Distinct Uptake Routes Participate in Silver Nanoparticle Engulfment by Earthworm and Human Immune Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticles
2.2. Earthworm Husbandry and Coelomocyte Isolations
2.3. Cell Culture Conditions
2.4. Endocytosis Inhibitor Treatments and In Vitro Exposure Conditions
2.5. Flow Cytometry Measurement of SSC Parameters
2.6. Transmission Electron Microscopy (TEM) for Intracellular AgNP Localization
2.7. Quantification of Intracellular Silver Content by Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.8. RNA Isolation, cDNA Synthesis, and Real-Time PCR
2.9. Quantification of Global Genomic DNA Methylation Levels
2.10. Bioenergetic Analysis of Mitochondria
2.11. Statistical Analysis
3. Results and Discussion
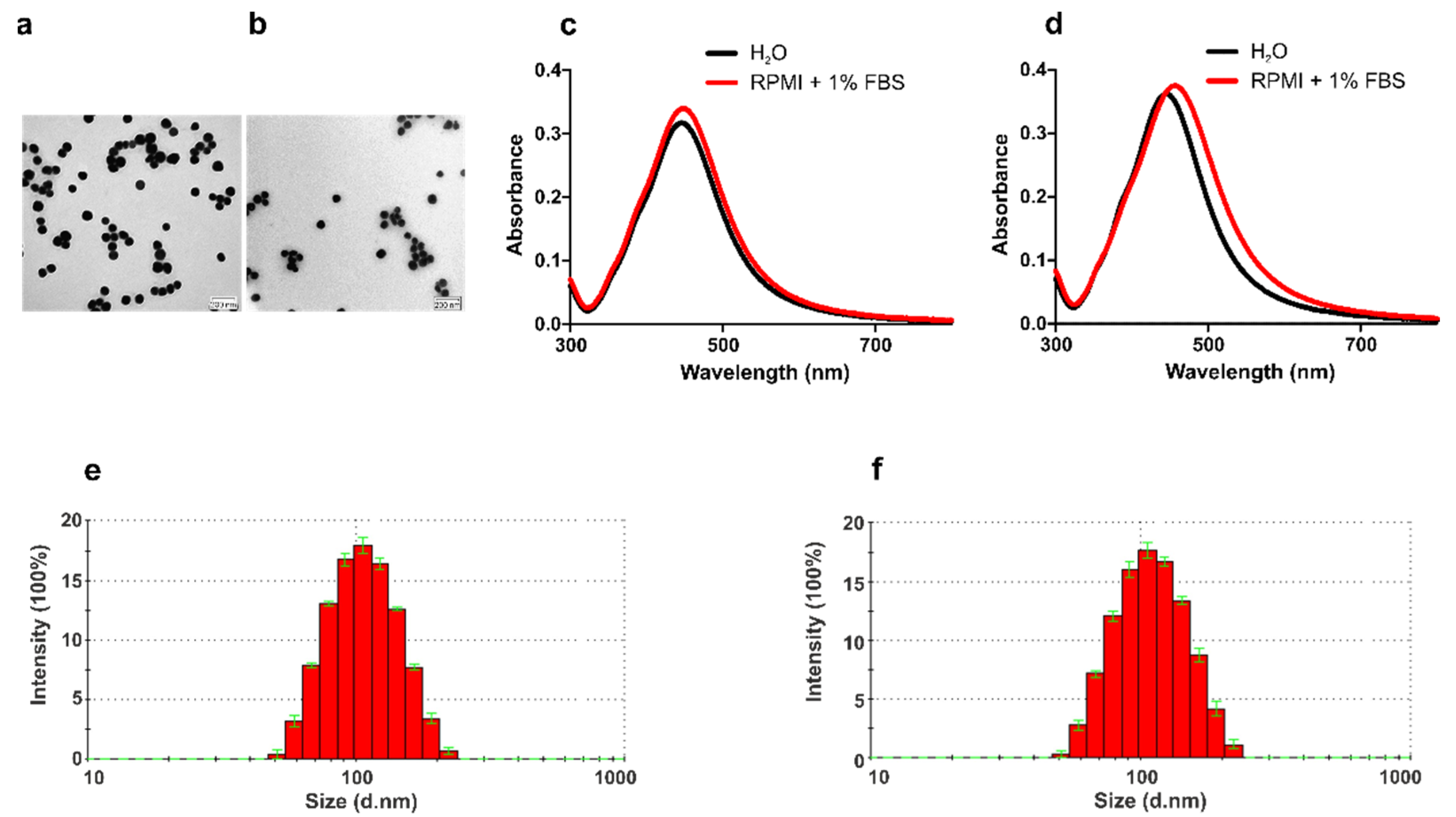
3.1. Physico-Chemical Characterization of AgNPs
3.2. Earthworm and Human Immunocytes Possess Different Sensitivities towards AgNPs
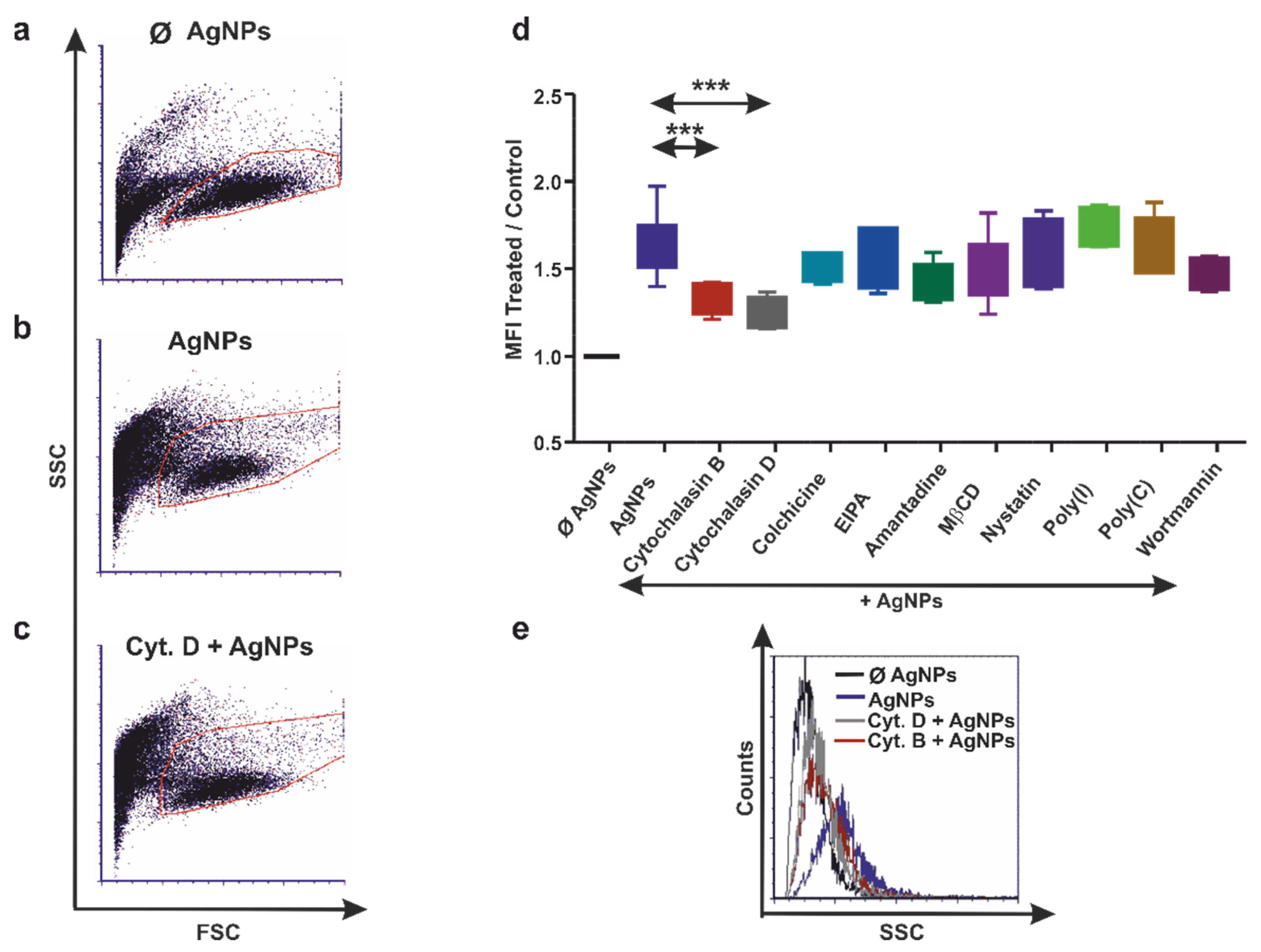
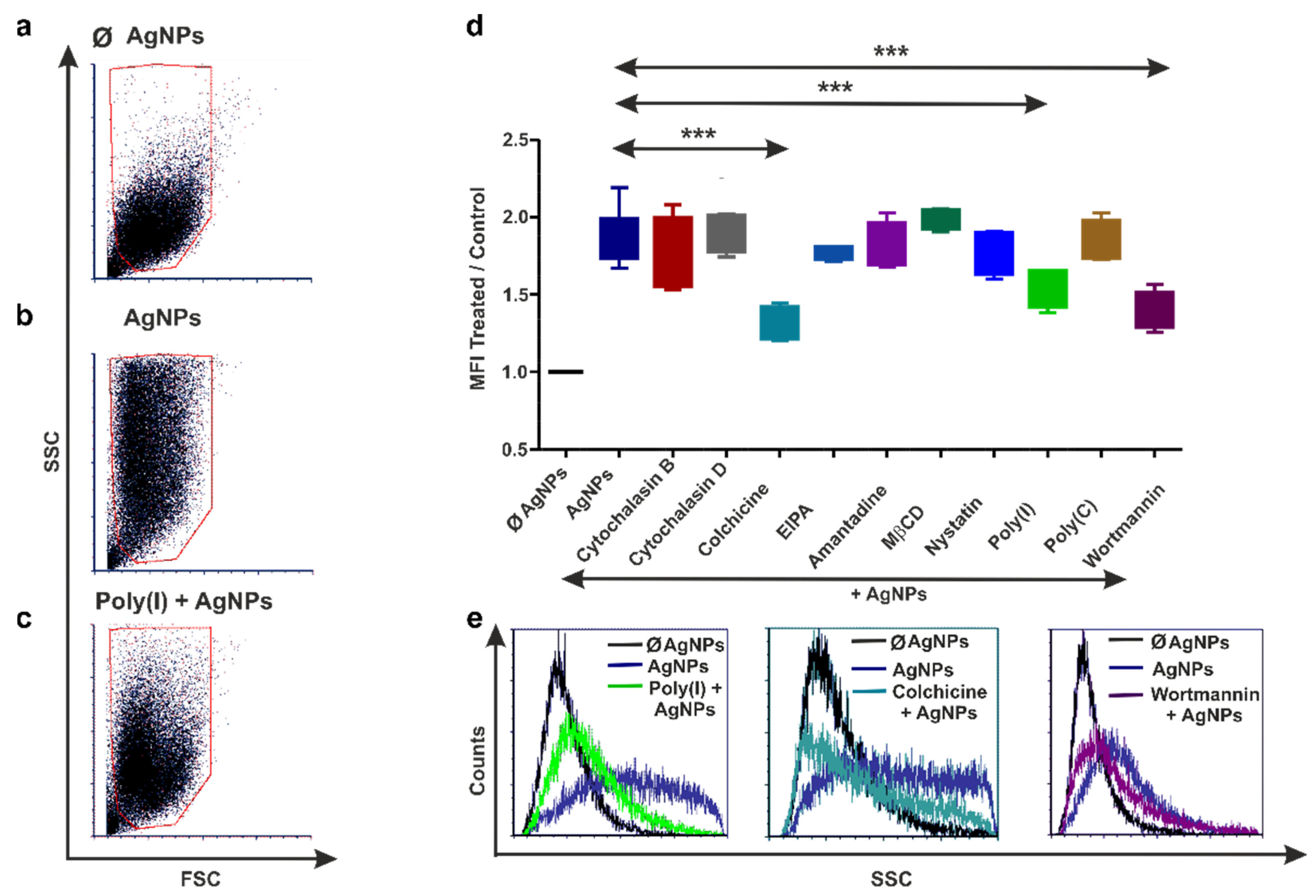
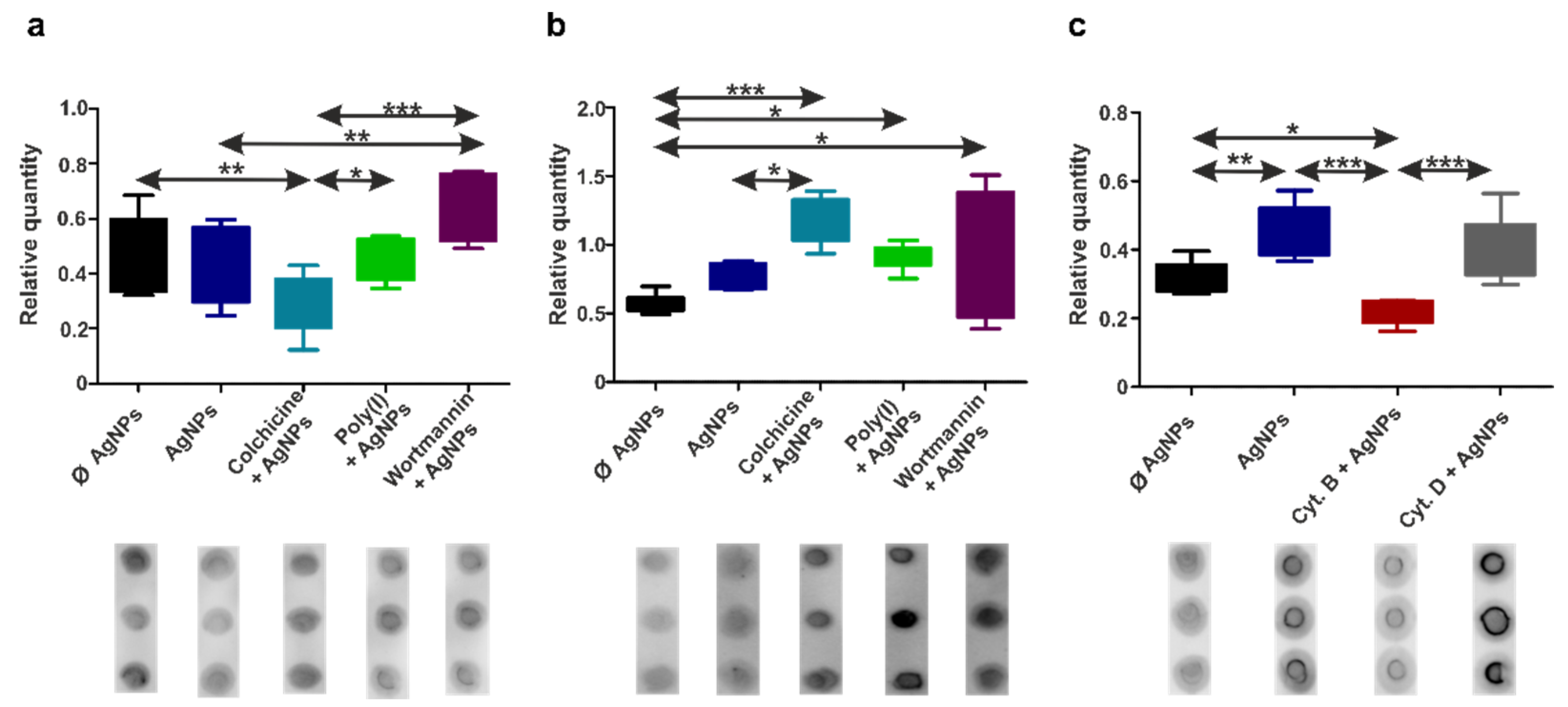
3.3. Human THP-1 and Diff. THP-1 Cells Utilize Similar Endocytosis Mechanisms for AgNP Uptake
3.4. Earthworm Coelomocytes Rely on Actin-Dependent Phagocytosis for AgNPs Uptake
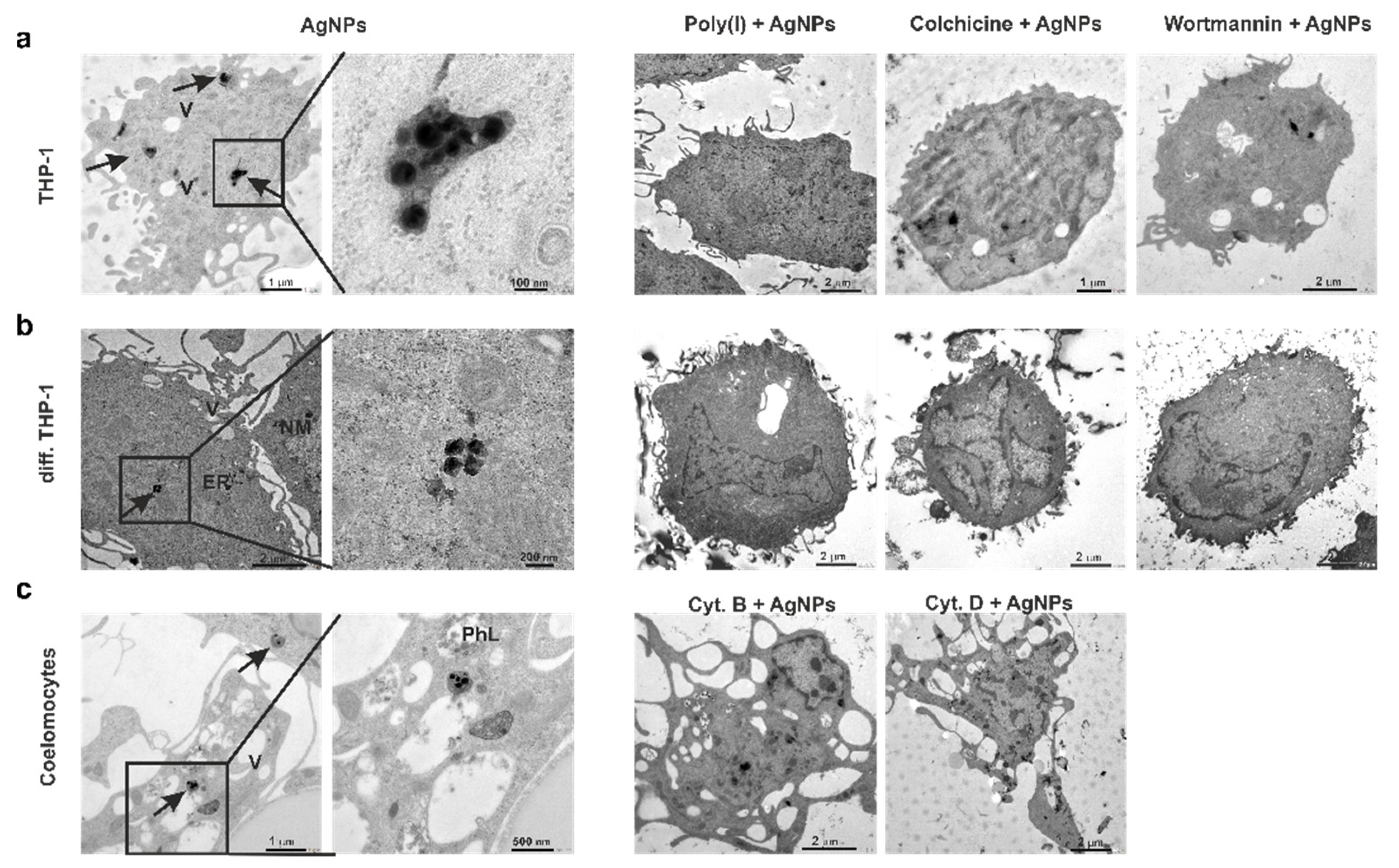
3.5. AgNPs Do Not Enter the Target Cell Nuclei
3.6. Quantification of Intracellular Silver Content
3.7. TLR/MyD88 Signaling Facilitates AgNPs Internalization
3.8. Earthworm Coelomocytes, but Not Human Immunocytes, Exhibit Global DNA Hypermethylation upon AgNPs’ Uptake
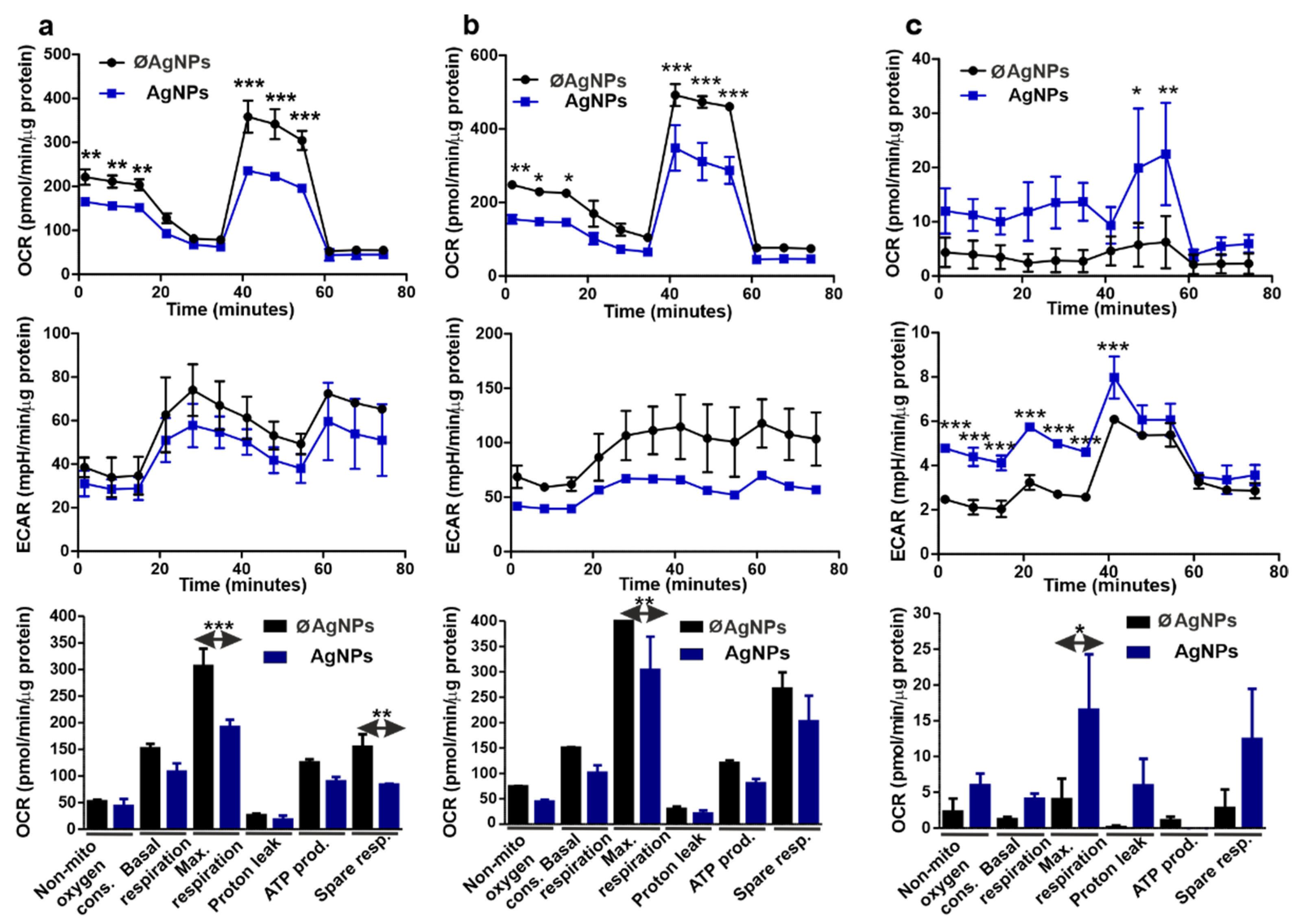
3.9. Cellular Respiration Parameters Changed in Response to AgNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambert, B.; Gillen, A.J.; Schuergers, N.; Wu, S.-J.; Boghossian, A.-A. Directed evolution of the optoelectronic properties of synthetic nanomaterials. Chem. Commun. 2019, 55, 3239–3242. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Hu, Z. Impact of metallic and metal oxide nanoparticles on wastewater treatment and anaerobic digestion. Environ. Sci. Process. Impacts 2012, 15, 39–48. [Google Scholar] [CrossRef]
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Possibilities and limitations of modelling environmental exposure to engineered nanomaterials by probabilistic material flow analysis. Environ. Toxicol. Chem. 2010, 29, 1036–1048. [Google Scholar] [CrossRef]
- Hayashi, Y.; Engelmann, P.; Foldbjerg, R.; Szabó, M.; Somogyi, I.; Pollák, E.; Molnár, L.; Autrup, H.; Sutherland, D.S.; Scott-Fordsmand, J.; et al. Earthworms and humans in vitro: Characterizing evolutionarily conserved stress and immune responses to silver nanoparticles. Environ. Sci. Technol. 2012, 46, 4166–4173. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Gottschalk, F.; Nowack, B. The release of engineered nanomaterials to the environment. J. Environ. Monit. 2011, 13, 1145–1155. [Google Scholar] [CrossRef]
- Tiede, K.; Hassellöv, M.; Breitbarth, E.; Chaudhry, Q.; Boxall, A.B.A. Considerations for environmental fate and ecotoxicity testing to support environmental risk assessments for engineered nanoparticles. J. Chromatogr. A 2009, 1216, 503–509. [Google Scholar] [CrossRef]
