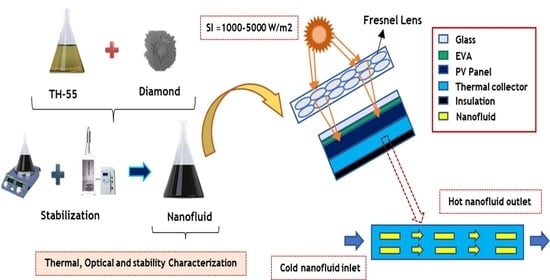
Insight into the Investigation of Diamond Nanoparticles Suspended Therminol®55 Nanofluids on Concentrated Photovoltaic/Thermal Solar Collector
Abstract
:1. Introduction
2. Methodology
2.1. Materials
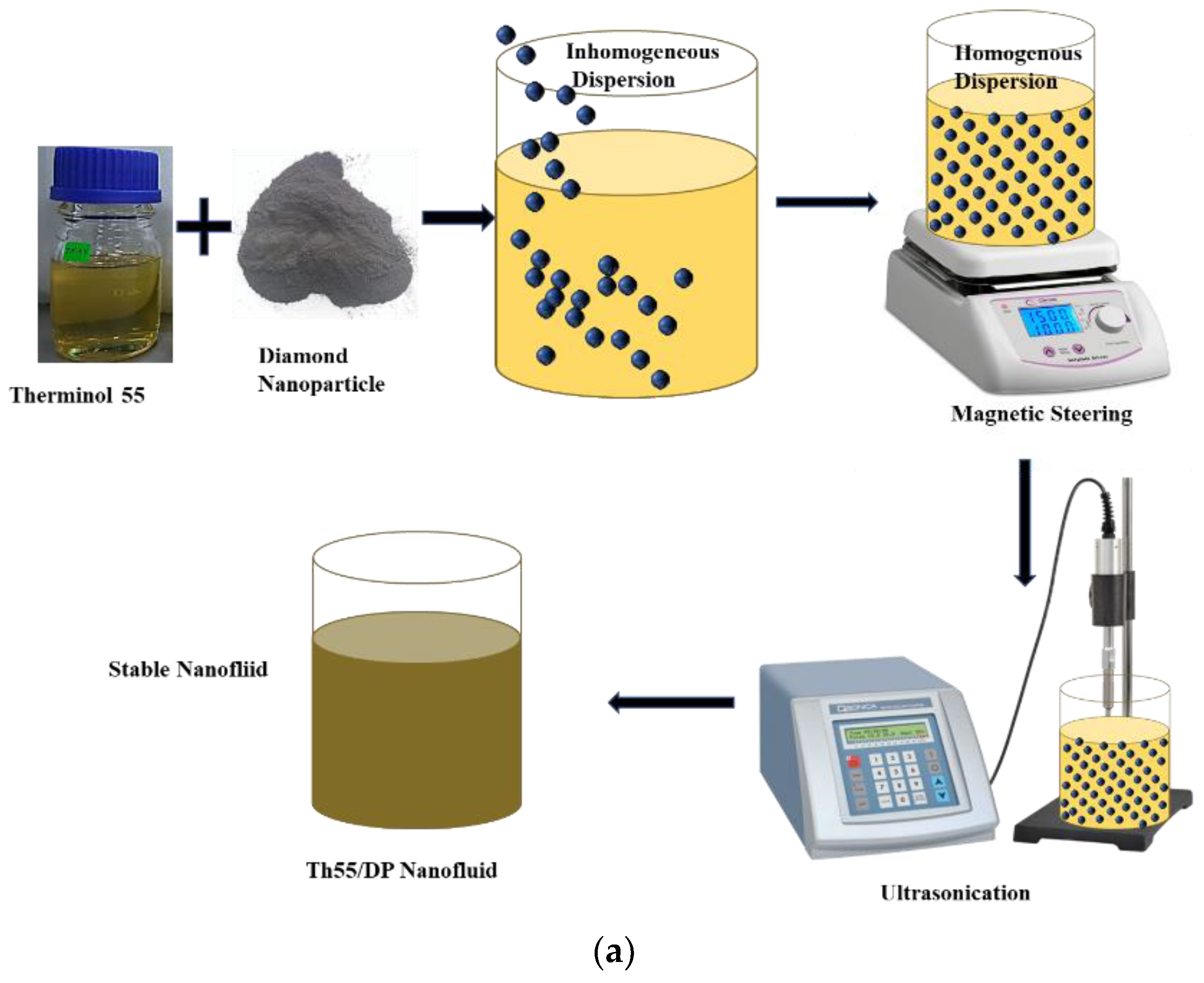
2.2. Formulation of Nanofluid
2.3. Characterization
2.3.1. Structural and Optical Properties Measurement
2.3.2. Thermo-Physical Properties Measurement
