Environmental Assessment of Solar Photo-Fenton Processes at Mild Condition in the Presence of Waste-Derived Bio-Based Substances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Goal and Scope Definition
2.2. Inventory Analysis
Laboratory Level
2.3. Pilot Plant Level
- -
- solar photo-Fenton at pH 2.8, caffeine concentration 1 mg/L
- -
- solar photo-Fenton at pH 2.8, caffeine concentration 50 mg/L
- -
- solar photo-Fenton at pH 5.0, caffeine concentration 1 mg/L
- -
- solar photo-Fenton at pH 5.0 with BBS-OMW, and caffeine concentration 1 mg/L
- -
- solar photo-Fenton at pH 5. with BBS-OMW, caffeine concentration 50 mg/L and increase of the reactant’s concentration
3. Results
3.1. Results at Laboratory Level
3.2. Results at Pilot Plant Level
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, S.Y.; Tan, X.; Show, P.L.; Rambabu, K.; Banat, F.; Veeramuthu, A.; Lau, B.F.; Ng, E.P.; Ling, T.C. Incorporating biowaste into circular bioeconomy: A critical review of current trend and scaling up feasibility. Environ. Technol. Innov. 2020, 19, 101034. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Montoneri, E.; Mainero, D.; Boffa, V.; Perrone, D.G.; Montoneri, C. Biochemenergy: A project to turn an urban wastes treatment plant into biorefinery for the production of energy, chemicals and consumer’s products with friendly environmental impact. Int. J. Glob. Environ. Issues 2011, 11, 170–196. [Google Scholar] [CrossRef]
- Fava, F.; Totaro, G.; Diels, L.; Reis, M.; Duarte, J.; Carioca, O.B.; Poggi-Varaldo, H.M.; Ferreira, B.S. Biowaste biorefinery in Europe: Opportunities and research & development needs. New Biotechnol. 2015, 32, 100–108. [Google Scholar] [CrossRef]
- Hingsamer, M.; Jungmeier, G. Biorefineries. In The Role of Bioenergy in the Emerging Bioeconomy: Resources, Technologies, Sustainability and Policy; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar] [CrossRef]
- Rolewicz-Kalińska, A.; Lelicińska-Serafin, K.; Manczarski, P. The circular economy and organic fraction of municipal solid waste recycling strategies. Energies 2020, 13, 4366. [Google Scholar] [CrossRef]
- Bioeconomy and EU Green Deal. Available online: https://ec.europa.eu/knowledge4policy/bioeconomy/bioeconomy-european-green-deal_en (accessed on 18 November 2020).
- Bianco Prevot, A.; Avetta, P.; Berto, S.; Daniele, P.G.; Tabasso, S.; Mainero, D.; Montoneri, E. Soluble Bio-Based Substances Obtained from Urban Wastes: Isolation and Characterization; Springer: Cham, Switzerland, 2015; pp. 1–16. [Google Scholar] [CrossRef]
- Montoneri, E. Municipal waste treatment, technological scale up and commercial exploitation: The case of bio-waste lignin to soluble lignin-like polymers. In Food Waste Reduction and Valorisation: Sustainability Assessment and Policy Analysis; Springer: Cham, Switzerland, 2017; pp. 79–120. [Google Scholar] [CrossRef]
- Avetta, P.; Bella, F.; Bianco Prevot, A.; Laurenti, E.; Montoneri, E.; Arques, A.; Carlos, L. Waste cleaning waste: Photodegradation of monochlorophenols in the presence of waste-derived photosensitizer. ACS Sustain. Chem. Eng. 2013, 1, 1545–1550. [Google Scholar] [CrossRef]
- Bianco Prevot, A.; Baino, F.; Fabbri, D.; Franzoso, F.; Magnacca, G.; Nisticò, R.; Arques, A. Urban biowaste-derived sensitizing materials for caffeine photodegradation. Environ. Sci. Pollut. Res. 2017, 24, 12599–12607. [Google Scholar] [CrossRef]
