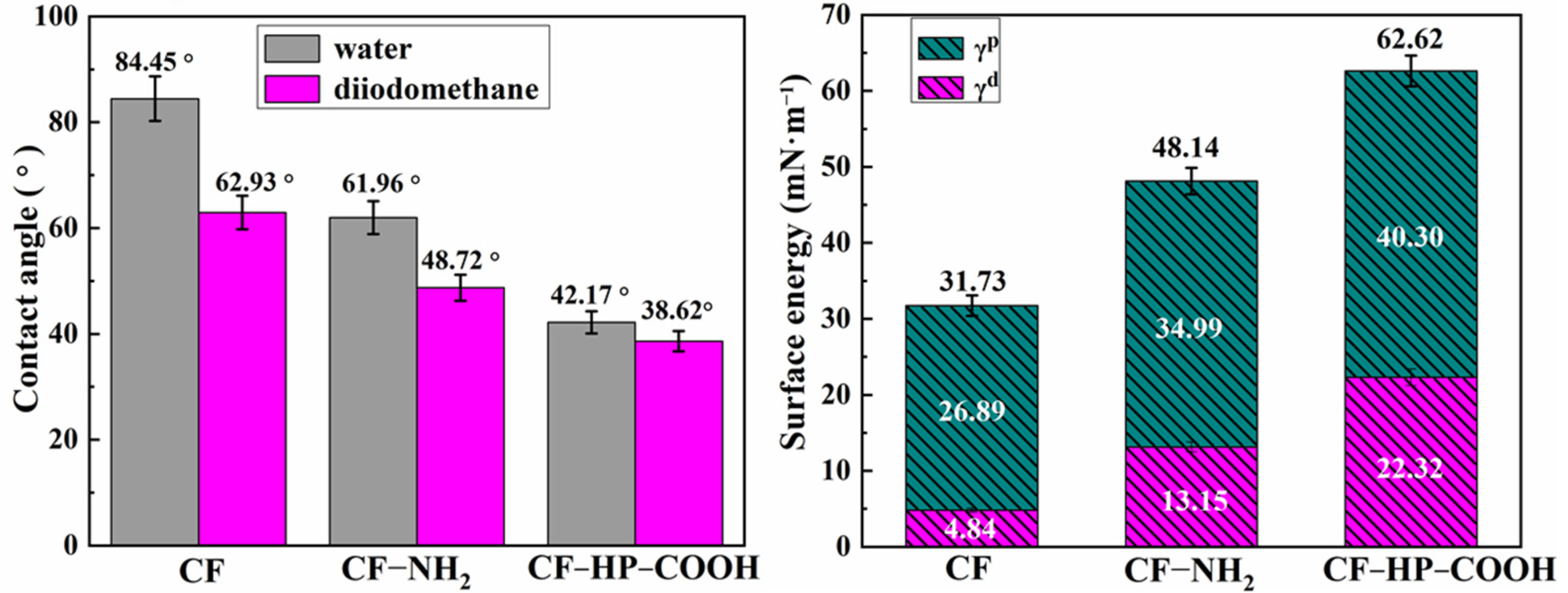
3.3. Surface Wettability of Carbon Fibers
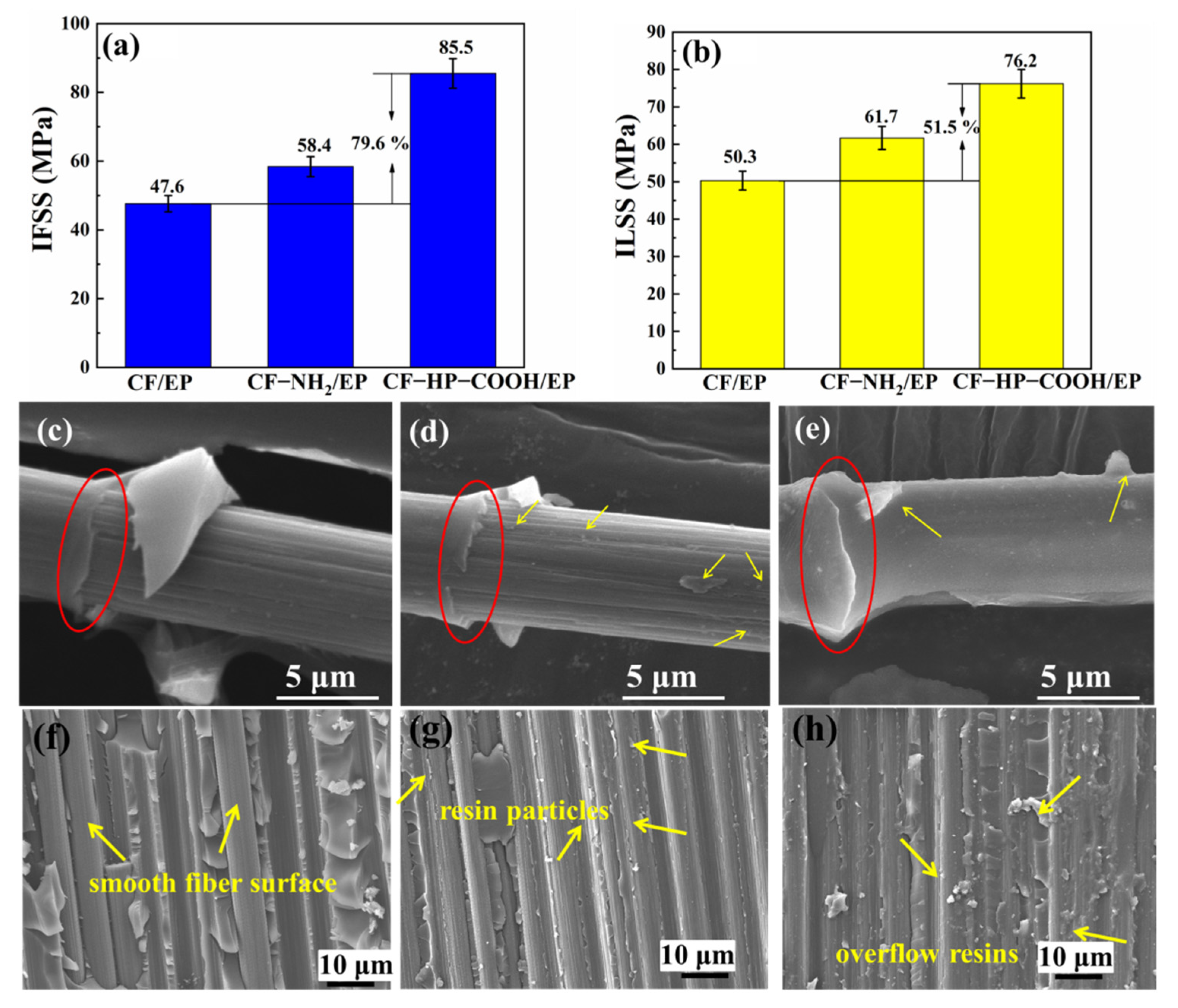
3.4. Interfacial Properties of the CF/EP Composites
3.5. Mechanical Properties of the Composites
3.6. Dynamic Mechanical Thermal Properties of the Composites
3.7. Interface Analysis of the Composites
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Deng, X.; Sui, G.; Yang, X. Improving interfacial and mechanical properties of carbon nanotube-sized carbon fiber/epoxy composites. Carbon 2019, 145, 629–639. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, G.; Yu, B.; Peng, M. New reactive rigid-rod aminated aromatic polyamide for the simultaneous strengthening and toughening of epoxy resin and carbon fiber/epoxy composites. Compos. Part B Eng. 2020, 197, 108044. [Google Scholar] [CrossRef]
- Feng, P.; Ma, L.; Wu, G.; Li, X.; Zhao, M.; Shi, L.; Wang, M.; Wang, X.; Song, G. Establishment of multistage gradient modulus intermediate layer between fiber and matrix via designing double “rigid-flexible” structure to improve interfacial and mechanical properties of carbon fiber/resin composites. Compos. Sci. Technol. 2020, 200, 108336. [Google Scholar] [CrossRef]
- Ma, L.; Meng, L.; Wu, G.; Wang, Y.; Zhao, M.; Zhang, C.; Zhang, C.; Huang, Y. Improving the interfacial properties of carbon fiber-reinforced epoxy composites by grafting of branched polyethyleneimine on carbon fiber surface in supercritical methanol. Compos. Sci. Technol. 2015, 114, 64–71. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jung, U.; Choi, S.; Jung, Y.C.; Lee, H.-S.; Kim, J. Effect of plasma gas and Ar incorporation on the shear strength between carbon fiber-reinforced thermoplastic polymer and Al. Compos. A Appl. Sci. Manuf. 2020, 138, 106041. [Google Scholar] [CrossRef]
