Microwave-Assisted Synthesis of Sulfur Quantum Dots for Detection of Alkaline Phosphatase Activity
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Apparatus
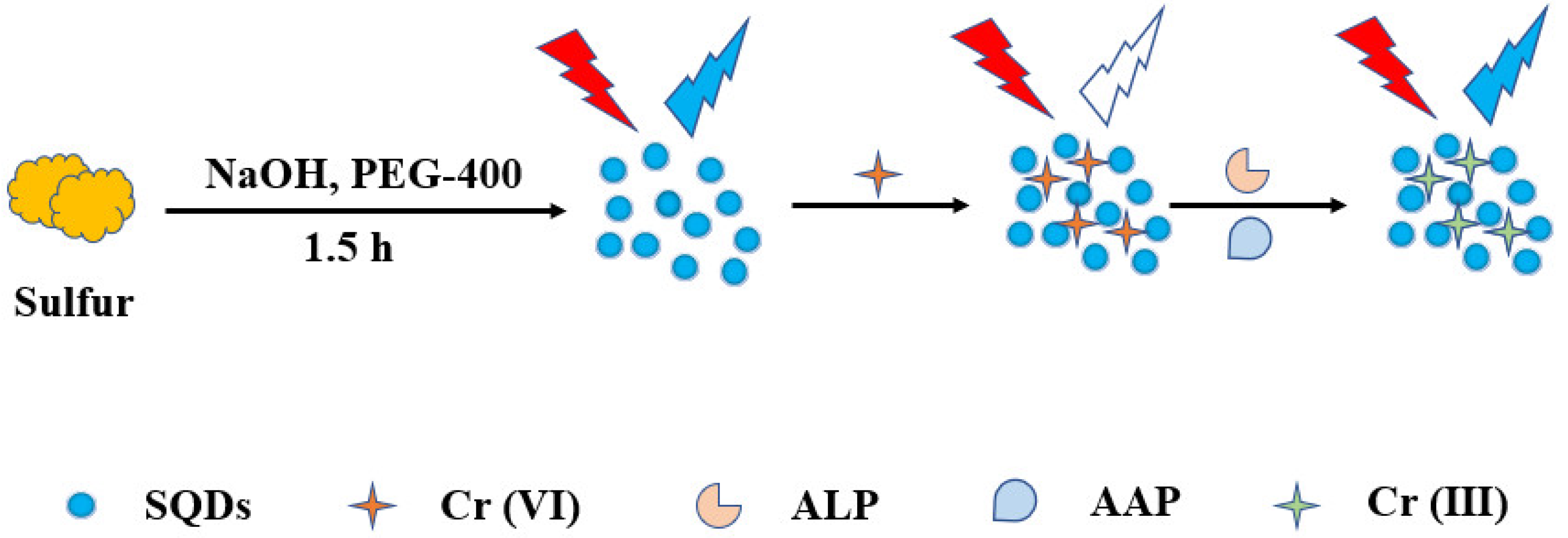
2.2. Synthesis of SQDs
2.3. Determination of ALP
3. Results and Discussion
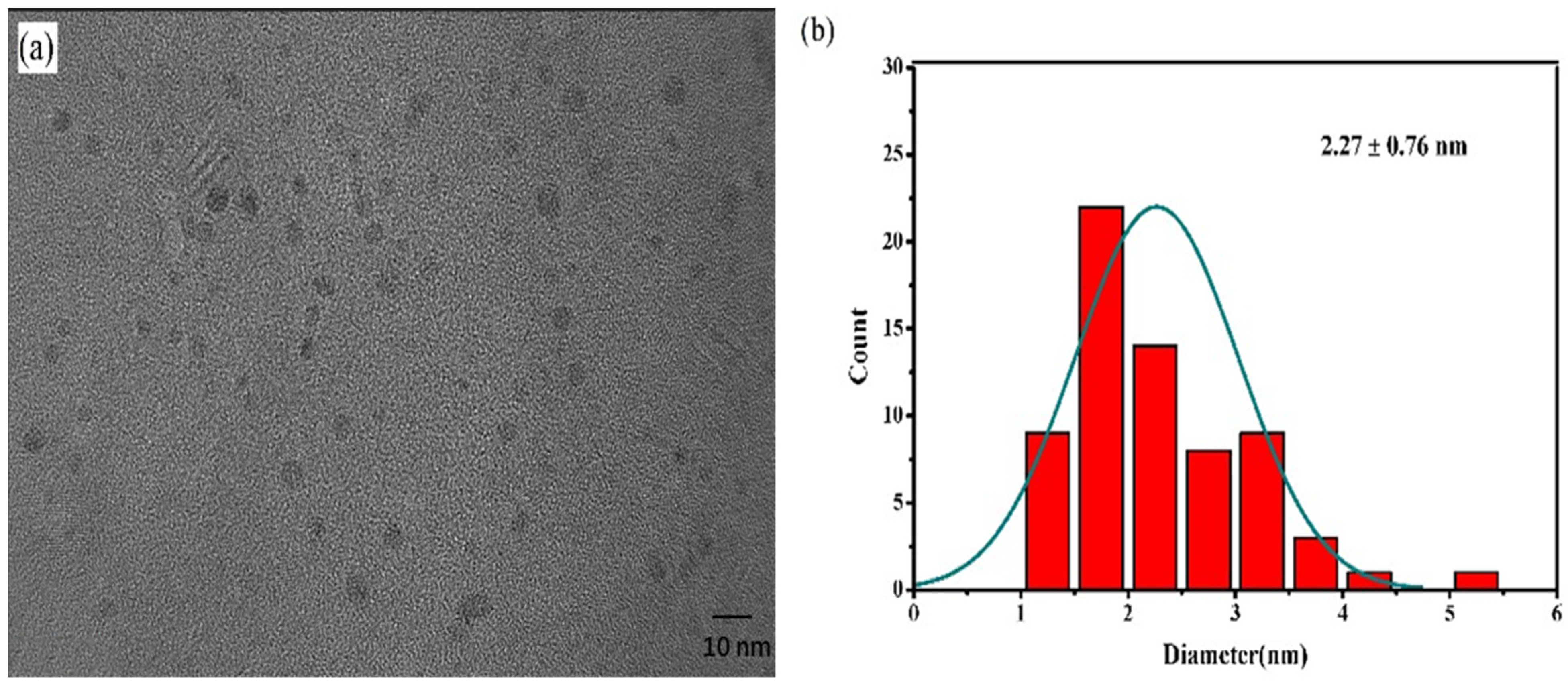
3.1. Preparation and Characterization of the SQDs
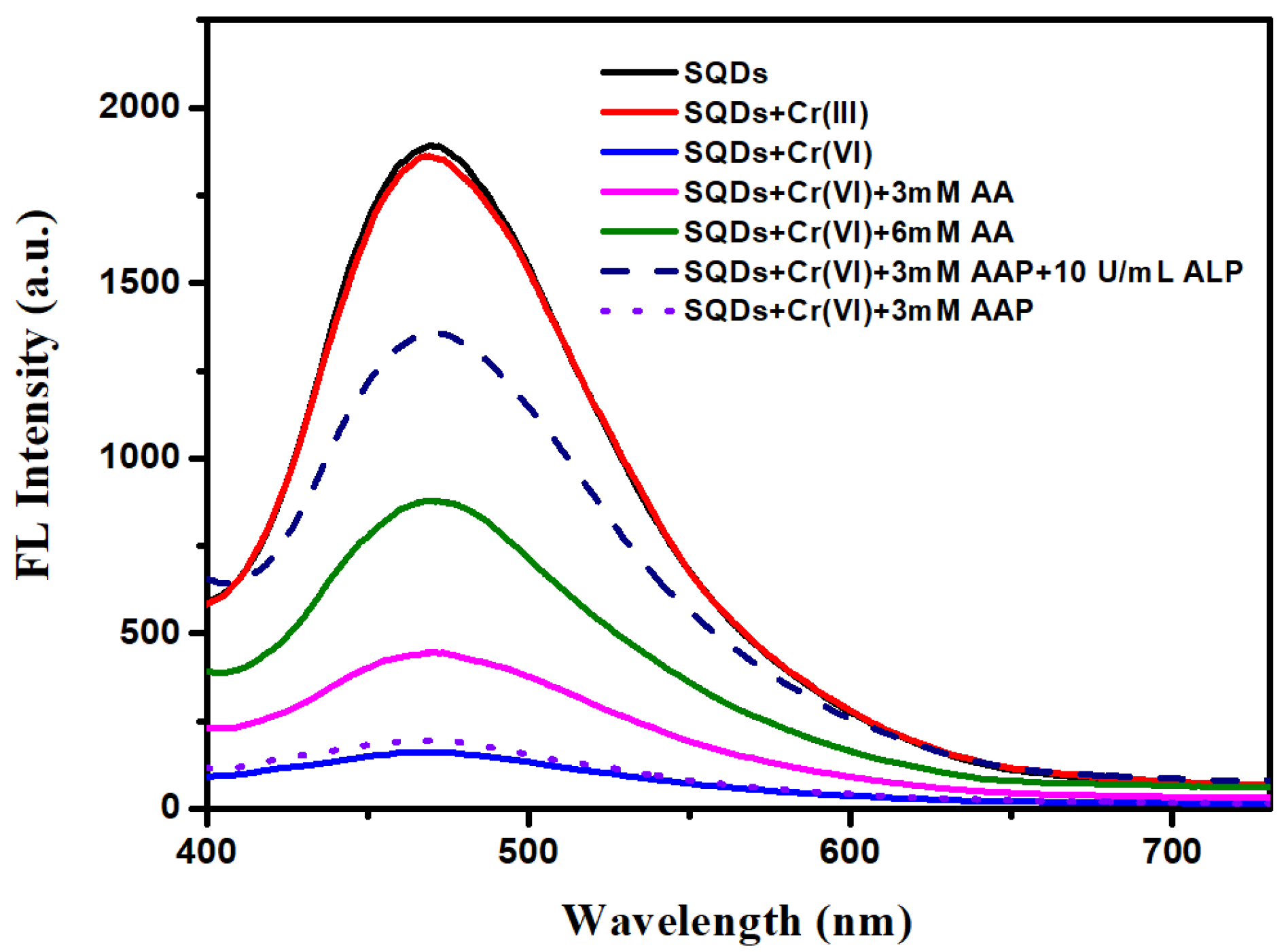
3.2. Feasibility of the Assay for ALP Analysis
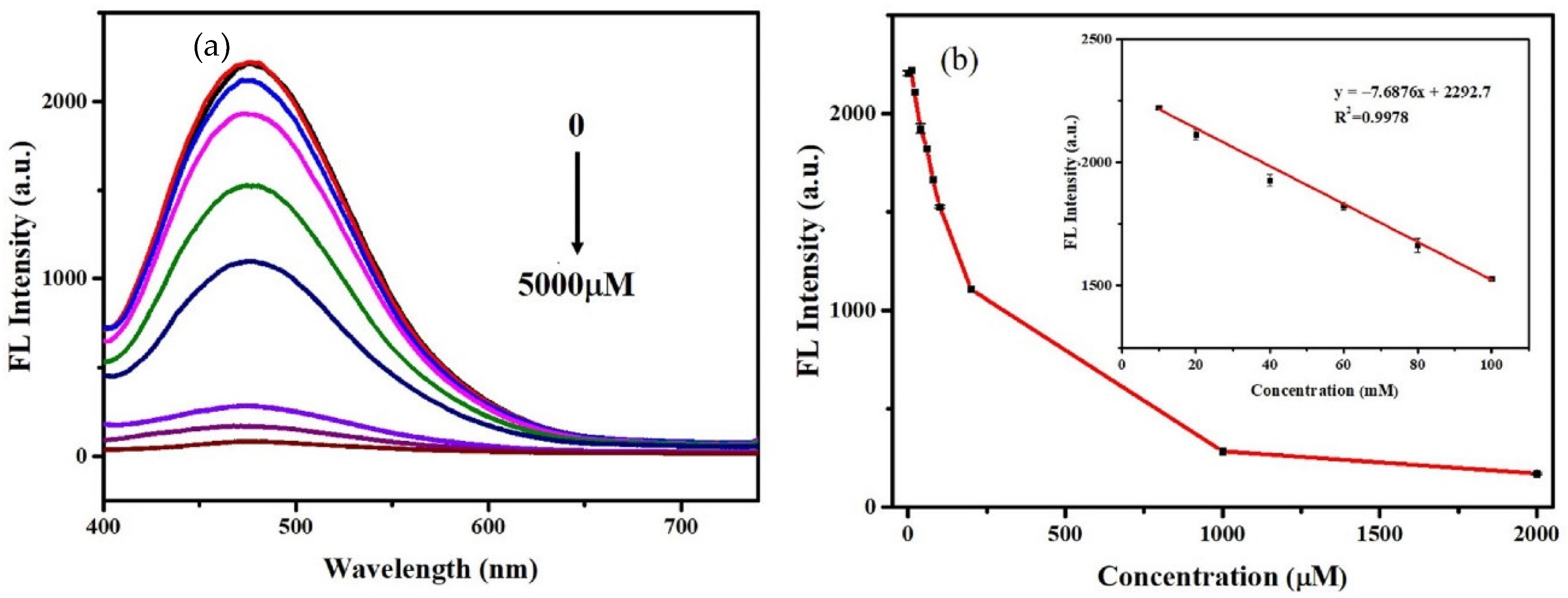
3.3. Detection of Cr (VI)
3.4. Detection of ALP
| Probe | Linear Range/U·mL−1 | LOD/U·mL−1 | Ref. |
|---|---|---|---|
| Luminol– Tb–GMP CPNPs | 0.00005–0.1 | 0.00002 | [46] |
| Hydrogelator | 0−2.8 | 0.06 | [31] |
| NIR-Phos-1,NIR-Phos-2 | 0–1.0 | 10−5–10−3 | [47] |
| 5-bromo-4-chloro-3-indolyl phosphate | 10–1000 | 0.87 | [48] |
| CuNPs | 0.1–40 | 0.05 | [49] |
| SQDs | 1.5–5.0 | 0.13 | This work |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, H.J.; Jin, H.; Chua, B.; Son, A. Clustered detection of eleven phthalic acid esters by fluorescence of graphene quantum dots displaced from gold nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 4186–4196. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Yang, X.; Li, C.; Zhang, Y.; Yang, H.; Chen, G.; Wang, Q. Tumor microenvironment-activated nir-ii nanotheranostic system for precise diagnosis and treatment of peritoneal metastasis. Angew. Chem. Int. Ed. 2020, 59, 7219–7223. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Feng, S.; Park, C.Y.; Park, K.Y.; Song, J.; Park, J.P.; Chun, H.S.; Park, T.J. Fluorescence detection of histamine based on specific binding bioreceptors and carbon quantum dots. Biosens. Bioelectron. 2020, 167, 112519. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Z.; Yao, Z.; Zhao, K.; Shao, F.; Su, J.; Liu, S. Black phosphorus quantum dots encapsulated biodegradable hollow mesoporous mno2: Dual-modality cancer imaging and synergistic chemo-phototherapy. Adv. Funct. Mater. 2021, 31, 2104643. [Google Scholar] [CrossRef]
