Spectrally Stable Blue Light-Emitting Diodes Based on All-Inorganic Halide Perovskite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Perovskite Solution Preparation
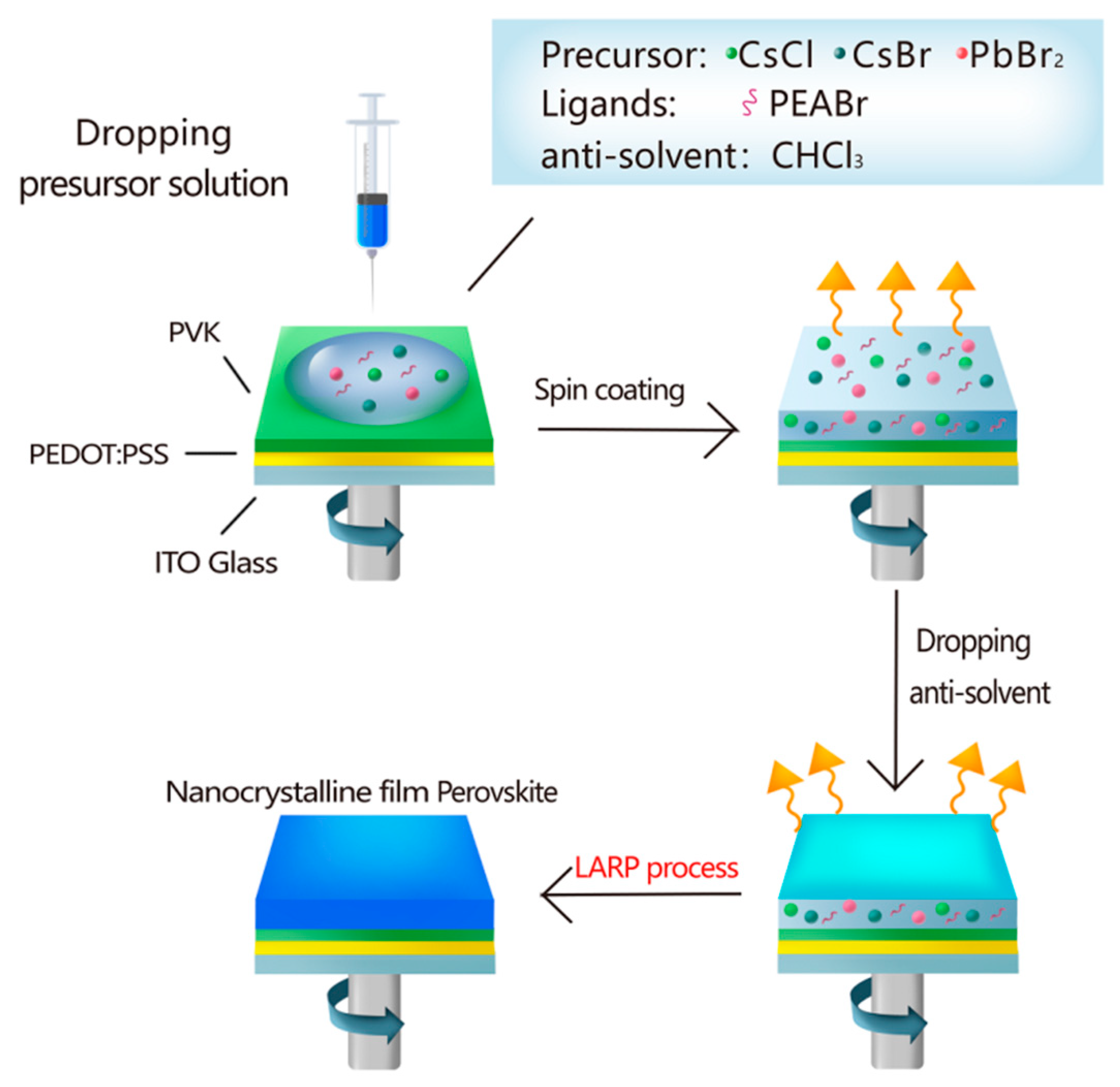
2.3. CsPbCl0.75Br2.25 Perovskite Nanocrystals (PNCs) Film Fabrication
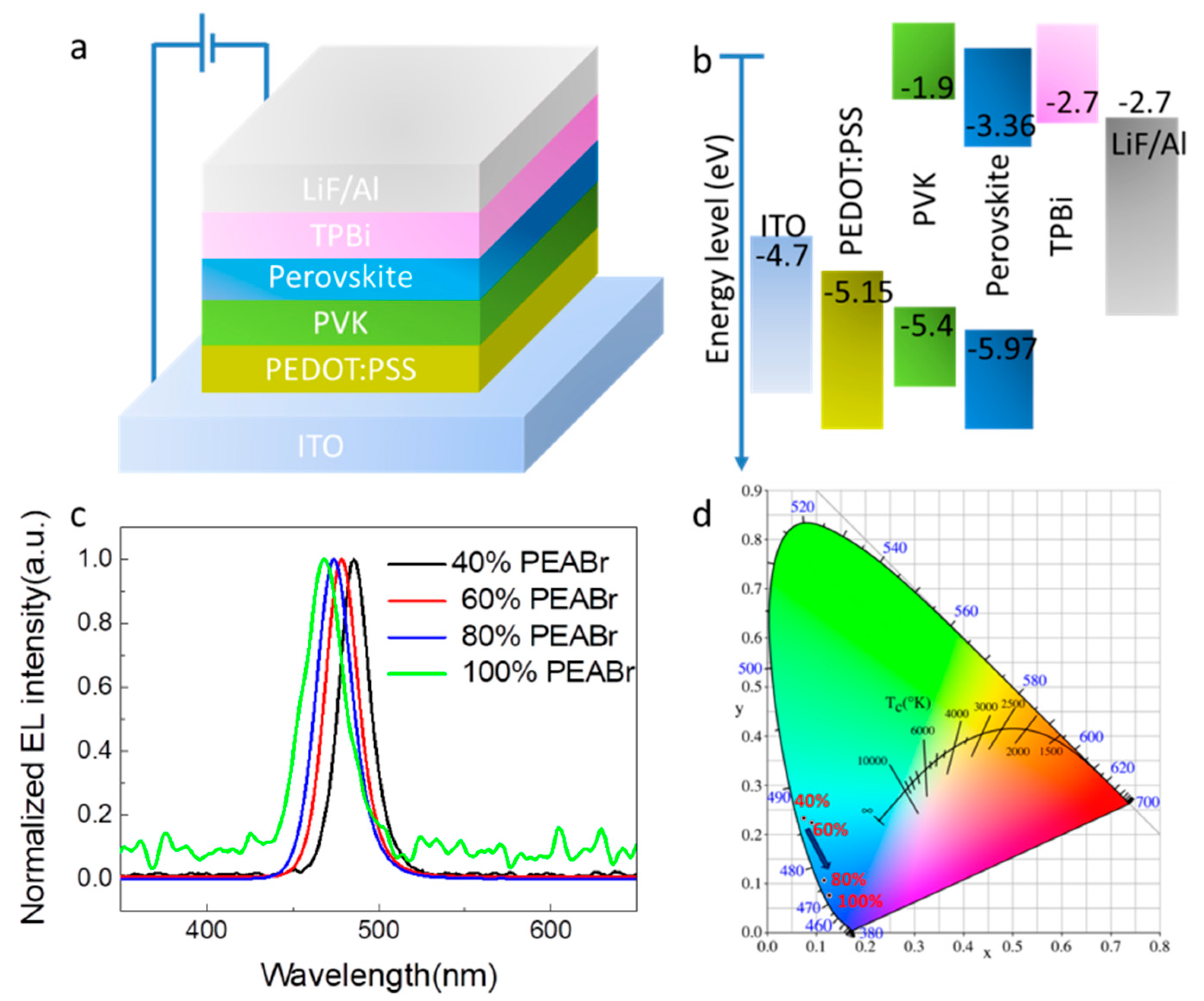
2.4. LED Device Fabrication
2.5. Device Measurements and Film Characterization
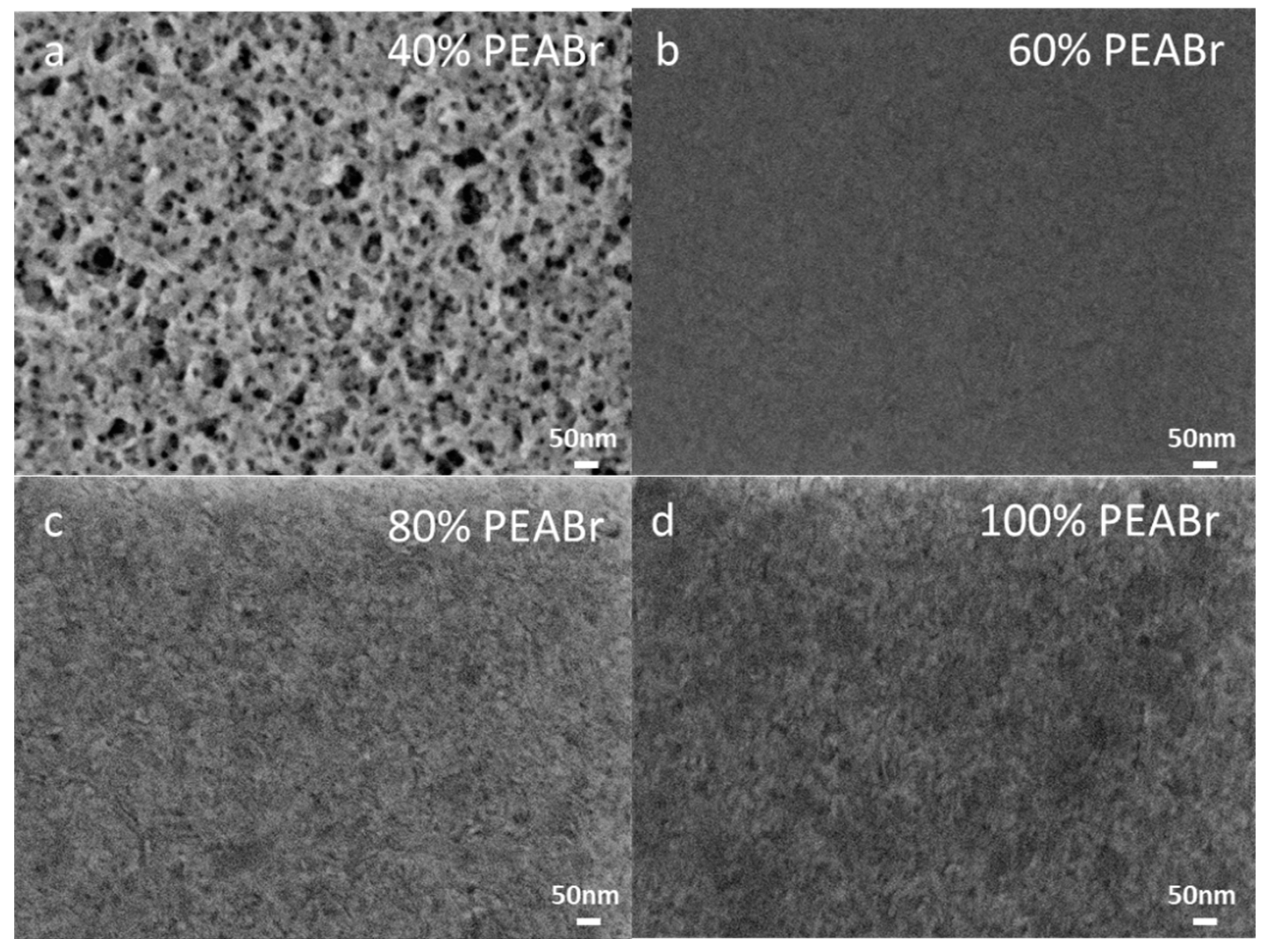
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX(3), X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Chen, X.; Ji, W.; Zeng, Q.; Ren, Z.; Su, Z.; Liu, L. Highly Controllable and Efficient Synthesis of Mixed-Halide CsPbX3 (X = Cl, Br, I) Perovskite QDs toward the Tunability of Entire Visible Light. ACS Appl. Mater. Interfaces 2017, 9, 33020–33028. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3Quantum Dots for Lighting and Displays: Room-Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Deng, J.; Chu, Z.; Jiang, Q.; Meng, J.; Wang, P.; Zhang, L.; Yin, Z.; You, J. Efficient green light-emitting diodes based on quasi-two-dimensional composition and phase engineered perovskite with surface passivation. Nat. Commun. 