Green, Sustainable Synthesis of γ-Fe2O3/MWCNT/Ag Nano-Composites Using the Viscum album Leaf Extract and Waste Car Tire for Removal of Sulfamethazine and Bacteria from Wastewater Streams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
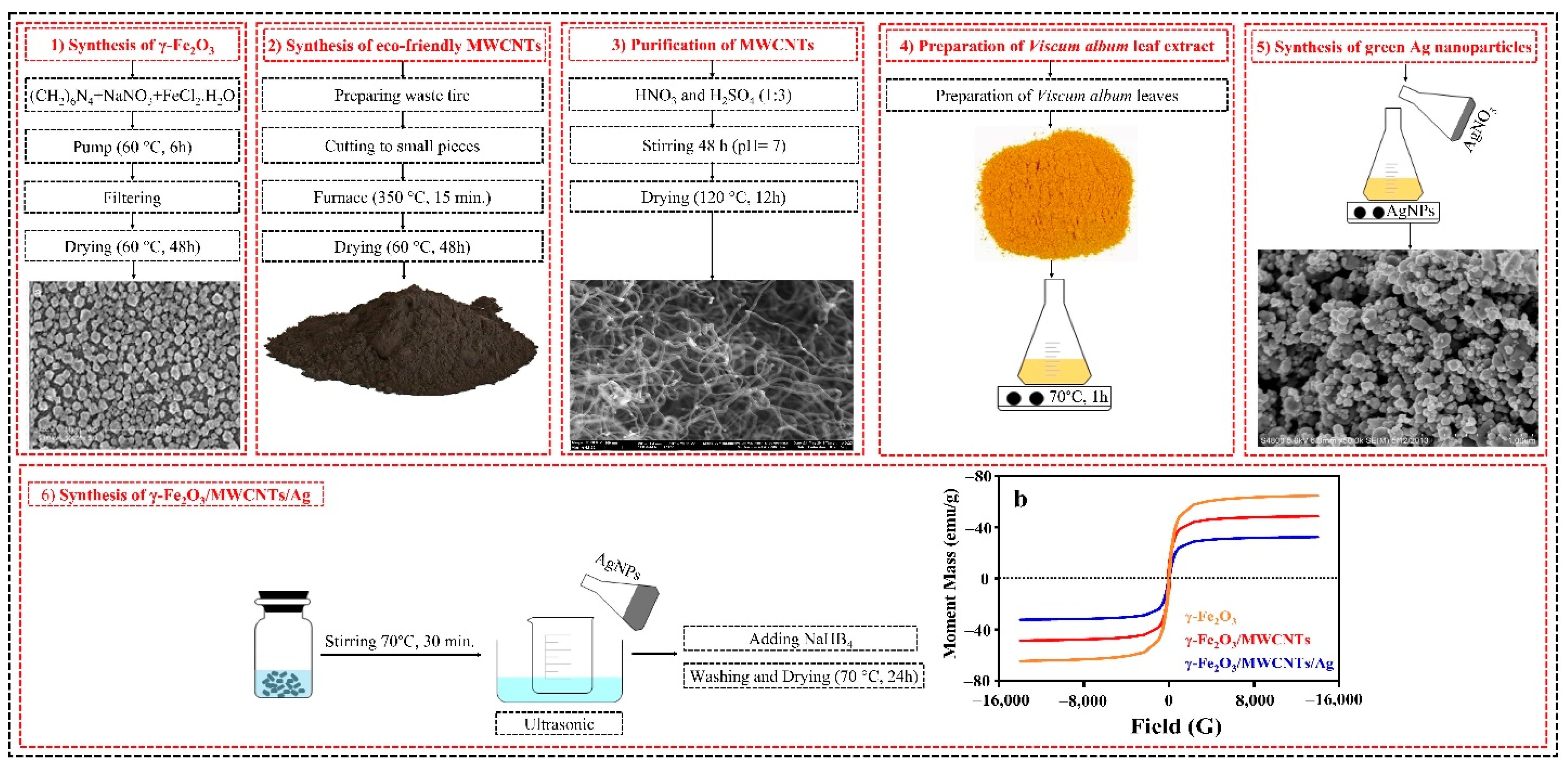
2.2. Synthesis of γ-Fe2O3 NPs
2.3. Green Synthesis of MWCNTs
2.4. Synthesis of γ-Fe2O3/ MWCNTs
2.5. Preparation of Viscum Album Leaf Extract
2.6. Biosynthesis of Ag NPs
2.7. Synthesis of the γ-Fe2O3/MWCNT/Ag NC Particles
2.8. Characterization of the γ-Fe2O3/MWCNT/Ag NC Particles
2.9. Adsorption Experiments
2.10. Design of Experiments by the Taguchi Method
2.11. Adsorption Isotherm
2.12. Adsorption Kinetics
2.13. Adsorption Thermodynamic
2.14. Antibacterial Test
2.15. Data Analyses Using Computer Software
3. Results and Discussion
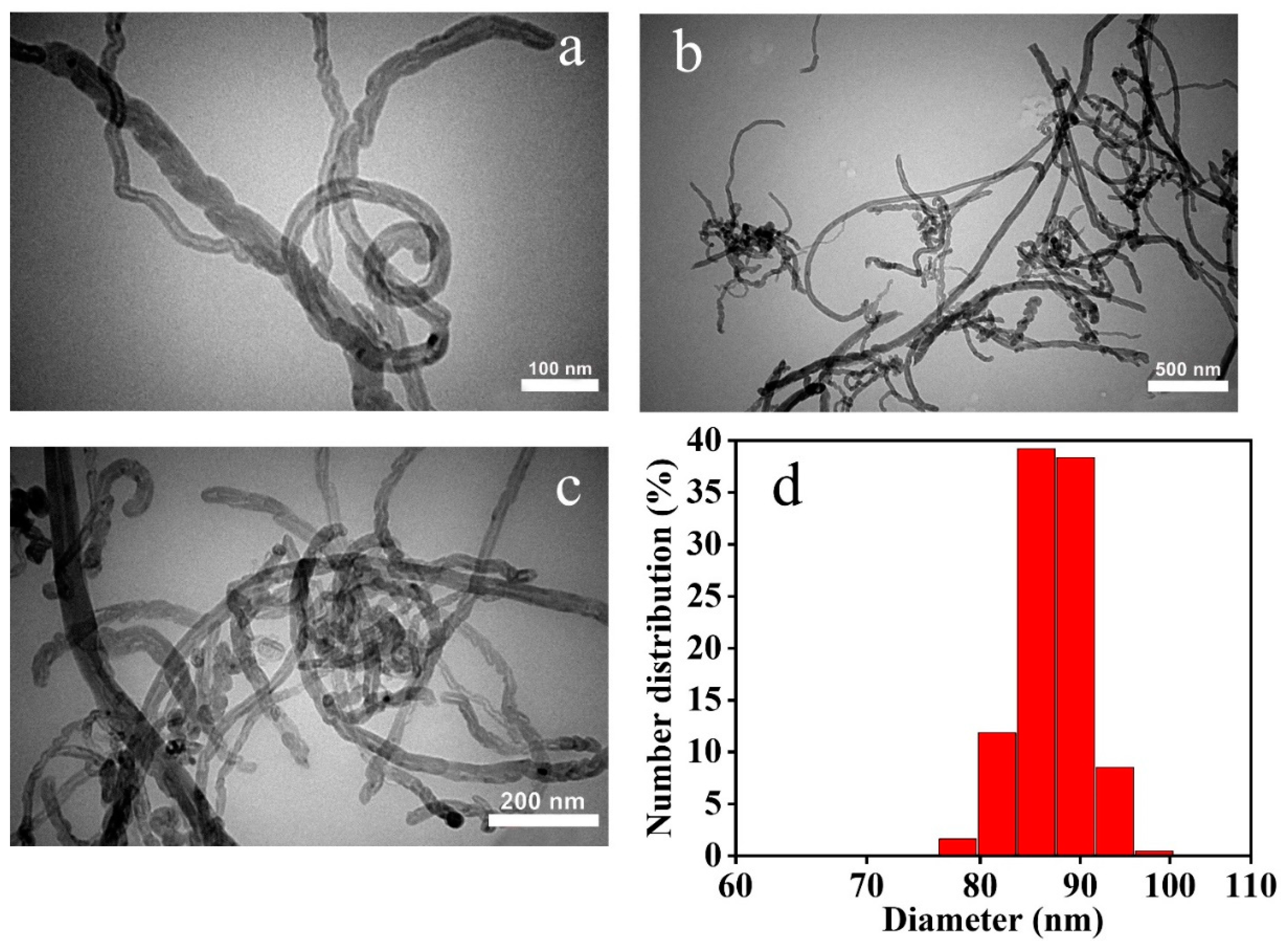
3.1. Structural and Morphological Characteristics of the γ-Fe2O3/MWCNTs/Ag NC Particles
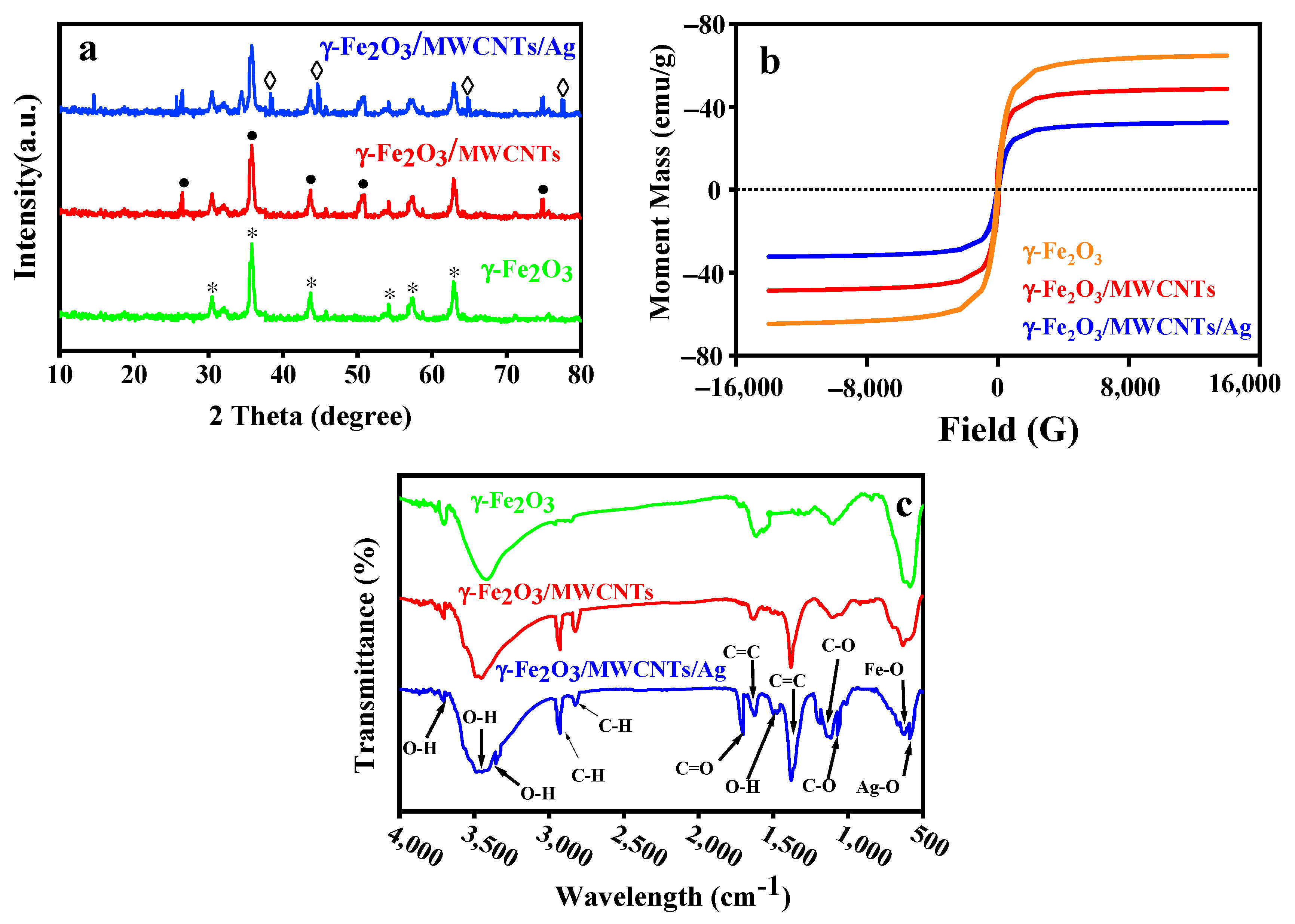
3.2. Crystallinity of the γ-Fe2O3/MWCNT/Ag NC Particles
3.3. Magnetic Properties of the γ-Fe2O3/MWCNT/Ag NC Particles
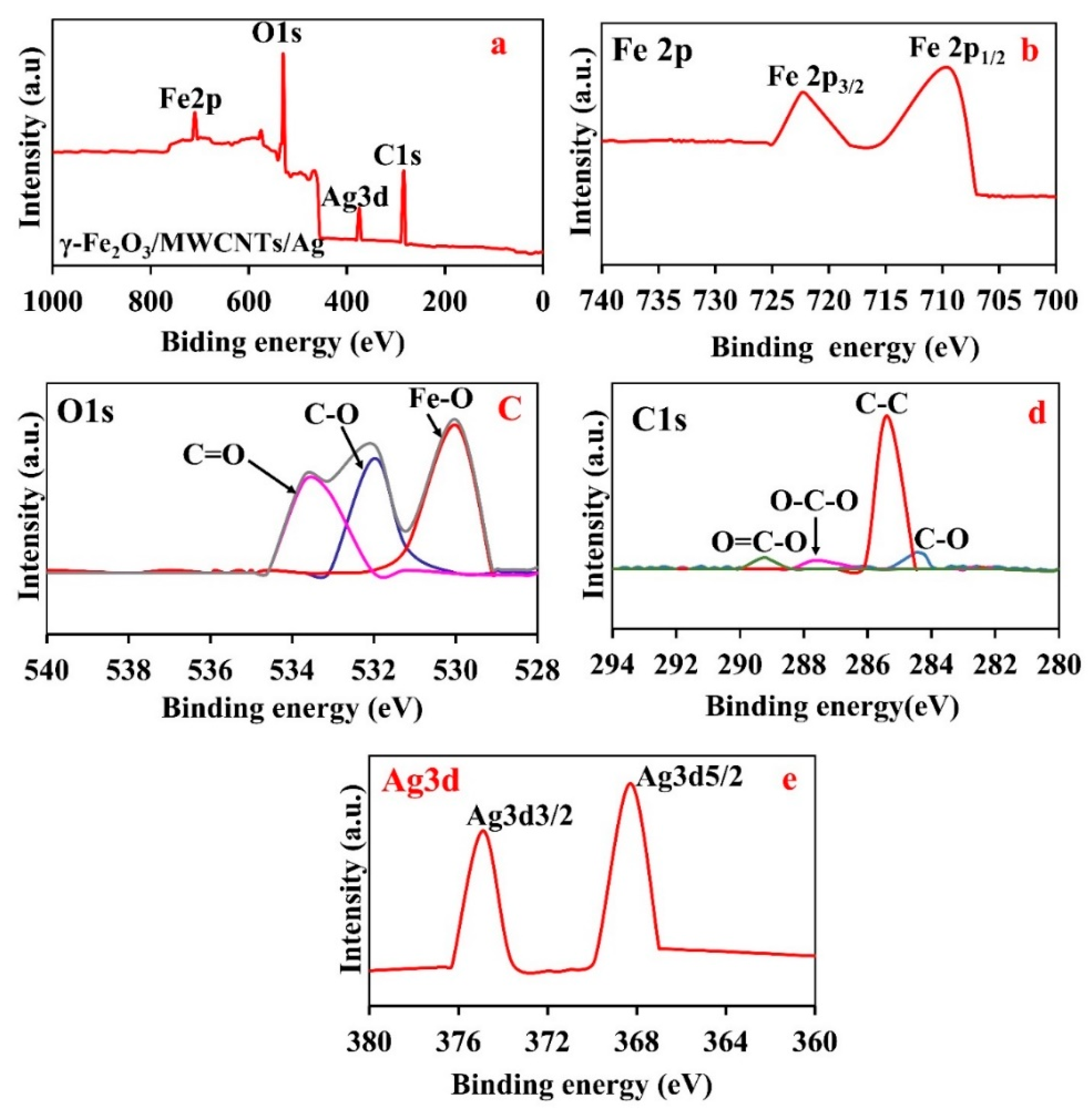
3.4. Surface Chemistry of the γ-Fe2O3/MWCNT/Ag NC Particles
3.5. Specific Surface Area of the γ-Fe2O3/MWCNT/Ag NC Particles
3.6. Thermal Properties of the γ-Fe2O3/MWCNT/Ag NC Particles
3.7. Adsorption Behavior
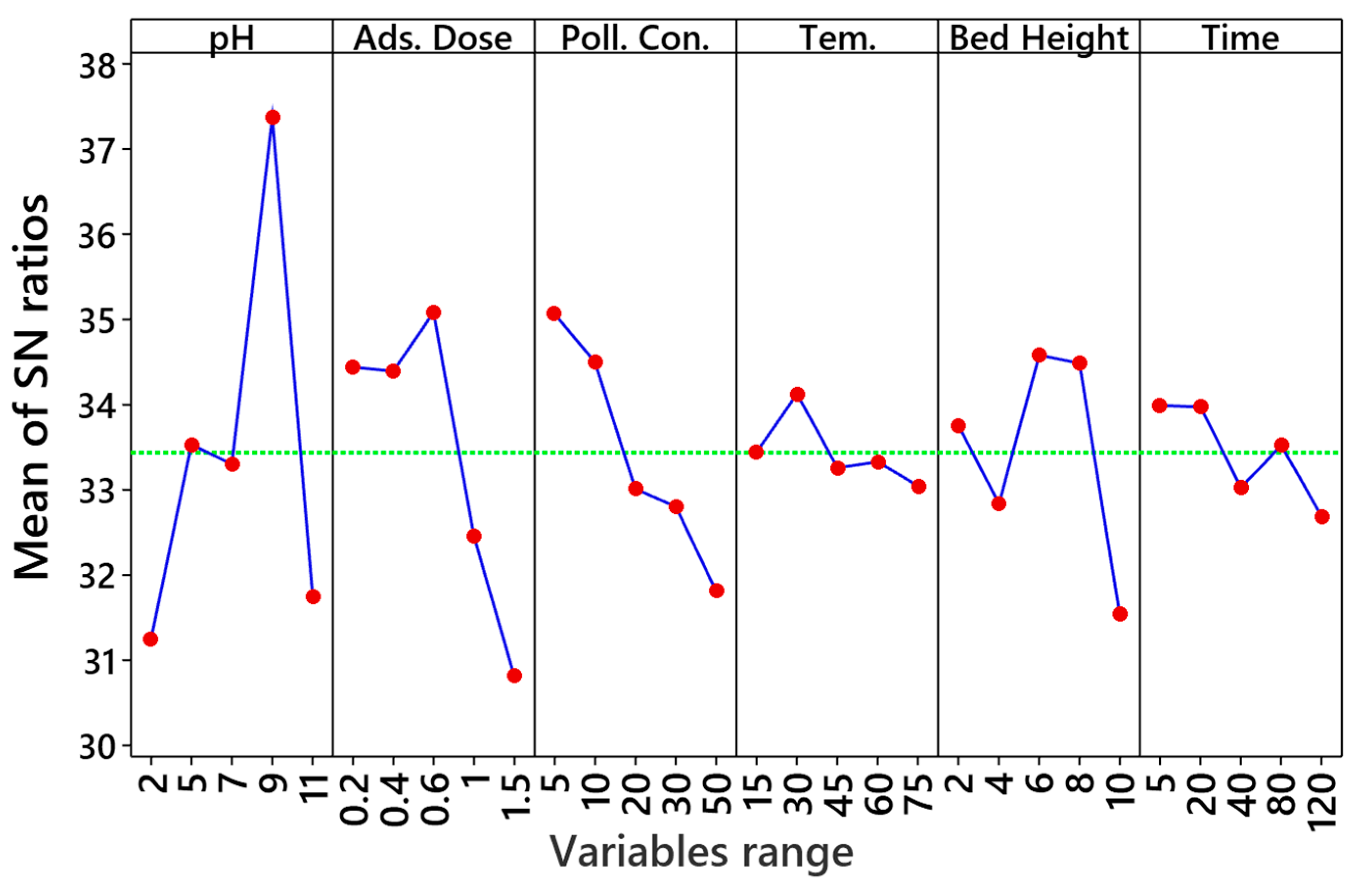
3.7.1. Taguchi Design of the Adsorption Experiment
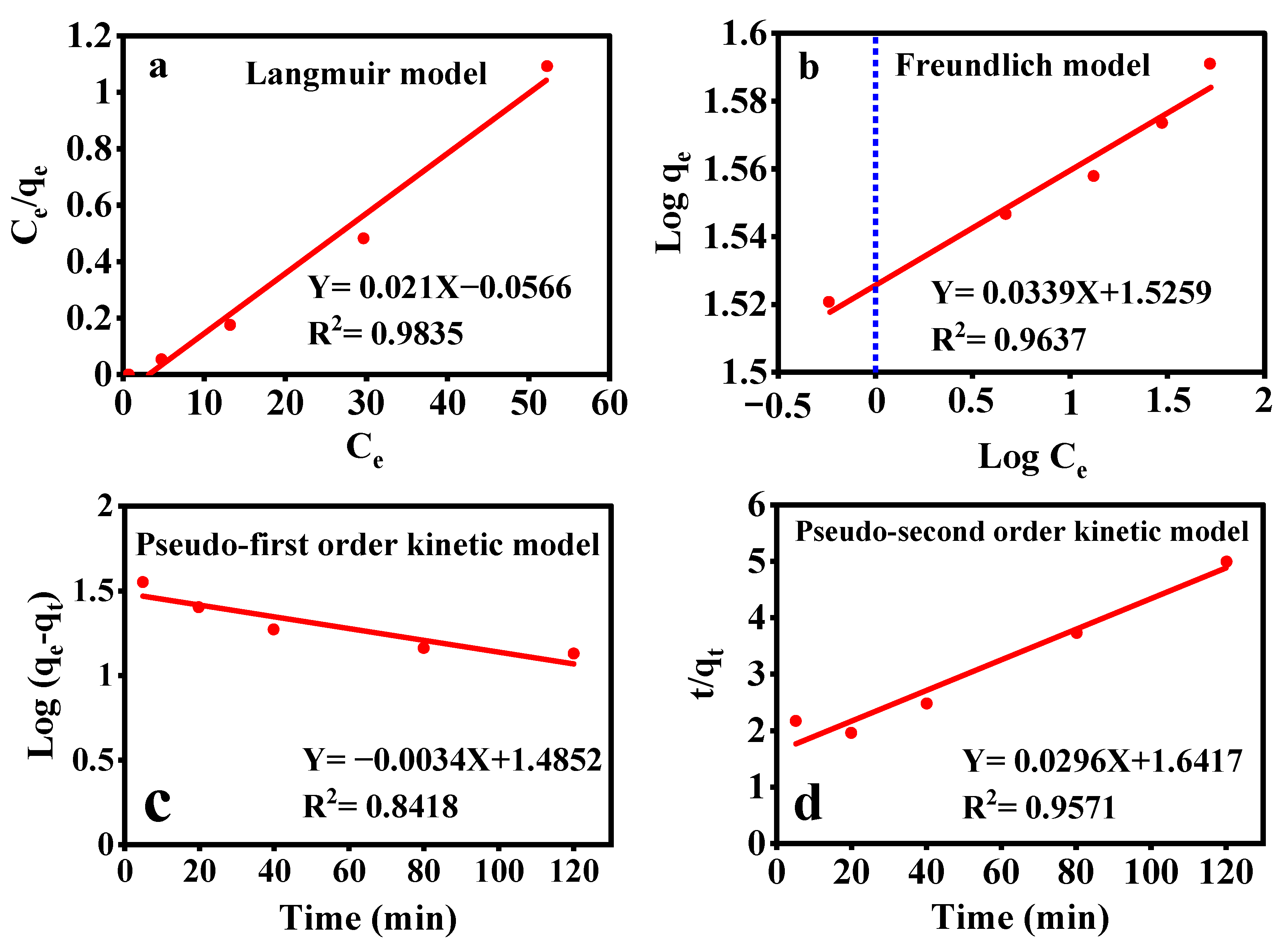
3.7.2. Adsorption Isotherms
3.7.3. Adsorption Kinetics
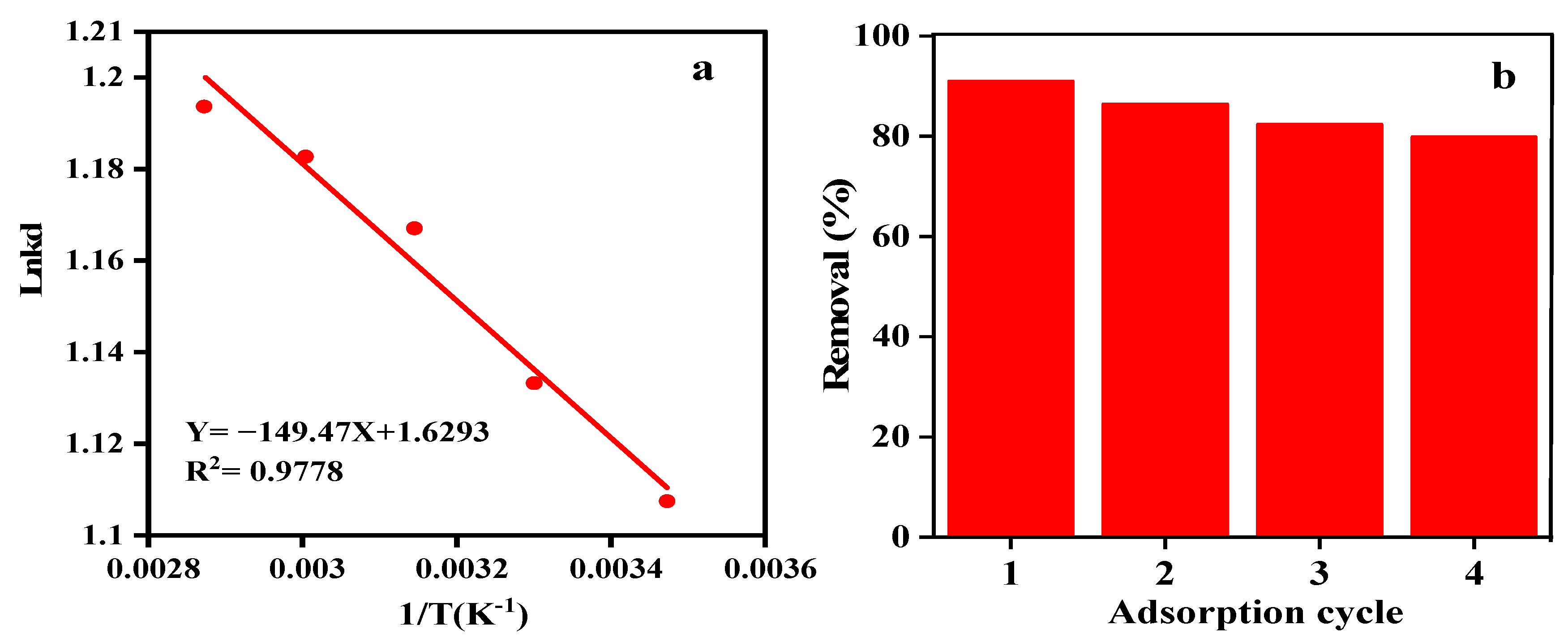
3.7.4. Adsorption Thermodynamics
3.7.5. Adsorbent Reusability
3.7.6. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Yang, Y.; Liu, X.; Zhao, J.; Liu, R.; Xing, B. Interaction of microplastics with antibiotics in aquatic environment: Distribution, adsorption, and toxicity. Environ. Sci. Technol. 2021, 55, 15579–15595. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.L.; Boxall, A.B.; Kolpin, D.W.; Leung, K.M.; Lai, R.W.; Galbán-Malagón, C.; Adell, A.D.; Mondon, J.; Metian, M.; Marchant, R.A. Pharmaceutical pollution of the world’s rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef]
- Yahiaoui, I.; Aissani-Benissad, F.; Fourcade, F.; Amrane, A. Removal of tetracycline hydrochloride from water based on direct anodic oxidation (Pb/PbO2 electrode) coupled to activated sludge culture. Chem. Eng. J. 2013, 221, 418–425. [Google Scholar] [CrossRef]
