Ultrathin Carbon-Coated Porous TiNb2O7 Nanosheets as Anode Materials for Enhanced Lithium Storage
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of TNO@C Nanosheets
2.2. Characterization of Materials
2.3. Electrochemical Measurements
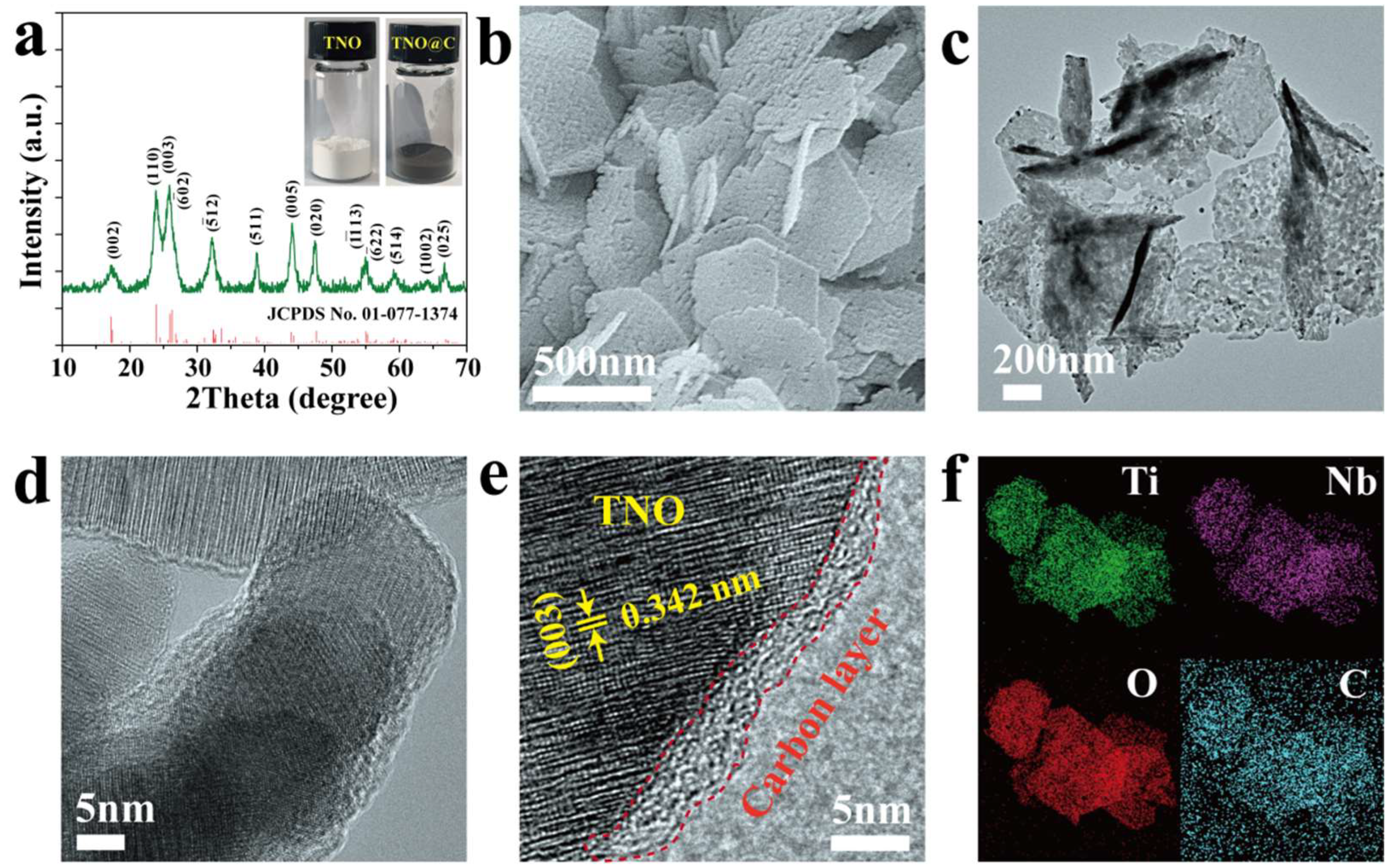
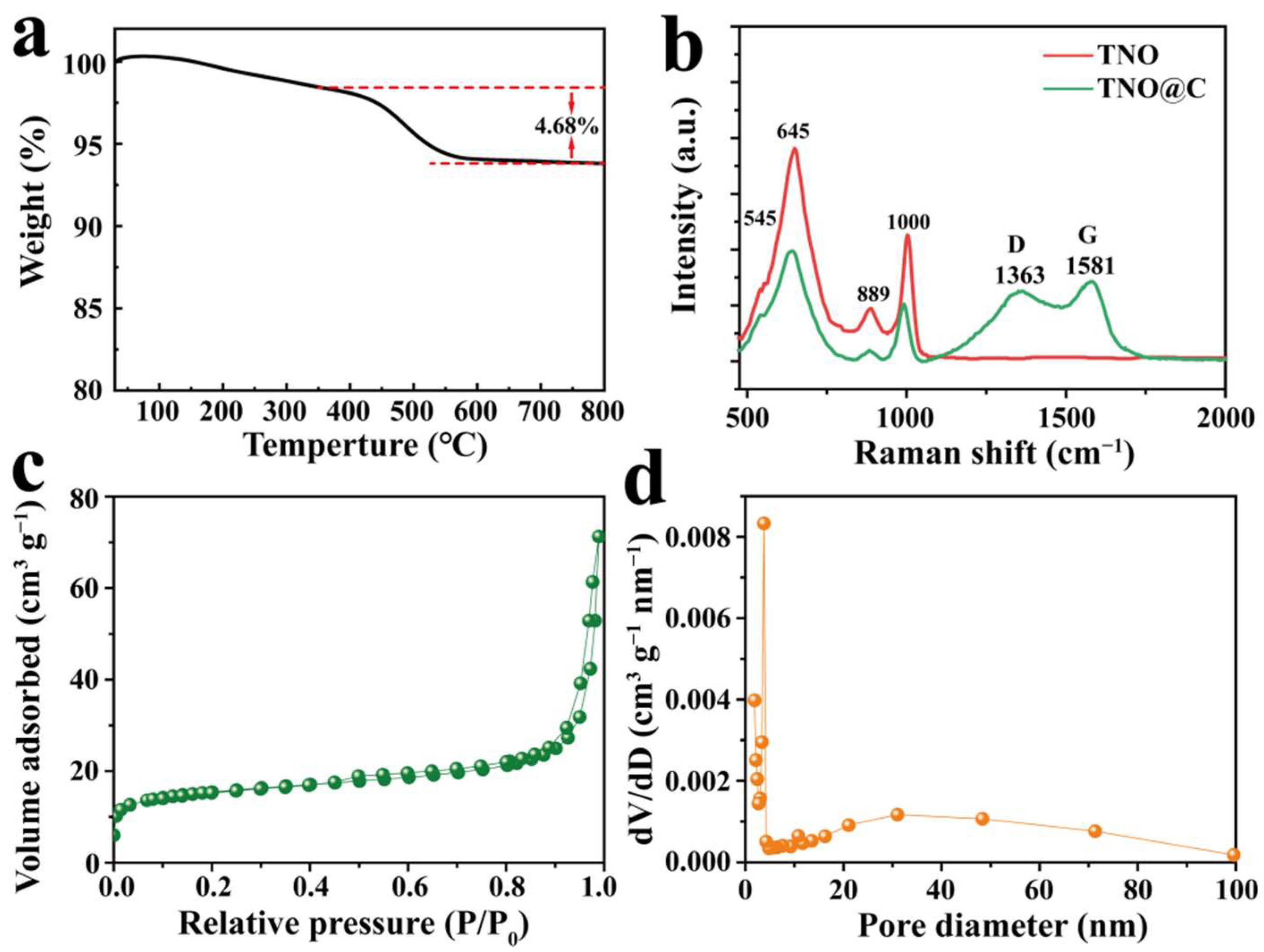
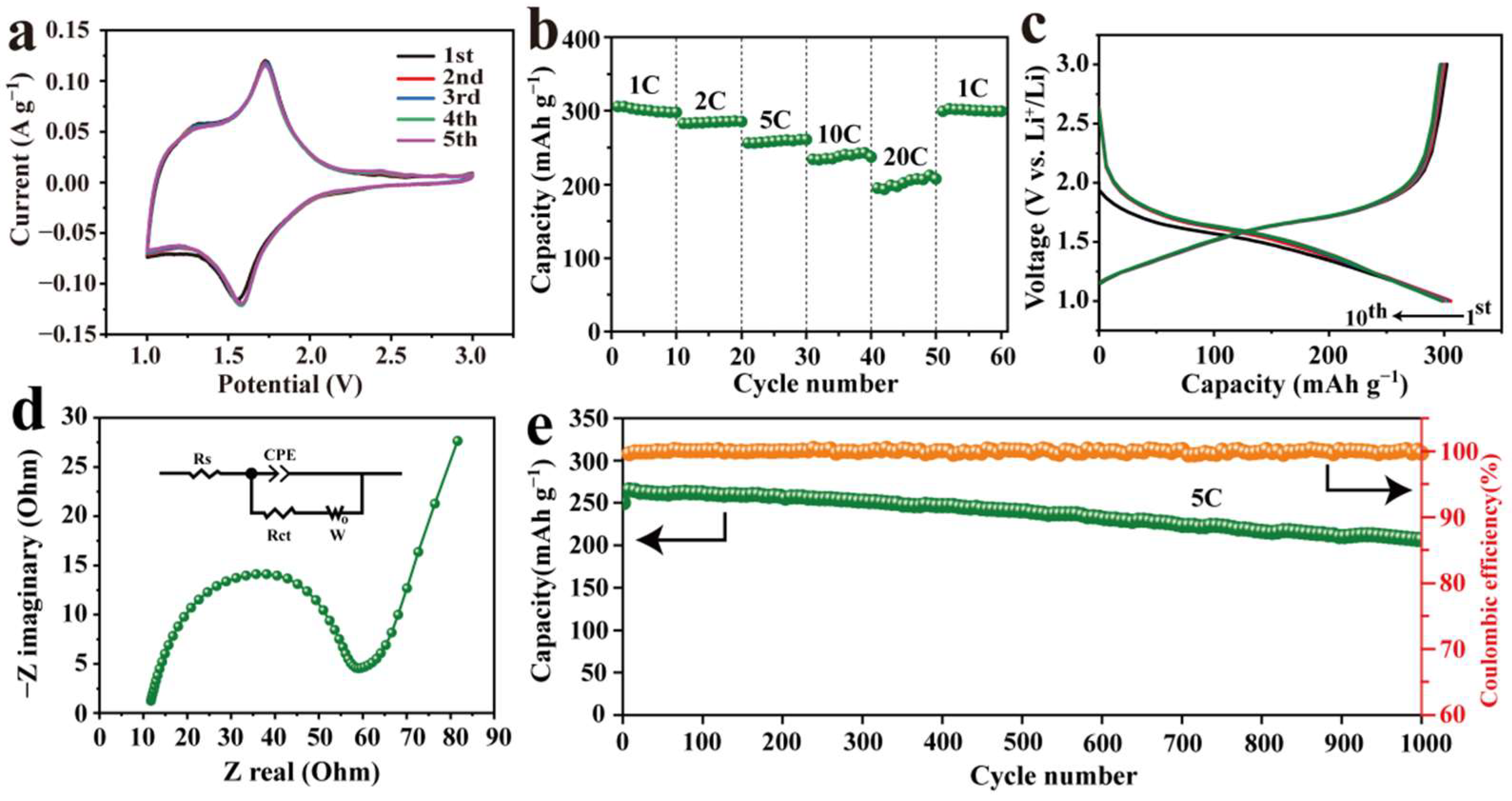
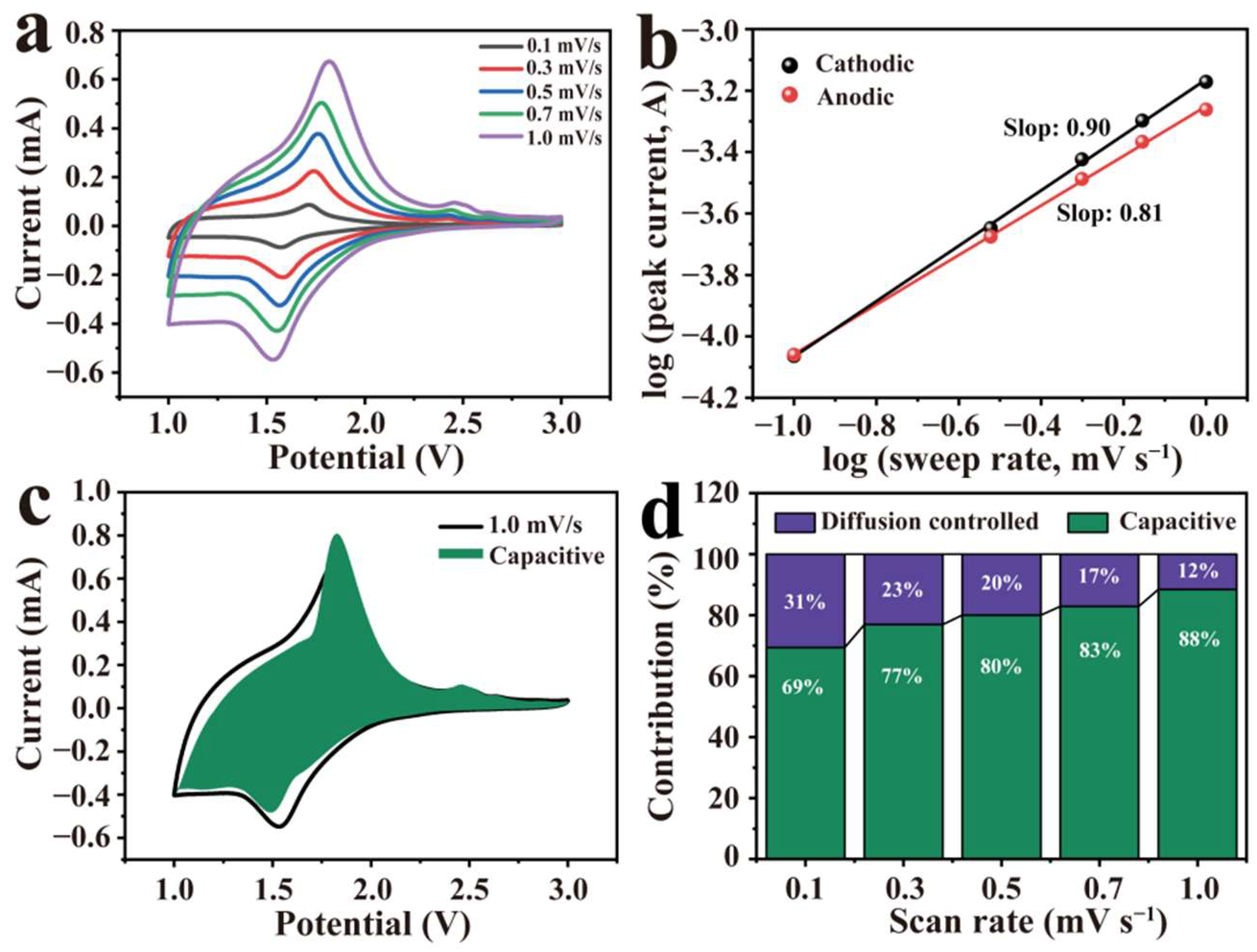
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, X.; Ran, F.; Huang, Y.; Zheng, R.; Yu, H.; Shu, J.; Xie, Y.; He, Y.B. Insight into the Synergistic Effect of N, S Co-Doping for Carbon Coating Layer on Niobium Oxide Anodes with Ultra-Long Life. Adv. Funct. Mater. 2021, 31, 2100311. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, J.; Wang, J.; Pathiranage, S.; Oncel, N.; Robert Ilango, P.; Zhang, X.; Mann, M.; Hou, X. In Situ Synthesis of Graphene-Coated Silicon Monoxide Anodes from Coal-Derived Humic Acid for High-Performance Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101645. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, H.; Liu, H.; Zhang, Q.; He, X.; Yu, S.; Petrova, V.; Feng, J.; Kostecki, R.; Liu, P.; et al. Achieving Fast and Durable Lithium Storage through Amorphous FeP Nanoparticles Encapsulated in Ultrathin 3D P-Doped Porous Carbon Nanosheets. ACS Nano 2020, 14, 9545–9561. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Dong, H.; Zhang, S.; Chen, X.; Sun, Y.; Gao, S.; Xu, J.; Wu, X.; Yuan, A.; Lu, W. Three-Dimensional Porous TiNb2O7/CNT-KB Composite Microspheres as Lithium-Ion Battery Anode Material. Chemelectrochem 2019, 6, 3959–3965. [Google Scholar] [CrossRef]
