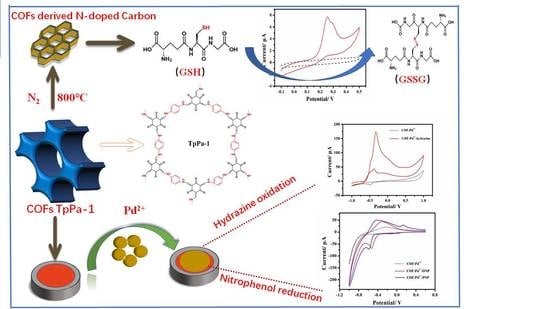
Covalent Organic Frameworks-TpPa-1 as an Emerging Platform for Electrochemical Sensing
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Solutions
2.2. Apparatus
2.3. Electrode Preparation and Modification
2.3.1. Preparation of Pd2+/COFs/GCE
2.3.2. Preparation of PCs/CPE
3. Results and Discussion
3.1. Electrochemical Sensing of Hydrazine
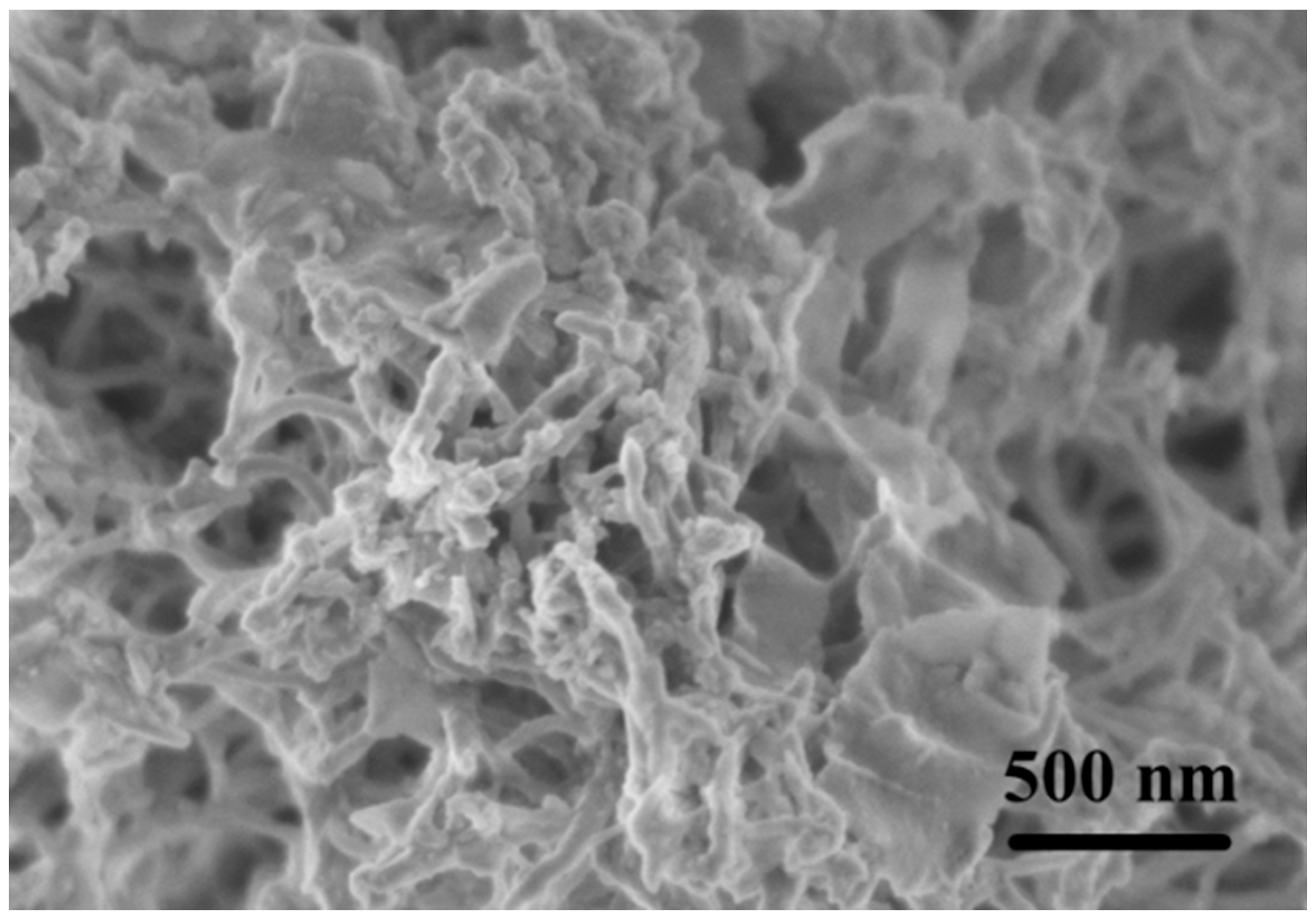
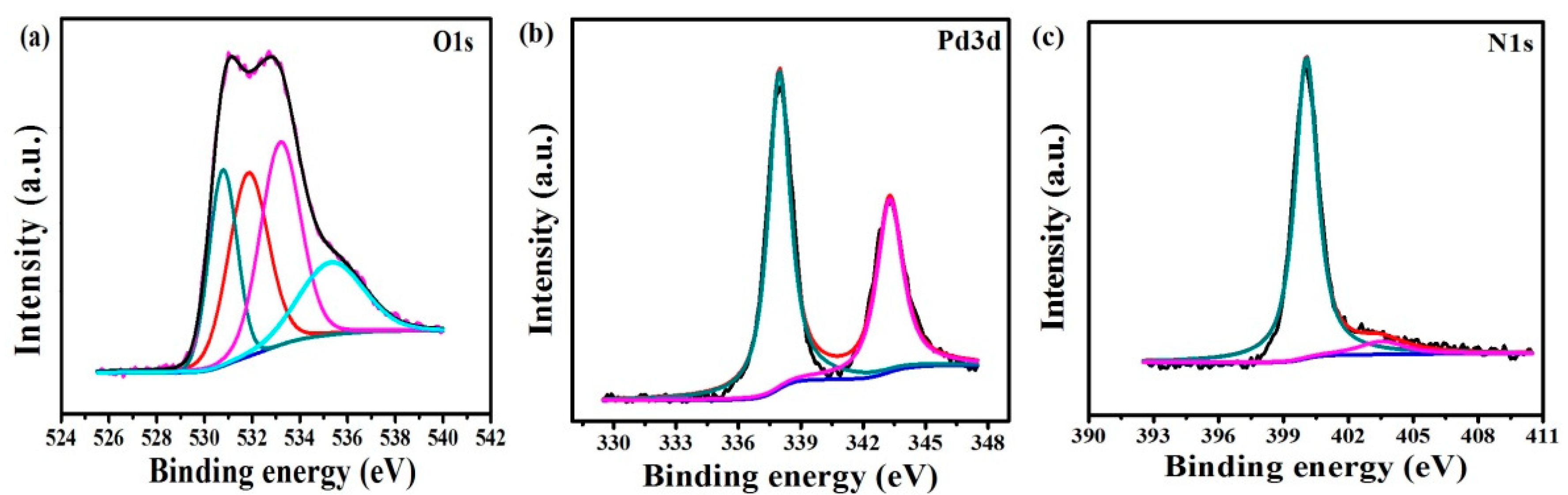
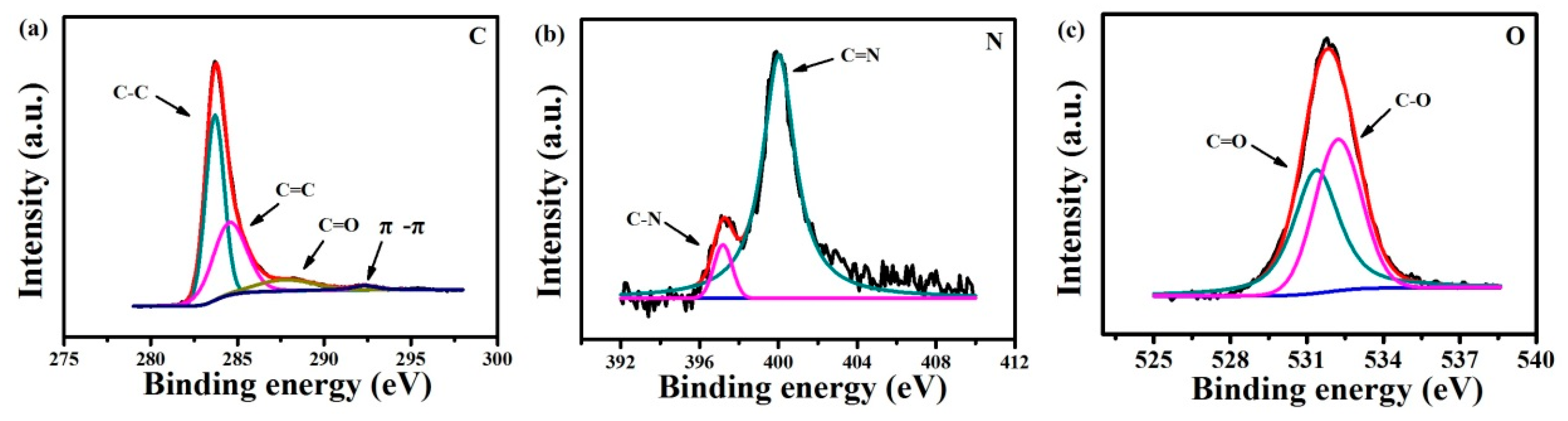
3.1.1. Characterization of Pd2+/COFs/GCE
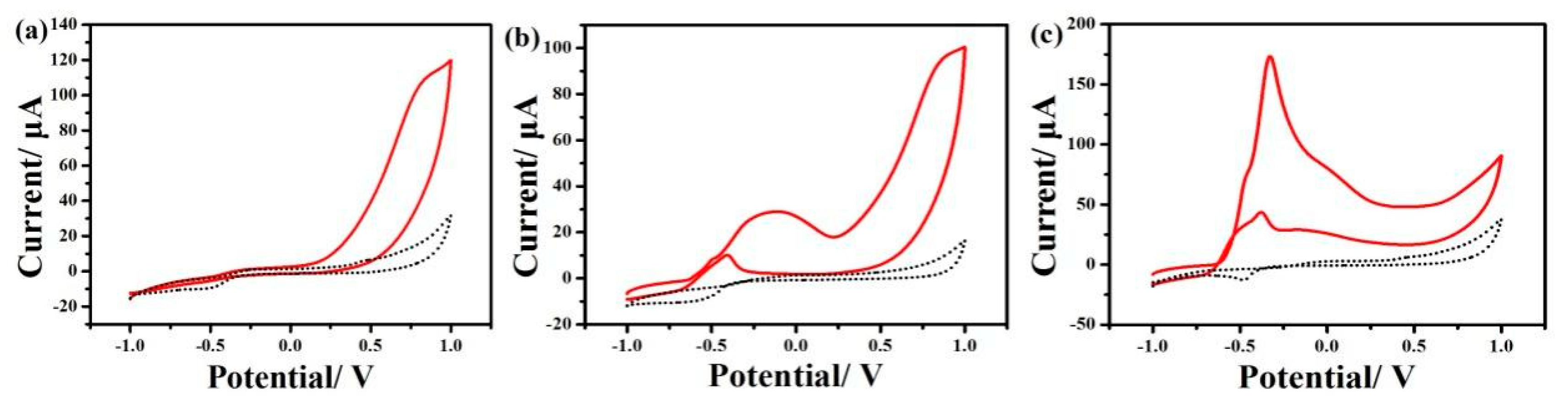
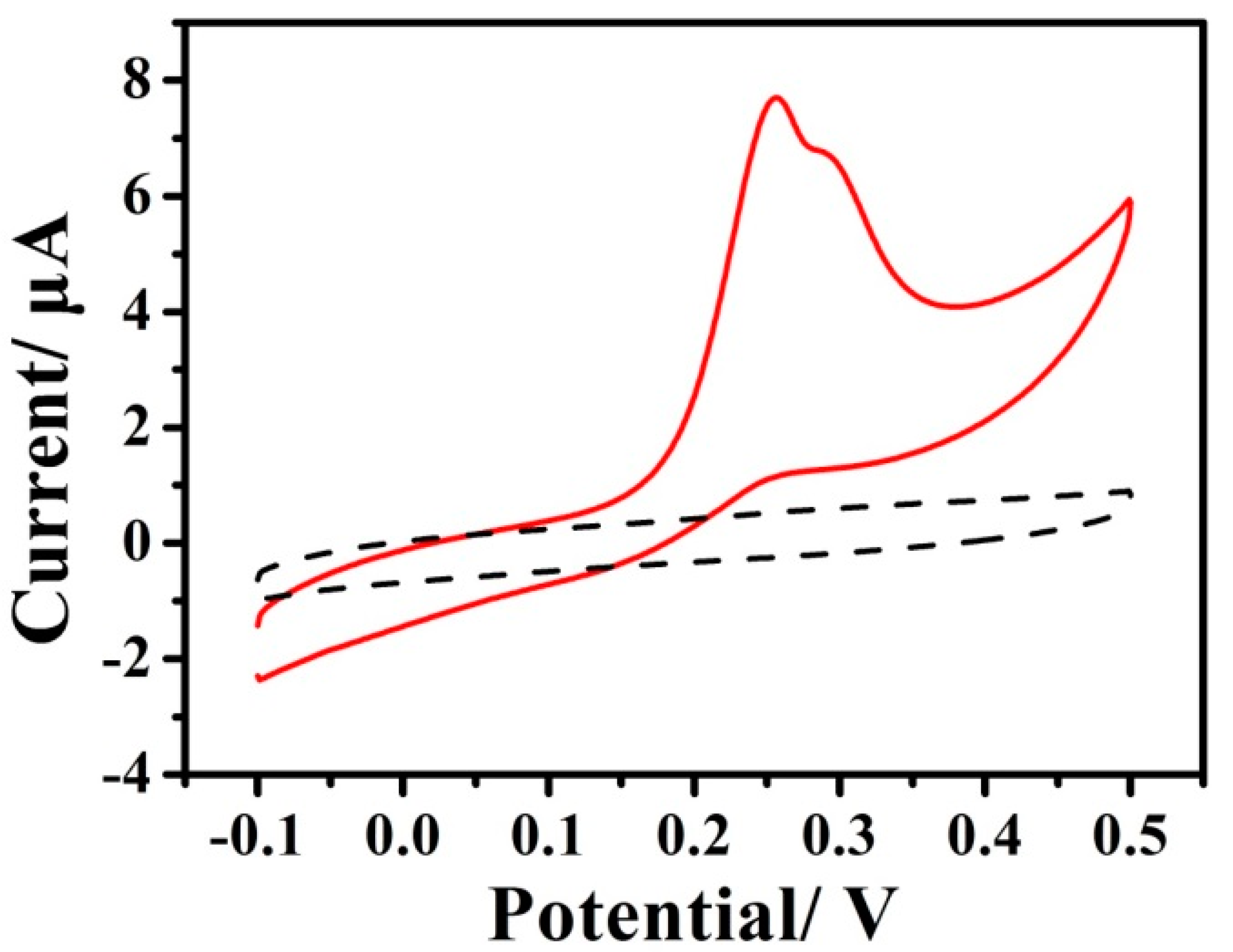
3.1.2. Electrochemical Oxidation of Hydrazine
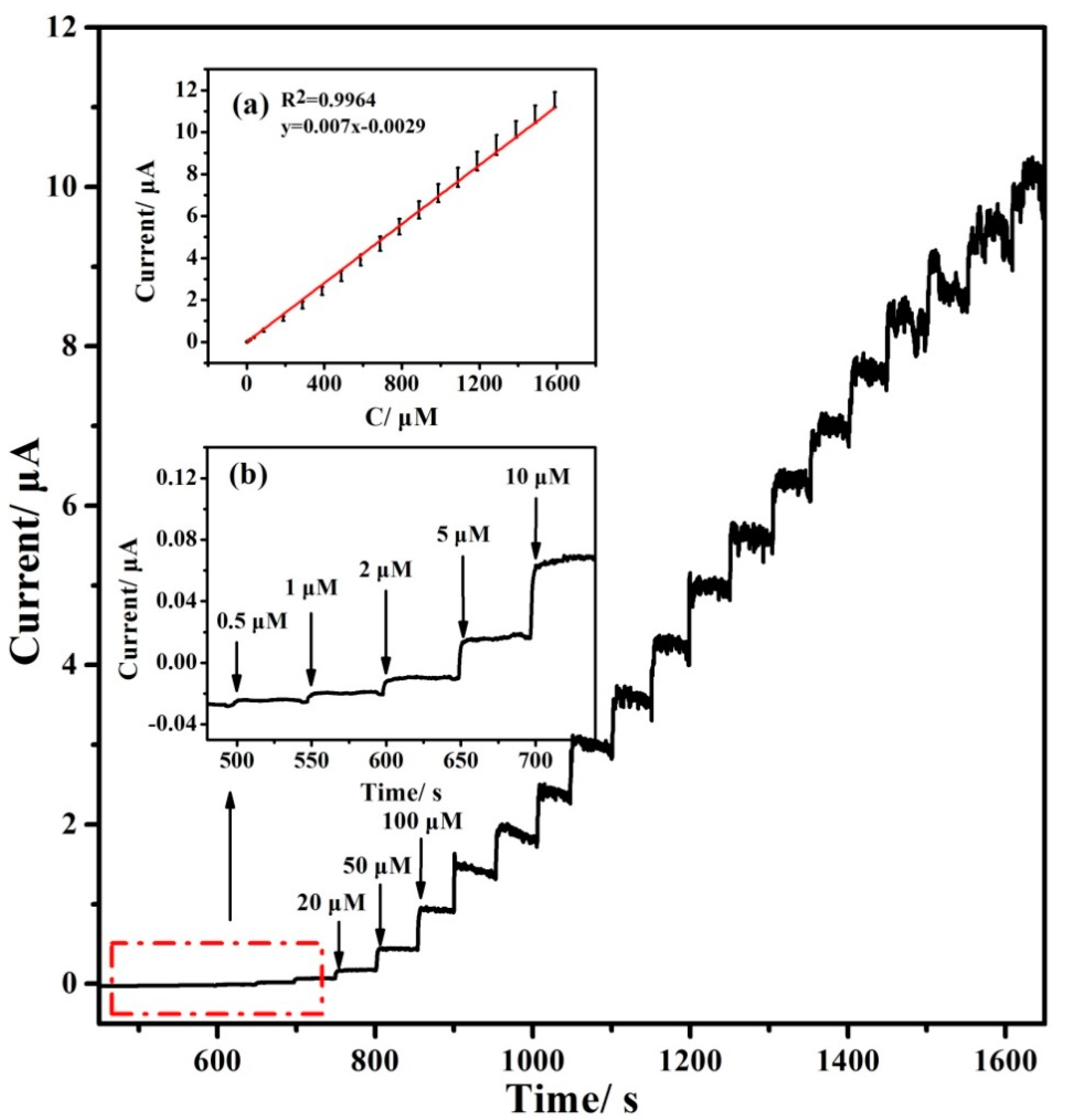
3.1.3. Analytical Performances for Hydrazine
3.1.4. Sample Analysis of Hydrazine
3.2. Electrochemical Sensing of ONP and PNP
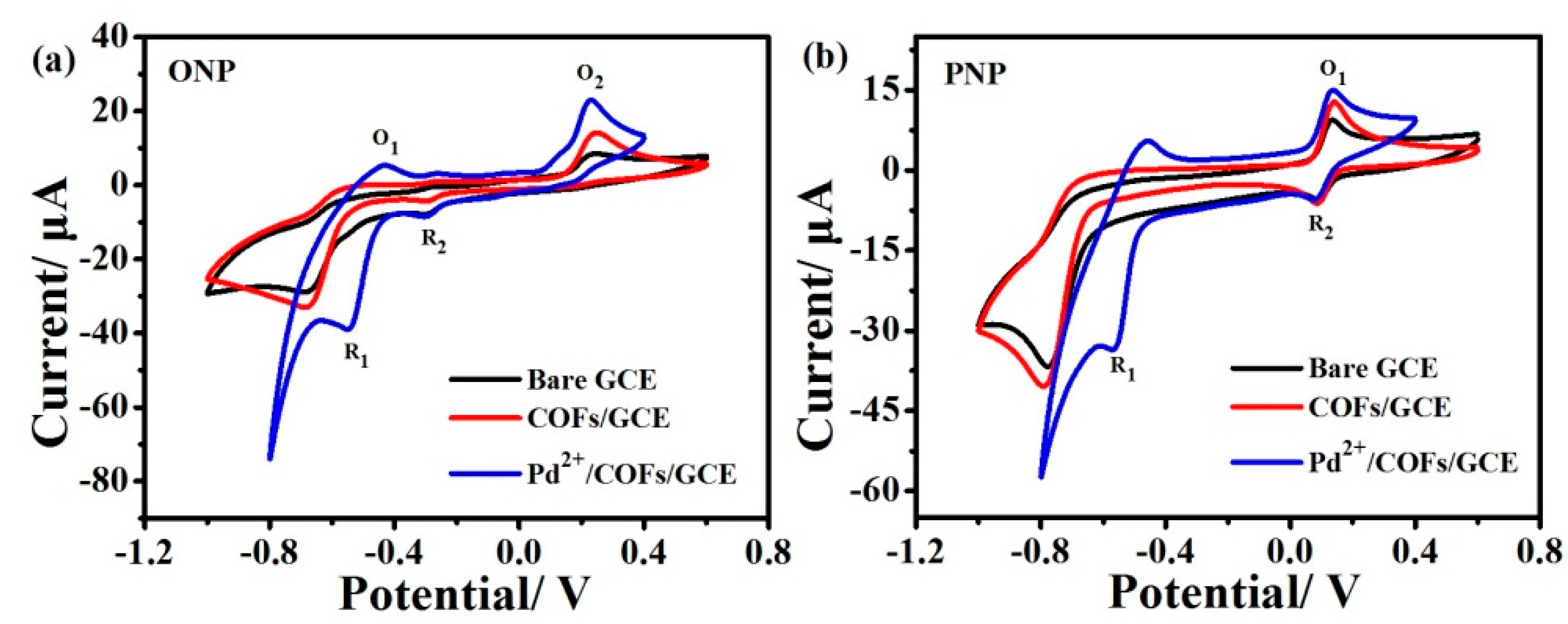
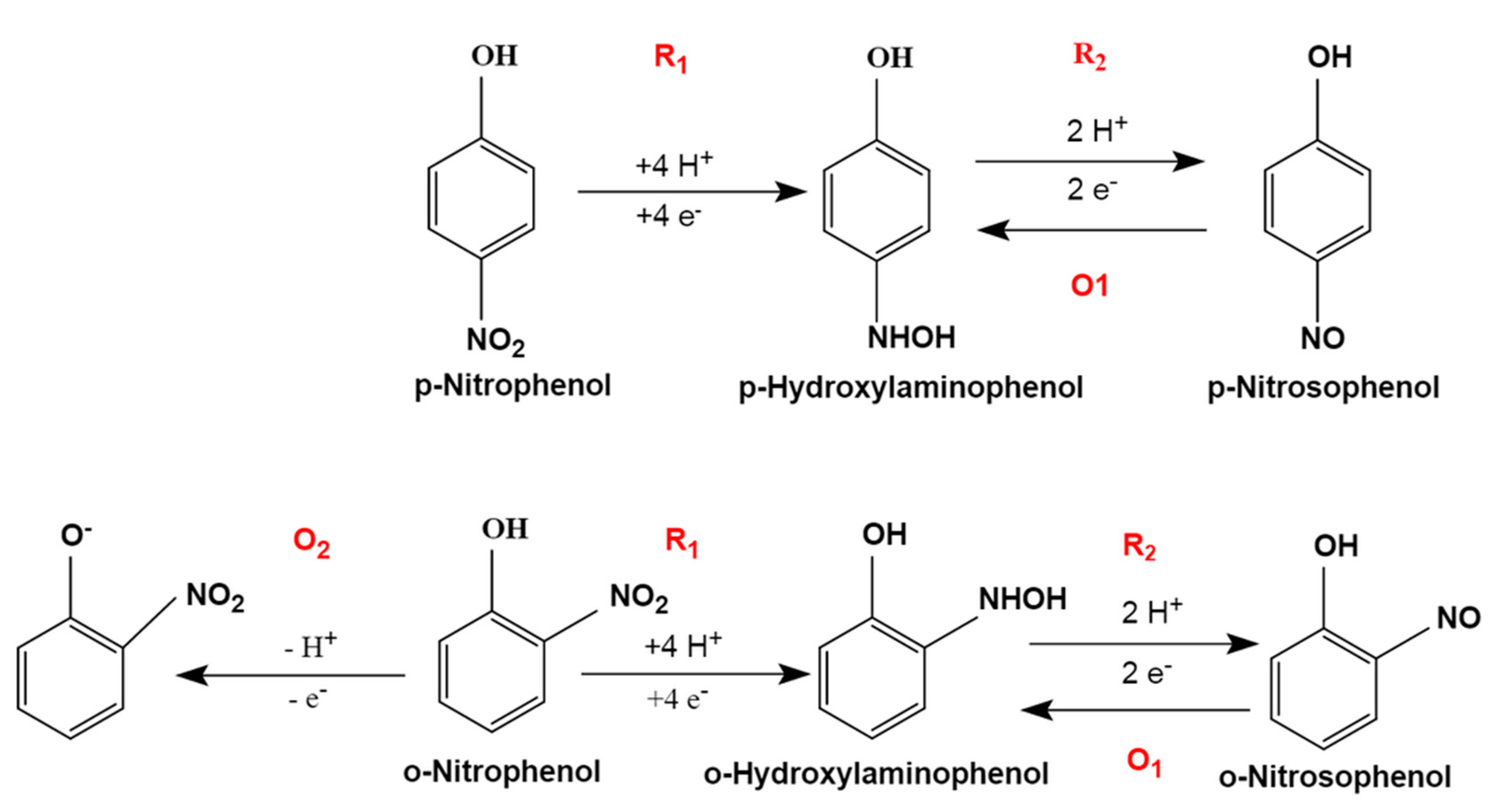
3.2.1. Electrochemical Behaviors of ONP and PNP
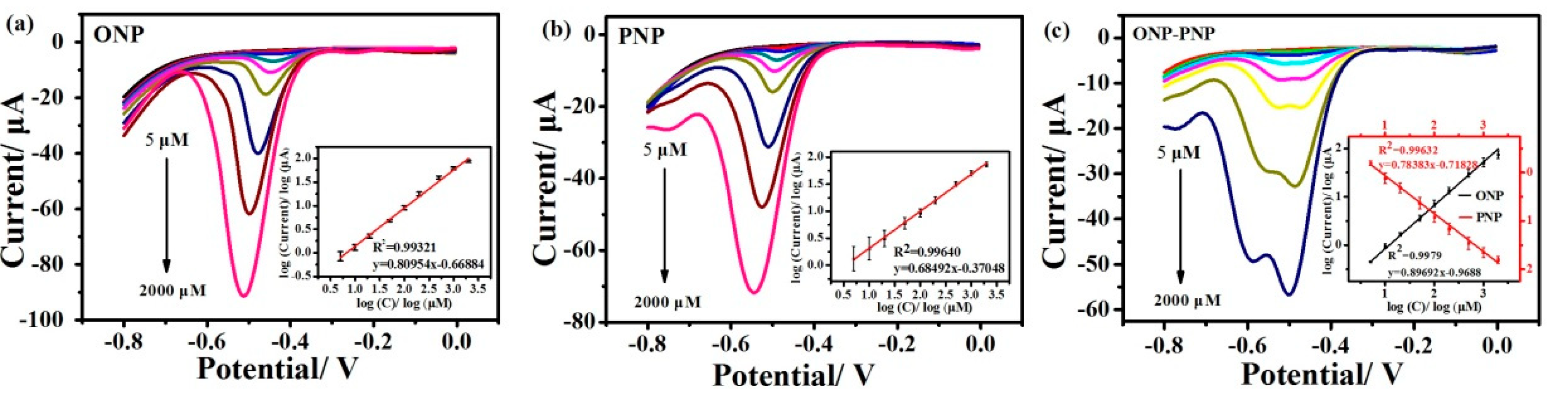
3.2.2. Analytical Performances for ONP and PNP
3.3. Electrochemical Sensing of GSH
3.3.1. Characterization of PC
3.3.2. Electrochemical Oxidation of GSH
3.3.3. Analytical Performances for GSH
3.3.4. Sample Analysis of GSH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.Y.; Weng, L.Y.; Yang, C. A review on nanomaterial-based electrochemical sensors for H2O2, H2S and NO inside cells or released by cells. Microchim. Acta 2017, 184, 1267–1283. [Google Scholar] [CrossRef]
- Liu, C.S.; Li, J.J.; Pang, H. Metal-organic framework-based materials as an emerging platform for advanced electrochemical sensing. Coordin. Chem. Rev. 2020, 410, 213222. [Google Scholar] [CrossRef]
- Yan, Y.; He, T.; Zhao, B.; Qi, K.; Liu, H.F.; Xia, B.Y. Metal/covalent-organic frameworks-based electrocatalysts for water splitting. J. Mater. Chem. A 2018, 6, 15905–15926. [Google Scholar] [CrossRef]
- Yuan, B.Q.; Liu, D.; Yin, H.J.; Zhang, D.J. Materials for Electroanalysis Based on Advanced Frameworks. Front. Chem. 2021, 3, 638338. [Google Scholar] [CrossRef]
- Chuang, C.H.; Kung, C.W. Metal-organic frameworks toward electrochemical sensors: Challenges and opportunities. Electroanalysis 2020, 32, 1885–1895. [Google Scholar] [CrossRef]
