Antimicrobial Effectiveness of Innovative Photocatalysts: A Review
Abstract
:1. Introduction
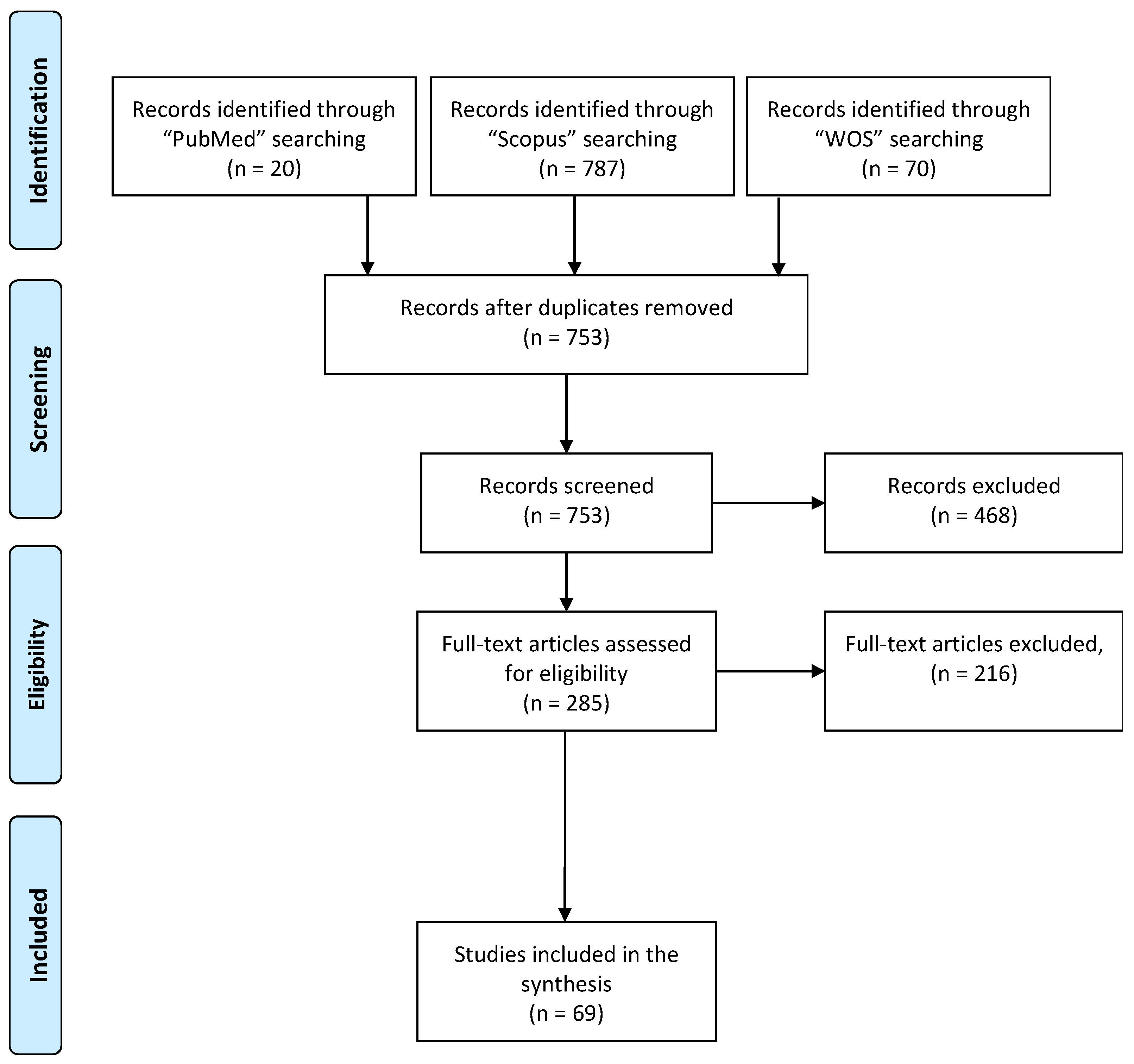
2. Methodology
2.1. Selection Protocol and Search Strategy
2.2. Inclusion and Exclusion Criteria
3. Preparation and Characterization of Photocatalysts
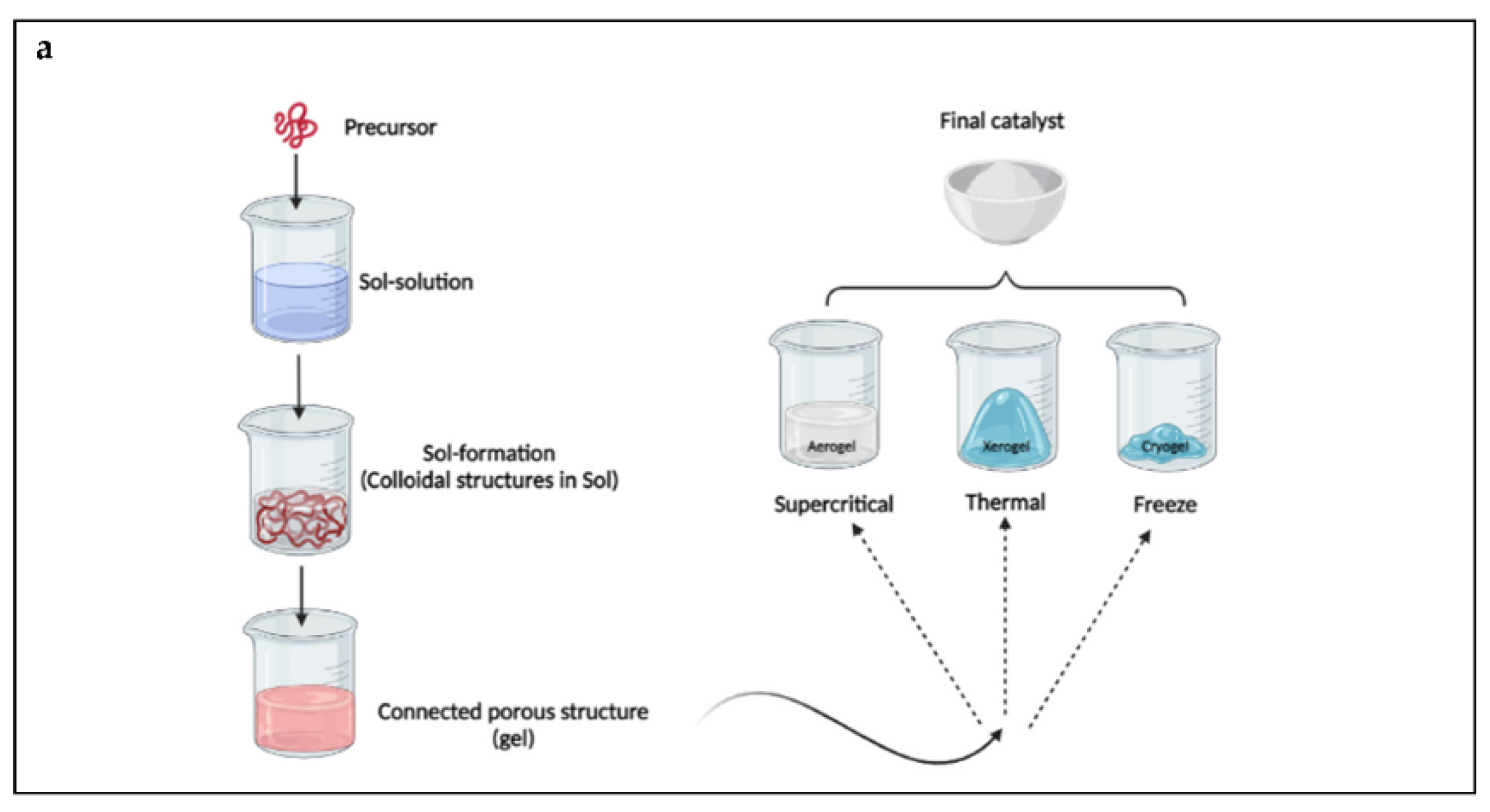
3.1. Sol–Gel Method
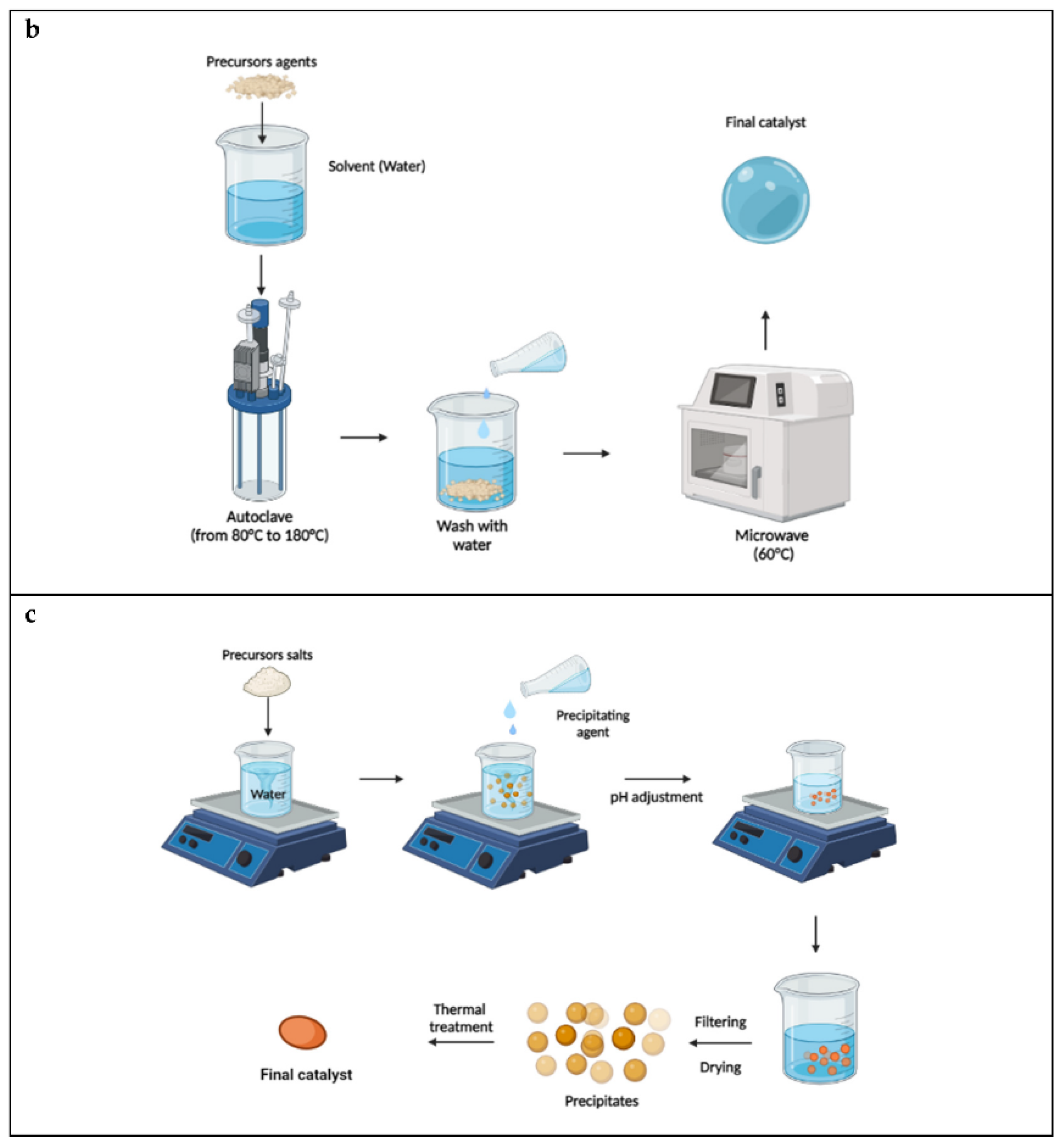
3.2. Hydrothermal Synthesis
3.3. Precipitation Method
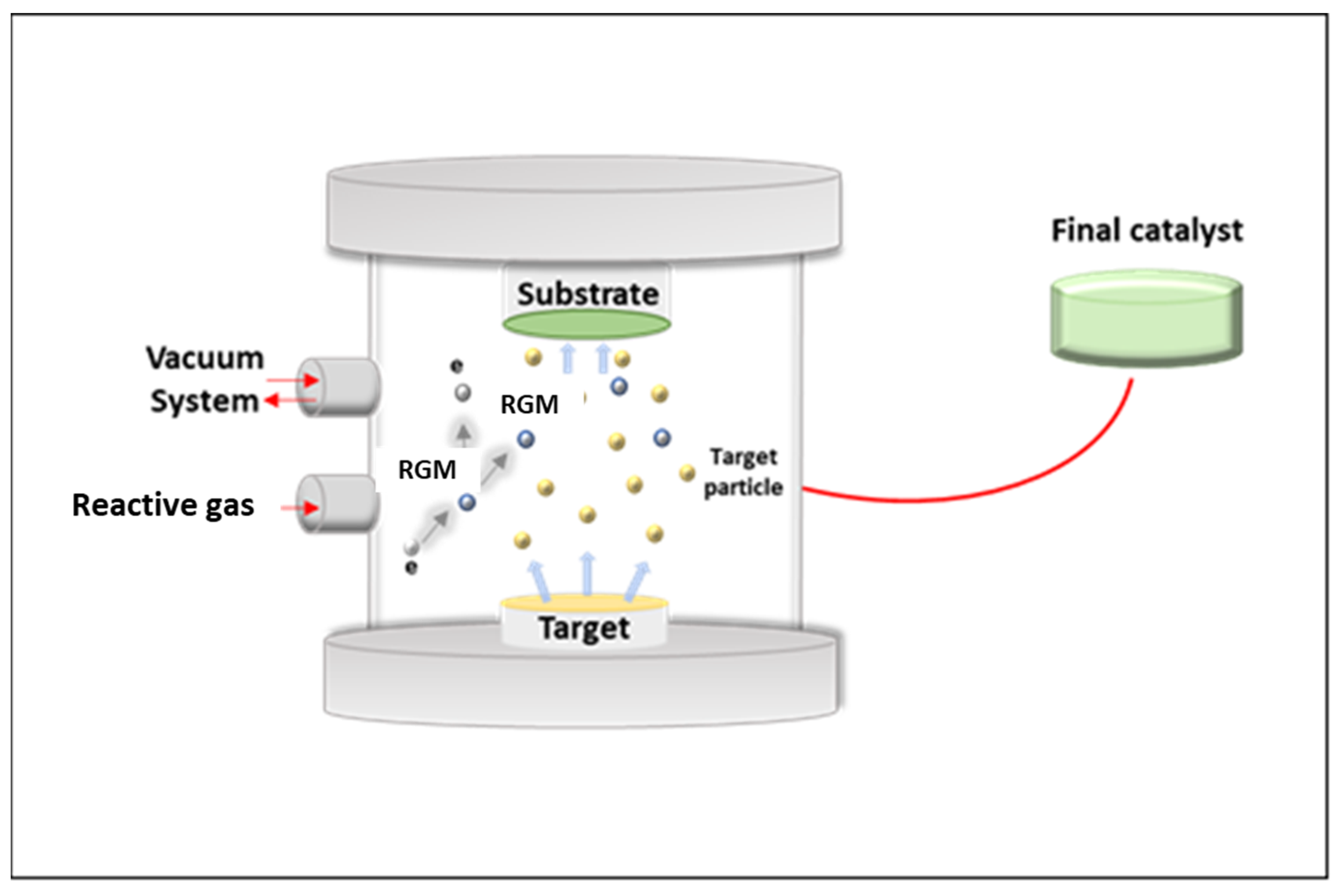
3.4. Microemulsion
3.5. Characterization of Photocatalysts
4. Antimicrobial Efficiencies
4.1. Photocatalyst Dose
4.2. Effect of pH
4.3. Effect of Temperature
4.4. Target
4.5. Effect of Water Matrix
4.6. Role of Direct Contact
4.7. Influence of Light
5. Discussion
5.1. Synthesis Methods
5.2. Regrowth
5.3. Reusability of Photocatalysts
5.4. Toxicity Evaluation
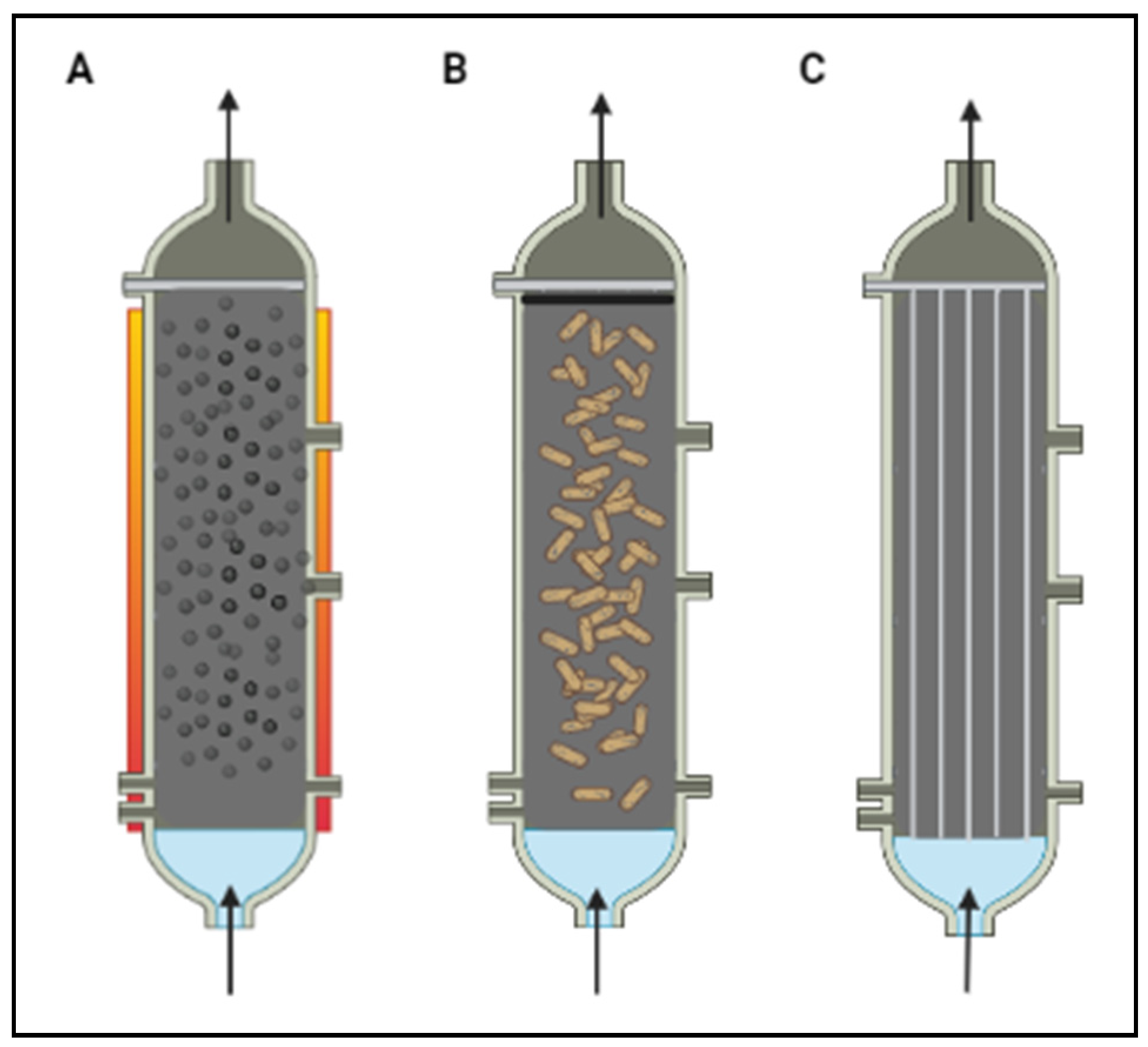
5.5. Photoreactor Configurations
5.6. Electric Energy Consumption
6. Conclusions
- Toxicological and ecotoxicological aspects have not been fully investigated and should be carefully assessed before planning full-scale production;
- Greening production and minimizing the use of solvents should be considered essential for large-scale application;
- Pilot-scale plant experiments are necessary to carry out a realistic cost evaluation per unit volume;
- Regrowth and reuse have to be considered for a complete assessment of behaviors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sethi, D.; Sakthivel, R. ZnO/TiO2 composites for photocatalytic inactivation of Escherichia coli. J. Photochem. Photobiol. B Biol. 2017, 168, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Misra, A.J.; Behera, M.; Behera, S.K.; Nayak, A.K.; Dhal, N.K.; Mishra, A.; Satpathy, B.K.; Lundborg, C.S.; Tripathy, S.K. Photocatalytic disinfection of extended-spectrum beta-lactamase producing Escherichia coli using Alumina/ZnO heterostructures. J. Environ. Chem. Eng. 2021, 9, 106334. [Google Scholar] [CrossRef]
- Wang, J.; Huang, K.; Wu, Z.; Yu, Y. Effects of ultrasound-assisted low-concentration chlorine washing on ready-to-eat winter jujube (Zizyphus jujuba Mill. cv. Dongzao): Cross-contamination prevention, decontamination efficacy, and fruit quality. Ultrason. Sonochem. 2022, 82, 105905. [Google Scholar] [CrossRef] [PubMed]
- da Costa, J.B.; Rodgher, S.; Daniel, L.A.; Espíndola, E.L.G. Toxicity on aquatic organisms exposed to secondary effluent disinfected with chlorine, peracetic acid, ozone and UV radiation. Ecotoxicology 2014, 23, 1803–1813. [Google Scholar] [CrossRef]
- Yuan, Q.B.; Guo, M.T.; Yang, J. Fate of antibiotic resistant bacteria and genes during wastewater chlorination: Implication for antibiotic resistance control. PLoS ONE 2015, 10, e0119403. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Liu, X.; Fang, C.; Chu, W.; Xu, Z. Ecotoxicological effects of disinfected wastewater effluents: A short review of: In vivo toxicity bioassays on aquatic organisms. Environ. Sci. Water Res. Technol. 2020, 6, 2275–2286. [Google Scholar] [CrossRef]
- Tang, H.; Zhong, H.; Pan, Y.; Zhou, Q.; Huo, Z.; Chu, W.; Xu, B. A New Group of Heterocyclic Nitrogenous Disinfection Byproducts (DBPs) in Drinking Water: Role of Extraction pH in Unknown DBP Exploration. Environ. Sci. Technol. 2021, 55, 6764–6772. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res.-Rev. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef]
- Brungs, W.A. Effects of residual chlorine on aquatic life. J. Water Pollut. Control Fed. 1973, 45, 2180–2193. [Google Scholar]
- Ferro, G.; Fiorentino, A.; Alferez, M.C.; Polo-López, M.I.; Rizzo, L.; Fernández-Ibáñez, P. Urban wastewater disinfection for agricultural reuse: Effect of solar driven AOPs in the inactivation of a multidrug resistant E. coli strain. Appl. Catal. B Environ. 2015, 178, 65–73. [Google Scholar] [CrossRef]
- Margarucci, L.M.; Romano Spica, V.; Gianfranceschi, G.; Valeriani, F. Untouchability of natural spa waters: Perspectives for treatments within a personalized water safety plan. Environ. Int. 2019, 133, 105095. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, B.; Liu, X.; Li, Z.; Zhu, S.; Liang, Y.; Wu, S. Recent progress in photocatalytic antibacterial. ACS Appl. Bio Mater. 2021, 4, 3909–3936. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Byrne, C.; Breen, A.; Pillai, S.C. Antimicrobial activity of photocatalysts: Fundamentals, mechanisms, kinetics and recent advances. Appl. Catal. B Environ. 2018, 225, 51–75. [Google Scholar] [CrossRef]
- Cho, E.-a.; Bailer, J.; Oris, J.T. Effect of methyl tert-butyl ether on the bioconcentration and photoinduced toxicity of fluoranthene in fathead minnow larvae (Pimephales promelas). Environ. Sci. Technol. 2003, 37, 1306–1310. [Google Scholar] [CrossRef]
- Raoufi, D. Transparent thin films of pure anatase Titania nanoparticles with low surface roughness prepared by electron beam deposition method. Mater. Res. Express 2019, 6, 096406. [Google Scholar] [CrossRef]
- Cantarella, M.; Sanz, R.; Buccheri, M.A.; Ruffino, F.; Rappazzo, G.; Scalese, S.; Impellizzeri, G.; Romano, L.; Privitera, V. Immobilization of nanomaterials in PMMA composites for photocatalytic removal of dyes, phenols and bacteria from water. J. Photochem. Photobiol. A Chem. 2016, 321, 1–11. [Google Scholar] [CrossRef]
- Mudhoo, A.; Paliya, S.; Goswami, P.; Singh, M.; Lofrano, G.; Carotenuto, M.; Carraturo, F.; Libralato, G.; Guida, M.; Usman, M.; et al. Fabrication, Functionalization and Performance of Doped Photocatalysts for Dye Degradation and Mineralization: A Review. Environ. Chem. Lett. 2020, 18, 1825–1903. [Google Scholar] [CrossRef]
- Covidence-Better Systematic Review Management. Available online: https://www.covidence.org/ (accessed on 1 January 2021).
- Khaki, M.; Ait-El-Fquih, B.; Hoteit, I.; Forootan, E.; Awange, J.; Kuhn, M. A two-update ensemble Kalman filter for land hydrological data assimilation with an uncertain constraint. J. Hydrol. 2017, 555, 447–462. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, X.; Chen, X.; Jia, Q.; Yu, R.; Ma, T. Effect of heat treatment conditions on the growth of MgAl2O4 nanoparticles obtained by sol-gel method. Ceram. Int. 2017, 43, 15246–15253. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. The advancements in sol-gel method of doped-TiO2 photocatalysts. Appl. Catal. A Gen. 2010, 375, 1–11. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 1–21. [Google Scholar] [CrossRef]