2018, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Wu, Q.; Cao, F.; Yang, D.; Shang, Y.; Ning, Z.; Zhang, W.; Zheng, W.; Yan, Y.; et al. Trifluoroacetate induced small-grained CsPbBr3 perovskite films result in efficient and stable light-emitting devices. Nat. Commun. 2019, 10, 665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef]
- Jeong, C.H.; Kim, P.S.H.; Wolf, M.H.; Lee, Y.H.; Heo, C.; Sadhanala, C.L.; Myoung, J.H.; Yoo, A.; SeunghyupIm, N.S.; Friend, S.H.; et al. Overcoming the electroluminescence efficiency limitations of perovskite lightemitting diodes. Science 2015, 350, 1222–1225. [Google Scholar]
- Chiba, T.; Hayashi, Y.; Ebe, H.; Hoshi, K.; Sato, J.; Sato, S.; Pu, Y.-J.; Ohisa, S.; Kido, J. Anion-exchange red perovskite quantum dots with ammonium iodine salts for highly efficient light-emitting devices. Nat. Photonics 2018, 12, 681–687. [Google Scholar] [CrossRef]
- Han, D.; Imran, M.; Zhang, M.; Chang, S.; Wu, X.G.; Zhang, X.; Tang, J.; Wang, M.; Ali, S.; Li, X.; et al. Efficient Light-Emitting Diodes Based on in Situ Fabricated FAPbBr3 Nanocrystals: The Enhancing Role of the Ligand-Assisted Reprecipitation Process. ACS Nano 2018, 12, 8808–8816. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, N.; Tian, H.; Guo, J.; Wei, Y.; Chen, H.; Miao, Y.; Zou, W.; Pan, K.; He, Y.; et al. Perovskite light-emitting diodes based on spontaneously formed submicrometre-scale structures. Nature 2018, 562, 249–253. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Bai, S.; Kim, V.; Lamboll, R.; Shivanna, R.; Auras, F.; Richter, J.M.; Yang, L.; Dai, L.; Alsari, M.; et al. High-efficiency perovskite–polymer bulk heterostructure light-emitting diodes. Nat. Photonics 2018, 12, 783–789. [Google Scholar] [CrossRef]
- Xu, W.; Hu, Q.; Bai, S.; Bao, C.; Miao, Y.; Yuan, Z.; Borzda, T.; Barker, A.J.; Tyukalova, E.; Hu, Z.; et al. Rational molecular passivation for high-performance perovskite light-emitting diodes. Nat. Photonics 2019, 13, 418–424. [Google Scholar] [CrossRef]
- Shen, Y.; Cheng, L.P.; Li, Y.Q.; Li, W.; Chen, J.D.; Lee, S.T.; Tang, J.X. High-Efficiency Perovskite Light-Emitting Diodes with Synergetic Outcoupling Enhancement. Adv. Mater. 2019, 31, e1901517. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Rivarola, F.W.; Davis, N.J.; Bai, S.; Jellicoe, T.C.; de la Pena, F.; Hou, S.; Ducati, C.; Gao, F.; Friend, R.H.; et al. Highly Efficient Perovskite Nanocrystal Light-Emitting Diodes Enabled by a Universal Crosslinking Method. Adv. Mater. 