- Bianco Prevot, A.; Avetta, P.; Fabbri, D.; Laurenti, E.; Marchis, T.; Perrone, D.G.; Montoneri, E.; Boffa, V. Waste-derived bioorganic substances for light-induced generation of reactive oxygenated species. ChemSusChem 2011, 4, 85–90. [Google Scholar] [CrossRef]
- Gomis, J.; Carlos, L.; Bianco Prevot, A.; Teixeira, A.C.S.C.; Mora, M.; Amat, A.M.; Vicente, R.; Arques, A. Bio-based substances from urban waste as auxiliaries for solar photo-Fenton treatment under mild conditions: Optimization of operational variables. Catal. Today 2015, 240, 39–45. [Google Scholar] [CrossRef]
- Gomis Juan Gonçalves, M.G.; Vercher, R.F.; Sabater, C.; Castillo, M.A.; Prevot, A.B.; Amat, A.M.; Arques, A. Determination of photostability, biocompatibility and efficiency as photo-Fenton auxiliaries of three different types of soluble bio-based substances (SBO). Catal. Today 2015, 252, 177–183. [Google Scholar] [CrossRef]
- Lastre-Acosta, A.M.; Vicente, R.; Mora, M.; Jáuregui-Haza, U.J.; Arques, A.; Teixeira, A.C.S.C. Photo-Fenton reaction at mildly acidic conditions: Assessing the effect of bio-organic substances of different origin and characteristics through experimental design. J. Environ. Sci. Health Part A 2019, 54, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Michael-Kordatou, I.; Andreou, R.; Iacovou, M.; Frontistis, Z.; Hapeshi, E.; Michael, C.; Fatta-Kassinos, D. On the capacity of ozonation to remove antimicrobial compounds, resistant bacteria and toxicity from urban wastewater effluents. J. Hazard. Mater. 2017, 323, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Stefan, M.I. Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications; IWA Publishing: London, UK, 2017; p. 16. ISBN 9781780407197. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Pesqueira, J.F.J.R.; Pereira, M.F.R.; Silva, A.M.T. Environmental impact assessment of advanced urban wastewater treatment technologies for the removal of priority substances and contaminants of emerging concern: A review. J. Clean. Prod. 2020, 261, 121078. [Google Scholar] [CrossRef]
- Sbardella, L.; Gala, I.V.; Comas, J.; Carbonell, S.M.; Rodríguez-Roda, I.; Gernjak, W. Integrated assessment of sulfate-based AOPs for pharmaceutical active compound removal from wastewater. J. Clean. Prod. 2020, 260, 121014. [Google Scholar] [CrossRef]
- Serra, A.; Brillas, E.; Domènech, X.; Peral, J. Treatment of biorecalcitrant α-methylphenylglycine aqueous solutions with a solar photo-Fenton-aerobic biological coupling: Biodegradability and environmental impact assessment. Chem. Eng. J. 2011, 172, 654–664. [Google Scholar] [CrossRef]
- Gallego-Schmid, A.; Tarpani, R.R.Z.; Miralles-Cuevas, S.; Cabrera-Reina, A.; Malato, S.; Azapagic, A. Environmental assessment of solar photo-Fenton processes in combination with nanofiltration for the removal of micro-contaminants from real wastewaters. Sci. Total Environ. 2019, 650, 2210–2220. [Google Scholar] [CrossRef]
- García-Ballesteros, S.; Grimalt, J.; Berto, S.; Minella, M.; Laurenti, E.; Vicente, R.; López-Pérez, M.F.; Amat, A.M.; Bianco Prevot, A.; Arques, A. New Route for Valorization of Oil Mill Wastes: Isolation of Humic-Like Substances to be Employed in Solar-Driven Processes for Pollutants Removal. ACS Omega 2018, 3, 13073–13080. [Google Scholar] [CrossRef]
- SimaPro. BV, P.C.; SimaPro: Amersfoort, The Netherlands, 2021.