- Wu, G.; Chen, L.; Liu, L. Effects of silanization and silica enrichment of carbon fibers on interfacial properties of methylphenylsilicone resin composites. Compos. A Appl. Sci. Manuf. 2017, 98, 159–165. [Google Scholar] [CrossRef]
- Gangineni, P.K.; Yandrapu, S.; Ghosh, S.K.; Anand, A.; Prusty, R.K.; Ray, B.C. Mechanical behavior of Graphene decorated carbon fiber reinforced polymer composites, An assessment of the influence of functional groups. Compos. A Appl. Sci. Manuf. 2019, 122, 36–44. [Google Scholar] [CrossRef]
- Chu, C.; Heyi, G.; Gu, N.; Zhang, K.; Jin, C. Interfacial microstructure and mechanical properties of carbon fiber composite modified with carbon dots. Compos. Sci. Technol. 2019, 184, 107856. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, G. Interfacial strength and mechanisms of silicone resin composites reinforced with 2 different polyhedral oligomericsilsesquioxanes/carbon fiber hybrids. Polym. Adv. Technol. 2018, 29, 2373–2380. [Google Scholar] [CrossRef]
- Song, B.; Wang, T.; Wang, L.; Liu, H.; Mai, X.; Wang, X.; Wang, N.; Huang, Y.; Ma, Y.; Liu, Y.; et al. Interfacially reinforced carbon fiber/epoxy composite laminates via in-situ synthesized graphitic carbon nitride (g-C3N4). Compos. B Eng. 2019, 158, 259–268. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, Y.; Zhang, C.; Liu, L.; Zhang, Y.; Wang, L. Effect of γ-Ray irradiation grafting on the carbon fibers and interfacial adhesion of epoxy composites. Compo. Sci. Technol. 2007, 67, 3261–3270. [Google Scholar] [CrossRef]