2016, 28, 3528–3534. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Yang, Y.; Xue, Q.; Yip, H.L.; Cao, Y. Modulation of recombination zone position for quasi-two-dimensional blue perovskite light-emitting diodes with efficiency exceeding 5. Nat. Commun. 2019, 10, 1027. [Google Scholar] [CrossRef]
- Hoye, R.L.Z.; Lai, M.L.; Anaya, M.; Tong, Y.; Galkowski, K.; Doherty, T.; Li, W.; Huq, T.N.; Mackowski, S.; Polavarapu, L.; et al. Identifying and Reducing Interfacial Losses to Enhance Color-Pure Electroluminescence in Blue-Emitting Perovskite Nanoplatelet Light-Emitting Diodes. ACS Energy Lett. 2019, 4, 1181–1188. [Google Scholar] [CrossRef]
- Fang, T.; Zhang, F.; Yuan, S.; Zeng, H.; Song, J. Recent advances and prospects toward blue perovskite materials and light-emitting diodes. InfoMat 2019, 1, 211–233. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Cao, Y.; Ge, R.; Wei, Y.-Q.; Wang, N.-N.; Wang, J.-P.; Huang, W. Sky-blue perovskite light-emitting diodes based on quasi-two-dimensional layered perovskites. Chin. Chem. Lett. 2017, 28, 29–31. [Google Scholar] [CrossRef]