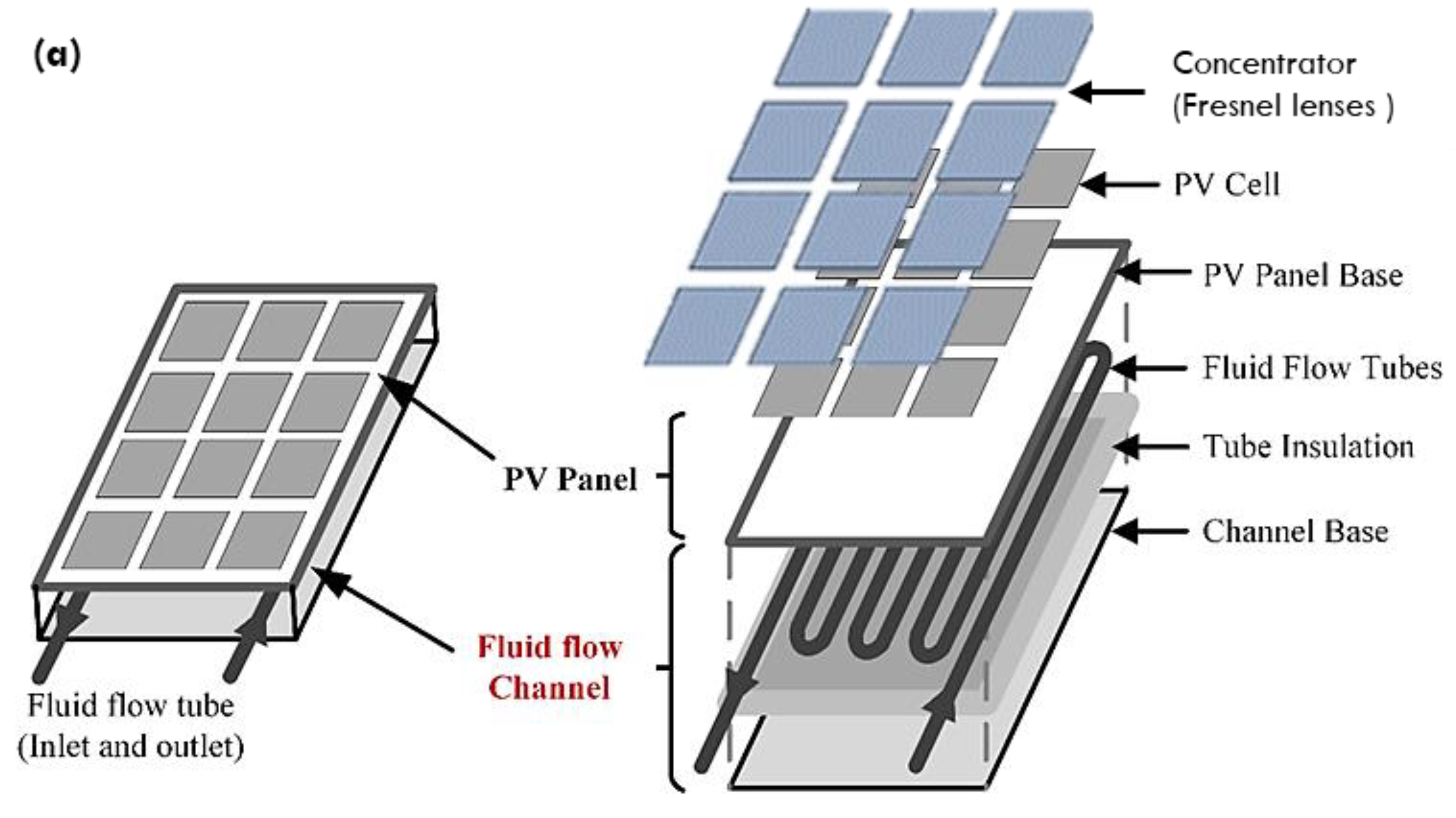
2.4. Numerical Implementation on Concentrated PV/T Solar Collector
2.4.1. Numerical Modelling of CPV/T Solar System
2.4.2. Boundary Conditions
2.4.3. Meshing and Grid Independency Test
3. Results and Discussion
3.1. Stability of the Nanofluids
3.2. Optical Properties
3.3. Thermo-Physical Properties
3.3.1. Thermal Conductivity (k)
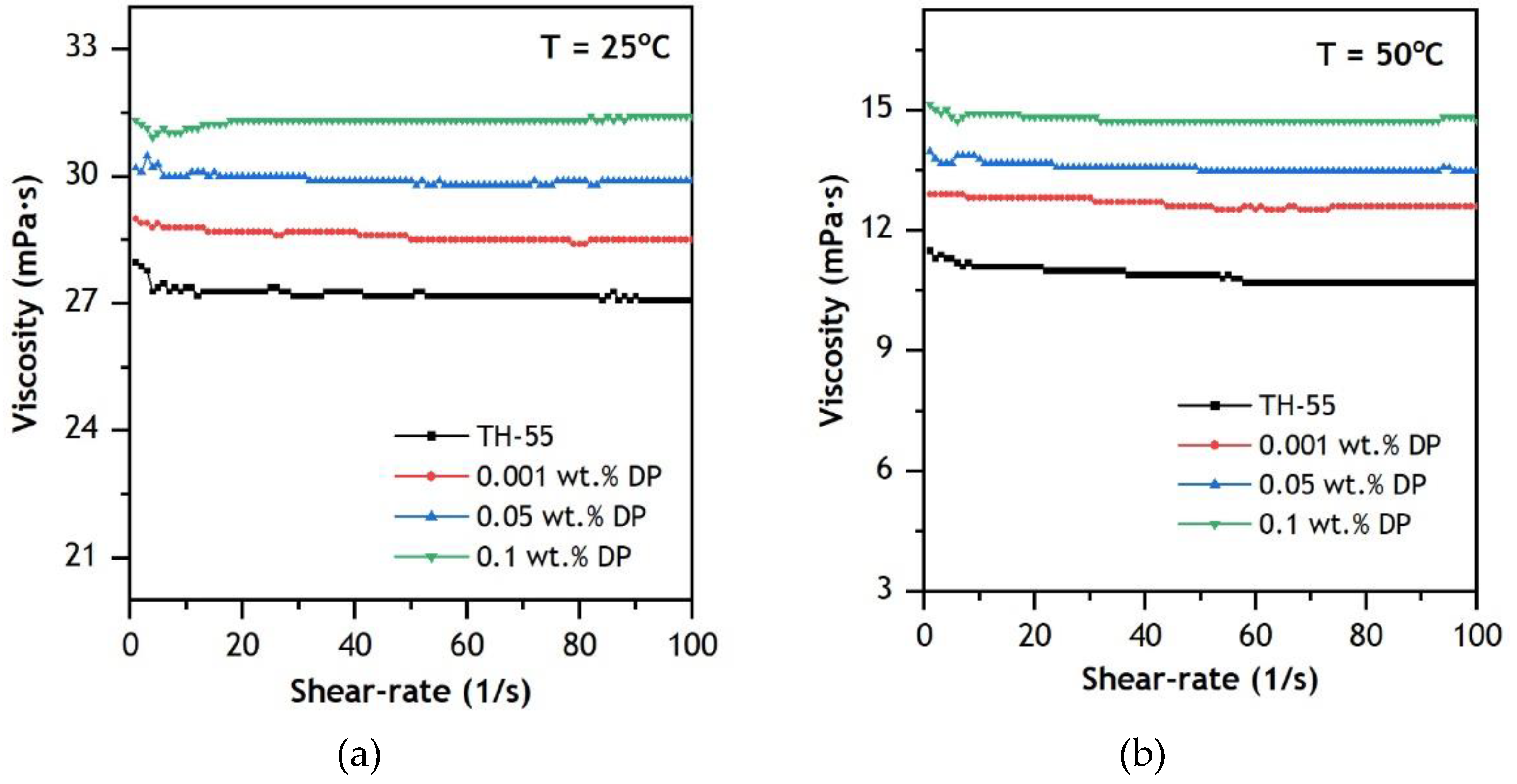
3.3.2. Dynamic Viscosity
3.3.3. Numerical Results
4. Conclusions
- Zeta potential (ζ) analysis confirms good suspension stability of the formulated NFs as absolute ζ values are found to be above ±30 mV. At 25 °C, 45.13, 41.21 and 34.81 mV of ζ are measured at 0.001, 0.05 and 0.1 wt.%, respectively. UV-vis profiles of the NFs showed enhanced absorbance of the NFs. The photo-thermal energy conversion efficiency of TH-55 oil has increased by 120.80% due to the dispersion of diamond nanoparticles.