- Yang, H.; Huang, H.; Ma, X.; Zhang, Y.; Yang, X.; Yu, M.; Sun, Z.; Li, C.; Wu, F.; Wang, Q. Au-doped ag2te quantum dots with bright nir-iib fluorescence for in situ monitoring of angiogenesis and arteriogenesis in a hindlimb ischemic model. Adv. Mater. 2021, 33, 2103953. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, X.; Zhang, C.; Zhao, P.; Ragauskas, A.J.; Song, X. Fluorescence enhancement of lignin-based carbon quantum dots by concentration-dependent and electron-donating substituent synergy and their cell imaging applications. ACS Appl. Mater. Interfaces 2021, 13, 61565–61577. [Google Scholar] [CrossRef]
- Das, P.; Maruthapandi, M.; Saravanan, A.; Natan, M.; Jacobi, G.; Banin, E.; Gedanken, A. Carbon dots for heavy-metal sensing, ph-sensitive cargo delivery, and antibacterial applications. ACS Appl. Nano Mater. 2020, 3, 11777–11790. [Google Scholar] [CrossRef]
- Wang, H.; Li, T.; Hashem, A.M.; Abdel-Ghany, A.E.; El-Tawil, R.S.; Abuzeid, H.M.; Coughlin, A.; Chang, K.; Zhang, S.; El-Mounayri, H.; et al. Nanostructured molybdenum-oxide anodes for lithium-ion batteries: An outstanding increase in capacity. Nanomaterials 2021, 12, 13. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Tailor made magnetic nanolights: Fabrication to cancer theranostics applications. Nanoscale Adv. 2021, 3, 6762–6796. [Google Scholar] [CrossRef]
- Yan, X.; Pei, Y.; Chen, H.; Zhao, J.; Zhou, Z.; Wang, H.; Zhang, L.; Wang, J.; Li, X.; Qin, C.; et al. Self-assembled networked pbs distribution quantum dots for resistive switching and artificial synapse performance boost of memristors. Adv. Mater. 2019, 31, 1805284. [Google Scholar] [CrossRef]
