Reduced Graphene Oxide/Carbon Paper for the Anode Diffusion Layer of a Micro Direct Methanol Fuel Cell
Abstract
:1. Introduction
2. Materials and Methods
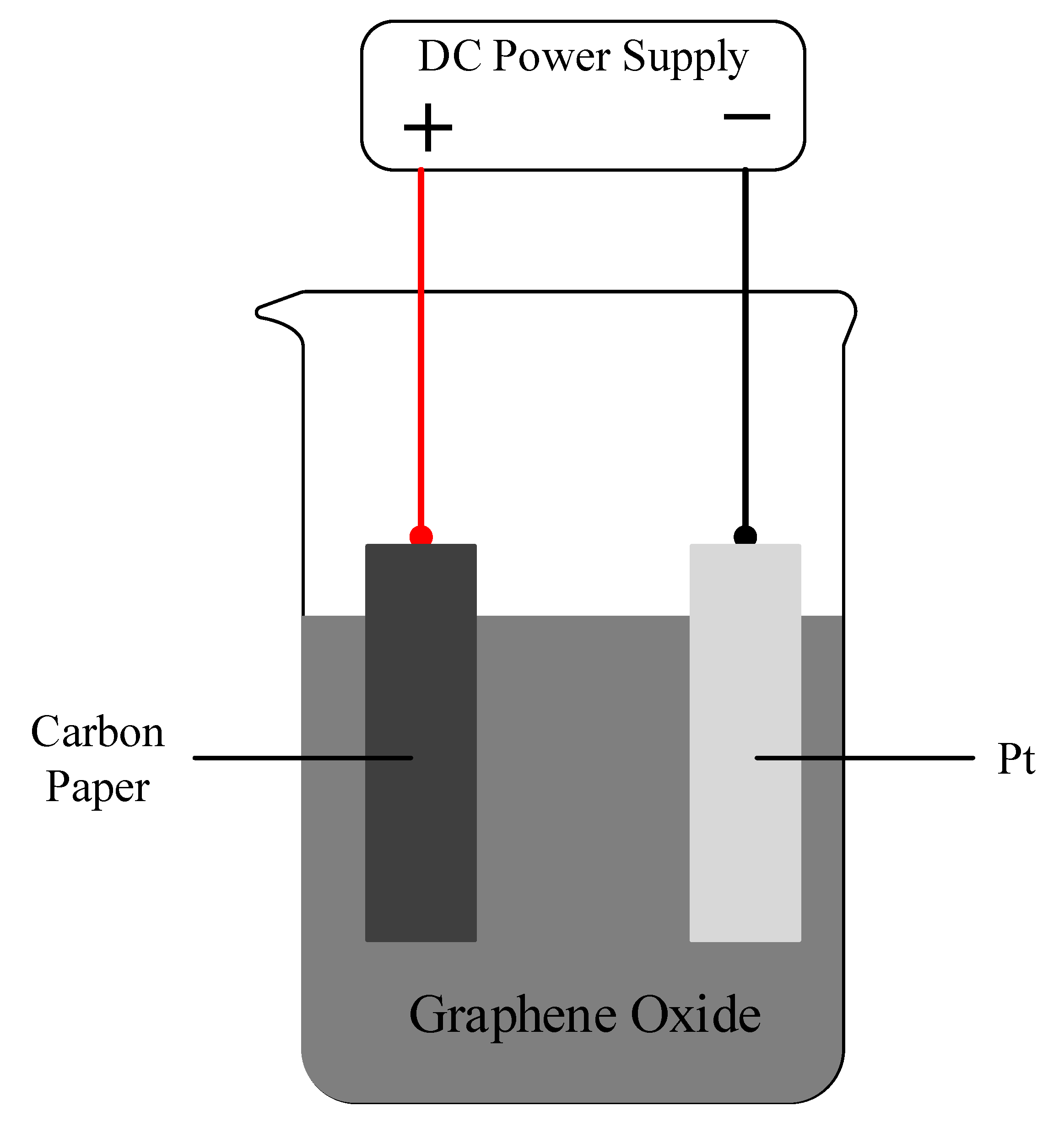
2.1. Reduced Graphene Oxide/Carbon Paper Preparation
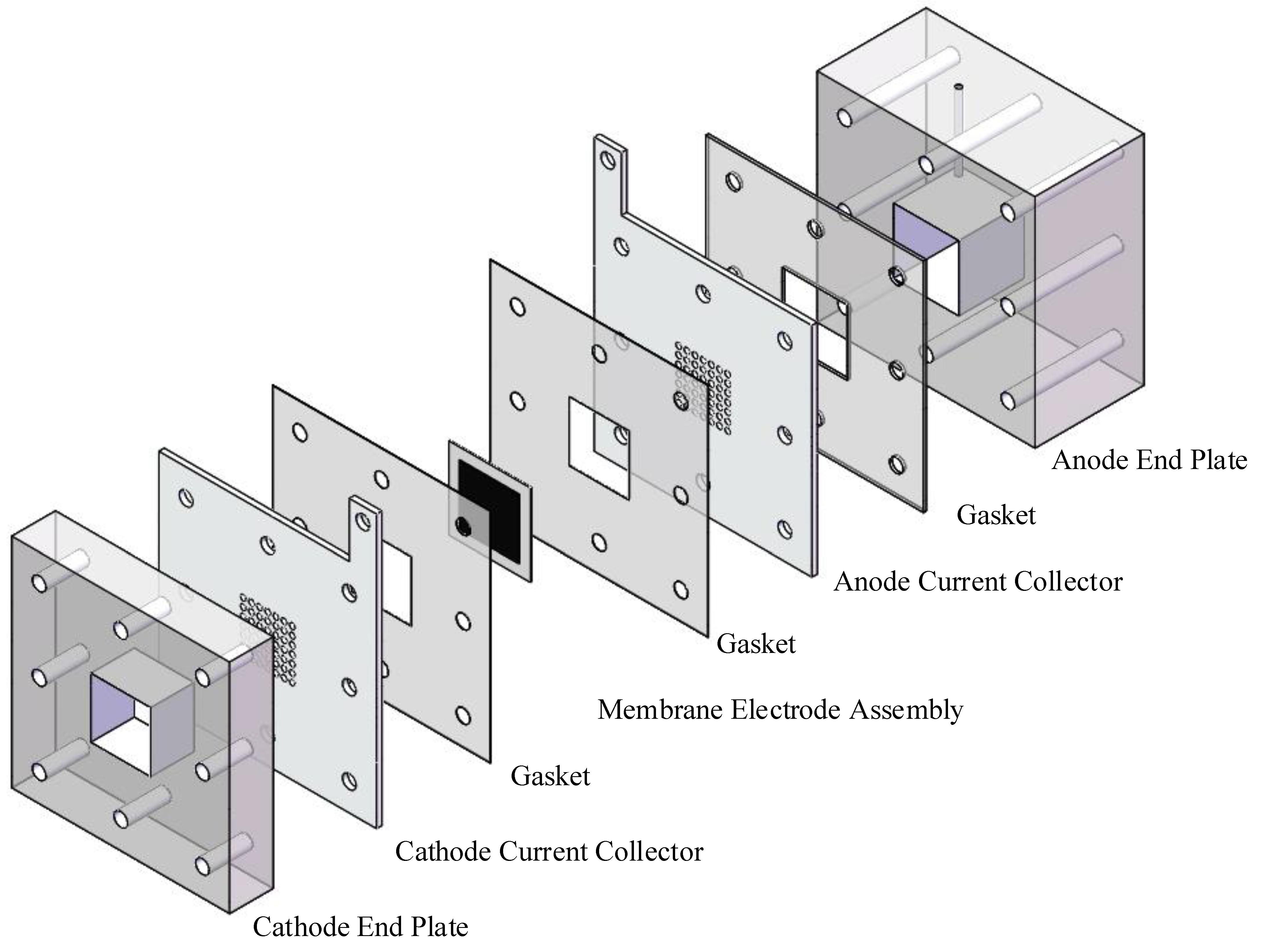
2.2. Single Cell Preparation
2.3. Electrochemical Tests
2.3.1. Performance Measurements
2.3.2. Electrochemical Impedance Spectroscopy (EIS)
2.3.3. Linear Sweep Voltammetry (LSV)
2.4. Methanol Mass Transfer Analysis
3. Results and Discussion
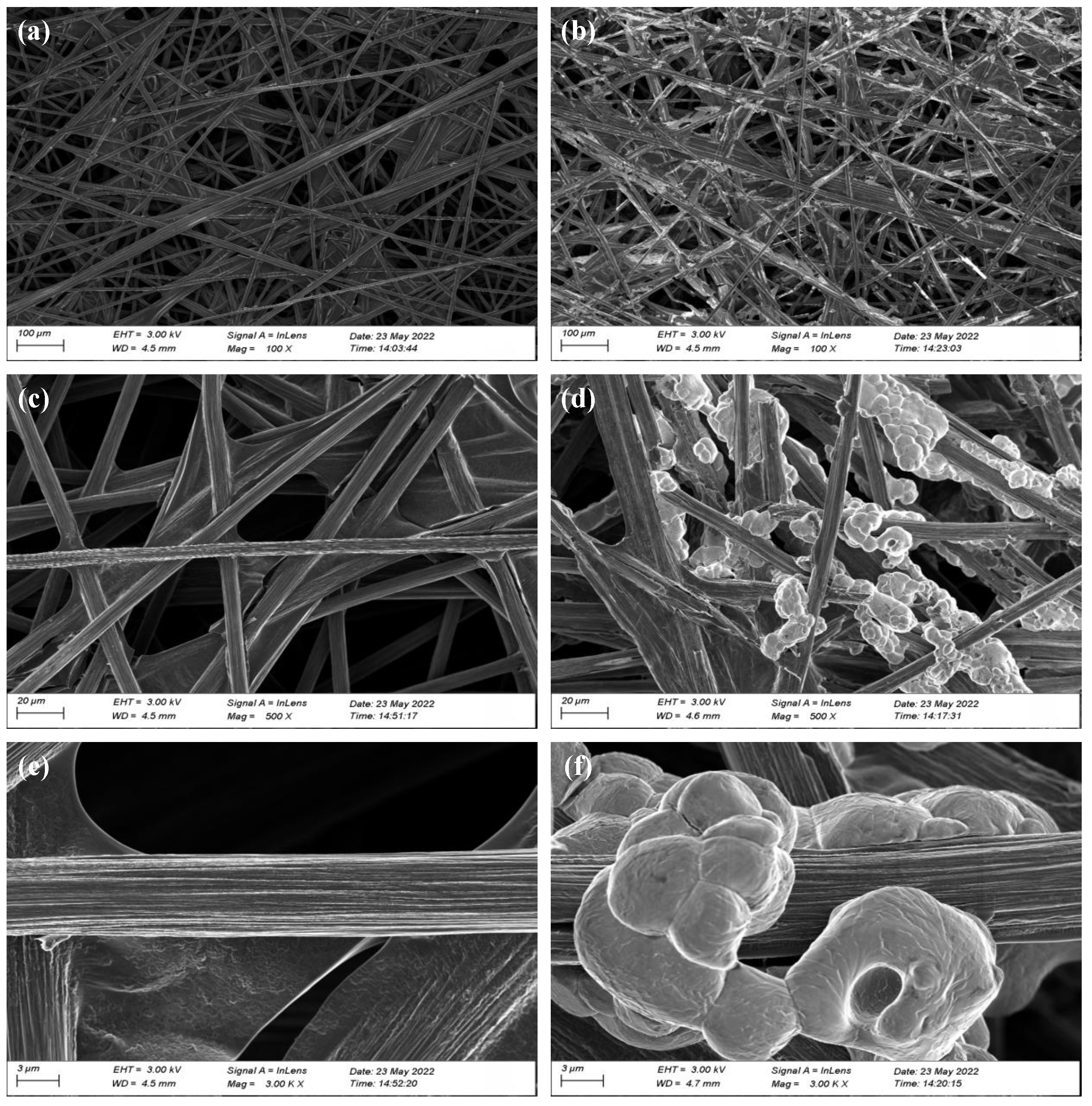
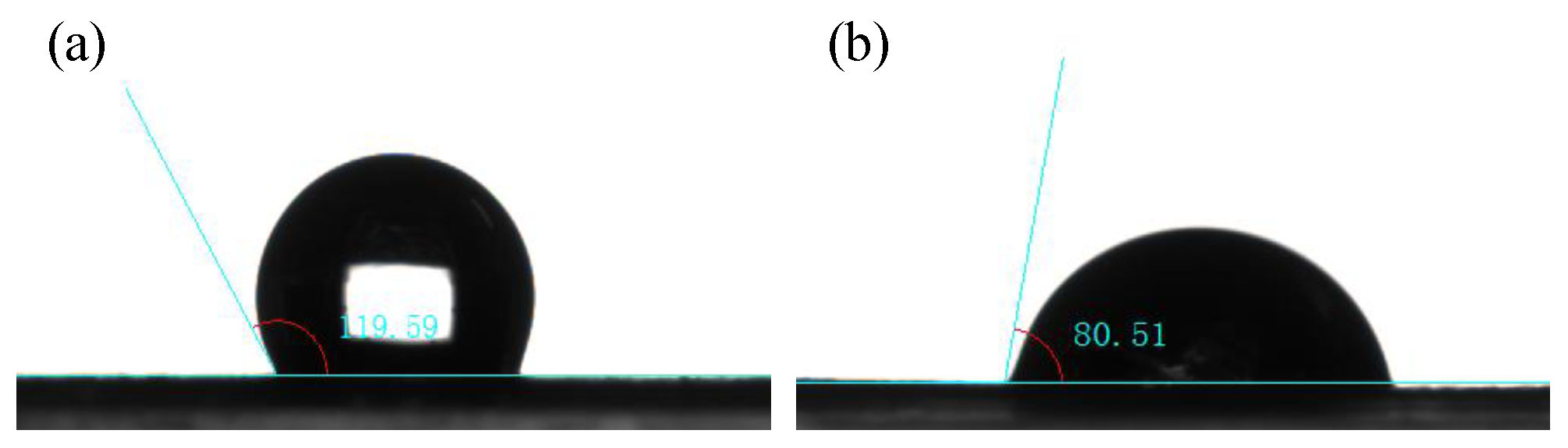
3.1. Physical Characterization
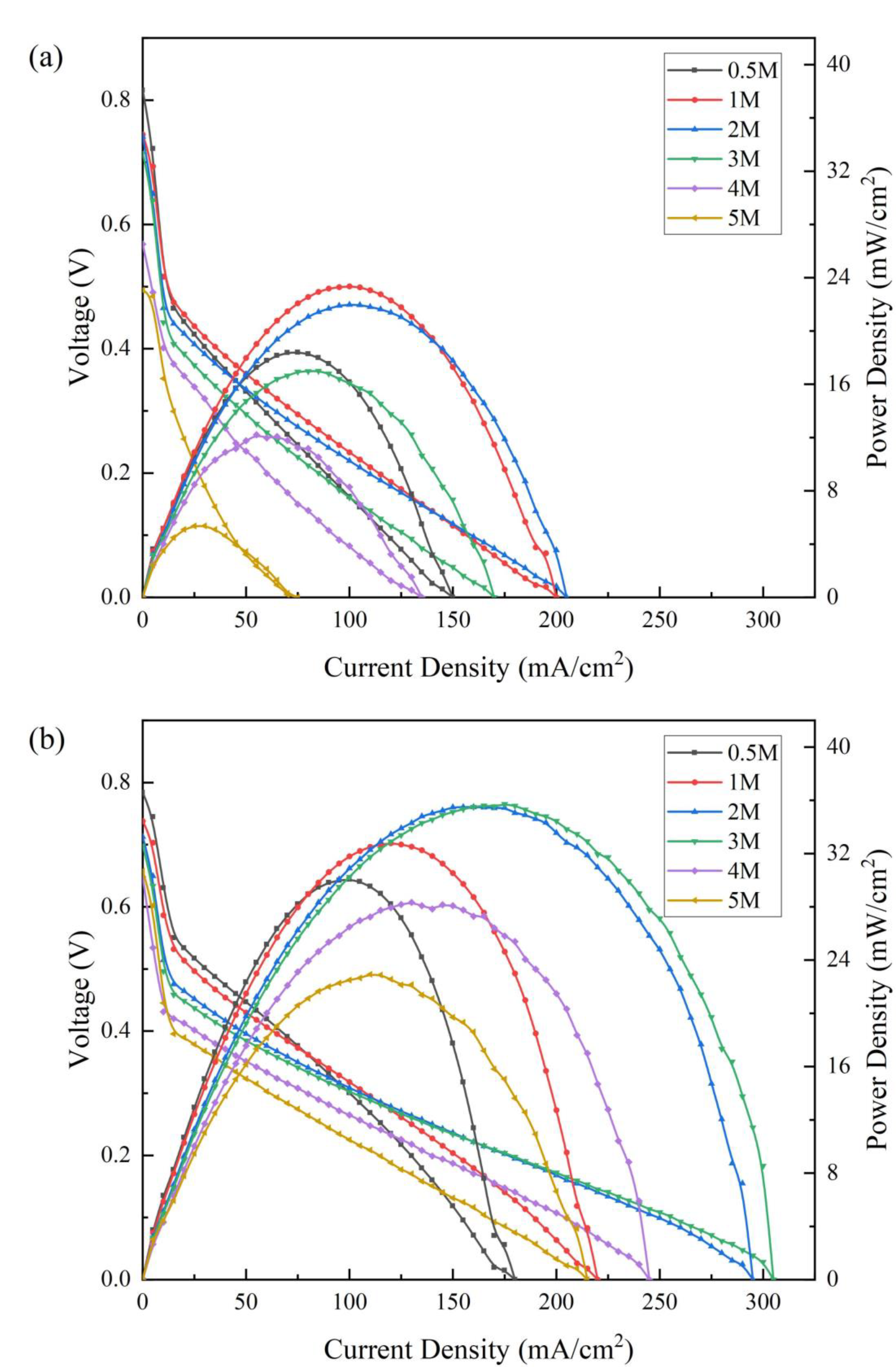
3.2. DMFC Performance
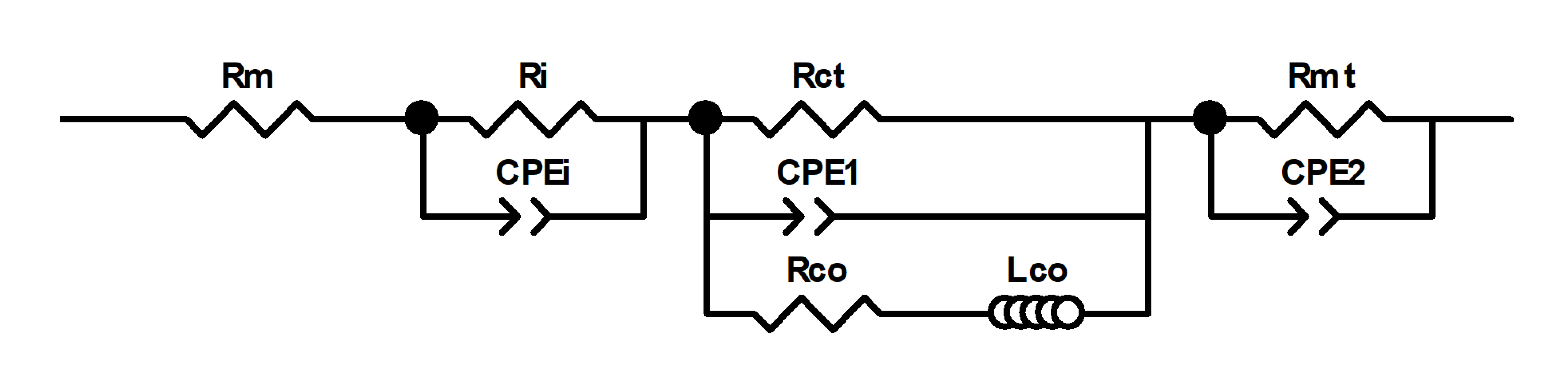
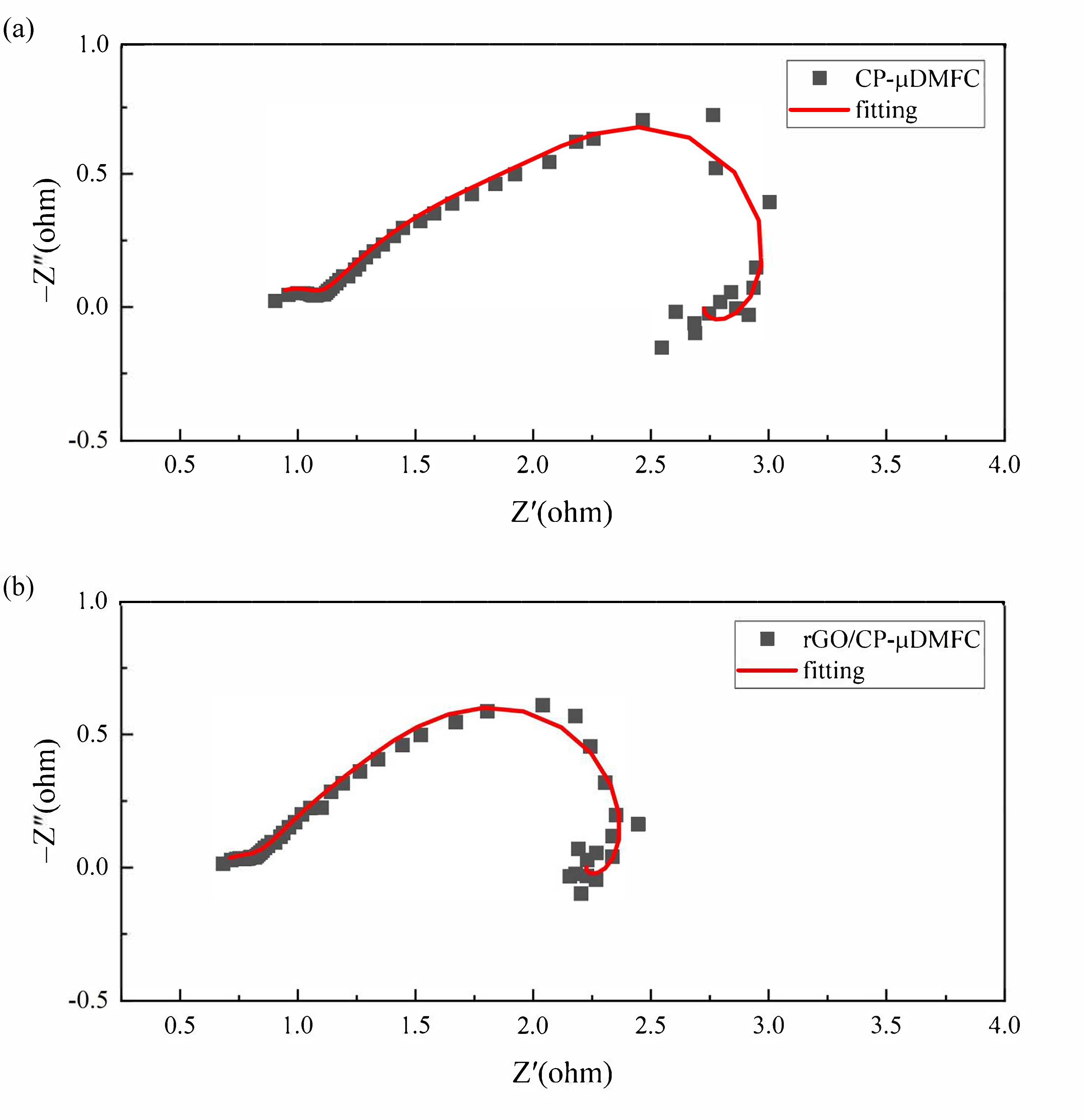
3.3. Electrochemical Impedance Spectroscopy
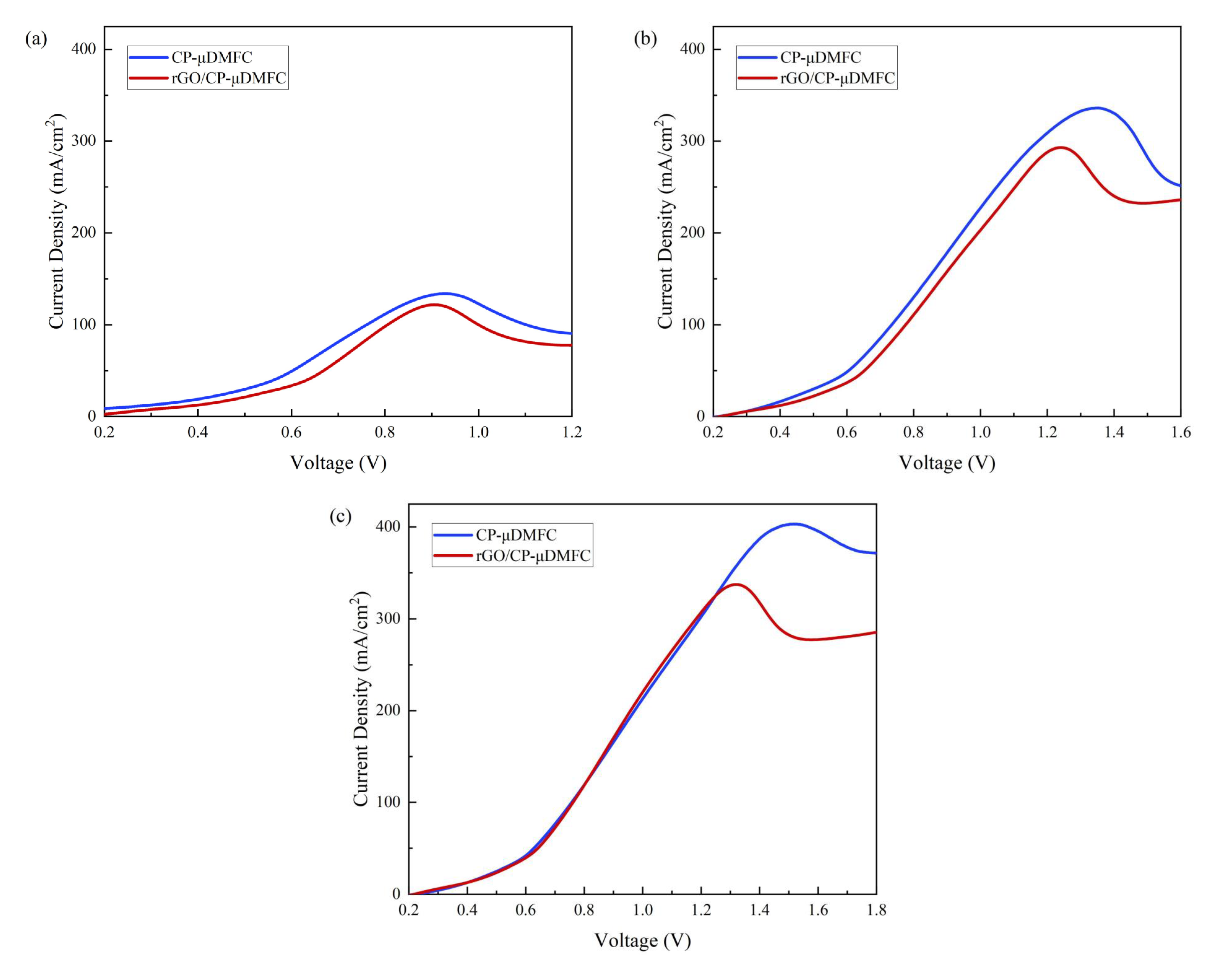
3.4. Methanol Crossover
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DL | Diffusion layer |
| ADL | Anode diffusion layer |
| DMFC | Micro direct methanol fuel cell |
| rGO/CP | Reduced graphene oxide/carbon paper |
| CL | Catalytic layer |