- Montoneri, E.; Boffa, V.; Savarino, P.; Perrone, D.; Ghezzo, M.; Montoneri, C.; Mendichi, R. Acid soluble bio-organic substances isolated from urban bio-waste. Chemical composition and properties of products. Waste Manag. 2011, 31, 10–17. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. From laboratory to industrial scale: A scale-up framework for chemical processes in life cycle assessment studies. J. Clean. Prod. 2016, 135, 1085–1097. [Google Scholar] [CrossRef]
- Shen, L.; Worrell, E.; Patel, M.K. Open-loop recycling: A LCA case study of PET bottle-to-fibre recycling. Resour. Conserv. Recycl. 2010, 55, 34–52. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Miao, X.S.; Koenig, B.G.; Struger, J. Distribution of acidic and neutral drugs in surface waters near sewage treatment plants in the lower Great Lakes, Canada. Environ. Toxicol. Chem. 2003, 22, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Malato, S.; Blanco, J.; Campos, A.; Cáceres, J.; Guillard, C.; Herrmann, J.M.; Fernández-Alba, A.R. Effect of operating parameters on the testing of new industrial titania catalysts at solar pilot plant scale. Appl. Catal. B Environ. 2003, 42, 349–357. [Google Scholar] [CrossRef]
- Fazio, S.; Biganzioli, F.; De Laurentiis, V.; Zampori, L.; Sala, S.; Diaconu, E. Supporting Information to the Characterisation Factors of Recommended EF Life Cycle Impact Assessment Methods; Version 2, from ILCD to EF 3.0, EUR 29600 EN, PUBSY No. JRC114822, New Models and Differences with ILCD, EUR; European Commission: Ispra, Italy, 2018; ISBN 978-92-79-98584-3. [CrossRef]


| Category | Laboratory Level | Pilot Plant Level |
|---|---|---|
| Goal | To identify the main environmental hotspots of near neutral pH photo-Fenton process and to analyse in detail the environmental behaviour and performance of two waste derived BBS used as auxiliary agents: green compost-derived and olive oil mill waste-derived BBS. | To evaluate the effect of performing, at different pH (pH 2.8, pH 5.0), solar photo-Fenton process, using waste derived BBS as auxiliary agent. |
| Functional unit | The removal of 90% caffeine from 250 mL of Milli-Q water | Treatment of 1 m3 of water contaminated with caffeine per day. |