- Lu, M.X.; Deng, Y.J.; Luo, Y.; Lv, J.P.; Li, T.B.; Xu, J.; Chen, S.W.; Wang, J.Y. Graphene aerogel–metal–organic framework-based electrochemical method for simultaneous detection of multiple heavy-metal ions. Anal. Chem. 2019, 91, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Waterhouse, G.I.; Xu, L.H.; Qiao, X.G.; Xu, Z.X. Three-dimensional electrochemical sensor with covalent organic framework decorated carbon nanotubes signal amplification for the detection of furazolidone. Sens. Actuators B Chem. 2020, 321, 128501. [Google Scholar] [CrossRef]
- He, W.H.; Ifraemov, R.; Raslin, A.; Hod, I. Room-Temperature Electrochemical Conversion of Metal-Organic Frameworks into Porous Amorphous Metal Sulfides with Tailored Composition and Hydrogen Evolution Activity. Adv. Funct. Mater. 2018, 28, 1707244. [Google Scholar] [CrossRef]
- Beitollahi, H.; Movahedifar, F.; Tajik, S.; Jahani, S. A review on the effects of introducing CNTs in the modification process of electrochemical sensors. Electroanalysis 2019, 31, 1195–1203. [Google Scholar] [CrossRef]
- Hu, X.W.; Long, Y.; Fan, M.Y.; Yuan, M.; Zhao, H.; Ma, J.T.; Dong, Z.P. Two-dimensional covalent organic frameworks as self-template derived nitrogen-doped carbon nanosheets for eco-friendly metal-free catalysis. Appl. Catal. B Environ. 2019, 244, 25–35. [Google Scholar] [CrossRef]
- Yang, L.; Cai, Z.; Hao, L.; Ran, L.L.; Xu, X.; Dai, Y.; Pan, S.Y.; Jing, B.J.; Zhou, J.L. Increase of structural defects by N doping in MoS2 cross-linked with N-doped CNTs/carbon for enhancing charge transfer in oxygen reduction. Electrochim. Acta 2018, 283, 448–458. [Google Scholar] [CrossRef]
- Sun, F.; Liu, X.Y.; Wu, H.B.; Wang, L.J.; Gao, J.H.; Li, H.X.; Lu, Y.F. In situ high-level nitrogen doping into carbon nanospheres and boosting of capacitive charge storage in both anode and cathode for a high-energy 4.5 V full-carbon lithium-ion capacitor. Nano Lett. 2018, 18, 3368–3376. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, D.G.; Guo, X.D.; Luo, Z.Y.; Tao, L.Y.; Ren, J.J.; Zhang, Y. Fabrication of a Covalent Organic Framework-Based Heterojunction via Coupling with ZnAgInS Nanosphere with High Photocatalytic Activity. Langmuir 2022, 38, 4680–4691. [Google Scholar] [CrossRef]