- Habte, L.; Shiferaw, N.; Mulatu, D.; Thenepalli, T.; Chilakala, R.; Ahn, J.W. Synthesis of nano-calcium oxide fromwaste eggshell by sol-gel method. Sustainability 2019, 11, 3196. [Google Scholar] [CrossRef]
- Ferraz, E.; Gamelas, J.A.F.; Coroado, J.; Monteiro, C.; Rocha, F. Recycling Waste Seashells to Produce Calcitic Lime: Characterization and Wet Slaking Reactivity. Waste Biomass Valorization 2019, 10, 2397–2414. [Google Scholar] [CrossRef]
- Tizo, M.S.; Blanco, L.A.V.; Cagas, A.C.Q.; Dela Cruz, B.R.B.; Encoy, J.C.; Gunting, J.V.; Arazo, R.O.; Mabayo, V.I.F. Efficiency of calcium carbonate from eggshells as an adsorbent for cadmium removal in aqueous solution. Sustain. Environ. Res. 2018, 28, 326–332. [Google Scholar] [CrossRef]
- Gombac, V.; De Rogatis, L.; Gasparotto, A.; Vicario, G.; Montini, T.; Barreca, D.; Balducci, G.; Fornasiero, P.; Tondello, E.; Graziani, M. TiO2 nanopowders doped with boron and nitrogen for photocatalytic applications. Chem. Phys. 2007, 339, 111–123. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q. Preparation and characterization of titania photocatalyst co-doped with boron, nickel, and cerium. Mater. Lett. 2008, 62, 2589–2592. [Google Scholar] [CrossRef]
- Huang, D.G.; Liao, S.J.; Liu, J.M.; Dang, Z.; Petrik, L. Preparation of visible-light responsive N-F-codoped TiO2 photocatalyst by a sol-gel-solvothermal method. J. Photochem. Photobiol. A Chem. 2006, 184, 282–288. [Google Scholar] [CrossRef]
- Srinivasan, S.S.; Wade, J.; Stefanakos, E.K.; Goswami, Y. Synergistic effects of sulfation and co-doping on the visible light photocatalysis of TiO2. J. Alloys Compd. 2006, 424, 322–326. [Google Scholar] [CrossRef]
- Wilke, K.; Breuer, H.D. The influence of transition metal doping on the physical and photocatalytic properties of titania. J. Photochem. Photobiol. A Chem. 1999, 121, 49–53. [Google Scholar] [CrossRef]
- Macías-Sánchez, J.J.; Hinojosa-Reyes, L.; Caballero-Quintero, A.; De La Cruz, W.; Ruiz-Ruiz, E.; Hernández-Ramírez, A.; Guzmán-Mar, J.L. Synthesis of nitrogen-doped ZnO by sol-gel method: Characterization and its application on visible photocatalytic degradation of 2,4-D and picloram herbicides. Photochem. Photobiol. Sci. 2015, 14, 536–542. [Google Scholar] [CrossRef]
- Pal, M.; Bera, S.; Sarkar, S.; Jana, S. Influence of Al doping on microstructural, optical and photocatalytic properties of sol-gel based nanostructured zinc oxide films on glass. RSC Adv. 2014, 4, 11552–11563. [Google Scholar] [CrossRef]
- Thongsuriwong, K.; Amornpitoksuk, P.; Suwanboon, S. Photocatalytic and antibacterial activities of Ag-doped ZnO thin films prepared by a sol-gel dip-coating method. J. Sol-Gel Sci. Technol. 2012, 62, 304–312. [Google Scholar] [CrossRef]
- Fu, M.; Li, Y.; Wu, S.; Lu, P.; Liu, J.; Dong, F. Sol-gel preparation and enhanced photocatalytic performance of Cu-doped ZnO nanoparticles. Appl. Surf. Sci. 2011, 258, 1587–1591. [Google Scholar] [CrossRef]
- Lima, M.K.; Fernandes, D.M.; Silva, M.F.; Baesso, M.L.; Neto, A.M.; de Morais, G.R.; Nakamura, C.V.; de Oliveira Caleare, A.; Hechenleitner, A.A.W.; Pineda, E.A.G. Co-doped ZnO nanoparticles synthesized by an adapted sol–gel method: Effects on the structural, optical, photocatalytic and antibacterial properties. J. Sol-Gel Sci. Technol. 2014, 72, 301–309. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P. Nanostructured N-doped TiO2 coated on glass spheres for the photocatalytic removal of organic dyes under UV or visible light irradiation. Appl. Catal. B Environ. 2015, 170–171, 153–161. [Google Scholar] [CrossRef]
- Sōmiya, S.; Roy, R. Hydrothermal synthesis of fine oxide powders. Bull. Mater. Sci. 2000, 23, 453–460. [Google Scholar] [CrossRef]
- Suo, G.; Li, J. Growth and application of TIO2 nanowires. In Titanium Dioxide Nanoparticles Characterizations, Properties, and Syntheses; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; pp. 207–226. ISBN 978-1-53611-073-9. [Google Scholar]
- Zhou, J.k.; Lv, L.; Yu, J.; Li, H.L.; Guo, P.-Z.; Sun, H.; Zhao, X.S. Synthesis of fluorinated TiO2 hollow microspheres and their photocatalytic activity under visible light. J. Phys. Chem. A 2008, 112, 5316–5321. [Google Scholar]
- Wu, D.; Long, M.; Cai, W.; Chen, C.; Wu, Y. Low temperature hydrothermal synthesis of N-doped TiO2 photocatalyst with high visible-light activity. J. Alloys Compd. 2010, 502, 289–294. [Google Scholar] [CrossRef]
- Amano, F.; Yamakata, A.; Nogami, K.; Osawa, M.; Ohtani, B. Visible light responsive pristine metal oxide photocatalyst: Enhancement of activity by crystallization under hydrothermal treatment. J. Am. Chem. Soc. 2008, 130, 17650–17651. [Google Scholar] [CrossRef]
- Li, W.; Carrete, J.; Mingo, N. Thermal conductivity and phonon linewidths of monolayer MoS2 from first principles. Appl. Phys. Lett. 2013, 103, 253103. [Google Scholar] [CrossRef]
- Regalbuto, J. Catalyst Preparation: Science and Engineering; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780849370885. [Google Scholar]
- Raoufi, D. Synthesis and microstructural properties of ZnO nanoparticles prepared by precipitation method. Renew. Energy 2013, 50, 932–937. [Google Scholar] [CrossRef]
- Nejati, K.; Rezvani, Z.; Pakizevand, R. Synthesis of ZnO Nanoparticles and Investigation of the Ionic Template Effect on Their Size and Shape. Int. Nano Lett. 2011, 1, 75–81. [Google Scholar]
- Mittal, M.; Sharma, M.; Pandey, O.P. UV-Visible light induced photocatalytic studies of Cu doped ZnO nanoparticles prepared by co-precipitation method. Sol. Energy 2014, 110, 386–397. [Google Scholar] [CrossRef]
- Amornpitoksuk, P.; Suwanboon, S.; Sangkanu, S.; Sukhoom, A.; Muensit, N.; Baltrusaitis, J. Synthesis, characterization, photocatalytic and antibacterial activities of Ag-doped ZnO powders modified with a diblock copolymer. Powder Technol. 2012, 219, 158–164. [Google Scholar] [CrossRef]
- Kubacka, A.; Caudillo-Flores, U.; Barba-Nieto, I.; Muñoz-Batista, M.J.; Fernández-García, M. Microemulsion: A versatile synthesis tool for photocatalysis. Curr. Opin. Colloid Interface Sci. 2020, 49, 42–59. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials. 1st Nano Update. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar] [CrossRef]
- Eastoe, J.; Hollamby, M.J.; Hudson, L. Recent advances in nanoparticle synthesis with reversed micelles. Adv. Colloid Interface Sci. 2006, 128–130, 5–15. [Google Scholar] [CrossRef]