- Dang, F.; Huang, Y.; Wang, Y.; Zhou, D.; Xing, B. Transfer and toxicity of silver nanoparticles in the food chain. Environ. Sci. Nano 2021, 8, 1519–1535. [Google Scholar] [CrossRef]
- Engelmann, P.; Bodó, K.; Najbauer, J.; Németh, P. Oligochaetes: Quo vadis earthworm immunity? In Advances in Comparative Immunology; Cooper, E.L., Ed.; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Engelmann, P.; Hayashi, Y.; Bodó, K.; Ernszt, D.; Somogyi, I.; Steib, A.; Orbán, J.; Pollák, E.; Nyitrai, M.; Németh, P.; et al. Phenotypic and functional characterization of earthworm coelomocyte subsets: Linking light scatter-based cell typing and imaging of the sorted populations. Dev. Comp. Immunol. 2016, 65, 41–52. [Google Scholar] [CrossRef]
- Kokhanyuk, K.; Bodó, K.; Sétáló, G., Jr.; Németh, P.; Engelmann, P. Bacterial engulfment mechanism is strongly conserved in evolution between earthworm and human immune cells. Front. Immunol. 2021, 12, 733541. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Greulich, C.; Diendorf, J.; Simon, T.; Eggeler, G.; Epple, M.; Köller, M. Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomater. 2011, 7, 347–354. [Google Scholar] [CrossRef]
- Bouallegui, Y.; Younes, R.B.; Turki, F.; Mezni, A.; Oueslati, R. Effect of exposure time, particle size and uptake pathways in immune cell lysosomal cytotoxicity of mussels exposed to silver nanoparticles. Drug Chem. Toxicol. 2018, 41, 169–174. [Google Scholar] [CrossRef]
- Khan, F.R.; Misra, S.K.; Bury, N.R.; Smith, B.D.; Rainbow, P.S.; Luoma, S.N.; Valsami-Jones, E. Inhibition of potential uptake pathways for silver nanoparticles in the estuarine snail Peringia ulvae. Nanotoxicology 2015, 9, 493–501. [Google Scholar] [CrossRef]
- Maurer, L.L.; Yang, X.; Schindler, A.J.; Taggart, R.K.; Jiang, C.; Hsu-Kim, H.; Sherwood, D.R.; Meyer, J.N. Intracellular trafficking pathways in silver nanoparticle uptake and toxicity in Caenorhabditis elegans. Nanotoxicology 2016, 10, 831–835. [Google Scholar] [CrossRef][Green Version]
- Shao, Z.; Guagliardo, P.; Jiang, H.; Wang, W.-X. Intra- and intercellular silver nanoparticle translocation and transformation in oyster gill filaments: Coupling Nanoscale secondary ion mass spectrometry and dual stable isotope tracing study. Environ. Sci. Technol. 2021, 55, 433–446. [Google Scholar] [CrossRef]
- Engelmann, P.; Molnár, L.; Pálinkás, L.; Cooper, E.L.; Németh, P. Earthworm leukocyte populations specifically harbor lysosomal enzymes that may respond to bacterial challenge. Cell Tissue Res. 2004, 316, 391–401. [Google Scholar] [CrossRef]
- Park, E.K.; Jung, H.S.; Yang, H.I.; Yoo, M.C.; Kim, C.; Kim, K.S. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm. Res. 2007, 56, 45–50. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Kim, J.-H.; Cho, J.Y. Cytochalasin B modulates macrophage-mediated inflammatory responses. Biomol. Ther. 2014, 22, 295–300. [Google Scholar] [CrossRef]
- DeFife, K.M.; Jenney, C.R.; Colton, E.; Anderson, J.M. Cytoskeletal and adhesive structural polarizations accompany IL-13-induced human macrophage fusion. J. Histochem. Cytochem. 1999, 47, 65–74. [Google Scholar] [CrossRef]
