Deposition Mechanism and Characterization of Plasma-Enhanced Atomic Layer-Deposited SnOx Films at Different Substrate Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and PEALD Process
2.2. Characteristic Measurements
3. Results and Discussion
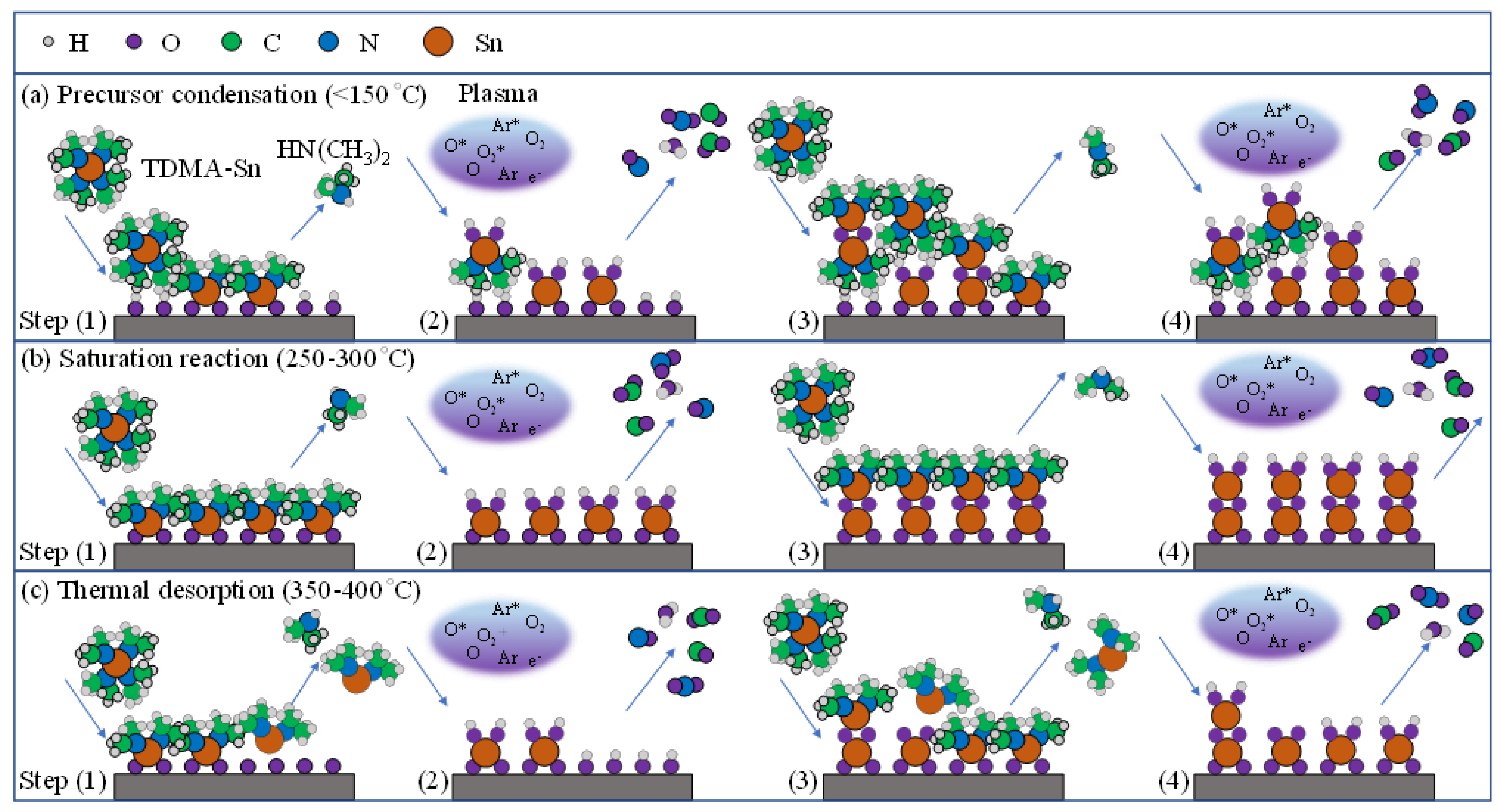
3.1. Deposition Mechanism
3.2. Chemical and Electronic State of the Sn and O
3.3. Structural Properties of the SnOx film
3.4. Photoelectric Properties of the SnOx film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellmer, K. Past Achievements and Future Challenges in the Development of Optically Transparent Electrodes. Nat. Photon. 2012, 6, 809–817. [Google Scholar] [CrossRef]
- Battaglia, C.; Cuevas, A.; De Wolf, S. High-Efficiency Crystalline Silicon Solar Cells: Status and Perspectives. Energy Environ. Sci. 2016, 9, 1552–1576. [Google Scholar] [CrossRef]
- So, F.; Kido, J.; Burrows, P. Organic Light-Emitting Devices for Solid-State Lighting. MRS Bull. 2008, 33, 7. [Google Scholar] [CrossRef]
- Klein, A. Transparent Conducting Oxides: Electronic Structure-Property Relationship from Photoelectron Spectroscopy with in Situ Sample Preparation. J. Am. Ceram. Soc. 2013, 96, 331–345. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Hultåker, A. Transparent and Conducting ITO Films: New Developments and Applications. Thin Solid Films 2002, 411, 1–5. [Google Scholar] [CrossRef]
- Gorley, P.M.; Khomyak, V.V.; Bilichuk, S.V.; Orletsky, I.G.; Horley, P.P.; Grechko, V.O. SnO2 Films: Formation, Electrical and Optical Properties. Mater. Sci. Eng. B 2005, 118, 160–163. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, G.; Liu, D.; Wang, X.; Ma, Y.; Wang, J.; Yin, J.; Yang, X.; Hou, X.; Yang, S. Crystal Growth of Undoped ZnO Films on Si Substrates under Different Sputtering Conditions. J. Cryst. Growth 2002, 243, 439–443. [Google Scholar] [CrossRef]
- Lee, K.E.; Wang, M.; Kim, E.J.; Hahn, S.H. Structural, Electrical and Optical Properties of Sol–Gel AZO Thin Films. Curre. Appl. Phys. 2009, 9, 683–687. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Polleux, J.; Dunn, B.; Tolbert, S.H. Templated Nanocrystal-Based Porous TiO2 Films for Next-Generation Electrochemical Capacitors. J. Am. Chem. Soc. 2009, 131, 1802–1809. [Google Scholar] [CrossRef]
- Park, B.-E.; Park, J.; Lee, S.; Lee, S.; Kim, W.-H.; Kim, H. Phase-Controlled Synthesis of SnOx Thin Films by Atomic Layer Deposition and Post-Treatment. Appl. Surf. Sci. 2019, 480, 472–477. [Google Scholar] [CrossRef]
- Bolotov, V.V.; Korusenko, P.M.; Nesov, S.N.; Povoroznyuk, S.N.; Roslikov, V.E.; Kurdyukova, E.A.; Sten’kin, Y.A.; Shelyagin, R.V.; Knyazev, E.V.; Kan, V.E.; et al. Nanocomposite Por-Si/SnOx Layers Formation for Gas Microsensors. Mater. Sci. Eng. B 2012, 177, 1–7. [Google Scholar] [CrossRef]