| ACL | Anode catalytic layer |
| MPL | Micro porous layer |
| MEA | Membrane electrode assembly |
| CP | Carbon paper |
| CC | Carbon cloth |
| 3DG | Three-dimensional graphene |
| EIS | Electrochemical impedance Spectroscopy |
| LSV | Linear scanning voltammetry |
| PEM | Proton exchange membrane |
| MOR | Methanol oxidation reaction |
| MIP | Mercury intrusion porosimetry |
| AMPL | Anode micro porous layer |
| ECM | Equivalent circuit model |
References
- Long, Z.; Gong, L.; Sun, Y.; Li, Y.; Xu, P.; Zhang, X.; Ge, J.; Liu, C.; Ma, S.; Jin, Z. In-situ precise electrocatalytic behaviors of Pt/C and PtRu/C for methanol oxidation of DMFCs via the designed micro-MEA. Int. J. Hydrogen Energy 2018, 43, 12413–12419. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, X.; Sun, H.; Wang, S.; Sun, G. Recent advances in multi-scale design and construction of materials for direct methanol fuel cells. Nano Energy 2019, 65. [Google Scholar] [CrossRef]
- Madaswamy, S.L.; Alothman, A.A.; Al-Anazy, M.M.; Ifseisi, A.A.; Alqahtani, K.N.; Natarajan, S.K.; Angaiah, S.; Ragupathy, D. Polyaniline-based nanocomposites for direct methanol fuel cells (DMFCs)—A Recent Review. J. Ind. Eng. Chem. 2021, 97, 79–94. [Google Scholar] [CrossRef]