| System boundaries | The system was modelled considering: (i) the production of each chemical reagent (H2SO4, H2O2, etc.); (ii) the isolation process to obtain the BBS; (iii) the degradation process itself. (The infrastructure and equipment used during the experimental analysis were excluded from the calculation). | Cradle-to-grave approach, encompassing the construction, the use for degradation processes and the decommissioning of the treatment plant. |
| Experimental conditions | See Supplementary Information—Table S1. | See Supplementary Information—Table S2. |
| Input | BBS-GC | BBS-OMW |
|---|---|---|
| Transport (tkm) | 0.40 | 0.15 |
| NaOH (g) | 133 | / |
| KOH (g) | / | 617 |
| Water (kg) | 37 | 110 |
| HCl (g) | 50 | / |
| Heat (extraction) (kJ) | 6084 | 2306 |
| Electricity (filtration) (kWh) | / | 0.036 |
| Electricity (centrifugation) (kWh) | 0.9 | / |
| Heat (drying) (kJ) | 608 | 608 |
| Process output (kg) | 1 | 1 |
| Input | Unit | Photo Fenton with BBS-OMW | Photo Fenton with BBS-GC |
|---|---|---|---|
| FeSO4 | mg | 6.2 | 6.2 |
| H2O2 | mg | 15 | 6.25 |
| H2SO4 | mg | 0.1 | 0.1 |
| NaOH | mg | 0.09 | 0.09 |
| BBS | mg | 2.5 | 2.43 |
| Electricity | kWh | 0.05 | 0.075 |
| Input | Amount | Unit |
|---|---|---|
| Stainless steel | 55 | kg |
| Aluminium | 9 | kg |
| Borosilicate glass | 5.57 | kg |
| Anodizing process | 2 | m2 |
| Polypropylene (PP) | 7 | kg |
| Pumps | 2 | items |
| Transport | 36 | tkm |
| Parameter | 1 mg/L Caffeine | 50 mg/L Caffeine | ||
|---|---|---|---|---|
| pH 2.8 | pH 5.0 | pH 2.8 | pH 5.0 | |
| Accumulated radiation (kJ) | 90 | 11,271 | 5713 | 16,635 |
| Required surface (m2) | 0.058 | 7.25 | 3.67 | 10.7 |
| N° of pilot plants equivalent | 1 * | 16.11 | 8.16 | 23.77 |
| Parameter | 1 mg/L Caffeine | 50 mg/L Caffeine | ||
|---|---|---|---|---|
| pH 2.8 | pH 5.0 | pH 2.8 | pH 5.0 | |
| H2SO4 (g) | 284.88 | 174.66 | 270.47 | 220.45 |
| H2O2 (g) | 100.90 | 100.90 | 100.90 | 303.03 |
| FeCl3·6H2O (g) | 20.00 | 20.00 | 20.00 | 50.00 |
| NaOH (g) | 56.80 | / | 57.60 | 18.00 |
| BBS-OMW (g) | / | / | / | 30 |
| Transport of chemicals (tkm) | 0.05 | 0.05 | 0.05 | 0.05 |
| N° of pilot plants equivalent normalized | 2.3 × 10−5 | 0.0037 | 0.0019 | 0.0054 |
| Electricity (kWh) | 0.44 | 7.08 | 3.59 | 10.45 |
| Impact Category | Unit | BBS-OMW | BBS-GC |
|---|---|---|---|
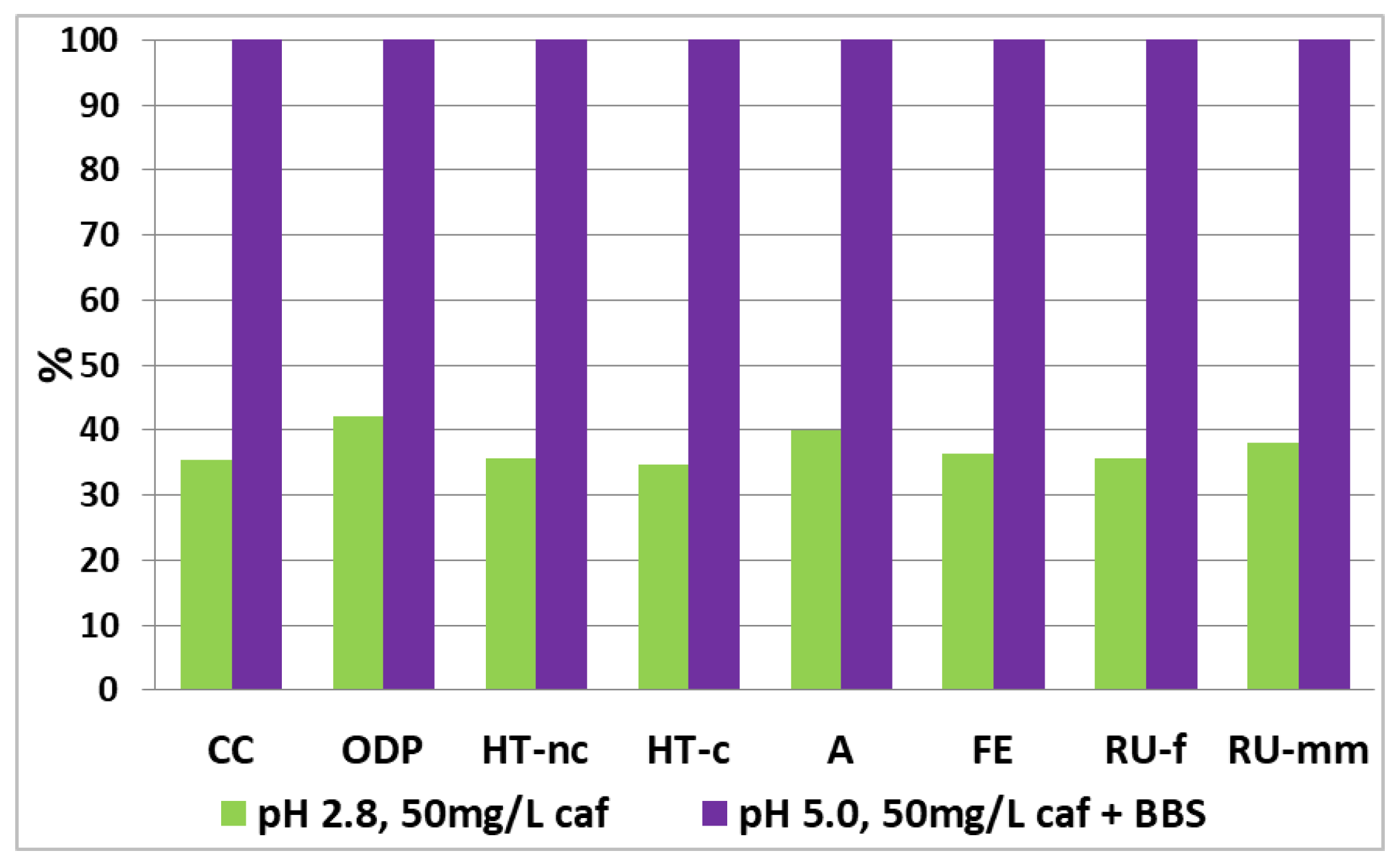
| Climate change (CC) | kg CO2 eq | 1.498 | 1.338 |
| Ozone depletion (OD) | kg CFC11 eq | 1.75 × 10−7 | 2.84 × 10−7 |