- Zhong, X.; Lu, Z.P.; Liang, W.; Hu, B.W. The magnetic covalent organic framework as a platform for high-performance extraction of Cr (VI) and bisphenol a from aqueous solution. J. Hazard. Mater. 2020, 393, 122353. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.N.; Zhang, J.X.; Shao, Y.H.; Jiang, H.; Liu, Y.F.; Chen, R.Z. Pd nanoparticles loaded on two-dimensional covalent organic frameworks with enhanced catalytic performance for phenol hydrogenation. Ind. Eng. Chem. Res. 2020, 59, 18489–18499. [Google Scholar] [CrossRef]
- Li, Z.D.; Zhang, H.Q.; Xiong, X.H.; Luo, F. U (VI) adsorption onto covalent organic frameworks-TpPa-1. J. Solid State Chem. 2019, 277, 484–492. [Google Scholar] [CrossRef]
- Choi, H.C.; Shim, M.; Bangsaruntip, S. Spontaneous reduction of metal ions on the sidewalls of carbon nanotubes. J. Am. Chem. Soc. 2002, 124, 9058–9059. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Li, R.; Zhao, Y.J.; Zhe, T.T.; Bu, T.; Liu, Y.N.; Sun, X.Y.; Hu, H.F.; Zhang, M.; Zheng, X.H. Surface morphology-controllable magnetic covalent organic frameworks: A novel electrocatalyst for simultaneously high-performance detection of p-nitrophenol and o-nitrophenol. Talanta 2020, 219, 121255. [Google Scholar] [CrossRef]
- Afzali, F.; Zavar, M.H.A.; Rounaghi, G.; Ashraf, N.J.E.A. Gold digital versatile disc platform modified with nano-porous mercury/gold amalgam as a solid-state disposable electrochemical sensor for detection of para-nitrophenol. Electrochim. Acta 2016, 209, 654–660. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Cao, S.; Yang, J.X.; Li, J.; Shi, K.; Li, X.K. Preparation of oxygen-rich hierarchical porous carbon for supercapacitors through the co-carbonization of pitch and biomass. Diamond Relat. Mater. 2019, 96, 118–125. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H.J.B. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Harfield, J.C.; Batchelor-McAuley, C.; Compton, R.G. Electrochemical determination of glutathione: A review. Analyst 2012, 137, 2285–2296. [Google Scholar] [CrossRef] [PubMed]
- Hanko, M.; Švorc, Ľ.; Planková, A.; Mikuš, P. Overview and recent advances in electrochemical sensing of glutathione—A review. Anal. Chim. Acta 2019, 1062, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Nesakumar, N.; Berchmans, S.; Alwarappan, S. Chemically modified carbon based electrodes for the detection of reduced glutathione. Sens. Actuators B Chem. 2018, 264, 448–466. [Google Scholar] [CrossRef]