- Fernández-García, M.; Wang, X.; Belver, C.; Hanson, J.C.; Rodriguez, J.A. Anatase-TiO2 nanomaterials: Morphological/size dependence of the crystallization and phase behavior phenomena. J. Phys. Chem. C 2007, 111, 674–682. [Google Scholar] [CrossRef]
- Iglesias-Juez, A.; Viñes, F.; Lamiel-García, O.; Fernández-García, M.; Illas, F. Morphology effects in photoactive ZnO nanostructures: Photooxidative activity of polar surfaces. J. Mater. Chem. A 2015, 3, 8782–8792. [Google Scholar] [CrossRef]
- Martin, D.J.; Qiu, K.; Shevlin, S.A.; Handoko, A.D.; Chen, X.; Guo, Z.; Tang, J. Highly efficient photocatalytic H2 evolution from water using visible light and structure-controlled graphitic carbon nitride. Angew. Chem. Int. Ed. 2014, 53, 9240–9245. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gasses in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Bragg, W. Crystal Structure. In Science; Bragg, W., Bragg, W.L., Eds.; Macmillan and Company: New York, NY, USA, 1934; Volume 80, pp. 290–291. [Google Scholar]
- Hart, M. X-ray diffraction by L. V. Azaroff, R. Kaplow, N. Kato, R.J. Weiss, A.J.C. Wilson and R. A. Young. Acta Crystallogr. Sect. A 1975, 31, 878. [Google Scholar] [CrossRef]
- Smith, E.; Dent, G. Modern Raman Spectroscopy—A Practical Approach; John Wiley & Sons, Ltd.: Chichester, UK, 2005; ISBN 0471496685. [Google Scholar]
- Anderson, C.; Bard, A.J. Improved Photocatalytic Activity and Characterization of Mixed TiO2/SiO2 and TiO2/Al2O3 Materials The characterization of mixed oxides of TiO2/SiO2 and TiO2/Al2O3 prepared by sol-gel methods is described. Application of the TiO2/Al2O3. J. Phys. Chem. B 1996, 5647, 2611–2616. [Google Scholar]
- Gionco, C.; Paganini, M.C.; Giamello, E.; Burgess, R.; Di Valentin, C.; Pacchioni, G. Cerium-doped zirconium dioxide, a visible-light-sensitive photoactive material of third generation. J. Phys. Chem. Lett. 2014, 5, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Paul, S.; Choudhury, A. Investigation of the optical property and photocatalytic activity of mixed phase nanocrystalline titania. Appl. Nanosci. 2014, 4, 839–847. [Google Scholar] [CrossRef]
- Shanmugam, V.; Sanjeevamuthu, S.; Jeyaperumal, K.S.; Vairamuthu, R. Fabrication of heterostructured vanadium modified g-C3N4/TiO2 hybrid photocatalyst for improved photocatalytic performance under visible light exposure and antibacterial activities. J. Ind. Eng. Chem. 2019, 76, 318–332. [Google Scholar] [CrossRef]
- Yu, X.; Wang, S.; Zhang, X.; Qi, A.; Qiao, X.; Liu, Z.; Wu, M.; Li, L.; Wang, Z.L. Heterostructured nanorod array with piezophototronic and plasmonic effect for photodynamic bacteria killing and wound healing. Nano Energy 2018, 46, 29–38. [Google Scholar] [CrossRef]
- Wu, X.; Cao, L.; Song, J.; Si, Y.; Yu, J.; Ding, B. Thorn-like flexible Ag2C2O4/TiO2 nanofibers as hierarchical heterojunction photocatalysts for efficient visible-light-driven bacteria-killing. J. Colloid Interface Sci. 2020, 560, 681–689. [Google Scholar] [CrossRef]
- Shi, H.; Fan, J.; Zhao, Y.; Hu, X.; Zhang, X.; Tang, Z. Visible light driven CuBi2O4/Bi2MoO6 p-n heterojunction with enhanced photocatalytic inactivation of E. coli and mechanism insight. J. Hazard. Mater. 2020, 381, 121006. [Google Scholar] [CrossRef]
- Song, J.; Yu, J.; Sun, G.; Si, Y.; Ding, B. Visible-light-driven, hierarchically heterostructured, and flexible silver/bismuth oxyiodide/titania nanofibrous membranes for highly efficient water disinfection. J. Colloid Interface Sci. 2019, 555, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Ma, S.; Wang, P.; Hou, Q.; Han, J.; Zhan, S. Efficient water disinfection with Ag2WO4-doped mesoporous g-C3N4 under visible light. J. Hazard. Mater. 2017, 338, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Baniamerian, H.; Safavi, M.; Alvarado-Morales, M.; Tsapekos, P.; Angelidaki, I.; Shokrollahzadeh, S. Photocatalytic inactivation of Vibrio fischeri using Fe2O3-TiO2-based nanoparticles. Environ. Res. 2018, 166, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, C.; Zhao, Y.; Liu, E.; Fan, J.; Ji, Z. Highly efficient visible light driven photocatalytic inactivation of E. coli with Ag QDs decorated Z-scheme Bi2S3/SnIn4S8 composite. Appl. Catal. B Environ. 2019, 254, 403–413. [Google Scholar] [CrossRef]
- Yan, H.; Liu, L.; Wang, R.; Zhu, W.; Ren, X.; Luo, L.; Zhang, X.; Luo, S.; Ai, X.; Wang, J. Binary composite MoS2/TiO2 nanotube arrays as a recyclable and efficient photocatalyst for solar water disinfection. Chem. Eng. J. 2020, 401, 126052. [Google Scholar] [CrossRef]
- Deng, J.; Liang, J.; Li, M.; Tong, M. Enhanced visible-light-driven photocatalytic bacteria disinfection by g-C3N4-AgBr. Colloids Surf. B Biointerfaces 2017, 152, 49–57. [Google Scholar] [CrossRef]
- Wanag, A.; Rokicka, P.; Kusiak-Nejman, E.; Kapica-Kozar, J.; Wrobel, R.J.; Markowska-Szczupak, A.; Morawski, A.W. Antibacterial properties of TiO2 modified with reduced graphene oxide. Ecotoxicol. Environ. Saf. 2018, 147, 788–793. [Google Scholar] [CrossRef]
- Liang, J.; Deng, J.; Li, M.; Tong, M. Bactericidal activity and mechanism of AgI/AgBr/BiOBr0.75I0.25 under visible light irradiation. Colloids Surf. B Biointerfaces 2016, 138, 102–109. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Shuai, D.; Zhang, W.; Niu, L.; Wang, L.; Zhang, H. Visible-light-driven, water-surface-floating antimicrobials developed from graphitic carbon nitride and expanded perlite for water disinfection. Chemosphere 2018, 208, 84–92. [Google Scholar] [CrossRef]
- Zhang, G.; Savateev, A.; Zhao, Y.; Li, L.; Antonietti, M. Advancing the n→π∗ electron transition of carbon nitride nanotubes for H2 photosynthesis. J. Mater. Chem. A 2017, 5, 12723–12728. [Google Scholar] [CrossRef]
- Rahimi, R.; Zargari, S.; Yousefi, A.; Yaghoubi Berijani, M.; Ghaffarinejad, A.; Morsali, A. Visible light photocatalytic disinfection of E. coli with TiO2-graphene nanocomposite sensitized with tetrakis(4-carboxyphenyl)porphyrin. Appl. Surf. Sci. 2015, 355, 1098–1106. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z.; Yu, M.; Xu, Z.; Liu, Y.; Li, F.; Wang, L. Preparation of enhanced AgI@MnO2 heterojunction photocatalysts for rapid sterilization under visible light. J. Alloys Compd. 2021, 887, 161431. [Google Scholar] [CrossRef]
- Xia, D.; An, T.; Li, G.; Wang, W.; Zhao, H.; Wong, P.K. Synergistic photocatalytic inactivation mechanisms of bacteria by graphene sheets grafted plasmonic AgAgX (X = Cl, Br, I) composite photocatalyst under visible light irradiation. Water Res. 2016, 99, 149–161. [Google Scholar] [CrossRef]