- Peng, Q.; Li, Y.; He, X.; Lv, H.; Hu, P.; Shang, Y.; Wang, C.; Wang, R.; Sritharan, T.; Du, S. Interfacial enhancement of carbon fiber composites by poly(amido amine) functionalization. Compo. Sci. Technol. 2013, 74, 37–42. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, J.; Hao, Z.; Huo, L.; Zhang, R.; Shao, L. In-situ modification of carbon fibers with hyperbranched polyglycerol via anionic ring opening polymerization for use in high-performance composites. Carbon 2017, 123, 548–557. [Google Scholar] [CrossRef]
- Eyckens, D.J.; Demir, B.; Randall, J.D.; Gengenbach, T.R.; Servinis, L.; Walsh, T.R.; Henderson, L.C. Using molecular entanglement as a strategy to enhance carbon fiber epoxy composite interfaces. Compos. Sci. Technol. 2020, 196, 108225. [Google Scholar] [CrossRef]
- Zhao, F.; Huang, Y.; Liu, L.; Bai, Y.; Xu, L. Formation of a carbon fiber/polyhedral oligomeric silsesquioxane/carbon nanotube hybrid reinforcement and its effect on the interfacial properties of carbon fiber/epoxy composites. Carbon 2011, 49, 2624–2632. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Li, J.; Sun, S.; Ma, L.; Wu, G.; Zhao, F.; Jiang, B.; Huang, Y. Enhancing the interfacial strength of carbon fiber reinforced epoxy composites by green grafting of poly(oxypropylene) diamines. Compos. A Appl. Sci. Manuf. 2017, 99, 58–64. [Google Scholar] [CrossRef]
- Sato, E.; Uehara, I.; Horibe, H.; Matsumoto, A. One-step synthesis of thermally curable hyperbranched polymers by addition-fragmentation chain transfer using divinyl monomers. Macromolecules 2014, 47, 937–943. [Google Scholar] [CrossRef]
- Popeney, C.S.; Lukowiak, M.C.; Böttcher, C.; Schade, B.; Welker, P.; Mangoldt, D.; Gunkel, G.; Guan, Z.; Haag, R. Tandem coordination, ring-opening, hyperbranched polymerization for the synthesis of water-soluble core-shell unimolecular transporters. ACS Macro Lett. 2012, 1, 564–567. [Google Scholar] [CrossRef]