- Duan, W.; Cui, H.; Jia, X.; Huang, X. Occurrence and ecotoxicity of sulfonamides in the aquatic environment: A review. Sci. Total Environ. 2022, 820, 153178. [Google Scholar] [CrossRef] [PubMed]
- Nafi, A.; Taseidifar, M. Removal of hazardous ions from aqueous solutions: Current methods, with a focus on green ion flotation. J. Environ. Manag. 2022, 319, 115666. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Sayadi, M.H.; Sobhani, S.; Hajiani, M. A potential natural solar light active photocatalyst using magnetic ZnFe2O4@ TiO2/Cu nanocomposite as a high performance and recyclable platform for degradation of naproxen from aqueous solution. J. Clean. Prod. 2020, 268, 122023. [Google Scholar] [CrossRef]
- Kargar, F.; Bemani, A.; Sayadi, M.H.; Ahmadpour, N. Synthesis of modified beta bismuth oxide by titanium oxide and highly efficient solar photocatalytic properties on hydroxychloroquine degradation and pathways. J. Photochem. Photobiol. A Chem. 2021, 419, 113453. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Botcha, N.; Zarie, E.; Elbahri, M. Ups and downs of water photodecolorization by nanocomposite polymer nanofibers. Nanomaterials 2019, 9, 250. [Google Scholar] [CrossRef]
- Homaeigohar, S. The nanosized dye adsorbents for water treatment. Nanomaterials 2020, 10, 295. [Google Scholar] [CrossRef]
- Ghadimi, M.; Zanganehtabar, S.; Homaeigohar, S. An overview of the water remediation potential of nanomaterials and their ecotoxicological impacts. Water 2020, 12, 1150. [Google Scholar] [CrossRef]
- Le, T.-H.; Ng, C.; Tran, N.H.; Chen, H.; Gin, K.Y.-H. Removal of antibiotic residues, antibiotic resistant bacteria and antibiotic resistance genes in municipal wastewater by membrane bioreactor systems. Water Res. 2018, 145, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Chinh, V.D.; Speranza, G.; Migliaresi, C.; van Chuc, N.; Tan, V.M.; Phuong, N.-T. Synthesis of gold nanoparticles decorated with multiwalled carbon nanotubes (Au-MWCNTs) via cysteaminium chloride functionalization. Sci. Rep. 2019, 9, 5667. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, M.; Zhang, S.; Pan, B. Application potential of carbon nanotubes in water treatment: A review. J. Environ. Sci. 2013, 25, 1263. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Strunskus, T.; Strobel, J.; Kienle, L.; Elbahri, M. A flexible oxygenated carbographite nanofilamentous buckypaper as an amphiphilic membrane. Adv. Mater. Interfaces 2018, 5, 1800001. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. An amphiphilic, graphitic buckypaper capturing enzyme biomolecules from water. Water 2019, 11, 2. [Google Scholar] [CrossRef]