- Li, M.; Liu, F.; Ma, Z.; Liu, W.; Liang, J.; Tong, M. Different mechanisms for E. coli disinfection and BPA degradation by CeO2-AgI under visible light irradiation. Chem. Eng. J. 2019, 371, 750–758. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Li, Y.; Shuai, D. Visible-light-driven photocatalytic disinfection of human adenovirus by a novel heterostructure of oxygen-doped graphitic carbon nitride and hydrothermal carbonation carbon. Appl. Catal. B Environ. 2019, 248, 11–21. [Google Scholar] [CrossRef]
- Feng, T.; Liang, J.; Ma, Z.; Li, M.; Tong, M. Bactericidal activity and mechanisms of BiOBr-AgBr under both dark and visible light irradiation conditions. Colloids Surf. B Biointerfaces 2018, 167, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.K.; Wu, T.; Huang, C.W.; Lai, C.Y.; Wu, M.Y.; Lin, Y.W. Enhanced photocatalytic performance of BiVO4 in aqueous AgNO3 solution under visible light irradiation. Appl. Surf. Sci. 2017, 399, 10–19. [Google Scholar] [CrossRef]
- Sharma, B.; Boruah, P.K.; Yadav, A.; Das, M.R. TiO2–Fe2O3 nanocomposite heterojunction for superior charge separation and the photocatalytic inactivation of pathogenic bacteria in water under direct sunlight irradiation. J. Environ. Chem. Eng. 2018, 6, 134–145. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Zheng, Y.; Li, C.; Kwok Yeung, K.W.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Wu, S. Ag3PO4 decorated black urchin-like defective TiO2 for rapid and long-term bacteria-killing under visible light. Bioact. Mater. 2021, 6, 1575–1587. [Google Scholar] [CrossRef]
- Mangayayam, M.; Kiwi, J.; Giannakis, S.; Pulgarin, C.; Zivkovic, I.; Magrez, A.; Rtimi, S. FeOx magnetization enhancing E. coli inactivation by orders of magnitude on Ag-TiO2 nanotubes under sunlight. Appl. Catal. B Environ. 2017, 202, 438–445. [Google Scholar] [CrossRef]
- Sun, L.; Du, T.; Hu, C.; Chen, J.; Lu, J.; Lu, Z.; Han, H. Antibacterial Activity of Graphene Oxide/g-C3N4 Composite through Photocatalytic Disinfection under Visible Light. ACS Sustain. Chem. Eng. 2017, 5, 8693–8701. [Google Scholar] [CrossRef]
- Rtimi, S.; Pulgarin, C.; Bensimon, M.; Kiwi, J. Evidence for TiON sputtered surfaces showing accelerated antibacterial activity under simulated solar irradiation. Sol. Energy 2013, 93, 55–62. [Google Scholar] [CrossRef]
- Baghriche, O.; Rtimi, S.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Effect of the spectral properties of TiO2, Cu, TiO2/Cu sputtered films on the bacterial inactivation under low intensity actinic light. J. Photochem. Photobiol. A Chem. 2013, 251, 50–56. [Google Scholar] [CrossRef]
- Ohtsu, N.; Yokoi, K.; Saito, A. Fabrication of a visible-light-responsive photocatalytic antibacterial coating on titanium through anodic oxidation in a nitrate/ethylene glycol electrolyte. Surf. Coat. Technol. 2015, 262, 97–102. [Google Scholar] [CrossRef]
- Gomez-Polo, C.; Larumbe, S.; Gil, A.; Muñoz, D.; Fernández, L.R.; Barquín, L.F.; García-Prieto, A.; Fdez-Gubieda, M.L.; Muela, A. Improved photocatalytic and antibacterial performance of Cr doped TiO2 nanoparticles. Surf. Interfaces 2021, 22, 100867. [Google Scholar] [CrossRef]
- Huang, S.M.; Weng, C.H.; Tzeng, J.H.; Huang, Y.Z.; Anotai, J.; Yen, L.T.; Chang, C.J.; Lin, Y.T. Kinetic study and performance comparison of TiO2-mediated visible-light-responsive photocatalysts for the inactivation of Aspergillus niger. Sci. Total Environ. 2019, 692, 975–983. [Google Scholar] [CrossRef]
- Sethi, D.; Jada, N.; Tiwari, A.; Ramasamy, S.; Dash, T.; Pandey, S. Photocatalytic destruction of Escherichia coli in water by V2O5/TiO2. J. Photochem. Photobiol. B Biol. 2015, 144, 68–74. [Google Scholar] [CrossRef]
- Rtimi, S.; Baghriche, O.; Pulgarin, C.; Lavanchy, J.C.; Kiwi, J. Growth of TiO2/Cu films by HiPIMS for accelerated bacterial loss of viability. Surf. Coat. Technol. 2013, 232, 804–813. [Google Scholar] [CrossRef]
- Gao, P.; Liu, J.; Zhang, T.; Sun, D.D.; Ng, W. Hierarchical TiO2/CdS “spindle-like” composite with high photodegradation and antibacterial capability under visible light irradiation. J. Hazard. Mater. 2012, 229–230, 209–216. [Google Scholar] [CrossRef]
- Alhussein, A.; Achache, S.; Deturche, R.; Sanchette, F.; Pulgarin, C.; Kiwi, J.; Rtimi, S. Beneficial effect of Cu on Ti-Nb-Ta-Zr sputtered uniform/adhesive gum films accelerating bacterial inactivation under indoor visible light. Colloids Surf. B Biointerfaces 2017, 152, 152–158. [Google Scholar] [CrossRef]
- Liang, J.; Deng, J.; Li, M.; Xu, T.; Tong, M. Bactericidal activity and mechanism of Ti-doped BiOI microspheres under visible light irradiation. Colloids Surf. B Biointerfaces 2016, 147, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Rtimi, S.; Pulgarin, C.; Robyr, M.; Aybush, A.; Shelaev, I.; Gostev, F.; Nadtochenko, V.; Kiwi, J. Insight into the catalyst/photocatalyst microstructure presenting the same composition but leading to a variance in bacterial reduction under indoor visible light. Appl. Catal. B Environ. 2017, 208, 135–147. [Google Scholar] [CrossRef]
- Rtimi, S.; Baghriche, O.; Sanjines, R.; Pulgarin, C.; Ben-Simon, M.; Lavanchy, J.C.; Houas, A.; Kiwi, J. Photocatalysis/catalysis by innovative TiN and TiN-Ag surfaces inactivate bacteria under visible light. Appl. Catal. B Environ. 2012, 123–124, 306–315. [Google Scholar] [CrossRef]
- Podporska-Carroll, J.; Myles, A.; Quilty, B.; McCormack, D.E.; Fagan, R.; Hinder, S.J.; Dionysiou, D.D.; Pillai, S.C. Antibacterial properties of F-doped ZnO visible light photocatalyst. J. Hazard. Mater. 2017, 324, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Thakur, I.; Verma, A.; Örmeci, B. Visibly active Fe-TiO2 composite: A stable and efficient catalyst for the catalytic disinfection of water using a once-through reactor. J. Environ. Chem. Eng. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Zammit, I.; Vaiano, V.; Ribeiro, A.R.; Silva, A.M.T.; Manaia, C.M.; Rizzo, L. Immobilised cerium-doped zinc oxide as a photocatalyst for the degradation of antibiotics and the inactivation of antibiotic-resistant bacteria. Catalysts 2019, 9, 222. [Google Scholar] [CrossRef]
- Suárez, L.; Baghriche, O.; Rtimi, S.; Pulgarin, C.; Kiwi, J. Sputtered Cu-polyethylene films inducing bacteria inactivation in the dark and under low intensity sunlight. J. Photochem. Photobiol. A Chem. 2016, 330, 163–168. [Google Scholar] [CrossRef]