- The addition of nanomaterial to the TH-55 increases thermal conductivity at elevated temperatures ranging from 20 to 70 °C, whereas the addition of nanoparticles increases dynamic viscosity marginally. However, it rapidly drops at elevated temperatures and behaves like a Newtonian fluid.
- Implementing the NFs in the CPV/T solar collector produced an improved cooling effect on the PV unit of the system as a maximum temperature drop of 21 °C was attained at 0.1 wt.% of DP. The highest augmentation in and of the nanofluid-based CPV/T system was obtained at about 11% and 1.8% at an optimum flow rate of 3 LPM, respectively.
- Future research could investigate the practical implementation of proposed nanofluids on CPV/T solar collectors by examining additional parameters, such as exergy analysis and estimation of the system’s pumping power.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Nomenclature | σ | Stefan Boltzmann Constant, W/(m2·K4) | |
| Ac | area of collector (m2) | Subscripts | |
| cp | Specific heat (J/K) | amb | ambient |
| FF | field factor | bf | base fluid |
| G | solar radiation intensity (W/m2) | el | electrical |
| H | convective heat transfer coefficient (W/m2·K) | in | inlet |
| Isc | short circuit current (A) | out | outlet |
| kbf | thermal conductivity of base fluid (W/m·K) | s | solid particle |
| knf | thermal conductivity of nanofluid (W/m·K) | th | thermal |
| ks | thermal conductivity of nanoparticle (W/m·K) | nf | Nanofluids |
| Pel | electrical power output (W) | Abbreviations | |
| Pth | thermal power output (W) | HPLC | high performance liquid chromatography |
| heat transfer due to convection (W) | NMR | nuclear magnetic resonance | |
| heat loss due to radiation (W) | IC | ion chromatographic | |
| T | temperature (K) | KF | Karl Fischer |
| Voc | open circuit voltage (V) | ND | Nano Diamond |
| Greeks | SEM | scanning electron microscopy | |
| ζ | zeta potential, mV | FT-IR | Fourier-transform infrared spectroscopy |
| Φ | nanoparticle weight fraction | UV-Vis | ultraviolet–visible spectroscopy |
| ρ | density, kg/m3 | TH-55 | Therminol 55 |
| η | efficiency | PV/T | photovoltaic thermal |
| ε | emissivity | UDF | user defined function |
References
- Rasih, R.A.; Sidik, N.A.C.; Samion, S. Recent progress on concentrating direct absorption solar collector using nanofluids. J. Therm. Anal. Calorim. 2019, 137, 903–922. [Google Scholar] [CrossRef]
- Das, L.; Habib, K.; Irshad, K.; Saidur, R.; Algarni, S.; Alqahtani, T. Thermo-Optical Characterization of Therminol55 Based MXene–Al2O3 Hybridized Nanofluid and New Correlations for Thermal Properties. Nanomaterials 2022, 12, 1862. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Rubbi, F.; Habib, K.; Aslfattahi, N.; Saidur, R.; Baran Saha, B.; Algarni, S.; Irshad, K.; Alqahtani, T. State-of-the-art ionic liquid & ionanofluids incorporated with advanced nanomaterials for solar energy applications. J. Mol. Liq. 2021, 336, 116563. [Google Scholar] [CrossRef]
- Das, L.; Rubbi, F.; Habib, K.; Saidur, R.; Islam, N.; Saha, B.B.; Aslfattahi, N.; Irshad, K. Hydrothermal performance improvement of an inserted double pipe heat exchanger with Ionanofluid. Case Stud. Therm. Eng. 2021, 28, 101533. [Google Scholar] [CrossRef]