- Yuan, B.Q.; Xu, C.Y.; Zhang, R.C.; Lv, D.H.; Li, S.J.; Zhang, D.J.; Liu, L.; Fernandez, C. Bioelectronics, Glassy carbon electrode modified with 7, 7, 8, 8-tetracyanoquinodimethane and graphene oxide triggered a synergistic effect: Low-potential amperometric detection of reduced glutathione. Biosens. Bioelectron. 2017, 96, 1–7. [Google Scholar] [CrossRef]
- Lee, P.; Compton, R.G. Selective electrochemical detection of thiol biomarkers in saliva using multiwalled carbon nanotube screen-printed electrodes. Sens. Actuators B Chem. 2015, 209, 983–988. [Google Scholar] [CrossRef]
- Zhou, M.; Ding, J.; Guo, L.P.; Shang, Q.K. Electrochemical behavior of L-cysteine and its detection at ordered mesoporous carbon-modified glassy carbon electrode. Anal. Chem. 2007, 79, 5328–5335. [Google Scholar] [CrossRef]
- Yuan, B.Q.; Zeng, X.Y.; Xu, C.Y.; Liu, L.; Ma, Y.H.; Zhang, D.J.; Fan, Y. Electrochemical modification of graphene oxide bearing different types of oxygen functional species for the electro-catalytic oxidation of reduced glutathione. Sens. Actuators B Chem. 2013, 184, 15–20. [Google Scholar] [CrossRef]
- Wu, S.; Lan, X.Q.; Huang, F.F.; Luo, Z.Z.; Ju, H.X.; Meng, C.G.; Duan, C.Y. Selective electrochemical detection of cysteine in complex serum by graphene nanoribbon. Biosens. Bioelectron. 2012, 32, 293–296. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.D.; Liu, M.X.; Zhou, H.; Zhang, Z.C.; Yu, C.; Wang, C.Y.; Wang, G.X. Improved the specificity of peroxidase-like carbonized polydopamine nanotubes with high nitrogen doping for glutathione detection. Sens. Actuators B Chem. 2021, 341, 129987. [Google Scholar] [CrossRef]
- Wang, Y.; de Carvalho, N.A.; Tan, S.S.; Gilbertson, L.M. Leveraging electrochemistry to uncover the role of nitrogen in the biological reactivity of nitrogen-doped graphene. Environ. Sci. Nano 2019, 6, 3525–3538. [Google Scholar] [CrossRef]
- Ndamanisha, J.C.; Bai, J.; Qi, B.; Guo, L.P. Application of electrochemical properties of ordered mesoporous carbon to the determination of glutathione and cysteine. Anal. Biochem. 2009, 386, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, T.; Iguchi, H.; Kikuchi, K.I. Separation of glutathione and its related amino acids by nanofiltration. Biochem. Eng. J. 2004, 19, 165–170. [Google Scholar] [CrossRef]
- Wang, Y.; Basdogan, Y.; Zhang, T.Y.; Lankone, R.S.; Wallace, A.N.; Fairbrother, D.H.; Keith, J.A.; Gilbertson, L.M. Interfaces, Unveiling the synergistic role of oxygen functional groups in the graphene-mediated oxidation of glutathione. ACS Appl. Mater. Interfaces 2020, 12, 45753–45762. [Google Scholar] [CrossRef]