- Rtimi, S.; Ballo, M.K.S.; Laub, D.; Pulgarin, C.; Entenza, J.M.; Bizzini, A.; Sanjines, R.; Kiwi, J. Duality in the Escherichia coli and methicillin resistant Staphylococcus aureus reduction mechanism under actinic light on innovative co-sputtered surfaces. Appl. Catal. A Gen. 2015, 498, 185–191. [Google Scholar] [CrossRef]
- Zammit, I.; Vaiano, V.; Iervolino, G.; Rizzo, L. Inactivation of an urban wastewater indigenous: Escherichia coli strain by cerium doped zinc oxide photocatalysis. RSC Adv. 2018, 8, 26124–26132. [Google Scholar] [CrossRef]
- Qi, K.; Xing, X.; Zada, A.; Li, M.; Wang, Q.; Liu, S.Y.; Wang, G. Transition metal doped ZnO nanoparticles with enhanced photocatalytic and antibacterial performances: Experimental and DFT studies. Ceram. Int. 2020, 46, 1494–1502. [Google Scholar] [CrossRef]
- Stoyanova, A.M.; Hitkova, H.Y.; Ivanova, N.K.; Bachvarova-Nedelcheva, A.D.; Iordanova, R.S.; Sredkova, M.P. Photocatalytic and antibacterial activity of Fe-doped TiO2 nanoparticles prepared by nonhydrolytic sol-gel method. Bulg. Chem. Commun. 2013, 45, 497–504. [Google Scholar]
- Wang, J.; Zhuang, H.; Hinton, A.; Bowker, B.; Zhang, J. Photocatalytic disinfection of spoilage bacteria Pseudomonas fluorescens and Macrococcus caseolyticus by nano-TiO2. LWT-Food Sci. Technol. 2014, 59, 1009–1017. [Google Scholar] [CrossRef]
- Raut, A.V.; Yadav, H.M.; Gnanamani, A.; Pushpavanam, S.; Pawar, S.H. Synthesis and characterization of chitosan-TiO2:Cu nanocomposite and their enhanced antimicrobial activity with visible light. Colloids Surf. B Biointerfaces 2016, 148, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zhang, X.; Zhang, W.; Zhang, S.; Su, H.; Tan, T. Visible-light-mediated synergistic photocatalytic antimicrobial effects and mechanism of Ag-nanoparticles@chitosan-TiO2 organic-inorganic composites for water disinfection. Appl. Catal. B Environ. 2015, 170–171, 255–262. [Google Scholar] [CrossRef]
- Wang, G.Z.; Chang, J.L.; Tang, W.; Xie, W.; Ang, Y.S. 2D materials and heterostructures for photocatalytic water-splitting: A theoretical perspective. J. Phys. D Appl. Phys. 2022, 55, 293002. [Google Scholar] [CrossRef]
- O’Dowd, K.; Nair, K.M.; Pillai, S.C. Photocatalytic degradation of antibiotic-resistant genes and bacteria using 2D nanomaterials: What is known and what are the challenges? Curr. Opin. Green Sustain. Chem. 2021, 30, 100471. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, J.; Liu, Z.; Wang, Z.; Yu, K.; Xing, B. Cleavage and transformation inhibition of extracellular antibiotic resistance genes by graphene oxides with different lateral sizes. Sci. Total Environ. 2019, 695, 133932. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Z.; Jiang, H.; Wang, X. Progress in Photocatalytic Semiconductor Hybrids for Bacterial Inactivation. Mater. Horiz. 2021, 8, 2964–3008. [Google Scholar] [CrossRef]
- Ortega-Gómez, E.; Martín, M.M.B.; García, B.E.; Pérez, J.A.S.; Ibáñez, P.F. Wastewater disinfection by neutral pH photo-Fenton: The role of solar radiation intensity. Appl. Catal. B Environ. 2016, 181, 1–6. [Google Scholar] [CrossRef]
- Zuliani, A.; Cova, C.M. Green Synthesis of Heterogeneous Visible-Light-Active Photocatalysts: Recent Advances. Photochem 2021, 1, 147–166. [Google Scholar] [CrossRef]
- Gao, D.; Li, H.; Wei, P.; Wang, Y.; Wang, G.; Bao, X. Electrochemical synthesis of catalytic materials for energy catalysis. Chin. J. Catal. 2022, 43, 1001–1016. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y.; Ghonchegi, Z. Fabrication and characterization of copper doped TiO2 nanotube arrays by in situ electrochemical method as efficient visible-light photocatalyst. Ceram. Int. 2015, 41, 8735–8741. [Google Scholar] [CrossRef]
- Ratova, M.; Sawtell, D.; Kelly, P.J. Micro-patterning of magnetron sputtered titanium dioxide coatings and their efficiency for photocatalytic applications. Coatings 2020, 10, 68. [Google Scholar] [CrossRef]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Takeda, S.; Suzuki, S.; Odaka, H.; Hosono, H. Photocatalytic TiO2 thin film deposited onto glass by DC magnetron sputtering. Thin Solid Films 2001, 392, 338–344. [Google Scholar] [CrossRef]
- Shaw, T.E.; Mathivathanan, L.; Jurca, T. One-Pot, One-Step Precatalysts through Mechanochemistry. Organometallics 2019, 38, 4066–4070. [Google Scholar] [CrossRef]
- Jia, X.; Han, Q.; Zheng, M.; Bi, H. One pot milling route to fabricate step-scheme AgI/I-BiOAc photocatalyst: Energy band structure optimized by the formation of solid solution. Appl. Surf. Sci. 2019, 489, 409–419. [Google Scholar] [CrossRef]
- Amrute, A.P.; De Bellis, J.; Felderhoff, M.; Schüth, F. Mechanochemical Synthesis of Catalytic Materials. Chem.–Eur. J. 2021, 27, 6819–6847. [Google Scholar] [CrossRef]
- Albarano, L.; Serafini, S.; Toscanesi, M.; Trifuoggi, M.; Zupo, V.; Costantini, M.; Vignati, D.A.L.; Guida, M.; Libralato, G. Genotoxicity Set Up in Artemia franciscana Nauplii and Adults. Water 2022, 14, 1594. [Google Scholar] [CrossRef]
- Bownik, A. Daphnia swimming behaviour as a biomarker in toxicity assessment: A review. Sci. Total Environ. 2017, 601–602, 194–205. [Google Scholar] [CrossRef]
- Forster, J.; Hirst, A.G.; Atkinson, D. Warming-induced reductions in body size are greater in aquatic than terrestrial species. Proc. Natl. Acad. Sci. USA 2012, 109, 19310–19314. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, Í.F.S.; Souza, T.M.; Vieira, L.R.; Marchi, F.C.; Nascimento, A.P.; Farias, D.F. Toxicity testing of pesticides in zebrafish—a systematic review on chemicals and associated toxicological endpoints. Environ. Sci. Pollut. Res. 2020, 27, 10185–10204. [Google Scholar] [CrossRef] [PubMed]
- Needleman, R.K.; Neylan, I.P.; Erickson, T. Potential Environmental and Ecological Effects of Global Climate Change on Venomous Terrestrial Species in the Wilderness. Wilderness Environ. Med. 2018, 29, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Guida, M.; Trifuoggi, M.; Thomas, P.; Palumbo, A.; Romano, G.; Oral, R. Sea Urchin Bioassays in Toxicity Testing: I. Inorganics, Organics, Complex Mixtures and Natural Products. Expert Opin. Environ. Biol. 2017, 6. [Google Scholar] [CrossRef]
- Chen, C.; Zeng, H.; Yi, M.; Xiao, G.; Xu, S.; Shen, S.; Feng, B. In-situ growth of Ag3PO4 on calcined Zn-Al layered double hydroxides for enhanced photocatalytic degradation of tetracycline under simulated solar light irradiation and toxicity assessment. Appl. Catal. B Environ. 2019, 252, 47–54. [Google Scholar] [CrossRef]