- Sun, R.; Liu, G.; Cao, S.; Dong, B.; Liu, X.; Hu, M.; Liu, M.; Duan, X. High rate capability performance of ordered mesoporous TiNb6O17 microsphere anodes for lithium ion batteries. Dalton. Trans. 2017, 46, 17061–17066. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.-K.; Lee, G.-W.; Haghighat-Shishavan, S.; Chung, K.Y.; Kim, K.-B. Polyol-mediated carbon-coated Li4Ti5O12 nanoparticle/graphene composites with long-term cycling stability for lithium and sodium ion storages. Chem. Eng. J. 2020, 385, 123984. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, J.; Xu, H.; Sun, S.; Xu, Y.; Zhou, M.; Gao, X.; Sun, Z. Plasma-Introduced Oxygen Defects Confined in Li4Ti5O12 Nanosheets for Boosting Lithium-Ion Diffusion. ACS Appl. Mater. Interfaces 2019, 11, 17384–17392. [Google Scholar] [CrossRef]
- Jiao, X.; Hao, Q.; Xia, X.; Yao, D.; Ouyang, Y.; Lei, W. Boosting long-cycle-life energy storage with holey graphene supported TiNb2O7 network nanostructure for lithium ion hybrid supercapacitors. J. Power Sources 2018, 403, 66–75. [Google Scholar] [CrossRef]
- Wang, H.; Qian, R.; Cheng, Y.; Wu, H.-H.; Wu, X.; Pan, K.; Zhang, Q. Micro/nanostructured TiNb2O7-related electrode materials for high-performance electrochemical energy storage: Recent advances and future prospects. J. Mater. Chem. A 2020, 8, 18425–18463. [Google Scholar] [CrossRef]