- Song, J.; Zheng, E.; Bian, J.; Wang, X.-F.; Tian, W.; Sanehira, Y.; Miyasaka, T. Low-Temperature SnO2-Based Electron Selective Contact for Efficient and Stable Perovskite Solar Cells. J. Mater. Chem. A 2015, 3, 10837–10844. [Google Scholar] [CrossRef]
- Tao, H.; Wang, H.; Bai, Y.; Long, H.; Zhao, H.; Fu, Q.; Ma, Z. Effects of Sputtering Power of SnO2 Electron Selective Layer on Perovskite Solar Cells. J. Mater. Sci. Mater. Electron. 2019, 30, 12036–12043. [Google Scholar] [CrossRef]
- Park, M.; Song, J.; An, M.; Lim, J.; Lee, C.; Roh, J.; Lee, D. Colloidal Quantum Dot Light-Emitting Diodes Employing Solution-Processable Tin Dioxide Nanoparticles in an Electron Transport Layer. RSC Adv. 2020, 10, 8261–8265. [Google Scholar] [CrossRef]
- Alfonso, C.; Charaï, A.; Armigliato, A.; Narducci, D. Transmission Electron Microscopy Investigation of Tin Sub-oxide Nucleation upon SnO2 Deposition on Silicon. Appl. Phys. Lett. 1996, 68, 1207–1208. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, Y.; Mei, J.; Tang, C.; Luo, K.; Li, S.; Zhan, H.; He, Z. Hierarchical SnO2–Sn3O4 Heterostructural Gas Sensor with High Sensitivity and Selectivity to NO2. Sens. Actuators B Chem. 2019, 301, 127010. [Google Scholar] [CrossRef]
- Hassan, M.A.M.; Salem, E.T.; Mohammed, N.J.; Agool, I.R. Tin Dioxide Nanostructure Using Rapid Thermal Oxidation Method and Hydrothermal Synthesis of CuO-SnO2-ZnO Nano Composite Oxides. Int. J. Nanosci. Nanoeng. 2014, 1, 22. [Google Scholar]
- Maleki, M.; Rozati, S.M. An Economic CVD Technique for Pure SnO2 Thin Films Deposition: Temperature Effects. Bull. Mater. Sci. 2013, 36, 217–221. [Google Scholar] [CrossRef]
- Kwoka, M.; Ottaviano, L.; Passacantando, M.; Santucci, S.; Czempik, G.; Szuber, J. XPS Study of the Surface Chemistry of L-CVD SnO2 Thin Films after Oxidation. Thin Solid Films 2005, 490, 36–42. [Google Scholar] [CrossRef]
- Huang, H.; Tan, O.K.; Lee, Y.C.; Tse, M.S. Preparation and Characterization of Nanocrystalline SnO2 Thin Films by PECVD. J. Cryst. Growth 2006, 288, 70–74. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Faglia, G.; Groppelli, S.; Nelli, P.; Taroni, A. A Novel PVD Technique for the Preparation of SnO2 Thin Films as C2H5OH Sensors. Sens. Actuators B Chem. 1992, 7, 721–726. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic Layer Deposition (ALD): From Precursors to Thin Film Structures. Thin Solid Films 2002, 409, 138–146. [Google Scholar] [CrossRef]
- Parsons, G.N.; George, S.M.; Knez, M. Progress and Future Directions for Atomic Layer Deposition and ALD-Based Chemistry. MRS Bull. 2011, 36, 865–871. [Google Scholar] [CrossRef]