- Valberg, P.A.; Brain, J.D.; Kane, D. Effects of colchicine or cytochalasin B on pulmonary macrophage endocytosis in vivo. J. Appl. Physiol. 1981, 50, 621–629. [Google Scholar] [CrossRef]
- Ivanov, A. Pharmacological inhibition of endocytic pathways: Is it specific enough to be useful? Exocytosis Endocytosis 2008, 440, 15–33. [Google Scholar] [CrossRef]
- Van Hamme, E.; Dewerchin, H.L.; Cornelissen, E.; Verhasselt, B.; Nauwynck, H.J. Clathrin- and caveolae-independent entry of feline infectious peritonitis virus in monocytes depends on dynamin. J. Gen. Virol. 2008, 89, 2147–2156. [Google Scholar] [CrossRef]
- Atger, V.M.; de la Llera Moya, M.; Stoudt, G.W.; Rodriguezal, W.V.; Phillips, M.C.; Rothblat, G.H. Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J. Clin. Investig. 1997, 99, 773–780. [Google Scholar] [CrossRef]
- Florian, P.; Macovei, A.; Sima, L.; Nichita, N.; Mattsby-Baltzer, I.; Roseanu, A. Endocytosis and trafficking of human lactoferrin in macrophage-like human THP-1 cells. Biochem. Cell Biol. 2012, 90, 449–455. [Google Scholar] [CrossRef]
- Raynal, I.; Prigent, P.; Peyramaure, S.; Najid, A.; Rebuzzi, C.; Corot, C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles. Investig. Radiol. 2004, 39, 56–63. [Google Scholar] [CrossRef]
- Kanno, S.; Furuyama, A.; Hirano, S. A murine scavenger receptor MARCO recognizes polystyrene nanoparticles. Toxicol. Sci. 2007, 97, 398–406. [Google Scholar] [CrossRef]
- Nakase, I.; Niwa, M.; Takeuchi, T.; Sonomura, K.; Kawabata, N.; Koike, Y.; Takehashi, M.; Tanaka, S.; Ueda, K.; Simpson, J.C.; et al. Cellular uptake of arginine-rich peptides: Roles for macropinocytosis and actin rearrangement. Mol. Ther. 2004, 10, 1011–1022. [Google Scholar] [CrossRef]
- Hansen, U.; Thünemann, A.F. Characterization of silver nanoparticles in cell culture medium containing fetal bovine serum. Langmuir 2015, 31, 6842–6852. [Google Scholar] [CrossRef]
- Bélteky, P.; Rónavári, A.; Igaz, N.; Szerencsés, B.; Tóth, I.Y.; Pfeiffer, I.; Kiricsi, M.; Kónya, Z. Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity. Int. J. Nanomed. 2019, 14, 667–687. [Google Scholar] [CrossRef]
- Pass, D.A.; Morgan, A.J.; Read, D.S.; Field, D.; Weightman, A.J.; Kille, P. The effect of anthropogenic arsenic contamination on the earthworm microbiome: The earthworm microbiome. Environ. Microbiol. 2015, 17, 1884–1896. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- Eom, H.J.; Choi, J. Clathrin-mediated endocytosis is involved in uptake and toxicity of silica nanoparticles in Caenorhabditis elegans. Chem.-Biol. Interact. 2019, 311, 108744. [Google Scholar] [CrossRef]
- Suzuki, H.; Toyooka, T.; Ibuki, Y. Simple and easy method to evaluate uptake potential of nanoparticles in mammalian cells using a flow cytometric light scatter analysis. Environ. Sci. Technol. 2007, 41, 3018–3024. [Google Scholar] [CrossRef]
- Schliwa, M.; Woehlke, G. Molecular motors. Nature 2003, 422, 759–765. [Google Scholar] [CrossRef]
- Clarke, M.; Köhler, J.; Heuser, J.; Gerisch, G. Endosome fusion and microtubule-based dynamics in the early endocytic pathway of Dictyostelium: Endosome fusion and dynamics. Traffic 2002, 3, 791–800. [Google Scholar] [CrossRef]