- George, M.; Pandey, A.K.; Abd Rahim, N.; Tyagi, V.V.; Shahabuddin, S.; Saidur, R. Concentrated photovoltaic thermal systems: A component-by-component view on the developments in the design, heat transfer medium and applications. Energy Convers. Manag. 2019, 186, 15–41. [Google Scholar] [CrossRef]
- Flitsanov, Y.; Kribus, A. A cooler for dense-array CPV receivers based on metal foam. Sol. Energy 2018, 160, 25–31. [Google Scholar] [CrossRef]
- Daneshazarian, R.; Cuce, E.; Cuce, P.M.; Sher, F. Concentrating photovoltaic thermal (CPVT) collectors and systems: Theory, performance assessment and applications. Renew. Sustain. Energy Rev. 2018, 81, 473–492. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hasanuzzaman, M.; Rahim, N.A. Effects of operational conditions on the energy efficiency of photovoltaic modules operating in Malaysia. J. Clean. Prod. 2017, 143, 912–924. [Google Scholar] [CrossRef]
- Chaabane, M.; Charfi, W.; Mhiri, H.; Bournot, P. Performance evaluation of concentrating solar photovoltaic and photovoltaic/thermal systems. Sol. Energy 2013, 98, 315–321. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, S.G.; Lee, G.H. Efficiency Improvement of a Photovoltaic Thermal (PVT) System Using Nanofluids. Energies 2019, 12, 3063. [Google Scholar] [CrossRef] [Green Version]
- Angayarkanni, S.A.; Philip, J. Review on thermal properties of nanofluids: Recent developments. Adv. Colloid Interface Sci. 2015, 225, 146–176. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.H.A.; Saidur, R.; Mahbubul, I.M.; Al-Sulaiman, F.A. Optical properties of various nanofluids used in solar collector: A review. Renew. Sustain. Energy Rev. 2017, 73, 1014–1030. [Google Scholar] [CrossRef]
- Sezer, N.; Atieh, M.A.; Koç, M. A comprehensive review on synthesis, stability, thermophysical properties, and characterization of nanofluids. Powder Technol. 2019, 344, 404–431. [Google Scholar] [CrossRef]
- Li, Z.; Lei, H.; Kan, A.; Xie, H.; Yu, W. Photothermal applications based on graphene and its derivatives: A state-of-the-art review. Energy 2021, 216, 119262. [Google Scholar] [CrossRef]
- Ghalandari, M.; Maleki, A.; Haghighi, A.; Safdari Shadloo, M.; Alhuyi Nazari, M.; Tlili, I. Applications of nanofluids containing carbon nanotubes in solar energy systems: A review. J. Mol. Liq. 2020, 313, 113476. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, N.; Feng, Y.; Michaelides, E.E.; Żyła, G.; Jing, D.; Zhang, X.; Norris, P.M.; Markides, C.N.; Mahian, O. A review of recent advances in thermophysical properties at the nanoscale: From solid state to colloids. Phys. Rep. 2020, 843, 1–81. [Google Scholar] [CrossRef]
- Parashar, N.; Yahya, S.M. Thermophysical and rheological properties of hybrid nanofluids: A review on recent studies. J. Therm. Anal. Calorim. 2021, 147, 4411–4449. [Google Scholar] [CrossRef]
- Arshad, A.; Jabbal, M.; Yan, Y.; Reay, D. A review on graphene based nanofluids: Preparation, characterization and applications. J. Mol. Liq. 2019, 279, 444–484. [Google Scholar] [CrossRef]