- Lee, B.K.; Jung, E.; Kim, S.H.; Moon, D.C.; Lee, S.S.; Park, B.K.; Hwang, J.H.; Chung, T.-M.; Kim, C.G.; An, K.-S. Physical/Chemical Properties of Tin Oxide Thin Film Transistors Prepared Using Plasma-Enhanced Atomic Layer Deposition. Mater. Res. Bull. 2012, 47, 3052–3055. [Google Scholar] [CrossRef]
- Kuang, Y.; Zardetto, V.; van Gils, R.; Karwal, S.; Koushik, D.; Verheijen, M.A.; Black, L.E.; Weijtens, C.; Veenstra, S.; Andriessen, R.; et al. Low-Temperature Plasma-Assisted Atomic-Layer-Deposited SnO2 as an Electron Transport Layer in Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 30367–30378. [Google Scholar] [CrossRef]
- Chistiakova, G.; Mews, M.; Wilks, R.G.; Bär, M.; Korte, L. In-System Photoelectron Spectroscopy Study of Tin Oxide Layers Produced from Tetrakis(Dimethylamino)Tin by Plasma Enhanced Atomic Layer Deposition. J. Vac. Sci. Technol. A Vac. Surf. Films 2018, 36, 02D401. [Google Scholar] [CrossRef]
- Kim, H.Y.; Nam, J.H.; George, S.M.; Park, J.-S.; Park, B.K.; Kim, G.H.; Jeon, D.J.; Chung, T.-M.; Han, J.H. Phase-Controlled SnO2 and SnO Growth by Atomic Layer Deposition Using Bis(N-Ethoxy-2,2-Dimethyl Propanamido)Tin Precursor. Ceram. Int. 2019, 45, 5124–5132. [Google Scholar] [CrossRef]
- Choi, D.; Maeng, W.J.; Park, J.-S. The Conducting Tin Oxide Thin Films Deposited via Atomic Layer Deposition Using Tetrakis-Dimethylamino Tin and Peroxide for Transparent Flexible Electronics. Appl. Surf. Sci. 2014, 313, 585–590. [Google Scholar] [CrossRef]
- Elam, J.W.; Baker, D.A.; Hryn, A.J.; Martinson, A.B.F.; Pellin, M.J.; Hupp, J.T. Atomic Layer Deposition of Tin Oxide Films Using Tetrakis(Dimethylamino) Tin. J. Vac. Sci. Technol. A Vac. Surf. Films 2008, 26, 244–252. [Google Scholar] [CrossRef]
- Lee, D.-K.; Wan, Z.; Bae, J.-S.; Lee, H.-B.-R.; Ahn, J.-H.; Kim, S.-D.; Kim, J.; Kwon, S.-H. Plasma-Enhanced Atomic Layer Deposition of SnO2 Thin Films Using SnCl4 and O2 Plasma. Mater. Lett. 2016, 166, 163–166. [Google Scholar] [CrossRef]
- Mullings, M.N.; Hägglund, C.; Bent, S.F. Tin Oxide Atomic Layer Deposition from Tetrakis(Dimethylamino)Tin and Water. J. Vac. Sci. Technol. A Vac. Surf. Films 2013, 31, 061503. [Google Scholar] [CrossRef]
- Tanskanen, J.T.; Bent, S.F. Insights into the Surface Chemistry of Tin Oxide Atomic Layer Deposition from Quantum Chemical Calculations. J. Phys. Chem. C 2013, 117, 19056–19062. [Google Scholar] [CrossRef]