- Ostap, E.M.; Maupin, P.; Doberstein, S.K.; Baines, I.C.; Korn, E.D.; Pollard, T.D. Dynamic localization of myosin-I to endocytic structures in Acanthamoeba. Cell Motil. Cytoskelet. 2003, 54, 29–40. [Google Scholar] [CrossRef]
- Araki, N. Role of microtubules and myosins in Fc gamma receptor-mediated phagocytosis. Front. Biosci. 2006, 11, 1479. [Google Scholar] [CrossRef]
- Olazabal, M.; Caron, E.; May, R.C.; Schilling, K.; Knecht, D.A.; Machesky, L.M. Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis. Curr. Biol. 2002, 12, 1413–1418. [Google Scholar] [CrossRef]
- Tuxworth, R.I.; Weber, I.; Wessels, D.; Addicks, G.C.; Soll, D.R.; Gerisch, G.; Titus, M.A. A role for myosin VII in dynamic cell adhesion. Curr. Biol. 2001, 11, 318–329. [Google Scholar] [CrossRef]
- Fujimoto, L.M.; Roth, R.; Heuser, J.E.; Schmid, S.L. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis: Actin and receptor mediated endocytosis. Traffic 2000, 1, 161–171. [Google Scholar] [CrossRef]
- Pelkmans, L.; Püntener, D.; Helenius, A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 2002, 296, 535–539. [Google Scholar] [CrossRef]
- Saffarian, S.; Cocucci, E.; Kirchhausen, T. Distinct dynamics of endocytic clathrin-coated pits and coated plaques. PLoS Biol. 2009, 7, e1000191. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Matveev, S.; van der Westhuyzen, D.R.; Smart, E.J. Co-expression of scavenger receptor-BI and caveolin-1 is associated with enhanced selective cholesteryl ester uptake in THP-1 macrophages. J. Lipid Res. 1999, 40, 1647–1654. [Google Scholar] [CrossRef]
- Peiser, L.; Gordon, S. The function of scavenger receptors expressed by macrophages and their role in the regulation of inflammation. Microbes Infect. 2001, 3, 149–159. [Google Scholar] [CrossRef]
- Yao, W.; Li, K.; Liao, K. Macropinocytosis contributes to the macrophage foam cell formation in RAW264.7 cells. Acta Biochim. Biophys. Sin. 2009, 41, 773–780. [Google Scholar] [CrossRef]
- Zhu, X.-D.; Zhuang, Y.; Ben, J.-J.; Qian, L.-L.; Huang, H.-P.; Bai, H.; Sha, J.-H.; He, Z.-G.; Chen, Q. Caveolae-dependent endocytosis is required for class A macrophage scavenger receptor-mediated apoptosis in macrophages. J. Biol. Chem. 2011, 286, 8231–8239. [Google Scholar] [CrossRef]
- Wang, H.; Wu, L.; Reinhard, B.M. Scavenger receptor mediated endocytosis of silver nanoparticles into J774A.1 macrophages is heterogeneous. ACS Nano 2012, 6, 7122–7132. [Google Scholar] [CrossRef]
- Singh, R.P.; Ramarao, P. Cellular uptake, intracellular trafficking and cytotoxicity of silver nanoparticles. Toxicol. Lett. 2012, 213, 249–259. [Google Scholar] [CrossRef]
- Hamilton, R.; Buckingham, S.; Holian, A. The effect of size on Ag nanosphere toxicity in macrophage cell models and lung epithelial cell lines is dependent on particle dissolution. Int. J. Mol. Sci. 2014, 15, 6815–6830. [Google Scholar] [CrossRef]
- Cholewa, J.; Nikolic, D.M.; Post, S.R. Regulation of Class A scavenger receptor-mediated cell adhesion and surface localization by PI3K: Identification of a regulatory cytoplasmic motif. J. Leukoc. Biol. 2010, 87, 443–449. [Google Scholar] [CrossRef]
- Nikolic, D.M.; Cholewa, J.; Gass, C.; Gong, M.C.; Post, S.R. Class A scavenger receptor-mediated cell adhesion requires the sequential activation of Lyn and PI3-kinase. Am. J. Physiol.-Cell Physiol. 2007, 292, C1450–C1458. [Google Scholar] [CrossRef]