- Li, X.; Miao, Z.; Marten, L.; Blankenau, I. Experimental measurements of fuel and water crossover in an active DMFC. Int. J. Hydrogen Energy 2021, 46, 4437–4446. [Google Scholar] [CrossRef]
- Braz, B.A.; Moreira, C.S.; Oliveira, V.B.; Pinto, A.M.F.R. Electrochemical impedance spectroscopy as a diagnostic tool for passive direct methanol fuel cells. Energy Rep. 2022, 8, 7964–7975. [Google Scholar] [CrossRef]
- Xing, L.; Shi, W.; Su, H.; Xu, Q.; Das, P.K.; Mao, B.; Scott, K. Membrane electrode assemblies for PEM fuel cells: A review of functional graded design and optimization. Energy 2019, 177, 445–464. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, Y.; Zheng, X.; Li, Y.; Zhang, X.; Liu, X. A CNT (carbon nanotube) paper as cathode gas diffusion electrode for water management of passive μDMFC (micro-direct methanol fuel cell) with highly concentrated methanol. Energy 2015, 82, 236–241. [Google Scholar] [CrossRef]
- Laoun, B.; Kasat, H.A.; Ahmad, R.; Kannan, A.M. Gas diffusion layer development using design of experiments for the optimization of a proton exchange membrane fuel cell performance. Energy 2018, 151, 689–695. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Sayed, E.T.; Mohamed, H.O.; Obaid, M.; Rezk, H.; Chae, K.J. Nonprecious anodic catalysts for low-molecular-hydrocarbon fuel cells: Theoretical consideration and current progress. Prog. Energy Combust. Sci. 2020, 77. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, Y.; Liu, X. A novel cathode gas diffusion layer for water management of passive μDMFC. Energy 2017, 139, 535–541. [Google Scholar] [CrossRef]
- Braz, B.A.; Oliveira, V.B.; Pinto, A.M.F.R. Experimental Evaluation of the Effect of the Anode Diffusion Layer Properties on the Performance of a Passive Direct Methanol Fuel Cell. Energies 2020, 13, 5198. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, X.; Li, J.; Qi, G. Three-dimensional graphene as gas diffusion layer for micro direct methanol fuel cell. Int. J. Mod. Phys. B 2018, 32, 1850145. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, L.; Li, J. A Novel Button-Type Micro Direct Methanol Fuel Cell with Graphene Diffusion Layer. Micromachines 2019, 10, 658. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Sayed, E.T.; Nakagawa, N. Significance of diffusion layers on the performance of liquid and vapor feed passive direct methanol fuel cells. Energy 2020, 209, 118492. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, X.; Hou, C.; Zhang, Y.; Wang, H.; Liu, X. Enhanced water management via the optimization of cathode microporous layer using 3D graphene frameworks for direct methanol fuel cell. J. Power Sources 2020, 451, 227800. [Google Scholar] [CrossRef]
- Yan, X.H.; Gao, P.; Zhao, G.; Shi, L.; Xu, J.B.; Zhao, T.S. Transport of highly concentrated fuel in direct methanol fuel cells. Appl. Therm. Eng. 2017, 126, 290–295. [Google Scholar] [CrossRef]
- Alrashidi, A.; Liu, H. Laser-perforated anode gas diffusion layers for direct methanol fuel cells. Int. J. Hydrogen Energy 2021, 46, 17886–17896. [Google Scholar] [CrossRef]
- Xue, S.; Wang, Q.; Dai, G.; Zhao, M.; Sun, S.; Yu, N.; Huang, Q.; Zhu, Y.; Fu, L.; Wu, Y. Titanium carbide/carbon nanofibers film as flexible gas diffusion layers for passive direct methanol fuel cells. Int. J. Energy Res. 2022, 46, 10919–10929. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Zhou, X.; Wang, F.; Luo, Z.; Wu, Q. Characterizations of carbonized electrospun mats as diffusion layers for direct methanol fuel cells. J. Power Sources 2020, 448, 227410. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Piela, P.; Fields, R.; Zelenay, P. Electrochemical Impedance Spectroscopy for Direct Methanol Fuel Cell Diagnostics. J. Electrochem. Soc. 2006, 153, A1902. [Google Scholar] [CrossRef]
- Braz, B.A.; Moreira, C.S.; Oliveira, V.B.; Pinto, A.M.F.R. Effect of the current collector design on the performance of a passive direct methanol fuel cell. Electrochim. Acta 2019, 300, 306–315. [Google Scholar] [CrossRef]
- Braz, B.A.; Oliveira, V.B.; Pinto, A.M.F.R. Experimental studies of the effect of cathode diffusion layer properties on a passive direct methanol fuel cell power output. Int. J. Hydrogen Energy 2019, 44, 19334–19343. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Shang, Y.; Guo, C. A simple and efficient electrochemical reductive method for graphene oxide. Bull. Mater. Sci. 2014, 37, 1529–1533. [Google Scholar] [CrossRef]
- Jamil, M.F.; Biçer, E.; Yarar Kaplan, B.; Alkan Gürsel, S. One-step fabrication of new generation graphene-based electrodes for polymer electrolyte membrane fuel cells by a novel electrophoretic deposition. Int. J. Hydrogen Energy 2021, 46, 5653–5663. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Nie, L.; Zhang, Y.; Liu, X. Stainless steel fiber felt as cathode diffusion backing and current collector for a micro direct methanol fuel cell with low methanol crossover. J. Power Sources 2014, 245, 520–528. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, F.; Zhang, Y.; Zhang, D. Performance Optimization of μDMFC with Foamed Stainless Steel Cathode Current Collector. Energies 2021, 14, 6608. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Rangel, C.M.; Pinto, A.M.F.R. One-dimensional and non-isothermal model for a passive DMFC. J. Power Sources 2011, 196, 8973–8982. [Google Scholar] [CrossRef]
- Multiphysics, C. Diffusion Coefficient in Porous Media. Available online: https://www.comsol.com/multiphysics/diffusion-coefficient?parent=diffusion-0402-392-422 (accessed on 17 August 2022).