- Lin, C.; Yu, S.; Wu, S.; Lin, S.; Zhu, Z.; Li, J.; Lu, L. Ru0.01Ti0.99Nb2O7 as an intercalation-type anode material with a large capacity and high rate performance for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 8627–8635. [Google Scholar] [CrossRef]
- Gao, J.; Cheng, X.; Lou, S.; Ma, Y.; Zuo, P.; Du, C.; Gao, Y.; Yin, G. Self-doping Ti1-xNb2+xO7 anode material for lithium-ion battery and its electrochemical performance. J. Alloy. Compd. 2017, 728, 534–540. [Google Scholar] [CrossRef]
- Gao, J.; Cheng, X.; Lou, S.; Wu, X.; Ding, F.; Zuo, P.; Ma, Y.; Du, C.; Gao, Y.; Yin, G. Surface nitrided and carbon coated TiNb2O7 anode material with excellent performance for lithium-ion batteries. J. Alloy. Compd. 2020, 835, 155241. [Google Scholar] [CrossRef]
- Liu, K.; Wang, J.; Yang, J.; Zhao, D.; Chen, P.; Man, J.; Yu, X.; Wen, Z.; Sun, J. Interstitial and substitutional V5+-doped TiNb2O7 microspheres: A novel doping way to achieve high-performance electrodes. Chem. Eng. J. 2021, 407, 127190. [Google Scholar] [CrossRef]
- Hu, L.; Yang, X.; Chen, Y.; Wang, L.; Li, J.; Tang, Y.; Zhang, H. Facile Construction of Hierarchical TiNb2O7/rGO Nanoflower with Robust Charge Storage Properties for Li Ion Batteries via an Esterification Reaction. Front. Energy Res. 2021, 9, 13. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Liu, Y.; Zhu, H.; Wang, L.; Liu, Y.; Xue, M.; Li, B.; Tao, X. Oxygen vacancy regulated TiNb2O7 compound with enhanced electrochemical performance used as anode material in Li-ion batteries. Electrochim. Acta 2020, 330, 135299. [Google Scholar] [CrossRef]