- Hoffmann, L.; Theirich, D.; Schlamm, D.; Hasselmann, T.; Pack, S.; Brinkmann, K.O.; Rogalla, D.; Peters, S.; Räupke, A.; Gargouri, H.; et al. Atmospheric Pressure Plasma Enhanced Spatial Atomic Layer Deposition of SnOx as Conductive Gas Diffusion Barrier. J. Vac. Sci. Technol. A Vac. Surf. Films 2018, 36, 01A112. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Park, J.-S. Highly Conductive SnO2 Thin Films Deposited by Atomic Layer Deposition Using Tetrakis-Dimethyl-Amine-Tin Precursor and Ozone Reactant. Surf. Coat. Technol. 2014, 259, 238–243. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Z.; Yang, L.; Yang, J.; Wang, P.; Gao, G.; Sun, C.; Ralchenko, V.; Zhu, J. Comparison of Thermal, Plasma-Enhanced and Layer by Layer Ar Plasma Treatment Atomic Layer Deposition of Tin Oxide Thin Films. J. Cryst. Growth 2021, 572, 126264. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Zhang, Z.-X.; Huang, P.-H.; Wu, W.-Y.; Ou, S.-L.; Lien, S.-Y.; Huang, C.-J.; Lee, M.-K.; Zhu, W.-Z. Effect of Plasma Power on the Structural Properties of Tin Oxide Prepared by Plasma-Enhanced Atomic Layer Deposition. Ceram. Int. 2021, 47, 8634–8641. [Google Scholar] [CrossRef]
- Huang, P.-H.; Zhang, Z.-X.; Hsu, C.-H.; Wu, W.-Y.; Huang, C.-J.; Lien, S.-Y. Chemical Reaction and Ion Bombardment Effects of Plasma Radicals on Optoelectrical Properties of SnO2 Thin Films via Atomic Layer Deposition. Materials 2021, 14, 690. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Yin, S. Oxygen Vacancies Confined in SnO2 Nanoparticles for Desirable Electronic Structure and Enhanced Visible Light Photocatalytic Activity. Appl. Surf. Sci. 2017, 420, 399–406. [Google Scholar] [CrossRef]
- Ma, D.; Li, Y.; Zhang, P.; Lin, Z. Oxygen Vacancy Engineering in Tin(IV) Oxide Based Anode Materials toward Advanced Sodium-Ion Batteries. ChemSusChem 2018, 11, 3693–3703. [Google Scholar] [CrossRef]
- Kumar, V.; Swart, H.C.; Gohain, M.; Bezuidenhoudt, B.C.B.; van Vuuren, A.J.; Lee, M.; Ntwaeaborwa, O.M. The Role of Neutral and Ionized Oxygen Defects in the Emission of Tin Oxide Nanocrystals for near White Light Application. Nanotechnology 2015, 26, 295703. [Google Scholar] [CrossRef] [PubMed]
- Sapuan, S.M.; Ismail, H.; Zainudin, E.S. Natural Fibre Reinforced Vinyl Ester and Vinyl Polymer Composites: Characterization, Properties and Applications; Woodhead Publishing: Sawston, UK, 2018; ISBN 978-0-08-102161-3. [Google Scholar]
- Kamble, D.L.; Harale, N.S.; Patil, V.L.; Patil, P.S.; Kadam, L.D. Characterization and NO2 Gas Sensing Properties of Spray Pyrolyzed SnO2 Thin Films. J. Anal. Appl. Pyrolysis 2017, 127, 38–46. [Google Scholar] [CrossRef]
- Manh Hung, N.; Nguyen, C.V.; Arepalli, V.K.; Kim, J.; Duc Chinh, N.; Nguyen, T.D.; Seo, D.-B.; Kim, E.-T.; Kim, C.; Kim, D. Defect-Induced Gas-Sensing Properties of a Flexible SnS Sensor under UV Illumination at Room Temperature. Sensors 2020, 20, 5701. [Google Scholar] [CrossRef]