- Serrà, A.; Pip, P.; Gómez, E.; Philippe, L. Efficient magnetic hybrid ZnO-based photocatalysts for visible-light-driven removal of toxic cyanobacteria blooms and cyanotoxins. Appl. Catal. B Environ. 2020, 268, 118745. [Google Scholar] [CrossRef]
- Maniakova, G.; Kowalska, K.; Murgolo, S.; Mascolo, G.; Libralato, G.; Lofrano, G.; Sacco, O.; Guida, M.; Rizzo, L. Comparison between heterogeneous and homogeneous solar driven advanced oxidation processes for urban wastewater treatment: Pharmaceuticals removal and toxicity. Sep. Purif. Technol. 2020, 236, 116249. [Google Scholar] [CrossRef]
- Al-Kandari, H.; Younes, N.; Al-Jamal, O.; Zakaria, Z.Z.; Najjar, H.; Alserr, F.; Pintus, G.; Al-Asmakh, M.A.; Abdullah, A.M.; Nasrallah, G.K. Ecotoxicological assessment of thermally- and hydrogen-reduced graphene oxide/tio2 photocatalytic nanocomposites using the zebrafish embryo model. Nanomaterials 2019, 9, 488. [Google Scholar] [CrossRef]
- Kamble, G.S.; Ling, Y.C. Solvothermal synthesis of facet-dependent BiVO4 photocatalyst with enhanced visible-light-driven photocatalytic degradation of organic pollutant: Assessment of toxicity by zebrafish embryo. Sci. Rep. 2020, 10, 12993. [Google Scholar] [CrossRef]
- Louros, V.L.; Silva, V.; Silva, C.P.; Calisto, V.; Otero, M.; Esteves, V.I.; Freitas, R.; Lima, D.L.D. Sulfadiazine’s photodegradation using a novel magnetic and reusable carbon based photocatalyst: Photocatalytic efficiency and toxic impacts to marine bivalves. J. Environ. Manag. 2022, 313, 115030. [Google Scholar] [CrossRef]
- Abhilash, M.R.; Gangadhar, A.; Krishnegowda, J.; Chikkamadaiah, M.; Srikantaswamy, S. Hydrothermal synthesis, characterization and enhanced photocatalytic activity and toxicity studies of a rhombohedral Fe2O3 nanomaterial. RSC Adv. 2019, 9, 25158–25169. [Google Scholar] [CrossRef] [PubMed]
- Padervand, M.; Heidarpour, H.; Bargahi, A. A mechanistic study and in-vivo toxicity bioassay on acetamiprid photodegradation over the zeolite supported cerium-based photocatalyst. J. Photochem. Photobiol. A Chem. 2020, 395, 112526. [Google Scholar] [CrossRef]
- El Nahrawy, A.M.; Hammad, A.B.A.; Bakr, A.M.; Hemdan, B.A.; Wassel, A.R. Decontamination of ubiquitous harmful microbial lineages in water using an innovative Zn2Ti0.8Fe0.2O4 nanostructure: Dielectric and terahertz properties. Heliyon 2019, 5, e02501. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Anchun, M.; Zhu, Z.; Quan, Y. Antibacterial titanium plate deposited by silver nanoparticles exhibits cell compatibility. Int. J. Nanomed. 2010, 5, 337–342. [Google Scholar]
- Malankowska, A.; Mikołajczyk, A.; Mȩdrzycka, J.; Wysocka, I.; Nowaczyk, G.; Jarek, M.; Puzyn, T.; Mulkiewicz, E. The effect of Ag, Au, Pt, and Pd on the surface properties, photocatalytic activity and toxicity of multicomponent TiO2-based nanomaterials. Environ. Sci. Nano 2020, 7, 3557–3574. [Google Scholar] [CrossRef]
- Intarasuwan, K.; Amornpitoksuk, P.; Suwanboon, S.; Graidist, P. Photocatalytic dye degradation by ZnO nanoparticles prepared from X2C2O4 (X = H, Na and NH4) and the cytotoxicity of the treated dye solutions. Sep. Purif. Technol. 2017, 177, 304–312. [Google Scholar] [CrossRef]
- Choi, J.H.; Hong, J.A.; Son, Y.R.; Wang, J.; Kim, H.S.; Lee, H.; Lee, H. Comparison of enhanced photocatalytic degradation efficiency and toxicity evaluations of ceo2 nanoparticles synthesized through double-modulation. Nanomaterials 2020, 10, 1543. [Google Scholar] [CrossRef]
- Caratto, V.; Locardi, F.; Alberti, S.; Villa, S.; Sanguineti, E.; Martinelli, A.; Balbi, T.; Canesi, L.; Ferretti, M. Different sol–gel preparations of iron-doped TiO2 nanoparticles: Characterization, photocatalytic activity and cytotoxicity. J. Sol-Gel Sci. Technol. 2016, 80, 152–159. [Google Scholar] [CrossRef]
- Balbi, T.; Caratto, V.; Fabbri, R.; Camisassi, G.; Villa, S.; Ferretti, M.; Canesi, L. Photocatalytic Fe-doped n-TiO2: From synthesis to utilization of in vitro cell models for screening human and environmental nanosafety. Resour. Technol. 2017, 3, 158–165. [Google Scholar]
- Medina-Ramírez, I.; Liu, J.L.; Hernández-Ramírez, A.; Romo-Bernal, C.; Pedroza-Herrera, G.; Jáuregui-Rincón, J.; Gracia-Pinilla, M.A. Synthesis, characterization, photocatalytic evaluation, and toxicity studies of TiO2-Fe3+ nanocatalyst. J. Mater. Sci. 2014, 49, 5309–5323. [Google Scholar] [CrossRef]
- Qureshi, F.; Nawaz, M.; Rehman, S.; Almofty, S.A.; Shahzad, S.; Nissapatorn, V.; Taha, M. Synthesis and characterization of cadmium-bismuth microspheres for the catalytic and photocatalytic degradation of organic pollutants, with antibacterial, antioxidant and cytotoxicity assay. J. Photochem. Photobiol. B Biol. 2020, 202, 111723. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Li, J.; Sun, J.; Zhang, W.; Li, Y.; Cui, D.; Hu, W.; Chang, Y. The Impact of Chronic Heat Stress on the Growth, Survival, Feeding, and Differential Gene Expression in the Sea Urchin Strongylocentrotus intermedius. Front. Genet. 2019, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Baran, W.; Adamek, E.; Sobczak, A.; Makowski, A. Photocatalytic degradation of sulfa drugs with TiO2, Fe salts and TiO2/FeCl3 in aquatic environment-Kinetics and degradation pathway. Appl. Catal. B Environ. 2009, 90, 516–525. [Google Scholar] [CrossRef]
- Madhavan, J.; Kumar, P.S.S.; Anandan, S.; Zhou, M.; Grieser, F.; Ashokkumar, M. Ultrasound assisted photocatalytic degradation of diclofenac in an aqueous environment. Chemosphere 2010, 80, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Vautier, M.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation of dyes in water: Case study of indigo and of indigo carmine. J. Catal. 2001, 201, 46–59. [Google Scholar] [CrossRef]
- Baran, W.; Sochacka, J.; Wardas, W. Toxicity and biodegradability of sulfonamides and products of their photocatalytic degradation in aqueous solutions. Chemosphere 2006, 65, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Saggioro, E.M.; Oliveira, A.S.; Buss, D.F.; Magalhães, D.D.P.; Pavesi, T.; Jimenéz, M.; Maldonado, M.I.; Ferreira, L.F.V.; Moreira, J.C. Photo-decolorization and ecotoxicological effects of solar compound parabolic collector pilot plant and artificial light photocatalysis of indigo carmine dye. Dyes Pigments 2015, 113, 571–580. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, S.; Wang, F.; Li, Q.; He, C.; Duan, N.; Wang, Z. Assessing the toxicity in vitro of degradation products from deoxynivalenol photocatalytic degradation by using upconversion nanoparticles@TiO2 composite. Chemosphere 2020, 238, 124648. [Google Scholar] [CrossRef]