- Kumawat, N.K.; Dey, A.; Kumar, A.; Gopinathan, S.P.; Narasimhan, K.L.; Kabra, D. Band Gap Tuning of CH(3)NH(3)Pb(Br(1-x)Clx)(3) Hybrid Perovskite for Blue Electroluminescence. ACS Appl. Mater. Interfaces 2015, 7, 13119–13124. [Google Scholar] [CrossRef]
- Gangishetty, M.K.; Hou, S.; Quan, Q.; Congreve, D.N. Reducing Architecture Limitations for Efficient Blue Perovskite Light-Emitting Diodes. Adv. Mater. 2018, 30, e1706226. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Quan, L.N.; Zhao, Y.; Peng, W.; Murali, B.; Sarmah, S.P.; Yuan, M.; Sinatra, L.; Alyami, N.M.; Liu, J.; et al. Highly Efficient Perovskite-Quantum-Dot Light-Emitting Diodes by Surface Engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Gangishetty, M.K.; Quan, Q.; Congreve, D.N. Efficient Blue and White Perovskite Light-Emitting Diodes via Manganese Doping. Joule 2018, 2, 2421–2433. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Quan, L.N.; Comin, R.; Walters, G.; Sabatini, R.; Voznyy, O.; Hoogland, S.; Zhao, Y.; Beauregard, E.M.; Kanjanaboos, P.; et al. Perovskite energy funnels for efficient light-emitting diodes. Nat. Nanotechnol. 2016, 11, 872–877. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, L.; Ge, R.; Zhang, S.; Miao, Y.; Zou, W.; Yi, C.; Sun, Y.; Cao, Y.; Yang, R.; et al. Perovskite light-emitting diodes based on solution-processed self-organized multiple quantum wells. Nat. Photonics 2016, 10, 699–704. [Google Scholar] [CrossRef]