- Rubbi, F.; Das, L.; Habib, K.; Aslfattahi, N.; Saidur, R.; Alam, S.U. A comprehensive review on advances of oil-based nanofluids for concentrating solar thermal collector application. J. Mol. Liq. 2021, 338, 116771. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Wang, X.; Wang, X. Significant thermal conductivity enhancement for nanofluids containing graphene nanosheets. Phys. Lett. A 2011, 375, 1323–1328. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Shirazi, S.F.S.; Amiri, A.; Alehashem, M.S.; Dahari, M.; Kazi, S.N. Experimental investigation of thermo-physical properties, convective heat transfer and pressure drop of functionalized graphene nanoplatelets aqueous nanofluid in a square heated pipe. Energy Convers. Manag. 2016, 114, 38–49. [Google Scholar] [CrossRef]
- Chen, W.-L.; Chou, H.-M.; Yang, Y.-C. Inverse estimation of the unknown base heat flux in irregular fins made of functionally graded materials. Int. Commun. Heat Mass Transf. 2017, 87, 157–163. [Google Scholar] [CrossRef]
- Menbari, A.; Alemrajabi, A.A.; Ghayeb, Y. Investigation on the stability, viscosity and extinction coefficient of CuO–Al2O3/Water binary mixture nanofluid. Exp. Therm. Fluid Sci. 2016, 74, 122–129. [Google Scholar] [CrossRef]
- Deymi-Dashtebayaz, M.; Rezapour, M. The effect of using nanofluid flow into a porous channel in the CPVT under transient solar heat flux based on energy and exergy analysis. J. Therm. Anal. Calorim. 2021, 145, 507–521. [Google Scholar] [CrossRef]
- Alwan Sywan Alshaheen, A.; Kianifar, A.; Baradaran Rahimi, A. Experimental study of using nano-(GNP, MWCNT, and SWCNT)/water to investigate the performance of a PVT module. J. Therm. Anal. Calorim. 2020, 139, 3549–3561. [Google Scholar] [CrossRef]
- Sahin, A.Z.; Uddin, M.A.; Yilbas, B.S.; Al-Sharafi, A. Performance enhancement of solar energy systems using nanofluids: An updated review. Renew. Energy 2020, 145, 1126–1148. [Google Scholar] [CrossRef]
- Sardarabadi, M.; Hosseinzadeh, M.; Kazemian, A.; Passandideh-Fard, M. Experimental investigation of the effects of using metal-oxides/water nanofluids on a photovoltaic thermal system (PVT) from energy and exergy viewpoints. Energy 2017, 138, 682–695. [Google Scholar] [CrossRef]
- Rubbi, F.; Das, L.; Habib, K.; Aslfattahi, N.; Saidur, R.; Rahman, M.T. State-of-the-art review on water-based nanofluids for low temperature solar thermal collector application. Sol. Energy Mater. Sol. Cells 2021, 230, 111220. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, R.; Wang, Z.; Wang, Q. Photo-thermal conversion properties of hybrid CuO-MWCNT/H2O nanofluids for direct solar thermal energy harvest. Appl. Therm. Eng. 2019, 147, 390–398. [Google Scholar] [CrossRef]
- Hemmat Esfe, M.; Kamyab, M.H.; Valadkhani, M. Application of nanofluids and fluids in photovoltaic thermal system: An updated review. Sol. Energy 2020, 199, 796–818. [Google Scholar] [CrossRef]
- Nasrin, R.; Hasanuzzaman, M.; Rahim, N.A. Effect of nanofluids on heat transfer and cooling system of the photovoltaic/thermal performance. Int. J. Numer. Methods Heat Fluid Flow 2019, 29, 1920–1946. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C. Investigation of a nanofluid-based concentrating thermal photovoltaic with a parabolic reflector. Energy Convers. Manag. 2019, 180, 171–182. [Google Scholar] [CrossRef]