- Hudson, M.H.; Chen, M.; Kamysbayev, V.; Janke, E.M.; Lan, X.; Allan, G.; Delerue, C.; Lee, B.; Guyot-Sionnest, P.; Talapin, D.V. Conduction band fine structure in colloidal hgte quantum dots. ACS Nano 2018, 12, 9397–9404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, L.; Sun, Z.; Luo, J. Rational chemical doping of metal halide perovskites. Chem. Soc. Rev. 2019, 48, 517–539. [Google Scholar] [CrossRef]
- Pu, C.; Qin, H.; Gao, Y.; Zhou, J.; Wang, P.; Peng, X. Synthetic control of exciton behavior in colloidal quantum dots. J. Am. Chem. Soc. 2017, 139, 3302–3311. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Arshad, F.; Sk, M.P. Emergence of sulfur quantum dots: Unfolding their synthesis, properties, and applications. Adv. Colloid Interface Sci. 2020, 285, 102274. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.E.; Zhang, P.; Yang, D.; Wang, Z. Synthesis, photoluminescence properties and sensing applications of luminescent sulfur nanodots. Chem. Commun. 2020, 56, 10982–10988. [Google Scholar] [CrossRef]
- Song, Y.; Tan, J.; Wang, G.; Gao, P.; Lei, J.; Zhou, L. Oxygen accelerated scalable synthesis of highly fluorescent sulfur quantum dots. Chem. Sci. 2019, 11, 772–777. [Google Scholar] [CrossRef]
- Arshad, F.; Sk, M.P. Luminescent sulfur quantum dots for colorimetric discrimination of multiple metal ions. ACS Appl. Nano Mater. 2020, 3, 3044–3049. [Google Scholar] [CrossRef]
- Wang, C.; Wei, Z.; Pan, C.; Pan, Z.; Wang, X.; Liu, J.; Wang, H.; Huang, G.; Wang, M.; Mao, L. Dual functional hydrogen peroxide boosted one step solvothermal synthesis of highly uniform sulfur quantum dots at elevated temperature and their fluorescent sensing. Sens. Actuators B Chem. 2021, 344, 130326. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Y.; Luo, Z.; Zhang, B.; Mei, X.; Yang, X. Facile synthesis of fluorescent sulfur quantum dots for selective detection of p-nitrophenol in water samples. Microchem. J. 2021, 170, 106735. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, P.; Ji, X.; Wang, H.; Kuang, H.; Cao, W.; Pan, M.; Shi, Y.E.; Wang, Z. Ultrasonication-promoted synthesis of luminescent sulfur nano-dots for cellular imaging applications. Chem. Commun. 2019, 55, 13004–13007. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Guo, Y.; Gu, L.; Wu, Y.; Bindra, A.K.; Teo, W.L.; Wu, F.G.; Zhao, Y. Thiolate-assisted route for constructing chalcogen quantum dots with photoinduced fluorescence enhancement. ACS Appl. Mater. Interfaces 2021, 13, 48449–48456. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.; Liu, L.; Hao, X.; Zheng, J.; Liu, W.; Gao, J.; Zhang, C.C.; Wang, Q. Signal transduction from small particles: Sulfur nanodots featuring mercury sensing, cell entry mechanism and in vitro tracking performance. Chem. Eng. J. 2020, 382, 122907. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Wu, J.; Li, M.; Tan, J.; Fu, W.; Tang, H.; Zhang, P. Negatively charged sulfur quantum dots for treatment of drug-resistant pathogenic bacterial infections. Nano Lett. 2021, 21, 9433–9441. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, Y.; Xu, B.; Zhang, W.; Xie, Y.; Chen, Y.; Wang, L.; Wang, X. A colorimetric and fluorescence dual-signal determination for iron (ii) and H2O2 in food based on sulfur quantum dots. Food Chem. 2022, 366, 130613. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Huang, Z.; Zhong, Q.; Deng, H.; Lai, M.; Yang, Y.; Chen, W.; Xia, X.; Peng, H. Size-focusing results in highly photoluminescent sulfur quantum dots with a stable emission wavelength. Nanoscale 2021, 13, 2519–2526. [Google Scholar] [CrossRef]