- Yin, L.; Pham-Cong, D.; Jeon, I.; Kim, J.-P.; Cho, J.; Jeong, S.-Y.; Woo Lee, H.; Cho, C.-R. Electrochemical performance of vertically grown WS2 layers on TiNb2O7 nanostructures for lithium-ion battery anodes. Chem. Eng. J. 2020, 382, 122800. [Google Scholar] [CrossRef]
- Yang, Y.; Yue, Y.; Wang, L.; Cheng, X.; Hu, Y.; Yang, Z.; Zhang, R.; Jin, B.; Sun, R. Facile synthesis of mesoporous TiNb2O7/C microspheres as long-life and high-power anodes for lithium-ion batteries. Int. J. Hydrog. Energy 2020, 45, 12583–12592. [Google Scholar] [CrossRef]
- Uceda, M.; Chiu, H.; Zhou, J.; Gauvin, R.; Zaghib, K.; Demopoulos, G. Nanoscale assembling of graphene oxide with electrophoretic deposition leads to superior percolation network in Li-ion electrodes: TiNb2O7/rGO composite anodes. Nanoscale 2020, 12, 23092–23104. [Google Scholar] [CrossRef]
- Zhou, J.; Dong, H.; Chen, Y.; Ye, Y.; Xiao, L.; Deng, B.; Liu, J. Carbon-coated TiNb2O7 nanosheet arrays as self-supported high mass-loading anodes for flexible Li-ion batteries. Chem. Commun. 2021, 57, 1822–1825. [Google Scholar] [CrossRef]
- Beladi-Mousavi, S.; Ying, Y.; Plutnar, J.; Pumera, M. Bismuthene Metallurgy: Transformation of Bismuth Particles to Ultrahigh-Aspect-Ratio 2D Microsheets. Small 2020, 16, 2002037. [Google Scholar] [CrossRef]
- Beladi-Mousavi, S.; Plutnar, J.; Pumera, M. Near-Atomic-Thick Bismuthene Oxide Microsheets for Flexible Aqueous Anodes: Boosted Performance upon 3D→2D Transition. ACS Appl. Mater. Interfaces 2020, 12, 55936–55944. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.; Ruoff, R.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhao, T. Recent advances in inorganic 2D materials and their applications in lithium and sodium batteries. J. Mater. Chem. A 2017, 5, 3735–3758. [Google Scholar] [CrossRef]
- Tian, R.; Breshears, M.; Horvath, D.; Coleman, J. The Rate Performance of Two-Dimensional Material-Based Battery Electrodes May Not Be as Good as Commonly Believed. ACS Nano 2020, 14, 3129–3140. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Z.; Zhang, R.; Ding, Y.; Xie, H.; Liu, G.; Fan, Y.; Yang, Z.; Liu, X. Polydopamine-derived N-doped carbon-coated porous TiNb2O7 microspheres as anode materials with superior rate performance for lithium-ion batteries. Electrochim. Acta 2021, 368, 137623. [Google Scholar] [CrossRef]
- Qian, R.; Yang, C.; Ma, D.; Li, K.; Feng, T.; Feng, J.; Pan, J.H. Robust lithium storage of block copolymer-templated mesoporous TiNb2O7 and TiNb2O7@C anodes evaluated in half-cell and full-battery configurations. Electrochim. Acta 2021, 379, 138179. [Google Scholar] [CrossRef]
- Zhu, G.; Li, Q.; Che, R. Hollow TiNb2O7@C Spheres with Superior Rate Capability and Excellent Cycle Performance as Anode Material for Lithium-Ion Batteries. Chemistry 2018, 24, 12932–12937. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Zhang, S.; Liu, G.; Xie, H.; Ma, J. Design of well-defined porous Ti2Nb10O29/C microspheres assembled from nanoparticles as anode materials for high-rate lithium ion batteries. Electrochim. Acta 2018, 292, 759–768. [Google Scholar] [CrossRef]