- Alous, S.; Kayfeci, M.; Uysal, A. Experimental investigations of using MWCNTs and graphene nanoplatelets water-based nanofluids as coolants in PVT systems. Appl. Therm. Eng. 2019, 162, 114265. [Google Scholar] [CrossRef]
- Rubbi, F.; Habib, K.; Saidur, R.; Aslfattahi, N.; Yahya, S.M.; Das, L. Performance optimization of a hybrid PV/T solar system using Soybean oil/MXene nanofluids as A new class of heat transfer fluids. Sol. Energy 2020, 208, 124–138. [Google Scholar] [CrossRef]
- Kazem, H.A.; Al-Waeli, A.H.A.; Chaichan, M.T.; Sopian, K. Numerical and experimental evaluation of nanofluids based photovoltaic/thermal systems in Oman: Using silicone-carbide nanoparticles with water-ethylene glycol mixture. Case Stud. Therm. Eng. 2021, 26, 101009. [Google Scholar] [CrossRef]
- Aslfattahi, N.; Samylingam, L.; Abdelrazik, A.S.; Arifutzzaman, A.; Saidur, R. MXene based new class of silicone oil nanofluids for the performance improvement of concentrated photovoltaic thermal collector. Sol. Energy Mater. Sol. Cells 2020, 211, 110526. [Google Scholar] [CrossRef]
- Huaxu, L.; Fuqiang, W.; Dong, Z.; Ziming, C.; Chuanxin, Z.; Bo, L.; Huijin, X. Experimental investigation of cost-effective ZnO nanofluid based spectral splitting CPV/T system. Energy 2020, 194, 116913. [Google Scholar] [CrossRef]
- Khanjari, Y.; Kasaeian, A.B.; Pourfayaz, F. Evaluating the environmental parameters affecting the performance of photovoltaic thermal system using nanofluid. Appl. Therm. Eng. 2017, 115, 178–187. [Google Scholar] [CrossRef]
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Clarendon Press: Oxford, UK, 1873; Volume 1. [Google Scholar]
- Das, L.; Habib, K.; Saidur, R.; Aslfattahi, N.; Yahya, S.M.; Rubbi, F. Improved Thermophysical Properties and Energy Efficiency of Aqueous Ionic Liquid/MXene Nanofluid in a Hybrid PV/T Solar System. Nanomaterials 2020, 10, 1372. [Google Scholar] [CrossRef]
- Nasrin, R.; Hasanuzzaman, M.; Rahim, N.A. Effect of high irradiation and cooling on power, energy and performance of a PVT system. Renew. Energy 2018, 116, 552–569. [Google Scholar] [CrossRef]
- Sardarabadi, M.; Passandideh-Fard, M.; Zeinali Heris, S. Experimental investigation of the effects of silica/water nanofluid on PV/T (photovoltaic thermal units). Energy 2014, 66, 264–272. [Google Scholar] [CrossRef]
- Hendricks, J.H.C.; van Sark, W.G.J.H.M. Annual performance enhancement of building integrated photovoltaic modules by applying phase change materials. Prog. Photovolt. Res. Appl. 2013, 21, 620–630. [Google Scholar] [CrossRef]
- Nasrin, R.; Hasanuzzaman, M.; Rahim, N.A. Effect of high irradiation on photovoltaic power and energy. Int. J. Energy Res. 2018, 42, 1115–1131. [Google Scholar] [CrossRef]
- Chakraborty, S.; Panigrahi, P.K. Stability of nanofluid: A review. Appl. Therm. Eng. 2020, 174, 115259. [Google Scholar] [CrossRef]
- Vandsburger, L. Synthesis and Covalent Surface Modification of Carbon Nanotubes for Preparation of Stabilized Nanofluid Suspensions. Master’s Thesis, McGill University, Montréal, QC, Canada, 2009. [Google Scholar]