- Sulahian, T.H.; Imrich, A.; DeLoid, G.; Winkler, A.R.; Kobzik, L. Signaling pathways required for macrophage scavenger receptor-mediated phagocytosis: Analysis by scanning cytometry. Respir. Res. 2008, 9, 59. [Google Scholar] [CrossRef]
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delacher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailänder, V.; Landfester, K.; et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano 2011, 5, 1657–1669. [Google Scholar] [CrossRef]
- van der Ploeg, M.J.; van den Berg, J.H.; Bhattacharjee, S.; de Haan, L.H.; Ershov, D.S.; Fokkink, R.G.; Zuilhof, H.; Rietjens, I.M.; van den Brink, N.W. In vitro nanoparticle toxicity to rat alveolar cells and coelomocytes from the earthworm Lumbricus rubellus. Nanotoxicology 2014, 8, 28–37. [Google Scholar] [CrossRef]
- Škanta, F.; Procházková, P.; Roubalová, R.; Dvořák, J.; Bilej, M. LBP/BPI homologue in Eisenia andrei earthworms. Dev. Comp. Immunol. 2016, 54, 1–6. [Google Scholar] [CrossRef]
- Škanta, F.; Roubalová, R.; Dvořák, J.; Procházková, P.; Bilej, M. Molecular cloning and expression of TLR in the Eisenia andrei earthworm. Dev. Comp. Immunol. 2013, 41, 694–702. [Google Scholar] [CrossRef]
- Bodó, K.; Kellermayer, Z.; László, Z.; Boros, Á.; Kokhanyuk, B.; Németh, P.; Engelmann, P. Injury-induced innate immune response during segment regeneration of the earthworm, Eisenia andrei. Int. J. Mol. Sci. 2021, 22, 2363. [Google Scholar] [CrossRef]
- Cooper, E.L.; Roch, P. Earthworm immunity: A model of immune competence. Pedobiologia 2003, 47, 676–688. [Google Scholar] [CrossRef]
- Berry, C.C.; de la Fuente, J.M.; Mullin, M.; Chu, S.W.L.; Curtis, A.S.G. Nuclear localization of HIV-1 Tat functionalized gold nanoparticles. IEEE Trans. Nanobiosci. 2007, 6, 262–269. [Google Scholar] [CrossRef]
- Hsiao, L.; Bierkandt, F.S.; Reichardt, P.; Luch, A.; Huang, Y.-L.; Jakubowski, N.; Tentshert, J.; Haase, A. Quantification and visualization of cellular uptake of TiO2 and Ag nanoparticles: Comparison of different ICP-MS techniques. J. Nanobiotechnol. 2016, 14, 50. [Google Scholar] [CrossRef]
- Aksoy, E.; Vanden Berghe, W.; Detienne, S.; Amraoui, Z.; Fitzgerald, K.A.; Haegeman, G.; Goldman, M.; Willems, F. Inhibition of phosphoinositide 3-kinase enhances TRIF-dependent NF-κB activation and IFN-β synthesis downstream of Toll-like receptor 3 and 4. Eur. J. Immunol. 2005, 35, 2200–2209. [Google Scholar] [CrossRef]
- Fukao, T.; Koyasu, S. PI3K and negative regulation of TLR signalling. Trends Immunol. 2003, 24, 358–363. [Google Scholar] [CrossRef]
- Park, Y.C.; Lee, C.H.; Kang, H.S.; Chung, H.T.; Kim, H.D. Wortmannin, a specific inhibitor of phosphatidylinositol-3-kinase, enhances LPS-induced NO production from murine peritoneal macrophages. Biochem. Biophys. Res. Commun. 1997, 240, 692–696. [Google Scholar] [CrossRef]
- Yu, H.; Ha, T.; Liu, L.; Wang, X.; Gao, M.; Kelley, J.; Kao, R.; Williams, D.; Li, C. Scavenger receptor A (SR-A) is required for LPS-induced TLR4 mediated NF-κB activation in macrophages. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2012, 1823, 1192–1198. [Google Scholar] [CrossRef]