- Wang, L.; Teng, Q.Q.; Sun, X.T.; Chen, Y.T.; Wang, Y.M.; Wang, H.; Zhang, Y.F. Facile synthesis of metal-organic frameworks/ordered mesoporous carbon composites with enhanced electrocatalytic ability for hydrazine. J. Colloid Interface Sci. 2018, 512, 127–133. [Google Scholar] [CrossRef]
- Wang, L.; Meng, T.J.; Jia, H.X.; Feng, Y.; Gong, T.; Wang, H.; Zhang, Y.F. Electrochemical study of hydrazine oxidation by leaf-shaped copper oxide loaded on highly ordered mesoporous carbon composite. J Colloid Interface Sci. 2019, 549, 98–104. [Google Scholar] [CrossRef]
- Gharani, M.; Bahari, A.; Ghasemi, S. Preparation of MoS2-reduced graphene oxide/Au nanohybrid for electrochemical sensing of hydrazine. J. Mater. Sci. Mater. Electron. 2021, 32, 7765–7777. [Google Scholar] [CrossRef]
- Yao, Y.Q.; Han, X.H.; Yang, X.H.; Zhao, J.; Chai, C.P. Detection of Hydrazine at MXene/ZIF-8 Nanocomposite Modified Electrode. Chin. J. Chem. 2021, 39, 330–336. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zheng, J.B. Amperometric hydrazine sensor based on the use of a gold nanoparticle-modified nanocomposite consisting of porous polydopamine, multiwalled carbon nanotubes and reduced graphene oxide. Mikrochim. Acta. 2020, 187, 89. [Google Scholar] [CrossRef]
- Vishnu, N.; Kumar, A.S.; Badhulika, S. Selective in-situ derivatization of intrinsic nickel to nickel hexacyanoferrate on carbon nanotube and its application for electrochemical sensing of hydrazine. J. Electroanal. Chem. 2019, 837, 60–66. [Google Scholar] [CrossRef]
- Li, S.; Feng, W.S.; Gao, X.H.; Guo, A.M.; Li, H.J. Copper-based materials derived from metal-organic frameworks for electrochemical sensing of hydrazine. Micro Nano Lett. 2021, 16, 478–483. [Google Scholar] [CrossRef]
- Asadi, F.; Azizi, S.N.; Ghasemi, S. Preparation of Ag nanoparticles on nano cobalt-based metal organic framework (ZIF-67) as catalyst support for electrochemical determination of hydrazine. J. Mater. Sci. Mater. Electron. 2019, 30, 5410–5420. [Google Scholar] [CrossRef]