- Rong, S.; Chen, Q.; Xu, G.; Wei, F.; Yang, J.; Ren, D.; Cheng, X.; Xia, X.; Li, J.; Gao, M.; et al. Novel and facile synthesis of heparin sulfur quantum dots via oxygen acceleration for ratiometric sensing of uric acid in human serum. Sens. Actuators B Chem. 2022, 353, 131146. [Google Scholar] [CrossRef]
- Arshad, F.; Sk, M.P.; Maurya, S.K.; Siddique, H.R. Mechanochemical synthesis of sulfur quantum dots for cellular imaging. ACS Appl. Nano Mater. 2021, 4, 3339–3344. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Xiong, Y.; Kershaw, S.V.; Li, T.; Wang, Y.; Zhai, Y.; Rogach, A.L. Hydrogen peroxide assisted synthesis of highly luminescent sulfur quantum dots. Angew. Chem. Int. Ed. Engl. 2019, 58, 7040–7044. [Google Scholar] [CrossRef]
- Li, S.; Chen, D.; Zheng, F.; Zhou, H.; Jiang, S.; Wu, Y. Water-soluble and lowly toxic sulphur quantum dots. Adv. Funct. Mater. 2014, 24, 7133–7138. [Google Scholar] [CrossRef]
- Niu, X.; Ye, K.; Wang, L.; Lin, Y.; Du, D. A review on emerging principles and strategies for colorimetric and fluorescent detection of alkaline phosphatase activity. Anal. Chim. Acta 2019, 1086, 29–45. [Google Scholar] [CrossRef]
- Dong, L.; Miao, Q.; Hai, Z.; Yuan, Y.; Liang, G. Enzymatic hydrogelation-induced fluorescence turn-off for sensing alkaline phosphatase in vitro and in living cells. Anal. Chem. 2015, 87, 6475–6478. [Google Scholar] [CrossRef] [PubMed]
- Haarhaus, M.; Brandenburg, V.; Kalantar-Zadeh, K.; Stenvinkel, P.; Magnusson, P. Alkaline phosphatase: A novel treatment target for cardiovascular disease in ckd. Nat. Rev. Nephrol. 2017, 13, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, D.; Jiang, Y.; Ni, P.; Zhang, C.; Wang, B.; Yang, F.; Lu, Y.; Sun, J. Logically regulating peroxidase-like activity of gold nanoclusters for sensing phosphate-containing metabolites and alkaline phosphatase activity. Anal. Chem. 2019, 91, 15017–15024. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, M.; Blazek, T.; Gorski, W. Electroanalysis of enzyme activity in small biological samples: Alkaline phosphatase. Anal. Chem. 2021, 93, 14280–14286. [Google Scholar] [CrossRef]