- Murshid, A.; Gong, J.; Prince, T.; Borges, T.J.; Calderwood, S.K. Scavenger receptor SREC-I mediated entry of TLR4 into lipid microdomains and triggered inflammatory cytokine release in RAW 264.7 cells upon LPS activation. PLoS ONE 2015, 10, e0122529. [Google Scholar] [CrossRef]
- Means, T.K.; Mylonakis, E.; Tampakakis, E.; Colvin, R.A.; Seung, E.; Puckett, L.; Tai, M.F.; Stewart, C.R.; Pukkila-Worley, R.; Hickman, S.E.; et al. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J. Exp. Med. 2009, 206, 637–653. [Google Scholar] [CrossRef]
- Limmon, G.V.; Arredouani, M.; McCann, K.L.; Minor, R.A.C.; Kobzik, L.; Imani, F. Scavenger receptor class-A is a novel cell surface receptor for double-stranded RNA. FASEB J. 2008, 22, 159–167. [Google Scholar] [CrossRef]
- Murshid, A.; Gong, J.; Ahmad, R.; Borges, T.J.; Calderwood, S.K. Scavenger receptor SREC-I promotes double stranded RNA-mediated TLR3 activation in human monocytes. Immunobiology 2015, 220, 823–832. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptor and RIG-1-like receptor signalling. Ann. N. Y. Acad. Sci. 2008, 1143, 1–20. [Google Scholar] [CrossRef]
- Hazeki, K.; Kinoshita, S.; Matsumura, T.; Nigorikawa, K.; Kubo, H.; Hazeki, O. Opposite effects of wortmannin and 2-(4-Morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride on Toll-like receptor-mediated nitric oxide production: Negative regulation of nuclear factor-κB by phosphoinositide 3-kinase. Mol. Pharmacol. 2006, 69, 1717–1724. [Google Scholar] [CrossRef]
- Hayashi, Y.; Heckmann, L.-H.; Simonsen, V.; Scott-Fordsmand, J.J. Time-course profiling of molecular stress responses to silver nanoparticles in the earthworm Eisenia fetida. Ecotoxicol. Environ. Saf. 2013, 98, 219–226. [Google Scholar] [CrossRef]
- Liew, F.Y.; Xu, D.; Brint, E.K.; O’Neill, L.A.J. Negative regulation of Toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005, 5, 446–458. [Google Scholar] [CrossRef]
- Hayashi, Y.; Miclaus, T.; Engelmann, P.; Autrup, H.; Sutherland, D.S.; Scott-Fordsmand, J.J. Nanosilver pathophysiology in earthworms: Transcriptional profiling of secretory proteins and the implication for the protein corona. Nanotoxicology 2016, 10, 303–311. [Google Scholar] [CrossRef]
- Bodó, K.; Hayashi, Y.; Gerencsér, G.; László, Z.; Kéri, A.; Galbács, G.; Telek, E.; Mészáros, M.; Deli, M.A.; Kokhanyuk, B.; et al. Species-specific sensitivity of Eisenia earthworms towards noble metal nanoparticles: A multiparametric in vitro study. Environ. Sci. Nano 2020, 7, 3509–3525. [Google Scholar] [CrossRef]
- Beschin, A.; Bilej, M.; Hanssens, F.; Raymakers, J.; Van Dyck, E.; Revets, H.; Brys, L.; Gomez, J.; De Baetselier, P.; Timmermans, M. Identification and cloning of a glucan- and lipopolysaccharide-binding protein from Eisenia foetida earthworm involved in the activation of prophenoloxidase cascade. J. Biol. Chem. 1998, 273, 24948–24954. [Google Scholar] [CrossRef]
- Suarez-Ulloa, V.; Gonzalez-Romero, R.; Eirin-Lopez, J.M. Environmental epigenetics: A promising venue for developing next-generation pollution biomonitoring tools in marine invertebrates. Mar. Pollut. Bull. 2015, 98, 5–13. [Google Scholar] [CrossRef]