- Khalatbary, M.; Sayadi, M.H.; Hajiani, M.; Norouzi, M. Adsorption studies on the removal of malachite green by γ-Fe2O3/MWCNTs/Cellulose as an eco-friendly nanoadsorbent. Biomass Convers. Biorefin. 2022, 25. [Google Scholar] [CrossRef]
- Jia, X.; Cheng, C.; Yu, S.; Yang, J.; Li, Y.; Song, H. Preparation and enhanced acetone sensing properties of flower-like α-Fe2O3/multi-walled carbon nanotube nanocomposites. Sens. Actuators B Chem. 2019, 300, 127012. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Eldesoky, G.E.; Al-Saeedi, S.I.; Nafady, A.; Al-Kadhi, N.S.; Khan, A. Effective and fast adsorptive removal of toxic cationic dye (MB) from aqueous medium using amino-functionalized magnetic multiwall carbon nanotubes. J. Mol. Liq. 2019, 282, 154–161. [Google Scholar] [CrossRef]
- Iqbal, A.; Saeed, A.; Kausar, A.; Arshad, M.; Mahar, J. Synthesis and characterization of DGEBA composites reinforced with Cu/Ag modified carbon nanotubes. Heliyon 2019, 5, e01733. [Google Scholar] [CrossRef]
- Olivi, M.; Zanni, E.; de Bellis, G.; Talora, C.; Sarto, M.S.; Palleschi, C.; Flahaut, E.; Monthioux, M.; Rapino, S.; Uccelletti, D. Inhibition of microbial growth by carbon nanotube networks. Nanoscale 2013, 5, 9023–9029. [Google Scholar] [CrossRef]
- Chamanehpour, E.; Sayadi, M.H.; Hajiani, M. A hierarchical graphitic carbon nitride supported by metal–organic framework and copper nanocomposite as a novel bifunctional catalyst with long-term stability for enhanced carbon dioxide photoreduction under solar light irradiation. Adv. Compos. Hybrid Mater. 2022. [Google Scholar] [CrossRef]
- Tomczyk, A.; Szewczuk-Karpisz, K.; Sokołowska, Z.; Kercheva, M.; Dimitrov, E. Purification of Aqueous Media by Biochars: Feedstock Type Effect on Silver Nanoparticles Removal. Molecules 2020, 25, 2930. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.M.; Westerhoff, P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci.Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Wodka, D.; Bielanska, E.; Socha, R.P.; Elzbieciak-Wodka, M.; Gurgul, J.; Nowak, P.; Warszyński, P.; Kumakiri, I. Photocatalytic activity of titanium dioxide modified by silver nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, H.; Abdel-Ghafar, H.; Mahmoud, M. Multi-walled carbon nanotubes decorated with silver nanoparticles for antimicrobial applications. J. Environ. Chem.Eng. 2021, 9, 105034. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-Álvarez, J.; Vega-Fernández, L.; de Oca-Vásquez, G.M.; Vega-Baudrit, J.R. Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as antimicrobial agents in the agricultural area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Ghollasimood, S.; Ahmadpour, N.; Homaeigohar, S. Biosynthesis of the ZnO/SnO2 nanoparticles and characterization of their photocatalytic potential for removal of organic water pollutants. J. Photochem. Photobiol. A Chem. 2022, 425, 113662. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, S.; Chen, Y.; Zhou, F.; Yan, C. Diatomite coated with Fe2O3 as an efficient heterogeneous catalyst for degradation of organic pollutant. J. Taiwan Inst. Chem. Eng. 2015, 49, 105–112. [Google Scholar] [CrossRef]
- Mwafy, E.A. Eco-friendly approach for the synthesis of MWCNTs from waste tires via chemical vapor deposition, Environmental Nanotechnology. Monit. Manag. 2020, 14, 100342. [Google Scholar] [CrossRef]
- Su, P.-G.; Yu, J.-H.; Chen, I.-C.; Syu, H.-C.; Chiu, S.-W.; Chou, T.-I. Detection of ppb-level NO2 gas using a portable gas-sensing system with a Fe2O3/MWCNTs/WO3 sensor using a pulsed-UV-LED. Anal. Methods 2019, 11, 973–979. [Google Scholar] [CrossRef]
- Chand, K.; Cao, D.; Fouad, D.E.; Shah, A.H.; Dayo, A.Q.; Zhu, K.; Lakhan, M.N.; Mehdi, G.; Dong, S. Green synthesis, characterization and photocatalytic application of silver nanoparticles synthesized by various plant extracts. Arab. J. Chem. 2020, 13, 8248–8261. [Google Scholar] [CrossRef]
- Moazzen, M.; Khaneghah, A.M.; Shariatifar, N.; Ahmadloo, M.; Eş, I.; Baghani, A.N.; Yousefinejad, S.; Alimohammadi, M.; Azari, A.; Dobaradaran, S. Multi-walled carbon nanotubes modified with iron oxide and silver nanoparticles (MWCNT-Fe3O4/Ag) as a novel adsorbent for determining PAEs in carbonated soft drinks using magnetic SPE-GC/MS method. Arab. J. Chem. 2019, 12, 476–488. [Google Scholar] [CrossRef]
- Bo, S.; Luo, J.; An, Q.; Xiao, Z.; Wang, H.; Cai, W.; Zhai, S.; Li, Z. Efficiently selective adsorption of Pb (II) with functionalized alginate-based adsorbent in batch/column systems: Mechanism and application simulation. J. Clean. Prod. 2020, 250, 119585. [Google Scholar] [CrossRef]