- Santara, B.; Giri, P.K.; Imakita, K.; Fujii, M. Microscopic Origin of Lattice Contraction and Expansion in Undoped Rutile TiO2 Nanostructures. J. Phys. D Appl. Phys. 2014, 47, 215302. [Google Scholar] [CrossRef]
- Li, T.-H.; Li, H.-T.; Pan, J.-H. Interplay between External Strain and Oxygen Vacancies on Raman Spectra of SnO 2. Chinese Phys. Lett. 2014, 31, 076201. [Google Scholar] [CrossRef]
- White, T.A.; Moreno, M.S.; Midgley, P.A. Structure Determination of the Intermediate Tin Oxide Sn3O4 by Precession Electron Diffraction. Z. Krist. 2010, 225, 56–66. [Google Scholar] [CrossRef]
- Wu, J.; Xie, Y.; Du, S.; Ren, Z.; Yu, P.; Wang, X.; Wang, G.; Fu, H. Heterophase Engineering of SnO2/Sn3O4 Drives Enhanced Carbon Dioxide Electrocatalytic Reduction to Formic Acid. Sci. China Mater. 2020, 63, 2314–2324. [Google Scholar] [CrossRef]
- Zhang, F.; Lian, Y.; Gu, M.; Yu, J.; Tang, T.B. Static and Dynamic Disorder in Metastable Phases of Tin Oxide. J. Phys. Chem. C 2017, 121, 16006–16011. [Google Scholar] [CrossRef]
- Murakami, K.; Nakajima, K.; Kaneko, S. Initial Growth of SnO2 Thin Film on the Glass Substrate Deposited by the Spray Pyrolysis Technique. Thin Solid Films 2007, 515, 8632–8636. [Google Scholar] [CrossRef]
- Khan, A.F.; Mehmood, M.; Rana, A.M.; Bhatti, M.T. Effect of Annealing on Electrical Resistivity of Rf-Magnetron Sputtered Nanostructured SnO2 Thin Films. Appl. Surface Sci. 2009, 255, 8562–8565. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Hsu, C.-H.; Lien, S.-Y.; Wu, W.-Y.; Ou, S.-L.; Chen, S.-Y.; Huang, W.; Zhu, W.-Z.; Xiong, F.-B.; Zhang, S. Temperature-Dependent HfO2/Si Interface Structural Evolution and Its Mechanism. Nanoscale Res. Lett. 2019, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Cho, Y.-S.; Wu, W.-Y.; Lien, S.-Y.; Zhang, X.-Y.; Zhu, W.-Z.; Zhang, S.; Chen, S.-Y. Enhanced Si Passivation and PERC Solar Cell Efficiency by Atomic Layer Deposited Aluminum Oxide with Two-Step Post Annealing. Nanoscale Res. Lett. 2019, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Sivaranjani, S.; Malathy, V.; Prince, J.J.; Subramanian, B.; Balasubramanian, T.; Sanjeeviraja, C.; Jayachandran, M.; Swaminathan, V. Thickness Dependence of Structural, Electrical and Optical Properties of Sputter Deposited Indium Tin Oxide Films. Adv. Sci. Lett. 2010, 3, 434–441. [Google Scholar] [CrossRef]
- Karthik, K.; Revathi, V.; Tatarchuk, T. Microwave-Assisted Green Synthesis of SnO2 Nanoparticles and Their Optical and Photocatalytic Properties. Mol. Cryst. Liquid Cryst. 2018, 671, 17–23. [Google Scholar] [CrossRef]
- Ágoston, P.; Albe, K.; Nieminen, R.M.; Puska, M.J. Intrinsic n-Type Behavior in Transparent Conducting Oxides: A Comparative Hybrid-Functional Study of In2O3, SnO2, and ZnO. Phys. Rev. Lett. 2009, 103, 245501. [Google Scholar] [CrossRef] [PubMed]