- Han, P.; Ma, L.; Song, G.; Shi, L.; Gu, Z.; Li, X.; Yang, C.; Wang, G. Strengthening and modulating interphases in carbon fiber/epoxy composites by grafting dendritic polyetheramine with different molecular weights onto carbon fiber. Polym. Compos. 2019, 40, 1525–1536. [Google Scholar] [CrossRef]
- Shi, L.; Song, G.; Li, P.; Li, X.; Pan, D.; Huang, Y.; Ma, L.; Guo, Z. Enhancing interfacial performance of epoxy resin composites via in-situ nucleophilic addition polymerization modification of carbon fibers with hyperbranched polyimidazole. Compos. Sci. Technol. 2021, 201, 108522. [Google Scholar] [CrossRef]
- Yang, L.; Han, P.; Gu, Z. Grafting of a novel hyperbranched polymer onto carbon fiber for interfacial enhancement of carbon fiber reinforced epoxy composites. Mater. Des. 2021, 200, 109456. [Google Scholar] [CrossRef]
- Arce, E.; Nieto, P.M.; Díaz, V.; Castro, R.G.; Bernad, A.; Rojo, J.J.B.C. Glycodendritic structures based on Boltornhyperbranched polymers and their interactions with Lens culinaris lectin. Bioconjugate Chem. 2003, 14, 817. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gao, S.; Chen, X.; Yang, L.; Wu, X.; Feng, S.; Hu, X.; Liu, J.; Xu, P.; Ding, Y. Effect of hydroxyl and carboxyl-functionalized carbon nanotubes on phase morphology, mechanical and dielectric properties of poly(lactide)/poly(butylene adipate-co-terephthalate) composites. J. Biol. Macromol. 2022, 206, 661–669. [Google Scholar] [CrossRef]
- Ma, P.; Kim, J.-K.; Tang, B. Functionalization of carbon nanotubes using a silane coupling agent. Carbon 2006, 44, 3232–3238. [Google Scholar] [CrossRef]
- Yu, J.; Meng, L.; Fan, D.; Zhang, C.; Yu, F.; Huang, Y. The oxidation of carbon fibers through K2S2O8/AgNO3 system that preserves fiber tensile strength. Compos. B Eng. 2014, 60, 261–267. [Google Scholar] [CrossRef]