- Yen, H.Y.; Lin, C.P. Adsorption of Cd (II) from wastewater using spent coffee grounds by Taguchi optimization. Desalin. Water Treat. 2016, 57, 11154–11161. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Bagtash, M.; Asanjarani, N. Hybrid central composite design approach for simultaneous optimization of removal of alizarin red S and indigo carmine dyes using cetyltrimethylammonium bromide-modified TiO2 nanoparticles. J. Environ. Chem. Eng. 2014, 2, 988–1000. [Google Scholar] [CrossRef]
- Googerdchian, F.; Moheb, A.; Emadi, R.; Asgari, M. Optimization of Pb (II) ions adsorption on nanohydroxyapatite adsorbents by applying Taguchi method. J. Hazard. Mater. 2018, 349, 186–194. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Zillohu, A.U.; Abdelaziz, R.; Hedayati, M.K.; Elbahri, M. A novel nanohybrid nanofibrous adsorbent for water purification from dye pollutants. Materials 2016, 9, 848. [Google Scholar] [CrossRef]
- Jiang, R.; Zhu, H.-Y.; Fu, Y.-Q.; Zong, E.-M.; Jiang, S.-T.; Li, J.-B.; Zhu, J.-Q.; Zhu, Y.-Y. Magnetic NiFe2O4/MWCNTs functionalized cellulose bioadsorbent with enhanced adsorption property and rapid separation. Carbohydr. Polym. 2021, 252, 117158. [Google Scholar] [CrossRef]
- Bhavani, P.; Reddy, N.R.; Reddy, I.V.S.; Sakar, M. Manipulation over phase transformation in iron oxide nanoparticles via calcination temperature and their effect on magnetic and dielectric properties. IEEE Trans. Magn. 2017, 53, 1–5. [Google Scholar] [CrossRef]
- Jiang, D.; Yang, Y.; Huang, C.; Huang, M.; Chen, J.; Rao, T.; Ran, X. Removal of the heavy metal ion nickel (II) via an adsorption method using flower globular magnesium hydroxide. J. Hazard. Mater. 2019, 373, 131–140. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Huang, F.; Yu, S.; Chen, G.; Kong, J. Magnetic removal of dyes from aqueous solution using multi-walled carbon nanotubes filled with Fe2O3 particles. J. Hazard. Mater. 2008, 160, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, N.; Sayadi, M.H.; Sobhani, S.; Hajiani, M. Photocatalytic degradation of model pharmaceutical pollutant by novel magnetic TiO2@ ZnFe2O4/Pd nanocomposite with enhanced photocatalytic activity and stability under solar light irradiation. J. Environ. Manag. 2020, 271, 110964. [Google Scholar] [CrossRef] [PubMed]
- Sahebian, S.; Zebarjad, S.; Khaki, J.V.; Lazzeri, A. The decoration of multi-walled carbon nanotubes with nickel oxide nanoparticles using chemical method. Int. Nano Lett. 2016, 6, 183–190. [Google Scholar] [CrossRef]