- Liu, X.; Mei, X.; Yang, J.; Li, Y. Hydrogel-involved colorimetric platforms based on layered double oxide nanozymes for point-of-care detection of liver-related biomarkers. ACS Appl. Mater. Interfaces 2022, 14, 6985–6993. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Chu, C.; Yang, M.; Huo, D.; Hou, C. Target-induced transcription amplification to trigger the trans-cleavage activity of crispr/cas13a (titac-cas) for detection of alkaline phosphatase. Biosens. Bioelectron. 2021, 185, 113281. [Google Scholar] [CrossRef]
- Peng, J.; Han, X.X.; Zhang, Q.C.; Yao, H.Q.; Gao, Z.N. Copper sulfide nanoparticle-decorated graphene as a catalytic amplification platform for electrochemical detection of alkaline phosphatase activity. Anal. Chim. Acta 2015, 878, 87–94. [Google Scholar] [CrossRef]
- Zhan, Y.; Yang, S.; Chen, L.; Zeng, Y.; Li, L.; Lin, Z.; Guo, L.; Xu, W. Ultrahigh efficient fret ratiometric fluorescence biosensor for visual detection of alkaline phosphatase activity and its inhibitor. ACS Sustain. Chem. Eng. 2021, 9, 12922–12929. [Google Scholar] [CrossRef]
- Hou, L.; Qin, Y.; Li, J.; Qin, S.; Huang, Y.; Lin, T.; Guo, L.; Ye, F.; Zhao, S. A ratiometric multicolor fluorescence biosensor for visual detection of alkaline phosphatase activity via a smartphone. Biosens. Bioelectron. 2019, 143, 111605. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Liu, K.; Zhang, Y.; Gao, G.; Huang, X.; Hou, S. Near-infrared ratiometric probe with a self-immolative spacer for rapid and sensitive detection of alkaline phosphatase activity and imaging in vivo. Anal. Chim. Acta 2020, 1094, 113–121. [Google Scholar] [CrossRef]