| Human toxicity, non-cancer (HT-nc) | CTUh | 4.351 × 10−8 | 1.31 × 10−8 |
| Human toxicity, cancer (HT-c) | CTUh | 7.85 × 10−10 | 4.85 × 10−10 |
| Acidification (A) | mol H+ eq | 7.49 × 10−3 | 6.62 × 10−3 |
| Eutrophication, freshwater (FE) | kg P eq | 7.94 × 10−4 | 3.29 × 10−4 |
| Resource use, fossils (RU-f) | MJ | 24.678 | 22.361 |
| Resource use, minerals and metals (RU-mm) | kg Sb eq | 3.803 × 10−5 | 1.76 × 10−5 |
| Heading | Total | FeSO4 | H2O2 | H2SO4 | NaOH | BBS-OMW | Electricity |
|---|---|---|---|---|---|---|---|
| CC kg CO2 eq | 1.69 × 10−2 | 1.07 × 10−6 | 1.77 × 10−5 | 1.13 × 10−8 | 1.20 × 10−7 | 3.75 × 10−6 | 1.69 × 10−2 |
| OD kg CFC-11 eq | 1.83 × 10−9 | 1.08 × 10−13 | 2.15 × 10−12 | 1.35 × 10−15 | 7.19 × 10−14 | 4.37 × 10−13 | 1.82 × 10−9 |
| HT-nc CTUh | 2.55 × 10−10 | 4.53 × 10−14 | 2.12 × 10−13 | 5.43 × 10−16 | 2.44 × 10−15 | 1.09 × 10−13 | 2.55 × 10−10 |
| HT-c CTUh | 7.91 × 10−12 | 1.60 × 10−15 | 4.40 × 10−14 | 1.70 × 10−17 | 6.36 × 10−17 | 1.96 × 10−15 | 7.86 × 10−12 |
| A mol H+ eq | 1.50 × 10−4 | 8.48 × 10−9 | 7.14 × 10−8 | 1.12 × 10−9 | 6.95 × 10−10 | 1.87 × 10−8 | 1.50 × 10−4 |
| FE kg P eq | 7.05 × 10−6 | 1.07 × 10−9 | 6.55 × 10−9 | 6.43 × 10−12 | 6.16 × 10−11 | 1.98 × 10−9 | 7.04 × 10−6 |
| RU-f MJ | 3.87 × 10−1 | 1.87 × 10−5 | 3.07 × 10−4 | 4.37 × 10−7 | 1.51 × 10−6 | 6.17 × 10−5 | 3.87 × 10−1 |
| RU-mm kg Sb eq | 1.37 × 10−7 | 1.06 × 10−10 | 3.27 × 10−10 | 1.51 × 10−12 | 2.74 × 10−12 | 9.51 × 10−11 | 1.36 × 10−7 |
| Impact Category (and Unit) | Total | FeSO4 | H2O2 | H2SO4 | NaOH | BBS-GC | Electricity |
|---|---|---|---|---|---|---|---|
| CC kg CO2 eq | 2.53 × 10−2 | 1.07 × 10−6 | 7.36 × 10−6 | 1.13 × 10−8 | 1.20 × 10−7 | 3.25 × 10−6 | 2.53 × 10−2 |
| OD kg CFC-11 eq | 2.74 × 10−9 | 1.08 × 10−13 | 8.94 × 10−13 | 1.35 × 10−15 | 7.19 × 10−14 | 6.90 × 10−13 | 2.73 × 10−9 |
| HT-nc CTUh | 3.83 × 10−10 | 4.53 × 10−14 | 8.82 × 10−14 | 5.43 × 10−16 | 2.44 × 10−15 | 3.18 × 10−14 | 3.83 × 10−10 |
| HT-c CTUh | 1.18 × 10−11 | 1.60 × 10−15 | 1.83 × 10−14 | 1.70 × 10−17 | 6.36 × 10−17 | 1.18 × 10−15 | 1.18 × 10−11 |
| A mol H+ eq | 2.25 × 10−4 | 8.48 × 10−9 | 2.97 × 10−8 | 1.12 × 10−9 | 6.95 × 10−10 | 1.61 × 10−8 | 2.25 × 10−4 |
| FE kg P eq | 1.06 × 10−5 | 1.07 × 10−9 | 2.73 × 10−9 | 6.43 × 10−12 | 6.16 × 10−11 | 7.99 × 10−10 | 1.06 × 10−5 |
| RU-f MJ | 5.81 × 10−1 | 1.87 × 10−5 | 1.28 × 10−4 | 4.37 × 10−7 | 1.51 × 10−6 | 5.43 × 10−5 | 5.81 × 10−1 |