- El-Khouly, S.M.; Fathy, N.A. Multi-walled carbon nanotubes supported amorphous Fe2O3 and Ag2O–Fe2O3 as F enton catalysts for degradation of m axilon red dye. Asia-Pac. J. Chem. Eng. 2018, 13, e2184. [Google Scholar] [CrossRef]
- Egbosiuba, T.C.; Abdulkareem, A.S.; Kovo, A.S.; Afolabi, E.A.; Tijani, J.O.; Bankole, M.T.; Bo, S.; Roos, W.D. Adsorption of Cr (VI), Ni (II), Fe (II) and Cd (II) ions by KIAgNPs decorated MWCNTs in a batch and fixed bed process. Sci. Rep. 2021, 11, 1–20. [Google Scholar] [CrossRef]
- Zadeh, R.J.; Sayadi, M.H.; Rezaei, M.R. Synthesis of Thiol modified magMCM-41 nanoparticles with rice husk ash as a robust, high effective, and recycling magnetic sorbent for the removal of herbicides. J. Environ. Chem. Eng. 2021, 9, 104804. [Google Scholar] [CrossRef]
- Lv, X.; Deng, J.; Wang, B.; Zhong, J.; Sham, T.-K.; Sun, X.; Sun, X. γ-Fe2O3@ CNTs anode materials for lithium ion batteries investigated by electron energy loss spectroscopy. Chem. Mater. 2017, 29, 3499–3506. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, W.; Li, L.; Jin, B.; Jin, E.; Jeong, S.; Jiang, Q. In-situ synthesized ZnFe2O4 firmly anchored to the surface of MWCNTs as a long-life anode material with high lithium storage performance. Appl. Surf. Sci. 2017, 425, 978–987. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, C.-F.; Chen, C.-M.; Chuang, F.-T. Modification of multi-walled carbon nanotubes by microwave digestion method as electrocatalyst supports for direct methanol fuel cell applications. Electrochem. Commun. 2007, 9, 159–163. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Ahmadpour, N.; Homaeigohar, S. Photocatalytic and Antibacterial Properties of Ag-CuFe2O4@ WO3 Magnetic Nanocomposite. Nanomaterials 2021, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Penki, T.R.; Shivakumara, S.; Minakshi, M.; Munichandraiah, N. Porous flower-like α-Fe2O3 nanostructure: A high performance anode material for lithium-ion batteries. Electrochim. Acta 2015, 167, 330–339. [Google Scholar] [CrossRef]
- Nowrouzi, M.; Younesi, H.; Bahramifar, N. High efficient carbon dioxide capture onto as-synthesized activated carbon by chemical activation of Persian Ironwood biomass and the economic pre-feasibility study for scale-up. J. Clean. Prod. 2017, 168, 499–509. [Google Scholar] [CrossRef]