- Pathak, A.K.; Borah, M.; Gupta, A.; Yokozeki, T.; Dhakate, S.R. Improved mechanical properties of carbon fiber/graphene oxide-epoxy hybrid composites. Compos. Sci. Technol. 2016, 135, 28–38. [Google Scholar] [CrossRef]
- Islam, M.S.; Deng, Y.; Tong, L.; Faisal, S.N.; Roy, A.K.; Minett, A.I.; Gomes, V.G. Grafting carbon nanotubes directly onto carbon fibers for superior mechanical stability, Towards next generation aerospace composites and energy storage applications. Carbon 2016, 96, 701–710. [Google Scholar] [CrossRef]
- Ramanathan, T.; Fisher, F.T.; Ruoff, R.S.; Brinson, L.C. Amino-functionalized carbon nanotubes for binding to polymers and biological systems. Chem. Mater. 2005, 17, 1290–1295. [Google Scholar] [CrossRef]
- Li, S.; Wu, Q.; Cui, C.; Zhu, H.; Hou, H.; Lin, Q.; Li, Y.; Lv, T. Synergetic reinforcements of epoxy composites with glass fibers and hyperbranched polymers. Polym. Compos. 2017, 39, 2871–2879. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, R.; Gao, F.; He, M.; Wang, C.; Liu, L.; Zhao, L.; Cui, H. Interfacial microstructure and enhanced mechanical properties of carbon fiber composites caused by growing generation 1-4 dendritic poly(amidoamine) on a fiber surface. Langmuir 2016, 32, 8339–8349. [Google Scholar] [CrossRef]
- Ni, Y.; Chen, L.; Teng, K.; Shi, J.; Qian, X.; Xu, Z.; Tian, X.; Hu, C.; Ma, M. Superior mechanical properties of epoxy composites reinforced by 3D interconnected graphene skeleton. ACS Appl. Mater. Interfaces 2015, 7, 11583–11591. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Zainuddin, S.; Hosur, M.V.; Malone, J.E.; Salam, M.B.A.; Kumar, A. Improvements in mechanical and thermo-mechanical properties of e-glass/epoxy composites using amino functionalized MWCNTs. Compos. Struct. 2012, 94, 2397–2406. [Google Scholar] [CrossRef]