- Buckeridge, J.; Catlow, C.R.A.; Farrow, M.R.; Logsdail, A.J.; Scanlon, D.O.; Keal, T.W.; Sherwood, P.; Woodley, S.M.; Sokol, A.A.; Walsh, A. Deep vs Shallow Nature of Oxygen Vacancies and Consequent n-Type Carrier Concentrations in Transparent Conducting Oxides. Phys. Rev. Mater. 2018, 2, 054604. [Google Scholar] [CrossRef]
- Sharma, M.; Aljawfi, R.N.; Kumari, K.; Chae, K.H.; Gautam, S.; Dalela, S.; Alvi, P.A.; Kumar, S. Investigation of Local Atomic Structure of Ni Doped SnO2 Thin Films via X-Ray Absorption Spectroscopy and Their Magnetic Properties. J. Mater. Sci. Mater. Electron. 2019, 30, 760–770. [Google Scholar] [CrossRef]
- Ribic, V.; Recnik, A.; Drazic, G.; Podlogar, M.; Brankovic, Z.; Brankovic, G. TEM and DFT Study of Basal-Plane Inversion Boundaries in SnO2-Doped ZnO. Sci. Sinter. 2021, 53, 237–252. [Google Scholar] [CrossRef]








| Parameter | Value |
|---|---|
| Bubbler temperature (°C) | 50 |
| Substrate temperature (°C) | 100–400 |
| TDMA-Sn pulse time (s) | 1.6 |
| TDMA-Sn purge time (s) | 6 |
| O2 pulse time (s) | 11 |
| O2 purge time (s) | 5 |
| Ar flow rate (sccm) | 80 |
| O2 flow rate (sccm) | 150 |
| O2 plasma power (W) | 2000 |
| TDMA-Sn carry gas flow rate (sccm) | 120 |
| TDMA-Sn dilute gas flow rate (sccm) | 400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.-H.; Zhang, Z.-X.; Hsu, C.-H.; Wu, W.-Y.; Ou, S.-L.; Huang, C.-J.; Wuu, D.-S.; Lien, S.-Y.; Zhu, W.-Z. Deposition Mechanism and Characterization of Plasma-Enhanced Atomic Layer-Deposited SnOx Films at Different Substrate Temperatures. Nanomaterials 2022, 12, 2859. https://doi.org/10.3390/nano12162859
Huang P-H, Zhang Z-X, Hsu C-H, Wu W-Y, Ou S-L, Huang C-J, Wuu D-S, Lien S-Y, Zhu W-Z. Deposition Mechanism and Characterization of Plasma-Enhanced Atomic Layer-Deposited SnOx Films at Different Substrate Temperatures. Nanomaterials. 2022; 12(16):2859. https://doi.org/10.3390/nano12162859
Chicago/Turabian StyleHuang, Pao-Hsun, Zhi-Xuan Zhang, Chia-Hsun Hsu, Wan-Yu Wu, Sin-Liang Ou, Chien-Jung Huang, Dong-Sing Wuu, Shui-Yang Lien, and Wen-Zhang Zhu. 2022. "Deposition Mechanism and Characterization of Plasma-Enhanced Atomic Layer-Deposited SnOx Films at Different Substrate Temperatures" Nanomaterials 12, no. 16: 2859. https://doi.org/10.3390/nano12162859
APA StyleHuang, P. -H., Zhang, Z. -X., Hsu, C. -H., Wu, W. -Y., Ou, S. -L., Huang, C. -J., Wuu, D. -S., Lien, S. -Y., & Zhu, W. -Z. (2022). Deposition Mechanism and Characterization of Plasma-Enhanced Atomic Layer-Deposited SnOx Films at Different Substrate Temperatures. Nanomaterials, 12(16), 2859. https://doi.org/10.3390/nano12162859