- Donia, A.; Atia, A.; Abouzayed, F. Preparation and characterization of nano-magnetic cellulose with fast kinetic properties towards the adsorption of some metal ions. Chem. Eng. J. 2012, 191, 22–30. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, H.; Wang, S.; Shi, W.; He, J.; Liu, H.; Huang, Y. Fe3O4–MWCNT magnetic nanocomposites as efficient peroxidase mimic catalysts in a Fenton-like reaction for water purification without pH limitation. RSC Adv. 2014, 4, 45809–45815. [Google Scholar] [CrossRef]
- Akbarzadeh, P.; Koukabi, N.; Kolvari, E. Anchoring of triethanolamine–Cu (II) complex on magnetic carbon nanotube as a promising recyclable catalyst for the synthesis of 5-substituted 1H-tetrazoles from aldehydes. Mol. Divers. 2020, 24, 319–333. [Google Scholar] [CrossRef]
- Pang, J.; Fu, F.; Ding, Z.; Lu, J.; Li, N.; Tang, B. Adsorption behaviors of methylene blue from aqueous solution on mesoporous birnessite. J. Taiwan Inst. Chem. Eng. 2017, 77, 168–176. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Boran, S.; Tanasie, C. A green approach for treatment of wastewater with manganese using wood ash. J. Chem. Technol. Biotechnol. 2020, 95, 1781–1789. [Google Scholar] [CrossRef]
- Alghamdi, W.M.; el Mannoubi, I. Investigation of Seeds and Peels of Citrullus colocynthis as Efficient Natural Adsorbent for Methylene Blue Dye. Processes 2021, 9, 1279. [Google Scholar] [CrossRef]
- Zahir, A.; Aslam, Z.; Kamal, M.S.; Ahmad, W.; Abbas, A.; Shawabkeh, R.A. Development of novel cross-linked chitosan for the removal of anionic Congo red dye. J. Mol. Liq. 2017, 244, 211–218. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Dong, W.; Zhang, L.; Kong, Q.; Wang, W. Efficient adsorption of sulfamethazine onto modified activated carbon: A plausible adsorption mechanism. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Mahanpoor, K.; Moradi, O. Removal of dye molecules from aqueous solution by carbon nanotubes and carbon nanotube functional groups: Critical review. Rsc Adv. 2017, 7, 47083–47090. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Rashki, O.; Shahri, E. Application of modified Spirulina platensis and Chlorella vulgaris powder on the adsorption of heavy metals from aqueous solutions. J. Environ. Chem. Eng. 2019, 7, 103169. [Google Scholar] [CrossRef]
- Rezaei, A.; Rezaei, M.R.; Sayadi, M.H. 3D network structure graphene hydrogel-Fe3O4@ SnO2/Ag via an adsorption/photocatalysis synergy for removal of 2, 4 dichlorophenol. J. Taiwan Inst. Chem.Eng. 2021, 121, 154–167. [Google Scholar] [CrossRef]
- Hadavifar, M.; Bahramifar, N.; Younesi, H.; Li, Q. Adsorption of mercury ions from synthetic and real wastewater aqueous solution by functionalized multi-walled carbon nanotube with both amino and thiolated groups. Chem. Eng. J. 2014, 237, 217–228. [Google Scholar] [CrossRef]