- Shen, S.; Guo, W.; Xie, D.; Wang, Y.; Deng, S.; Zhong, Y.; Wang, X.; Xia, X.; Tu, J. A synergistic vertical graphene skeleton and S–C shell to construct high-performance TiNb2O7-based core/shell arrays. J. Mater. Chem. A 2018, 6, 20195–20204. [Google Scholar] [CrossRef]
- Liang, D.; Lu, Y.; Hu, L.; Wang, L.; Liang, S.; Liang, X.; Liu, L.; Xu, Z.; Han, C.; Liang, C. Mesoporous TiNb2O7 nanosheets anode with excellent rate capability and cycling performance in lithium ion half/full batteries. J. Power Sources 2022, 544, 231897. [Google Scholar] [CrossRef]
- Lou, S.; Cheng, X.; Zhao, Y.; Lushington, A.; Gao, J.; Li, Q.; Zuo, P.; Wang, B.; Gao, Y.; Ma, Y.; et al. Superior performance of ordered macroporous TiNb2O7 anodes for lithium ion batteries: Understanding from the structural and pseudocapacitive insights on achieving high rate capability. Nano Energy 2017, 34, 15–25. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, L.; Sun, R.; Chen, W.; Hu, M.; Liu, M.; Duan, X.; Zhang, T. Mesoporous TiNb2O7 microspheres as high performance anode materials for lithium-ion batteries with high-rate capability and long cycle-life. Electrochim. Acta 2018, 259, 20–27. [Google Scholar] [CrossRef]
- Babu, B.; Shaijumon, M. Studies on kinetics and diffusion characteristics of lithium ions in TiNb2O7. Electrochim. Acta 2020, 345, 136208. [Google Scholar] [CrossRef]
- Wang, D.; Shan, Z.; Tian, J.; Chen, Z. Understanding the formation of ultrathin mesoporous Li4Ti5O12 nanosheets and their application in high-rate, long-life lithium-ion anodes. Nanoscale 2019, 11, 520–531. [Google Scholar] [CrossRef]