- Avanes, A.; Hasanzadeh-Karamjavan, M.; Shokri-Jarcheloo, G. Electrocatalytic oxidation and amperometric determination of hydrazine using a carbon paste electrode modified with beta-nickel hydroxide nanoplatelets. Mikrochim Acta 2019, 186, 441. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.Q.; Dong, Y.H.; Sheng, Q.L.; Zheng, J.B. A high-performance non-enzymatic electrochemical hydrazine sensor based on NiCo2S4 porous sphere. Talanta 2019, 198, 23–29. [Google Scholar] [CrossRef]
- Ghasemi, S.; Hosseini, S.R.; Hasanpoor, F.; Nabipour, S. Amperometric hydrazine sensor based on the use of Pt-Pd nanoparticles placed on reduced graphene oxide nanosheets. Mikrochim. Acta 2019, 186, 601. [Google Scholar] [CrossRef]
- Maleki, A.; Rezaee, R.; Daraei, H.; Shahmoradi, B.; Amini, N. Fabrication of a sensitive electrochemical sensor to environmental pollutant of hydrazine in real water samples based on synergistic catalysis of Ag@C core–shell and polyalizarin yellow R. J. Alloys Compd. 2018, 763, 997–1004. [Google Scholar] [CrossRef]
- Shahid, M.M.; Rameshkumar, P.; Basirunc, W.J.; Wijayantha, U.; Chiu, W.S.; Khiew, P.S.; Huang, N.M. An electrochemical sensing platform of cobalt oxide@gold nanocubes interleaved reduced graphene oxide for the selective determination of hydrazine. Electrochim. Acta 2018, 259, 606–616. [Google Scholar] [CrossRef]
- Mousavi-Majd, A.; Ghasemi, S.; Hosseini, S.R. Zeolitic imidazolate framework derived porous ZnO/Co3O4 incorporated with gold nanoparticles as ternary nanohybrid for determination of hydrazine. J. Alloys Compd. 2022, 896, 162922. [Google Scholar] [CrossRef]
- Vinoth, S.; Sampathkumar, P.; Giribabu, K.; Pandikumar, A. Ultrasonically assisted synthesis of barium stannate incorporated graphitic carbon nitride nanocomposite and its analytical performance in electrochemical sensing of 4-nitrophenol, Ultrasonics Sonochemistry. Ultrason. Sonochem. 2020, 62, 104855. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Zhao, J.; Li, S.H.; Guo, M.J.; Fan, Z. An electrochemical sensor for the detection of p-nitrophenol based on a cyclodextrin-decorated gold nanoparticle-mesoporous carbon hybrid. Analyst 2019, 144, 4400–4406. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A.; Alam, M.; Ali, M.A. Nanostructured CeO2: Ag platform for electrochemically sensitive detection of nitrophenol. Colloids Surf. A. 2021, 613, 126116. [Google Scholar] [CrossRef]
- Suresh, R.; Giribabu, K.; Manigandan, R.; Kumar, S.P.; Munusamy, S.; Muthamizh, S.; Narayanan, V. Polyaniline Nanorods: Synthesis, Characterization, and Application for the Determination ofpara-Nitrophenol. Anal. Lett. 2015, 49, 269–281. [Google Scholar] [CrossRef]