- Jiang, Y.; Qin, C.; Cui, M.; He, T.; Liu, K.; Huang, Y.; Luo, M.; Zhang, L.; Xu, H.; Li, S.; et al. Spectra stable blue perovskite light-emitting diodes. Nat. Commun. 2019, 10, 1868. [Google Scholar] [CrossRef] [Green Version]
- Yao, E.P.; Yang, Z.; Meng, L.; Sun, P.; Dong, S.; Yang, Y.; Yang, Y. High-Brightness Blue and White LEDs based on Inorganic Perovskite Nanocrystals and their Composites. Adv. Mater. 2017, 29, 1606859. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, G.H.; El-Demellawi, J.K.; Yin, J.; Pan, J.; Velusamy, D.B.; Hedhili, M.N.; Alarousu, E.; Bakr, O.M.; Alshareef, H.N.; Mohammed, O.F. Giant Photoluminescence Enhancement in CsPbCl3 Perovskite Nanocrystals by Simultaneous Dual-Surface Passivation. ACS Energy Lett. 2018, 3, 2301–2307. [Google Scholar] [CrossRef] [Green Version]
- Yong, Z.J.; Guo, S.Q.; Ma, J.P.; Zhang, J.Y.; Li, Z.Y.; Chen, Y.M.; Zhang, B.B.; Zhou, Y.; Shu, J.; Gu, J.L.; et al. Doping-Enhanced Short-Range Order of Perovskite Nanocrystals for Near-Unity Violet Luminescence Quantum Yield. J. Am. Chem. Soc. 2018, 140, 9942–9951. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhang, C.; Jiang, X.F.; Liu, M.; Xia, R.; Shi, T.; Chen, D.; Xue, Q.; Zhao, Y.J.; Su, S.; et al. High-Performance Color-Tunable Perovskite Light Emitting Devices through Structural Modulation from Bulk to Layered Film. Adv. Mater. 2017, 29, 1603157. [Google Scholar] [CrossRef]
- Xing, J.; Zhao, Y.; Askerka, M.; Quan, L.N.; Gong, X.; Zhao, W.; Zhao, J.; Tan, H.; Long, G.; Gao, L.; et al. Color-stable highly luminescent sky-blue perovskite light-emitting diodes. Nat. Commun. 2018, 9, 3541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.; Wang, K.; Sun, X.W.; Choy, W.C.H. Strategies Toward Efficient Blue Perovskite Light-Emitting Diodes. Adv. Funct. Mater. 2021, 31, 2100516. [Google Scholar] [CrossRef]
- Zhang, L.; Long, R. Developments and challenges ahead in blue perovskite light-emitting devices. J. Energy Chem. 2022, 71, 2095–4956. [Google Scholar] [CrossRef]
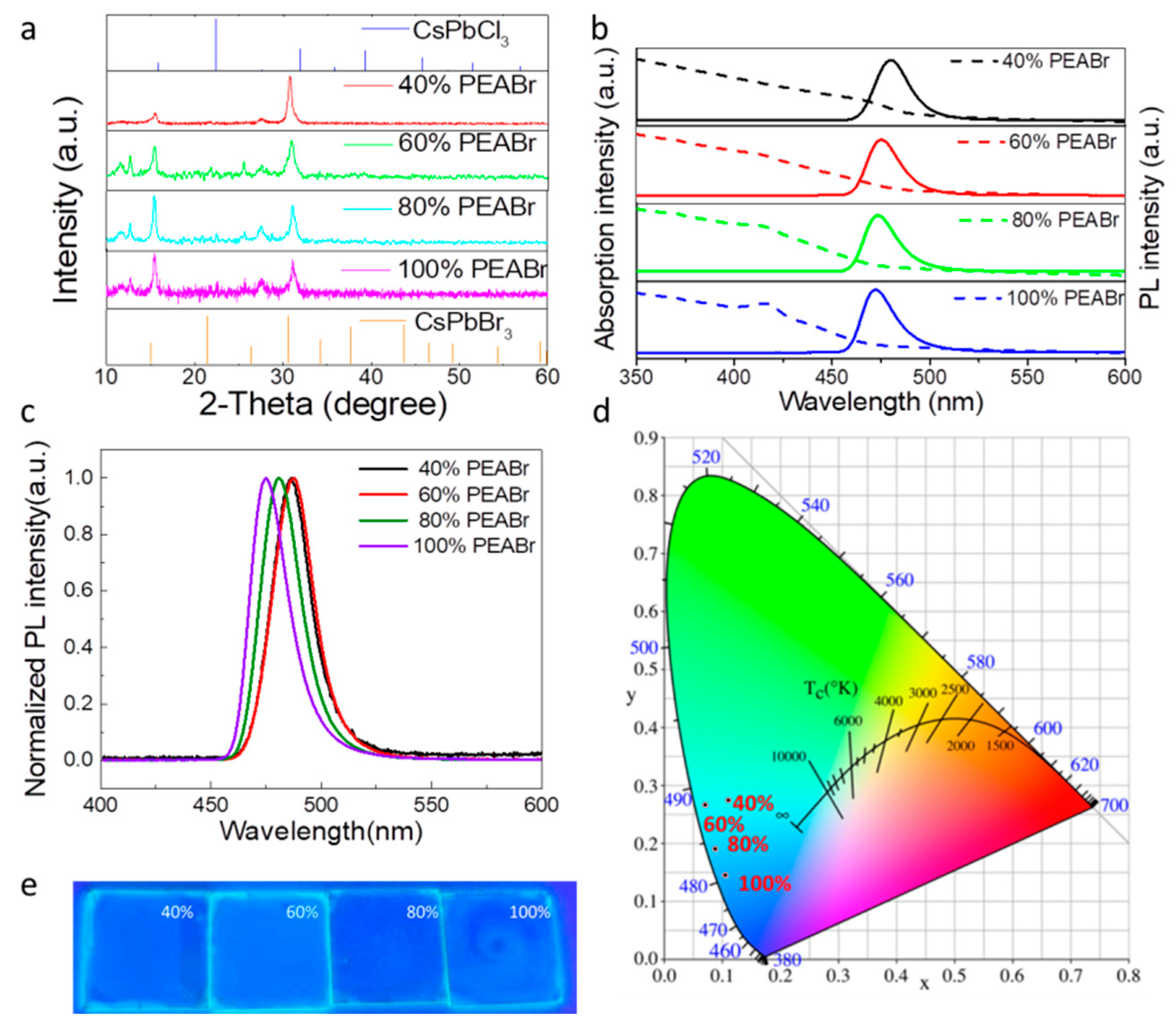
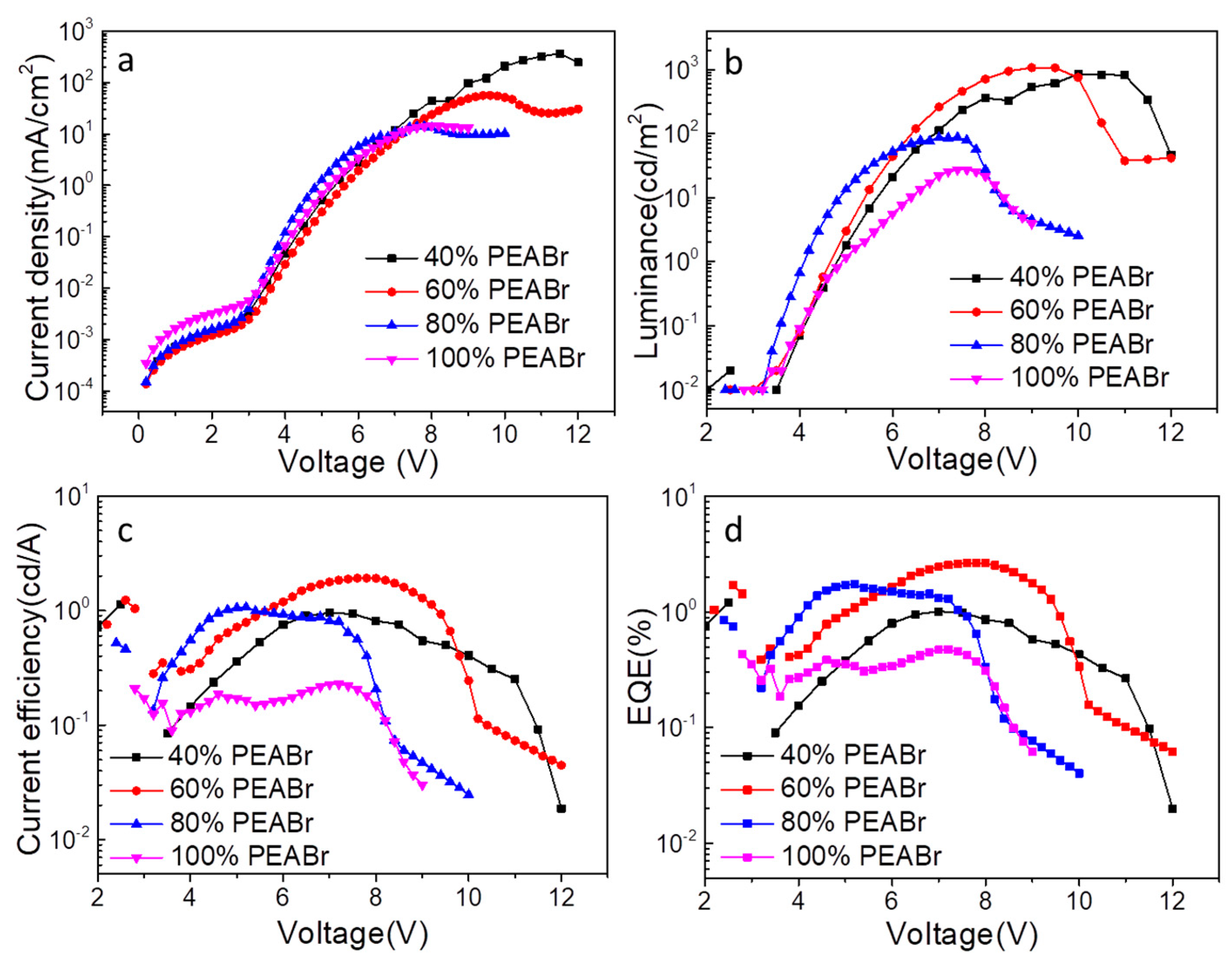
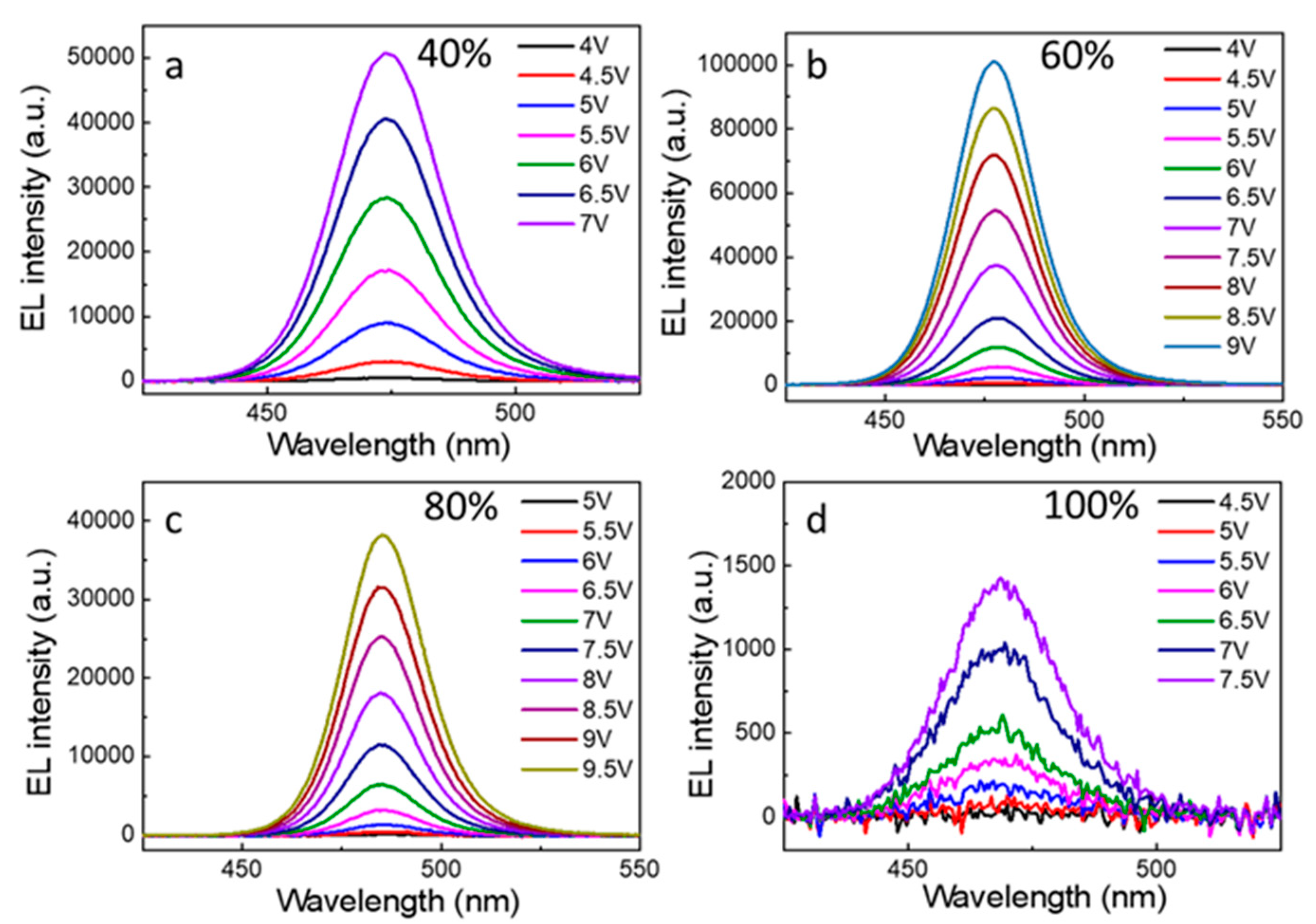
| PL (nm) | The Max PL Intensity | FWHM (nm) | |
|---|---|---|---|
| 40% PEABr | 480 | 33,886 | 21.8 |
| 60% PEABr | 475 | 56,537 | 20.4 |
| 80% PEABr | 473 | 49,984 | 20.7 |