- Hu, L.; Wu, F.; Lin, C.; Khlobystov, A.; Li, L. Graphene-modified LiFePO4 cathode for lithium ion battery beyond theoretical capacity. Nat. Commun. 2013, 4, 1687. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zou, B.; Zhang, C.; Ng, D.H.L.; El-Khodary, S.A.; Liu, X.; Li, G.; Qiu, J.; Zhao, Y.; Yang, S.; et al. Oxygen-Defective TiNb2O7-x Nanochains with Enlarged Lattice Spacing for High-Rate Lithium Ion Capacitor. Adv. Mater. Interfaces 2020, 7, 2000705. [Google Scholar] [CrossRef]
- Li, R.; Zhu, X.; Fu, Q.; Liang, G.; Chen, Y.; Luo, L.; Dong, M.; Shao, Q.; Lin, C.; Wei, R.; et al. Nanosheet-based Nb12O29 hierarchical microspheres for enhanced lithium storage. Chem. Commun. 2019, 55, 2493–2496. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, D.; Lu, Y.; Zhou, N.; Xu, Z. Ultrathin Carbon-Coated Porous TiNb2O7 Nanosheets as Anode Materials for Enhanced Lithium Storage. Nanomaterials 2022, 12, 2943. https://doi.org/10.3390/nano12172943
Liang D, Lu Y, Zhou N, Xu Z. Ultrathin Carbon-Coated Porous TiNb2O7 Nanosheets as Anode Materials for Enhanced Lithium Storage. Nanomaterials. 2022; 12(17):2943. https://doi.org/10.3390/nano12172943
Chicago/Turabian StyleLiang, Dewei, Yu Lu, Ningning Zhou, and Zezhong Xu. 2022. "Ultrathin Carbon-Coated Porous TiNb2O7 Nanosheets as Anode Materials for Enhanced Lithium Storage" Nanomaterials 12, no. 17: 2943. https://doi.org/10.3390/nano12172943