- Industries, T. TORAYC Carbon Paper Datasheet. Available online: https://www.cf-composites.toray/resources/data_sheets/ (accessed on 22 July 2022).
- Sun, J.; Guo, T.; Deng, H.; Jiao, K.; Huang, X. Effect of electrode variable contact angle on the performance and transport characteristics of passive direct methanol fuel cells. Int. J. Hydrogen Energy 2015, 40, 10568–10587. [Google Scholar] [CrossRef]
- Yuan, T.; Zou, Z.; Chen, M.; Li, Z.; Xia, B.; Yang, H. New anodic diffusive layer for passive micro-direct methanol fuel cell. J. Power Sources 2009, 192, 423–428. [Google Scholar] [CrossRef]
- Yuan, T.; Yang, J.; Wang, Y.; Ding, H.; Li, X.; Liu, L.; Yang, H. Anodic diffusion layer with graphene-carbon nanotubes composite material for passive direct methanol fuel cell. Electrochim. Acta 2014, 147, 265–270. [Google Scholar] [CrossRef]

| Concentration (mol/L) | Maximum Power Density (mW/cm2) | Open-Circuit Voltage (V) | ||
|---|---|---|---|---|
| CP-DMFC | rGO/CP-DMFC | CP-DMFC | rGO/CP-DMFC | |
| 0.5 | 18.40 | 30.05 | 0.82 | 0.78 |
| 1 | 23.36 | 32.77 | 0.75 | 0.74 |
| 2 | 21.98 | 35.50 | 0.74 | 0.71 |
| 3 | 17.00 | 35.72 | 0.71 | 0.70 |
| 4 | 12.21 | 28.33 | 0.57 | 0.65 |
| 5 | 5.37 | 22.93 | 0.49 | 0.66 |
| Materials and Methods | Pmax (Conventional DL) | Pmax (Novel DL) | Reference |
|---|---|---|---|
| (mW/cm2) | (mW/cm2) | ||
| rGO/CP | 23.36 (1 mol/L) | 35.72 (3 mol/L) | This work |
| rGO-SSFF | 31 (3 mol/L) | 35 (4 mol/L) | [10] |
| 3DG | 25 (1 mol/L) | 31.2 (1 mol/L) | [12] |
| Button-type dmfc with 3DG DL | 6.8 (1 mol/L) | 9.3 (1 mol/L) | [13] |
| Modified CP | 36.6 (6 mol/L) | 36.9 (10 mol/L) | [16] |
| Laser-perforated CC | 67.6 (2 mol/L) | 89.1 (2 mol/L) | [17] |
| TiC/CNFs film | 18.1 ( mol/L) | 20.2 ( mol/L) | [18] |
| Carbonized PAN mats | nearly 85.7 (2 mol/L) | 85.7 (2 mol/L) | [19] |
| Parameters | CP-DMFC () | rGO/CP-DMFC () |
|---|---|---|
| 0.848 | 0.634 | |
| 0.248 | 0.242 | |
| 1.687 | 0.793 | |
| 2.007 | 1.485 | |
| 0.725 | 0.844 |
| Concentration | CP-DMFC | rGO/CP-DMFC |
|---|---|---|
| (mol/L) | () | () |
| 1 | 133.9 | 121.9 |
| 3 | 336.2 | 293.1 |
| 5 | 403.3 | 337.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Li, K.; Wang, Z.; Zhao, Z. Reduced Graphene Oxide/Carbon Paper for the Anode Diffusion Layer of a Micro Direct Methanol Fuel Cell. Nanomaterials 2022, 12, 2941. https://doi.org/10.3390/nano12172941
Zhang D, Li K, Wang Z, Zhao Z. Reduced Graphene Oxide/Carbon Paper for the Anode Diffusion Layer of a Micro Direct Methanol Fuel Cell. Nanomaterials. 2022; 12(17):2941. https://doi.org/10.3390/nano12172941
Chicago/Turabian StyleZhang, Dacheng, Kang Li, Ziten Wang, and Zhengang Zhao. 2022. "Reduced Graphene Oxide/Carbon Paper for the Anode Diffusion Layer of a Micro Direct Methanol Fuel Cell" Nanomaterials 12, no. 17: 2941. https://doi.org/10.3390/nano12172941