- Li, X.; Cai, M.; Shen, Z.; Zhang, M.; Tang, Z.; Luo, S.; Lu, N. “Three-in-one” nanocomposite as multifunctional nanozyme for ultrasensitive ratiometric fluorescence detection of alkaline phosphatase. J. Mater. Chem. B 2022. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Liu, Q.; Su, X. Fluorometric determination of the activity of alkaline phosphatase based on a system composed of WS2 quantum dots and MnO2 nanosheets. Microchim. Acta 2019, 186, 839. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, H.; Liu, S.; Bai, Z.; Zhang, S.; Zhang, X.; Zhang, C. Assembling of sulfur quantum dots in fission of sublimed sulfur. J. Am. Chem. Soc. 2018, 140, 7878–7884. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Du, Q.; Huang, Y.; Wang, L.; Cheng, S.; Wang, Z.; Wong, T.N.; Yeow, E.K.L.; Sun, H. Rapid synthesis of sulfur nanodots by one-step hydrothermal reaction for luminescence-based applications. ACS Appl. Nano Mater. 2019, 2, 6622–6628. [Google Scholar] [CrossRef]
- Fan, S.; Li, X.; Ma, F.; Yang, M.; Su, J.; Chen, X. Sulfur quantum dot based fluorescence assay for lactate dehydrogenase activity detection. J. Photochem. Photobiol. A Chem. 2022, 430, 113989. [Google Scholar] [CrossRef]
- Tong, Y.J.; Yu, L.D.; Wu, L.L.; Cao, S.P.; Liang, R.P.; Zhang, L.; Xia, X.H.; Qiu, J.D. Aggregation-induced emission of luminol: A novel strategy for fluorescence ratiometric detection of alp and as(v) with high sensitivity and selectivity. Chem. Commun. 2018, 54, 7487–7490. [Google Scholar] [CrossRef]
- Park, C.S.; Ha, T.H.; Kim, M.; Raja, N.; Yun, H.S.; Sung, M.J.; Kwon, O.S.; Yoon, H.; Lee, C.S. Fast and sensitive near-infrared fluorescent probes for alp detection and 3d printed calcium phosphate scaffold imaging in vivo. Biosens. Bioelectron. 2018, 105, 151–158. [Google Scholar] [CrossRef]