- Karimi, P.; Javanshir, S.; Sayadi, M.H.; Arabyarmohammadi, H. Arsenic removal from mining effluents using plant-mediated, green-synthesized iron nanoparticles. Processes 2019, 7, 759. [Google Scholar] [CrossRef]
- Ain, Q.U.; Rasheed, U.; Yaseen, M.; Zhang, H.; Tong, Z. Superior dye degradation and adsorption capability of polydopamine modified Fe3O4-pillared bentonite composite. J. Hazard. Mater. 2020, 397, 122758. [Google Scholar] [CrossRef]
- Abo-Neima, S.E.; Motaweh, H.A.; Elsehly, E.M. Antimicrobial activity of functionalised carbon nanotubes against pathogenic microorganisms. IET Nanobiotechnol. 2020, 14, 457–464. [Google Scholar] [CrossRef]
- Mohan, R.; Shanmugharaj, A.; Hun, R.S. An efficient growth of silver and copper nanoparticles on multiwalled carbon nanotube with enhanced antimicrobial activity. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96, 119–126. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalatbary, M.; Sayadi, M.H.; Hajiani, M.; Nowrouzi, M.; Homaeigohar, S. Green, Sustainable Synthesis of γ-Fe2O3/MWCNT/Ag Nano-Composites Using the Viscum album Leaf Extract and Waste Car Tire for Removal of Sulfamethazine and Bacteria from Wastewater Streams. Nanomaterials 2022, 12, 2798. https://doi.org/10.3390/nano12162798
Khalatbary M, Sayadi MH, Hajiani M, Nowrouzi M, Homaeigohar S. Green, Sustainable Synthesis of γ-Fe2O3/MWCNT/Ag Nano-Composites Using the Viscum album Leaf Extract and Waste Car Tire for Removal of Sulfamethazine and Bacteria from Wastewater Streams. Nanomaterials. 2022; 12(16):2798. https://doi.org/10.3390/nano12162798
Chicago/Turabian StyleKhalatbary, Mansooreh, Mohammad Hossein Sayadi, Mahmood Hajiani, Mohsen Nowrouzi, and Shahin Homaeigohar. 2022. "Green, Sustainable Synthesis of γ-Fe2O3/MWCNT/Ag Nano-Composites Using the Viscum album Leaf Extract and Waste Car Tire for Removal of Sulfamethazine and Bacteria from Wastewater Streams" Nanomaterials 12, no. 16: 2798. https://doi.org/10.3390/nano12162798
APA StyleKhalatbary, M., Sayadi, M. H., Hajiani, M., Nowrouzi, M., & Homaeigohar, S. (2022). Green, Sustainable Synthesis of γ-Fe2O3/MWCNT/Ag Nano-Composites Using the Viscum album Leaf Extract and Waste Car Tire for Removal of Sulfamethazine and Bacteria from Wastewater Streams. Nanomaterials, 12(16), 2798. https://doi.org/10.3390/nano12162798