- Nyce, J. Drug-induced DNA hypermethylation and drug resistance in human tumors. Cancer Res. 1989, 49, 5829–5836. [Google Scholar]
- Conroy, J.; Byrne, S.J.; Gun’ko, Y.K.; Rakovich, Y.P.; Donegan, J.F.; Davies, A.; Kelleher, D.; Volkov, Y. CdTe nanoparticles display tropism to core histones and histone-rich cell organelles. Small 2008, 4, 2006–2015. [Google Scholar] [CrossRef]
- Lu, X.; Miousse, I.R.; Pirela, S.V.; Melnyk, S.; Koturbash, I.; Demokritou, P. Short-term exposure to engineered nanomaterials affects cellular epigenome. Nanotoxicology 2016, 10, 140. [Google Scholar] [CrossRef]
- Aigner, G.P.; Pittl, V.; Fiechtner, B.; Egger, B.; Šrut, M.; Höckner, M. Common mechanisms cannot explain time- and dose-dependent DNA methylation changes in earthworms exposed to cadmium. Sci. Total Environ. 2022, 812, 151468. [Google Scholar] [CrossRef]
- Šrut, M.; Drechsel, V.; Höckner, M. Low levels of Cd induce persisting epigenetic modifications and acclimation mechanisms in the earthworm Lumbricus terrestris. PLoS ONE 2017, 12, e0176047. [Google Scholar] [CrossRef]
- Bicho, R.C.; Roelofs, D.; Mariën, J.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Epigenetic effects of (nano)materials in environmental species—Cu case study in Enchytraeus crypticus. Environ. Int. 2020, 136, 105447. [Google Scholar] [CrossRef]
- Bicho, R.C.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Multigenerational exposure to WCCo nanomaterials—Epigenetics in the soil invertebrate Enchytraeus crypticus. Nanomaterials 2020, 10, 836. [Google Scholar] [CrossRef]
- Jeltsch, A. Phylogeny of methylomes. Science 2010, 328, 837–838. [Google Scholar] [CrossRef]
- Novo, M.; Andre, J.; Cunha, L.; Morgan, A.J.; Spurgeon, D.; Kille, P. The functional ghost in the genome machine: Holistic mapping of environmentally induced changes in the epigenome of a soil sentinel. Toxicol. Lett. 2014, 229, S18. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Clementi, E.; Brown, G.C.; Feelisch, M.; Moncada, S. Persistent inhibition of cell respiration by nitric oxide: Crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl. Acad. Sci. USA 1998, 95, 7631–7636. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Olesen, P.; Hougaard, M.; Dang, D.A.; Hoffmann, H.J.; Autrup, H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol. Lett. 2009, 190, 156–162. [Google Scholar] [CrossRef]
- Rivas-García, L.; Quiles, J.L.; Varela-López, A.; Giampieri, F.; Battino, M.; Bettmer, J.; Montes-Bayón, M.; Llopis, J.; Sánchez-González, C. Ultra-small iron nanoparticles target mitochondria inducing autophagy, acting on mitochondrial DNA and reducing respiration. Pharmaceutics 2021, 13, 90. [Google Scholar] [CrossRef]
- Saborano, R.; Wongpinyochit, T.; Totten, J.D.; Johnston, B.F.; Seib, F.P.; Duarte, I.F. Metabolic reprogramming of macrophages exposed to silk, poly(lactic-co-glycolic acid), and silica nanoparticles. Adv. Healthc. Mater. 2017, 6, 1601240. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef]
- Höckner, M.; Piechnik, C.A.; Fiechtner, B.; Weinberger, B.; Tomanek, L. Cadmium-related effects on cellular immunity comprises altered metabolism in earthworm coelomocytes. Int. J. Mol. Sci. 2020, 21, 599. [Google Scholar] [CrossRef]