- Hoelzer, K.; Wong, N.; Thomas, J.; Talkington, K.; Jungman, E.; Coukell, A. Antimicrobial drug use in food-producing animals and associated human health risks: What, and how strong, is the evidence? BMC Vet. Res. 2017, 13, 211. [Google Scholar] [CrossRef]
- Sridhara Chary, N.; Kamala, C.T.; Samuel Suman Raj, D. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf. 2008, 69, 513–524. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Rizzo, L.; Sannino, D. Photocatalytic activity of a visible light active structured photocatalyst developed for municipal wastewater treatment. J. Clean. Prod. 2018, 175, 38–49. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.K.; Mahalingam, H. An effective and low-cost TiO2/polystyrene floating photocatalyst for environmental remediation. Int. J. Environ. Res. 2015, 9, 535–544. [Google Scholar]
- Sacco, O.; Venditto, V.; Fittipaldi, R.; Vaiano, V.; Daniel, C. Composite polymeric films with photocatalytic properties. Chem. Eng. Trans. 2021, 86, 571–576. [Google Scholar]
- Sacco, O.; Vaiano, V.; Daniel, C.; Navarra, W.; Venditto, V. Highly robust and selective system for water pollutants removal: How to transform a traditional photocatalyst into a highly robust and selective system for water pollutants removal. Nanomaterials 2019, 9, 1509. [Google Scholar] [CrossRef]
- Azbar, N.; Yonar, T.; Kestioglu, K. Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere 2004, 55, 35–43. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D. Electric energy saving in photocatalytic removal of crystal violet dye through the simultaneous use of long-persistent blue phosphors, nitrogen-doped TiO2 and UV-light emitting diodes. J. Clean. Prod. 2019, 210, 1015–1021. [Google Scholar] [CrossRef]
| Photocatalyst | Form | Preparation Method | Dose (g/L) | Contact Time (min) | Target | UFC/mL | Light Source | Power of Light Source (W) | Results (%) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| g-C3N4-V-TiO2 | P | Hydrothermal calcination | 0.5 | 60 | E. coli | - | Vis | 500 | 99.5 | [62] |
| S. aureus | ||||||||||
| ZnO/TiO2 | P | Wet impregnation calcination | 0.5 | 10 | E. coli | 107 | UV Vis | 8 | 99.9 | [1] |
| Au/BiTiO3/TiO2 | P | Hydrothermal treatment Ion exchange treatment Physical vapor deposition (PVD) process | - | 40 | E. coli | 6 × 104 | Simulated sunlight | - | 99.5 | [63] |
| S. aureus | 99.7 | |||||||||
| Ag2C2O4/TiO2 | NF | Electrospinning Calcination | 0.1 | 30 | E. coli | 2 × 107 | Vis | 300 | 99.99 | [64] |
| CuBi2O4/Bi2MoO6 | P | Hydrothermal treatment Ultrasonication Heating | 0.8 | 240 | E. coli | 107 | Vis | 300 | 100 | [65] |
| Ag/BiOI/TiO2 | NF | Electrospinning ionic layer adsorption and reaction (SILAR) photodeposition | - | 30 | E. coli | 3 × 107 | Vis | 16 | 99.9 | [66] |
| Ag2WO4/g-C3N4 | P | Deposition Precipitation Ultrasonication | 4 | 90 | E. coli | 107 | Vis | 300 | 100 | [67] |
| Fe2O3-TiO2 | P | Ultrasonic co-precipitation | 1.05 | 30 | V. fischeri | 3 × 106 | UV | - | 100 | [68] |
| Ag QDs/Bi2S3/SnIn4S8 | P | Solvothermal method | - | 240 | E. coli | 2.5 × 107 | Vis | 300 | 100 | [69] |
| MoS2/TiO2 | NT | Two-step anodization Hydrothermal method | - | 150 | Staphylococcus aureus | >108 | Vis | - | 100 | [70] |
| E. coli | ||||||||||
| g-C3N4-AgBr | P | Adsorption–deposition | 0.1 | 150 | S. aureus | 3 × 106 | Vis | 300 | 100 | [71] |
| 60 | E. coli | |||||||||
| TiO2-rGO | P | Hydrothermal method | 0.1 | 75 | E. coli | 1.5 × 106 | Artificial solar light | - | 100 | [72] |
| AgI/AgBr/BiOBr0․75I0․25n | P | Solvothermal method | 0.08 | 30 | E. coli | 3 × 107 | Vis | 300 | 100 | [73] |
| g-C3N4/expanded perlite (EP-520) | P | Thermal method | - | 120 | E. coli | 1 × 108 | Vis | 300 | 100 | [74,75] |
| 240 | MS2 | |||||||||
| Al2O3/ZnO | P | Co-precipitation Calcination | 0.5 | 240 | E. coli | 106 | Vis | - | 100 | [2] |
| TGP (TiO2–graphene sensitized by tetrakis(4-carboxyphenyl)porphyrin (TCPP)) | P | Solvothermal method | - | 440 | E. coli | - | Vis | 450 | 64 | [76] |
| AgI@MnO2 | P | Deposition | 0.05 | 25 | S. aureus | - | Vis | 15 | 99.4 | [77] |
| E. coli | 92.2 | |||||||||
| Ag-AgX/RGOs | S | Deposition Precipitation | - | 35 min | E. coli | 2 × 107 | Vis | 300 | 100 | [78] |
| CeO2-AgI, | P | Hydrothermal method Deposition | 0.1 | 40 | E. coli | 107 | Vis | - | 100 | [79] |
| O-g-C3N4/HTCC-2 | MS | Solvothermal method Hydrothermal method | 0.15 | 120 | Viruses | 105 MPN/mL | Vis | - | 100 | [80] |
| BiOBr-AgBr | P | Precipitation Ion exchange | 0.08 | 24 | E. coli | 1 × 107 | Vis | - | 100 | [81] |
| BiVO4/Ag+ | P | Hydrothermal method | 0.1 | 15 | E. coli | 108 | Vis | - | >99 | [82] |
| TiO2–Fe2O3 | P | Ex situ synthetic route Ultrasonication | - | 120 | E. coli | 3.22 × 109 | Sunlight | - | 98.3 | [83] |
| TiO2-X/Ag3PO4 | P | Hydrothermal method | 0.2 | 20 | S. aureus | 107 | Simulated sunlight | - | 99.8 | [84] |
| E. coli | 99.8 | |||||||||
| Ag(3%)-TiO2 | NT | Hydrothermal method | 0.1 | 60 | E. coli | 106 | Sunlight | - | 100 | [85] |
| GO/g-C3N4 | P | Sonochemical method | 0.1 | 120 | E. coli | 107 | Vis | - | 100 | [86] |
| Photocatalyst | Form | Preparation Method | Dose (g/L) | Contact Time (min) | Target | UFC/mL | Light Source | Power of Light Source (W) | Results (%) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| TiON | F | Sputtering on polyester (4 min) | - | 40 | E. coli | 106 | Simulated sunlight | 128 | 100 | [87] |
| TiO2-Cu | F | Sputtering on cotton (1 min) | - | 120 | E. coli | 3.8 × 106 | Vis | 255 | 100 | [88] |
| N-TiO2 | F | Anodic oxidation | - | 240 | E. coli | 2 × 106 | UV | - | 33 | [89] |
| N-TiO2 | P | Sol–gel | 0.1 | 360 | E. coli | 105 | Vis | 90 | - | [90] |
| Cr-TiO2 | 0.1 | 70 | ||||||||
| Cr/N-TiO2 | 0.2 | - | ||||||||
| N-TiO2 | P | Hydrolisis calcination | 1% | 7800 | Aspergillus niger | 105 | Vis | - | 100 | [91] |
| N-T-TiO2 | 7200 | |||||||||
| C-TiO2 | 7200 | |||||||||
| Pd-CTiO2 | 5760 | |||||||||
| V2O5/TiO2 | P | Wet impregnation method | 0.5 | 30 | E. coli | 108 | UV-C Vis | 8 | 100 | [92] |
| TiO2/Cu | F | Sputtering on polyester | - | 10 | E. coli | 106 | Simulated sunlight | 87.5 | 100 | [93] |
| TiO2/CdS | P | Hydrothermal ultrasonication Hot injection | 0.1 | 10 | E. coli | 108 | Vis | - | 99 | [94] |
| TNTZ-Cu | F | Sputtering on glass | - | 75 | E. coli | 3 × 106 | Vis | 18 | 100 | [95] |