| 100% PEABr | 472 | 22,316 | 22.6 |
| CE (cd/A) | Luminance (cd/m2) | EQE | EL (nm) | FWHM (nm) | |
|---|---|---|---|---|---|
| 40% PEABr | 0.95 | 846.9@10 V | 1.01@7 V | 485 | 22 |
| 60% PEABr | 1.92 | 1069@9 V | [email protected] V | 478 | 23 |
| 80% PEABr | 1.06 | [email protected] V | [email protected] V | 474 | 24 |
| 100% PEABr | 0.23 | [email protected] V | 0.47@7 V | 468 | 26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Su, Y.; Xue, X.; Zeng, Q.; Sun, Y.; Zhu, K.; Ye, W.; Ji, W.; Leng, X. Spectrally Stable Blue Light-Emitting Diodes Based on All-Inorganic Halide Perovskite Films. Nanomaterials 2022, 12, 2906. https://doi.org/10.3390/nano12172906
Zhang H, Su Y, Xue X, Zeng Q, Sun Y, Zhu K, Ye W, Ji W, Leng X. Spectrally Stable Blue Light-Emitting Diodes Based on All-Inorganic Halide Perovskite Films. Nanomaterials. 2022; 12(17):2906. https://doi.org/10.3390/nano12172906
Chicago/Turabian StyleZhang, Huidan, Ying Su, Xulan Xue, Qinghui Zeng, Yifang Sun, Kai Zhu, Weiguang Ye, Wenyu Ji, and Xiangyang Leng. 2022. "Spectrally Stable Blue Light-Emitting Diodes Based on All-Inorganic Halide Perovskite Films" Nanomaterials 12, no. 17: 2906. https://doi.org/10.3390/nano12172906