- Alam, M.M.; Asiri, A.M.; Rahman, M.M. Electrochemical detection of 2-Nitrophenol using a glassy carbon electrode modified with BaO Nanorods. Chem. Asian J. 2021, 16, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Nejati, K.; Asadpour-Zeynali, K.; Rezvani, Z.; Peyghami, R. Determination of 2-nitrophenol by electrochemical synthesized Mg/Fe layered double hydroxide sensor. Int. J. Electrochem. Sci. 2014, 9, 5222–5234. [Google Scholar]
- Yin, H.S.; Zhou, Y.L.; Ai, S.Y.; Cui, L.; Zhu, L.S. Electrochemical determination of 2-Nitrophenol in Water Samples using Mg-Al-SDS Hydrotalcite-Like Clay modified glassy carbon electrode. Electroanalysis 2010, 22, 1136–1142. [Google Scholar] [CrossRef]
- Li, J.H.; He, L.Z.; Jiang, J.B.; Xu, Z.F.; Liu, M.Q.; Liu, X.; Tong, H.X.; Liu, Z.; Qian, D. Facile syntheses of bimetallic Prussian blue analogues (KxM [Fe(CN)6]•nH2O, M = Ni, Co, and Mn) for electrochemical determination of toxic 2-nitrophenol. Electrochim. Acta 2020, 353, 136579. [Google Scholar] [CrossRef]
- Chu, L.; Han, L.; Zhang, X.L. Electrochemical simultaneous determination of nitrophenol isomers at nano-gold modified glassy carbon electrode. J. Appl. Electrochem. 2011, 41, 687–694. [Google Scholar] [CrossRef]
- Zhang, J.J.; Cui, S.Q.; Ding, Y.P.; Yang, X.X.; Guo, K.; Zhao, J.T. Bioelectronics, Two-dimensional mesoporous ZnCo2O4 nanosheets as a novel electrocatalyst for detection of o-nitrophenol and p-nitrophenol. Biosens. Bioelectron. 2018, 112, 177–185. [Google Scholar] [CrossRef]
- Hryniewicz, B.M.; Orth, E.S.; Vidotti, M. Enzymeless PEDOT-based electrochemical sensor for the detection of nitrophenols and organophosphates. Sens. Actuators B Chem. 2018, 257, 570–578. [Google Scholar] [CrossRef]
- Wu, W.T.; Chen, X.; Jiao, Y.T.; Fan, W.T.; Liu, Y.L.; Huang, W.H. Versatile Construction of Biomimetic Nanosensors for Electrochemical Monitoring of Intracellular Glutathione. Electroanal. Chem. 2022, 134, e202115820. [Google Scholar]
- Zhao, L.Z.; Zhao, L.; Miao, Y.; Zhang, C.X. Selective electrochemical determination of glutathione from the leakage of intracellular GSH contents in HeLa cells following doxorubicin-induced cell apoptosis. Electrochim. Acta 2016, 206, 86–98. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.T.; Goncalves, L.M.; Compton, R.G. Electrochemical determination of free and total glutathione in human saliva samples. Sens. Actuators B Chem. 2015, 221, 962–968. [Google Scholar] [CrossRef]