| Ti- BiOI | P | Solvothermal method | 0.06 | 24 | E. coli | 3 × 107 | Vis | 300 | 100 | [96] |
| 45 | S. aureus | 3 × 106 | ||||||||
| CuOx-TiO2-PET | F | Sputtering on PET | - | 20 | E. coli | 4 × 106 | Actinic light | - | 100 | [97] |
| TiN/TiN-Ag | F | Sputtering on polyester | - | 15 | E. coli | 108 | Actinic light | 112 | 100 | [98] |
| F-ZnO | P | Sol–gel | - | 360 | S. aureus | - | Vis | 150 | 99.99 | [99] |
| E. coli | 99.87 | |||||||||
| Fe-TiO2 | P | Dip coating | Fixed bed | 120 | E. coli | 106 | Solar | - | >99 | [100] |
| Ce-ZnO | P | Precipitation | - | 120 | E. coli | 1 × 105 | UVA | 18 | 99.99 | [101] |
| P. aeruginosa | ||||||||||
| PECuOx | F | Sputtering on polyester | - | 15 | E. coli | >106 | Sunlight | 60 | 100 | [102] |
| TiO2/Cu-PES | F | Sputtering on polyester | - | 30 | E. coli | >106 | Actinic light | - | 100 | [103] |
| Ce-ZnO | P | Precipitation | 0.1 | 120 | E. coli | 106 | UVA | 125 | 100 | [104] |
| Cu-ZnO | NP | Precipitation | 0.5 | 240 | E. coli | 106.5 | Simulated sunlight | 300 | 100 | [105] |
| ZnO/TiO2 | NP | Sol–gel | 1 | 20 | E. coli | 105 | UV | 8 | 100 | [106] |
| ZnCl2/TiO2, Zn(Ac)2/TiO2, Zn(NO3)2/TiO2 ZnSO4/TiO2 | NP | Sol–gel calcination | 4 | 120 | Candida albicans | 105–106 | Vis | 270 | >95 | [107] |
| >87.5 | ||||||||||
| >87.5 | ||||||||||
| 100 | ||||||||||
| ZnCl2/TiO2, Zn(Ac)2/TiO2, Zn(NO3)2/TiO2 ZnSO4/TiO2 | NP | Sol–gel calcination | 4 | 120 | E. coli | 105–106 | Vis | 270 | >92.5 | [108] |
| >80 | ||||||||||
| >90 | ||||||||||
| 100 | ||||||||||
| ZnCl2/TiO2, Zn(Ac)2/TiO2, Zn(NO3)2/TiO2 ZnSO4/TiO2 | NP | Sol–gel calcination | 4 | 120 | S. aureus | 105–106 | Vis | 270 | >90 | [109] |
| >80 | ||||||||||
| >95 | ||||||||||
| 100 |
| Photocatalyst | Form | Type | Preparation Method | Dose (g/L) | Contact Time (min) | Target | UFC/mL | Light Source | Power of Light Source (W) | Results (%) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PMMA/TiO2 | F | PC | Sonication method | - | 60 | E. coli | 105 | UV-A | - | 70 | [16] |
| PMMA/TiO2/SWCNTs | UV-A | - | - | [16] | |||||||
| PMMA/TiO2-TCPP | Vis | - | 40 | [16] | |||||||
| Chitosan-TiO2:Cu (CS-CT) | P | PFNC | Sol–gel and ultra-sonication | 0.2 | 120 | E. coli | 3 × 104 | Vis | 8 | 100 | [108] |
| 150 | S. aureus | ||||||||||
| Ag-NPs@CTA | PF | PFNC | Active imprinting | 0.3 | 120 | E. coli | 108 | Vis | 40 | 99 | [109] |
| S. aureus | |||||||||||
| C. albicans |
| Photocatalyst | Target | Dose (g/L) | Endpoint | Effects | Reference |
|---|---|---|---|---|---|
| Ag3PO4 | Chlorella vulgaris | 0.04 | Growth inhibition | Beneficial effects | [130] |
| ZnO@ZnS | Spirulina platensis | 0.025–0.4 | Viability, biomass, and photosynthetic pigments | Weak effect | [131] |
| N- TiO2 | Vibrio fischeri, Raphidocelis subcapitata, Daphnia magna | 0.002 and 0.005 | Growth inhibition and mortality | Weak effect | [132] |
| Thermally (RGOTi) and hydrogen (H2RGOTi)-reduced graphene oxide/TiO2 | Zebrafish embryos | 0.1, 0.2, 0.3, 0.4, 0.6, 0.8, and 1 | Acutoxicity, cardiotoxicity, neurobehavioral toxicity, hematopoietic toxicity, and hatching rate | LC50 = 1 g/L and 0.7 g/L for H2RGOTi and RGOTi, respectively Decrease in body size from H2RGOTi Increase in eye, yolk, and pericardial size from RGOTi | [133] |
| Facet-dependent monoclinic scheelite BiVO4 | Zebrafish embryos | 0.02 | Mortality | Weak effect | [134] |
| Biochar functionalized with titanium dioxide (TiO2) | Mytilus galoprovincialis | 0.1 | Survival, neurotoxicity, and energy metabolism | n.e. | [135] |
| Alumina/ZnO | Mouse | 2 | Gut histopathology | n.e. | [2] |
| Fe2O3 | Wistar rats | 0.02 | Hearth histopathology | Cardiovascular damage | [136] |
| CeO/S | Laboratory rats | 0.05 | Biochemical effects and blood sampling | Increase in ALT and AST activity Decrease in blood cells and hemoglobin level | [137] |
| Zn2TiO4 | Hep-2 cell line | 0.3 | Cytotoxicity | n.e. | [138] |
| Ti-nAg | Human gingival fibroblast cells | n.a. | Cytotoxicity | n.e. | [139] |
| Ag @chitosan–TiO2 | Mammal cells | 15.2 | Cytotoxicity | Weak effect | [109] |
| TiO2:Cu | Mouse embryo fibroblast cells | 2 | Cytotoxicity | Beneficial effects | [108] |
| Multicomponent TiO2-based | Mouse embryo fibroblast cells Human lung cell line Human liver cell line | 2.56 | Cytotoxicity | EC50 = 0.1 g/L for mouse cell EC50 = 0.08 g/L for human liver cell EC50 => 0.3 g/L for human lung cell | [140] |
| O2-g-C3N4 | Human lung cell line | 0.15 | Cytotoxicity | n.e. | [80] |
| ZnO(H) | Human lung cell line | 0.08 | Cytotoxicity | n.e. | [141] |
| CeO2-Fe/Cr | Aneuploid immortal keratinocyte cell line | 0.025–0.1 | Cytotoxicity | Cells’ viability decreased | [142] |
| Fe-TiO2 | Human endothelial cells (HECV) | 0.01 | Cytotoxicity | Cells’ viability decreased | [143] |
| Fe- TiO2 | Human endothelial cells (HECVs) Mouse macrophages (RAW 247) Hemocytes of Mytilus galloprovincialis | 0.0001–0.001–0.01 | Cytotoxicity | Cells’ viability decreased in HECVs n.e. in RAW 247 and in hemocytes of Mytilus galloprovincialis | [144] |
| Fe-TiO2 | Human red blood cell | 0.0001–0.1 | Cytotoxicity | n.e. | [145] |
| Cd-Bi | Human colon colorectal tumor cell line | 0.25–5 | Cytotoxicity | Strong effect | [146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lofrano, G.; Ubaldi, F.; Albarano, L.; Carotenuto, M.; Vaiano, V.; Valeriani, F.; Libralato, G.; Gianfranceschi, G.; Fratoddi, I.; Meric, S.; et al. Antimicrobial Effectiveness of Innovative Photocatalysts: A Review. Nanomaterials 2022, 12, 2831. https://doi.org/10.3390/nano12162831
Lofrano G, Ubaldi F, Albarano L, Carotenuto M, Vaiano V, Valeriani F, Libralato G, Gianfranceschi G, Fratoddi I, Meric S, et al. Antimicrobial Effectiveness of Innovative Photocatalysts: A Review. Nanomaterials. 2022; 12(16):2831. https://doi.org/10.3390/nano12162831
Chicago/Turabian StyleLofrano, Giusy, Francesca Ubaldi, Luisa Albarano, Maurizio Carotenuto, Vincenzo Vaiano, Federica Valeriani, Giovanni Libralato, Gianluca Gianfranceschi, Ilaria Fratoddi, Sureyya Meric, and et al. 2022. "Antimicrobial Effectiveness of Innovative Photocatalysts: A Review" Nanomaterials 12, no. 16: 2831. https://doi.org/10.3390/nano12162831
APA StyleLofrano, G., Ubaldi, F., Albarano, L., Carotenuto, M., Vaiano, V., Valeriani, F., Libralato, G., Gianfranceschi, G., Fratoddi, I., Meric, S., Guida, M., & Romano Spica, V. (2022). Antimicrobial Effectiveness of Innovative Photocatalysts: A Review. Nanomaterials, 12(16), 2831. https://doi.org/10.3390/nano12162831