- Wan, Y.; Gong, L.; Tang, L.; Wu, L.; Jiang, J. Mechanical properties of epoxy composites filled with silane-functionalized graphene oxide. Compos. Part A Appl. Sci. Manuf. 2014, 64, 79–89. [Google Scholar] [CrossRef]

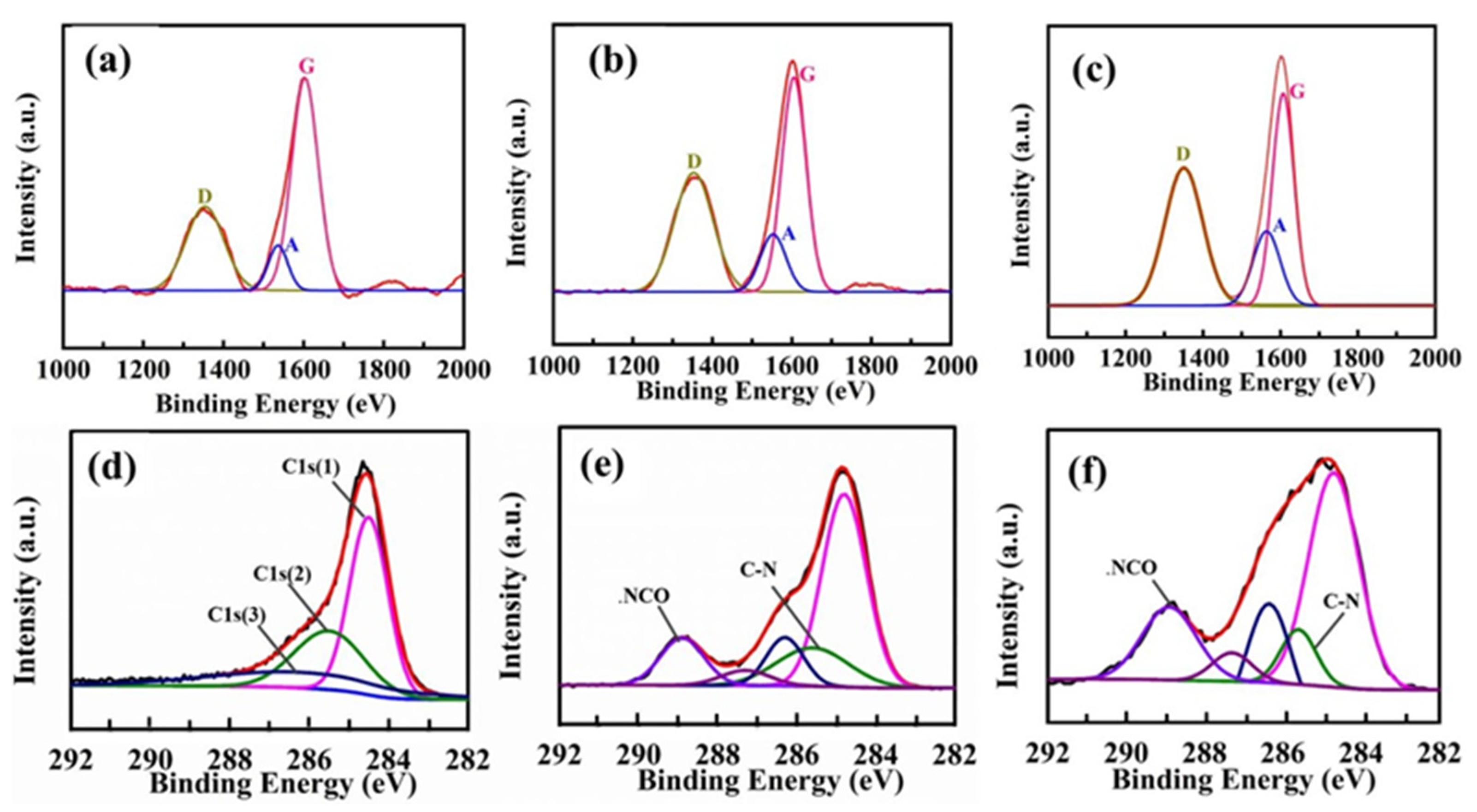



| Samples | D | G | A | ID/IG | IA/IG |
|---|---|---|---|---|---|
| W (cm−1) | W (cm−1) | W (cm−1) | |||
| Untreated CF | 1353 | 1600 | 1536 | 0.56 | 0.16 |
| CF–NH2 | 1353 | 1600 | 1550 | 0.87 | 0.28 |
| CF–HP–COOH | 1353 | 1600 | 1550 | 1.13 | 0.44 |
| Samples | Element Content (%) | ||||
|---|---|---|---|---|---|
| C | O | N | O/C | N/C | |
| Untreated CF | 92.31 | 5.26 | 2.43 | 5.7 | 2.63 |
| CF–NH2 | 69.63 | 12.53 | 17.84 | 18.00 | 25.62 |
| CF–HP–COOH | 71.10 | 25.31 | 3.59 | 35.60 | 5.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, P.; Yang, L.; Zhang, S.; Gu, Z. Constructing a Superior Interfacial Microstructure on Carbon Fiber for High Interfacial and Mechanical Properties of Epoxy Composites. Nanomaterials 2022, 12, 2778. https://doi.org/10.3390/nano12162778
Han P, Yang L, Zhang S, Gu Z. Constructing a Superior Interfacial Microstructure on Carbon Fiber for High Interfacial and Mechanical Properties of Epoxy Composites. Nanomaterials. 2022; 12(16):2778. https://doi.org/10.3390/nano12162778
Chicago/Turabian StyleHan, Ping, Lina Yang, Susu Zhang, and Zheng Gu. 2022. "Constructing a Superior Interfacial Microstructure on Carbon Fiber for High Interfacial and Mechanical Properties of Epoxy Composites" Nanomaterials 12, no. 16: 2778. https://doi.org/10.3390/nano12162778
APA StyleHan, P., Yang, L., Zhang, S., & Gu, Z. (2022). Constructing a Superior Interfacial Microstructure on Carbon Fiber for High Interfacial and Mechanical Properties of Epoxy Composites. Nanomaterials, 12(16), 2778. https://doi.org/10.3390/nano12162778