- Mahato, K.; Chandra, P. Paper-based miniaturized immunosensor for naked eye alp detection based on digital image colorimetry integrated with smartphone. Biosens. Bioelectron. 2019, 128, 9–16. [Google Scholar] [CrossRef]
- Yang, D.; Guo, Z.; Tang, Y.; Miao, P. Poly(thymine)-templated selective formation of copper nanoparticles for alkaline phosphatase analysis aided by alkyne–azide cycloaddition “click” reaction. ACS Appl. Nano Mater. 2017, 1, 168–174. [Google Scholar] [CrossRef]

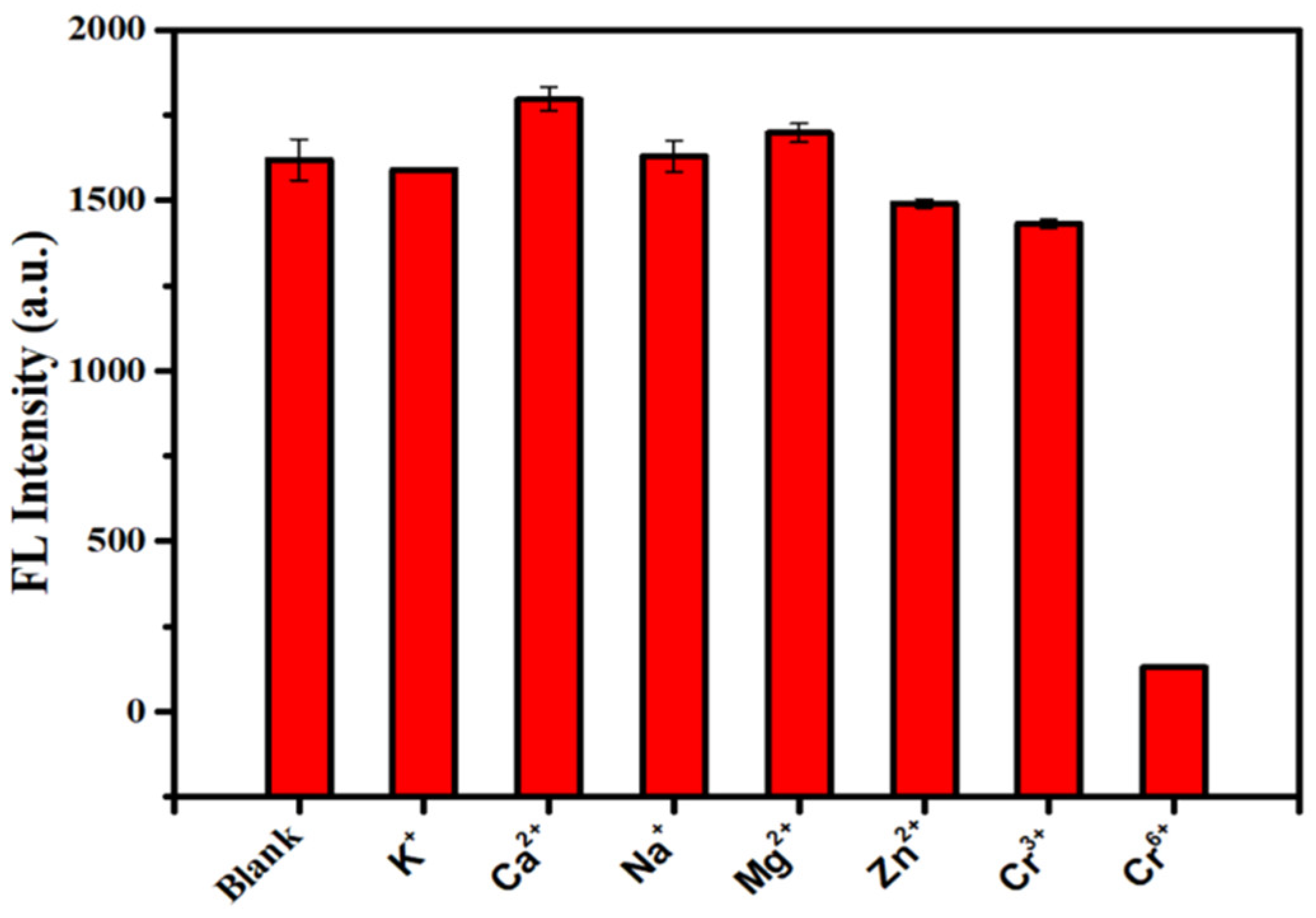
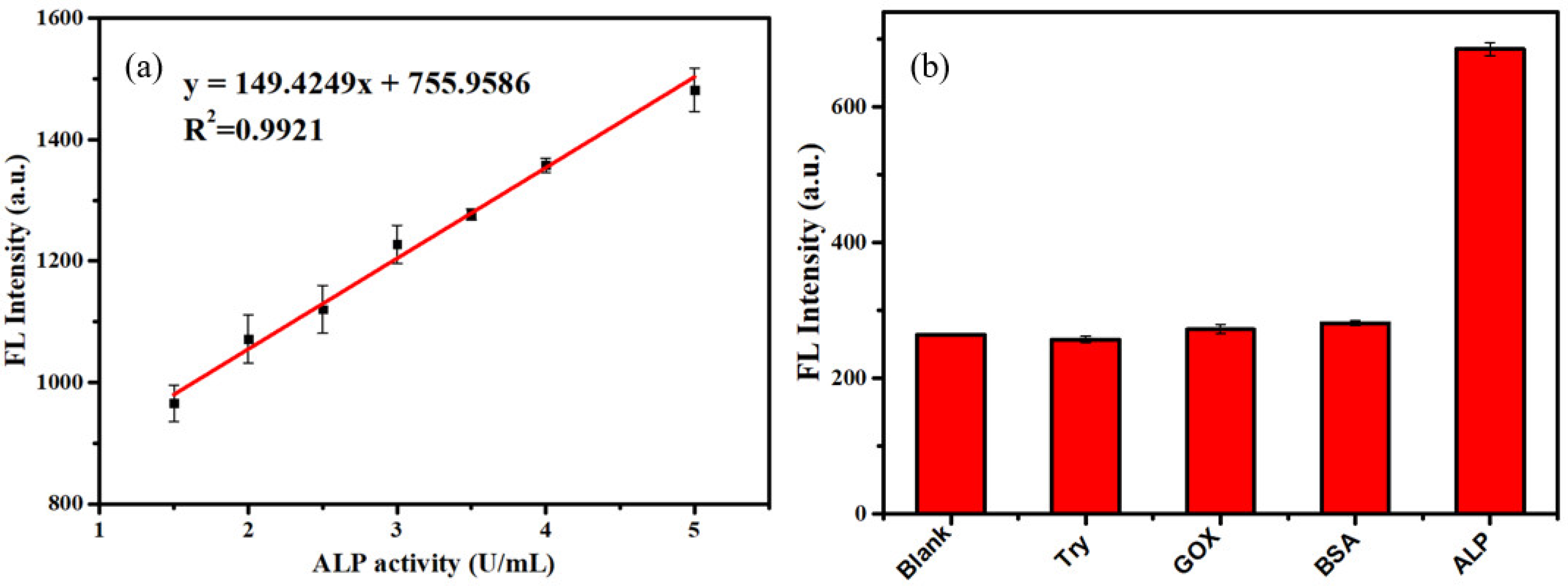
| Samples | Added (U/mL) | Found (U/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 1.43 ± 0.02 | - | 0.301 | |
| human serum | 2 | 2.63 ± 0.09 | 119.61 | 0.495 |
| 2.5 | 3.05 ± 0.20 | 108.15 | 2.497 | |
| 3.5 | 3.05 ± 0.20 | 102.01 | 1.181 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; Zhou, Q.; Yang, M.; Zhang, J.; Chen, X. Microwave-Assisted Synthesis of Sulfur Quantum Dots for Detection of Alkaline Phosphatase Activity. Nanomaterials 2022, 12, 2787. https://doi.org/10.3390/nano12162787
Ma F, Zhou Q, Yang M, Zhang J, Chen X. Microwave-Assisted Synthesis of Sulfur Quantum Dots for Detection of Alkaline Phosphatase Activity. Nanomaterials. 2022; 12(16):2787. https://doi.org/10.3390/nano12162787
Chicago/Turabian StyleMa, Fanghui, Qing Zhou, Minghui Yang, Jianglin Zhang, and Xiang Chen. 2022. "Microwave-Assisted Synthesis of Sulfur Quantum Dots for Detection of Alkaline Phosphatase Activity" Nanomaterials 12, no. 16: 2787. https://doi.org/10.3390/nano12162787
APA StyleMa, F., Zhou, Q., Yang, M., Zhang, J., & Chen, X. (2022). Microwave-Assisted Synthesis of Sulfur Quantum Dots for Detection of Alkaline Phosphatase Activity. Nanomaterials, 12(16), 2787. https://doi.org/10.3390/nano12162787