- Bertoli, F.; Garry, D.; Monopoli, M.P.; Salvati, A.; Dawson, K.A. The intracellular destiny of the protein corona: A study on its cellular internalization and evolution. ACS Nano 2016, 10, 10471–10479. [Google Scholar] [CrossRef]
- Manzanares, D.; Ceña, V. Endocytosis: The nanoparticle and submicron nanocompounds gateway into the cell. Pharmaceutics 2020, 12, 371. [Google Scholar] [CrossRef]


| Inhibitor | Target Pathway | Mode of Action | Applied Conditions | References |
|---|---|---|---|---|
| Cytochalasin B | Phagocytosis | F-actin depolymerization | 5 µM, 1 h | [22] |
| Cytochalasin D | 5 µM, 1 h | [23] | ||
| Colchicine | Pinocytosis | Disrupting microtubules | 100 µM, 2 h | [24] |
| 5-(N-ethyl-N-isopropyl) amiloride (EIPA) | Macropinocytosis | Blocking the Na+/H+ exchanger | 5 µM, 30 min | [25] |
| Amantadine | Clathrin-mediated endocytosis (CME) | Blocks the clathrin-coated pits | 500 µM, 30 min | [26] |
| Methyl-ß-cyclodextrin (MßCD) | Caveolae-mediated endocytosis (CvME) | Remove cholesterol from membrane | 1 mM, 30 min | [27] |
| Nystatin | CvME | Cholesterol sequestration | 54 µM, 30 min | [28] |
| Polyinosinic acid (Poly(I)) | Various pathways (Scavenger receptor (ScR)-mediated) | Binds SR | 287.2 µM, 1 h | [29] |
| Polycytidylic acid (PolyI) | None (Poly(I) antagonist) | Does not bind SR | 107.1 µM, 1 h | [30] |
| Wortmannin | Macropinocytosis | PI3K inhibition | 400 nM, 30 min | [31] |
| % Total Silver Mass in Cells | Ø Inhibitor | Poly(I) | Colchicine | Wortmannin | Cyt. B | Cyt. D |
|---|---|---|---|---|---|---|
| THP-1 | 19.72 ± 0.48 | 11.05 ± 7.99 | 8.94 ± 1.02 | 10.34 ± 3.39 | - | - |
| diff. THP-1 | 17.34 ± 10.11 | 12.51 ± 9.61 | 11.38 ± 10.55 | 13.3 ± 2.99 | - | - |
| Coelomocytes | 16.22 ± 0.4 | - | - | - | 16.62 ± 2.2 | 19.62 ± 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokhanyuk, B.; Vántus, V.B.; Radnai, B.; Vámos, E.; Kajner, G.; Galbács, G.; Telek, E.; Mészáros, M.; Deli, M.A.; Németh, P.; et al. Distinct Uptake Routes Participate in Silver Nanoparticle Engulfment by Earthworm and Human Immune Cells. Nanomaterials 2022, 12, 2818. https://doi.org/10.3390/nano12162818
Kokhanyuk B, Vántus VB, Radnai B, Vámos E, Kajner G, Galbács G, Telek E, Mészáros M, Deli MA, Németh P, et al. Distinct Uptake Routes Participate in Silver Nanoparticle Engulfment by Earthworm and Human Immune Cells. Nanomaterials. 2022; 12(16):2818. https://doi.org/10.3390/nano12162818
Chicago/Turabian StyleKokhanyuk, Bohdana, Viola Bagóné Vántus, Balázs Radnai, Eszter Vámos, Gyula Kajner, Gábor Galbács, Elek Telek, Mária Mészáros, Mária A. Deli, Péter Németh, and et al. 2022. "Distinct Uptake Routes Participate in Silver Nanoparticle Engulfment by Earthworm and Human Immune Cells" Nanomaterials 12, no. 16: 2818. https://doi.org/10.3390/nano12162818
APA StyleKokhanyuk, B., Vántus, V. B., Radnai, B., Vámos, E., Kajner, G., Galbács, G., Telek, E., Mészáros, M., Deli, M. A., Németh, P., & Engelmann, P. (2022). Distinct Uptake Routes Participate in Silver Nanoparticle Engulfment by Earthworm and Human Immune Cells. Nanomaterials, 12(16), 2818. https://doi.org/10.3390/nano12162818