- Yuan, B.Q.; Zhang, R.C.; Jiao, X.X.; Li, J.; Shi, H.Z.; Zhang, D.J. Amperometric determination of reduced glutathione with a new Co-based metal-organic coordination polymer modified electrode. Electrochem. Commun. 2014, 40, 92–95. [Google Scholar] [CrossRef]
- Kannappan, S.; Bhat, L.R.; Nesakumar, N.; Babu, K.J.; Kulandaisamy, A.J.; Rayappan, J.B.B. Design and development of a non-enzymatic electrochemical biosensor for the detection of Glutathione. Electroanalysis 2022, 34, 1–12. [Google Scholar] [CrossRef]
- Abbas, M.N.; Saeed, A.A.; Ali, M.B.; Errachid, A.; Zine, N.; Baraket, A.; Singh, B. Biosensor for the oxidative stress biomarker glutathione based on SAM of cobalt phthalocyanine on a thioctic acid modified gold electrode. J. Solid State Electrochem. 2019, 23, 1129–1144. [Google Scholar] [CrossRef]
- Xu, H.Y.; Xiao, J.J.; Liu, B.; Griveau, S.; Bedioui, F. Enhanced electrochemical sensing of thiols based on cobalt phthalocyanine immobilized on nitrogen-doped graphene. Biosens. Bioelectron. 2015, 66, 438–444. [Google Scholar] [CrossRef]
- Saranya, S.; Geetha, B.; Deepa, P.N. Simultaneous detection of glutathione, threonine, and glycine at electrodeposited RuHCF/rGO–modified electrode. Ionics 2019, 25, 5537–5550. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Yuan, B.; Chen, S.; Gan, L.; Xu, C. Covalent Organic Frameworks-TpPa-1 as an Emerging Platform for Electrochemical Sensing. Nanomaterials 2022, 12, 2953. https://doi.org/10.3390/nano12172953
Li G, Yuan B, Chen S, Gan L, Xu C. Covalent Organic Frameworks-TpPa-1 as an Emerging Platform for Electrochemical Sensing. Nanomaterials. 2022; 12(17):2953. https://doi.org/10.3390/nano12172953
Chicago/Turabian StyleLi, Gang, Baiqing Yuan, Sidi Chen, Liju Gan, and Chunying Xu. 2022. "Covalent Organic Frameworks-TpPa-1 as an Emerging Platform for Electrochemical Sensing" Nanomaterials 12, no. 17: 2953. https://doi.org/10.3390/nano12172953
APA StyleLi, G., Yuan, B., Chen, S., Gan, L., & Xu, C. (2022). Covalent Organic Frameworks-TpPa-1 as an Emerging Platform for Electrochemical Sensing. Nanomaterials, 12(17), 2953. https://doi.org/10.3390/nano12172953