| RU-mm kg Sb eq | 2.05 × 10−7 | 1.06 × 10−10 | 1.36 × 10−10 | 1.51 × 10−12 | 2.74 × 10−12 | 4.28 × 10−11 | 2.05 × 10−7 |
| Total | Steel | Aluminum | Boro- Silicate Glass | Polypropylene | Anodi- Zing Process | Pumps | Transport | |
|---|---|---|---|---|---|---|---|---|
| CC kg CO2 eq | 409.71 | 248.39 | 89.81 | 13.15 | 16.67 | 6.65 | 16.55 | 18.50 |
| OD kg CFC-11 eq | 2.68 × 10−5 | 1.26 × 10−5 | 7.42 × 10−6 | 8.81 × 10−7 | 3.40 × 10−7 | 7.05 × 10−7 | 9.57 × 10−7 | 3.96 × 10−6 |
| HT-nc CTUh | 2.01 × 10−5 | 1.18 × 10−5 | 3.25 × 10−6 | 1.73 × 10−7 | 1.19 × 10−7 | 9.74 × 10−8 | 4.45 × 10−6 | 2.50 × 10−7 |
| HT-c CTUh | 4.60 × 10−6 | 4.14 × 10−6 | 2.17 × 10−7 | 7.95 × 10−9 | 3.84 × 10−9 | 6.44 × 10−9 | 2.23 × 10−7 | 8.69 × 10−9 |
| A mol H+ eq | 2.88 | 1.60 | 0.65 | 0.11 | 0.07 | 0.04 | 0.36 | 0.05 |
| FE kg P eq | 1.87 × 10−1 | 9.42 × 10−2 | 4.08 × 10−2 | 4.21 × 10−3 | 2.95 × 10−3 | 5.25 × 10−3 | 3.80 × 10−2 | 1.95 × 10−3 |
| RU-f MJ | 5005.53 | 2642.93 | 1106.73 | 158.49 | 527.55 | 115.40 | 180.49 | 273.94 |
| RU-mm kg Sb eq | 1.47 × 10−2 | 9.11 × 10−3 | 3.39 × 10−4 | 1.94 × 10−3 | 1.55 × 10−4 | 7.23 × 10−5 | 2.14 × 10−3 | 9.10 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costamagna, M.; Arques, A.; Lo-Iacono-Ferreira, V.G.; Bianco Prevot, A. Environmental Assessment of Solar Photo-Fenton Processes at Mild Condition in the Presence of Waste-Derived Bio-Based Substances. Nanomaterials 2022, 12, 2781. https://doi.org/10.3390/nano12162781
Costamagna M, Arques A, Lo-Iacono-Ferreira VG, Bianco Prevot A. Environmental Assessment of Solar Photo-Fenton Processes at Mild Condition in the Presence of Waste-Derived Bio-Based Substances. Nanomaterials. 2022; 12(16):2781. https://doi.org/10.3390/nano12162781
Chicago/Turabian StyleCostamagna, Mattia, Antonio Arques, Vanesa G. Lo-Iacono-Ferreira, and Alessandra Bianco Prevot. 2022. "Environmental Assessment of Solar Photo-Fenton Processes at Mild Condition in the Presence of Waste-Derived Bio-Based Substances" Nanomaterials 12, no. 16: 2781. https://doi.org/10.3390/nano12162781
APA StyleCostamagna, M., Arques, A., Lo-Iacono-Ferreira, V. G., & Bianco Prevot, A. (2022). Environmental Assessment of Solar Photo-Fenton Processes at Mild Condition in the Presence of Waste-Derived Bio-Based Substances. Nanomaterials, 12(16), 2781. https://doi.org/10.3390/nano12162781