- Ilyas, S.U.; Pendyala, R.; Narahari, M.; Susin, L. Stability, rheology and thermal analysis of functionalized alumina- thermal oil-based nanofluids for advanced cooling systems. Energy Convers. Manag. 2017, 142, 215–229. [Google Scholar] [CrossRef]
- Samylingam, L.; Aslfattahi, N.; Saidur, R.; Yahya, S.M.; Afzal, A.; Arifutzzaman, A.; Tan, K.H.; Kadirgama, K. Thermal and energy performance improvement of hybrid PV/T system by using olein palm oil with MXene as a new class of heat transfer fluid. Sol. Energy Mater. Sol. Cells 2020, 218, 110754. [Google Scholar] [CrossRef]
- Kocsis, L.; Herman, P.; Eke, A. The modified Beer–Lambert law revisited. Phys. Med. Biol. 2006, 51, N91–N98. [Google Scholar] [CrossRef]
- Gulzar, O.; Qayoum, A.; Gupta, R. Photo-thermal characteristics of hybrid nanofluids based on Therminol-55 oil for concentrating solar collectors. Appl. Nanosci. 2019, 9, 1133–1143. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Luo, B.; Wei, K.; Zeng, G. The MXene/water nanofluids with high stability and photo-thermal conversion for direct absorption solar collectors: A comparative study. Energy 2021, 227, 120483. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An Effective 2D Light-to-Heat Conversion Material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef] [Green Version]
- Baby, T.T.; Ramaprabhu, S. Investigation of thermal and electrical conductivity of graphene based nanofluids. J. Appl. Phys. 2010, 108, 124308. [Google Scholar] [CrossRef]
- Shahil, K.M.F.; Balandin, A.A. Thermal properties of graphene and multilayer graphene: Applications in thermal interface materials. Solid State Commun. 2012, 152, 1331–1340. [Google Scholar] [CrossRef]
- Toghraie, D.; Chaharsoghi, V.A.; Afrand, M. Measurement of thermal conductivity of ZnO–TiO2/EG hybrid nanofluid. J. Therm. Anal. Calorim. 2016, 125, 527–535. [Google Scholar] [CrossRef]
- Ghaffarkhah, A.; Afrand, M.; Talebkeikhah, M.; Sehat, A.A.; Moraveji, M.K.; Talebkeikhah, F.; Arjmand, M. On evaluation of thermophysical properties of transformer oil-based nanofluids: A comprehensive modeling and experimental study. J. Mol. Liq. 2020, 300, 112249. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Pendyala, R.; Narahari, M. Experimental investigation of natural convection heat transfer characteristics in MWCNT-thermal oil nanofluid. J. Therm. Anal. Calorim. 2019, 135, 1197–1209. [Google Scholar] [CrossRef]
- Arthur, O.; Karim, M.A. An investigation into the thermophysical and rheological properties of nanofluids for solar thermal applications. Renew. Sustain. Energy Rev. 2016, 55, 739–755. [Google Scholar] [CrossRef]
- Dardan, E.; Afrand, M.; Meghdadi Isfahani, A.H. Effect of suspending hybrid nano-additives on rheological behavior of engine oil and pumping power. Appl. Therm. Eng. 2016, 109, 524–534. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Luo, B. The enhanced heat transfer of diathermic oil-based alumina-doped zinc oxide nanofluids for domestic solar heating systems. J. Therm. Anal. Calorim. 2021, 147, 3977–3988. [Google Scholar] [CrossRef]
- Nasrin, R.; Rahim, N.A.; Fayaz, H.; Hasanuzzaman, M. Water/MWCNT nanofluid based cooling system of PVT: Experimental and numerical research. Renew. Energy 2018, 121, 286–300. [Google Scholar] [CrossRef]



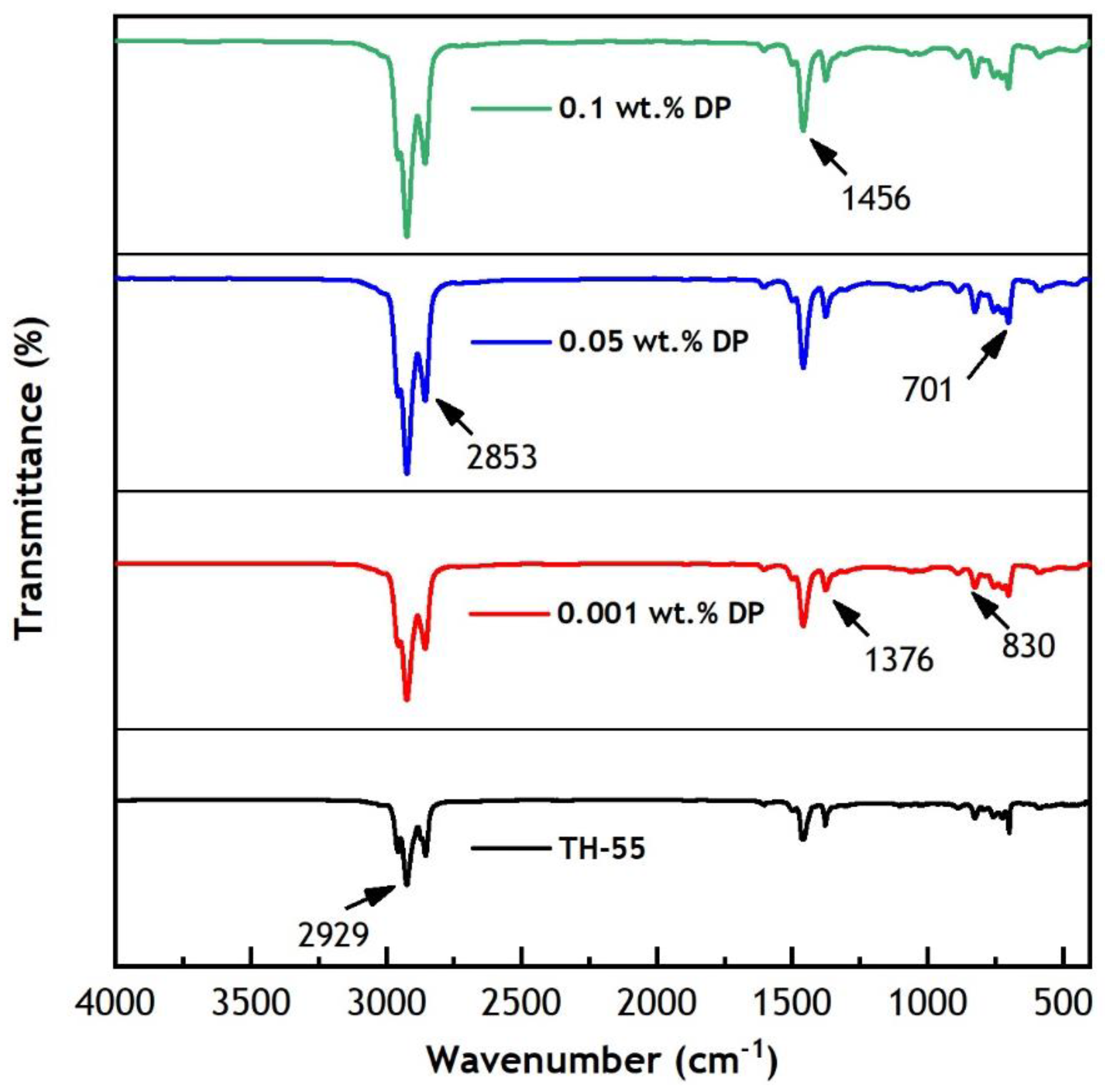
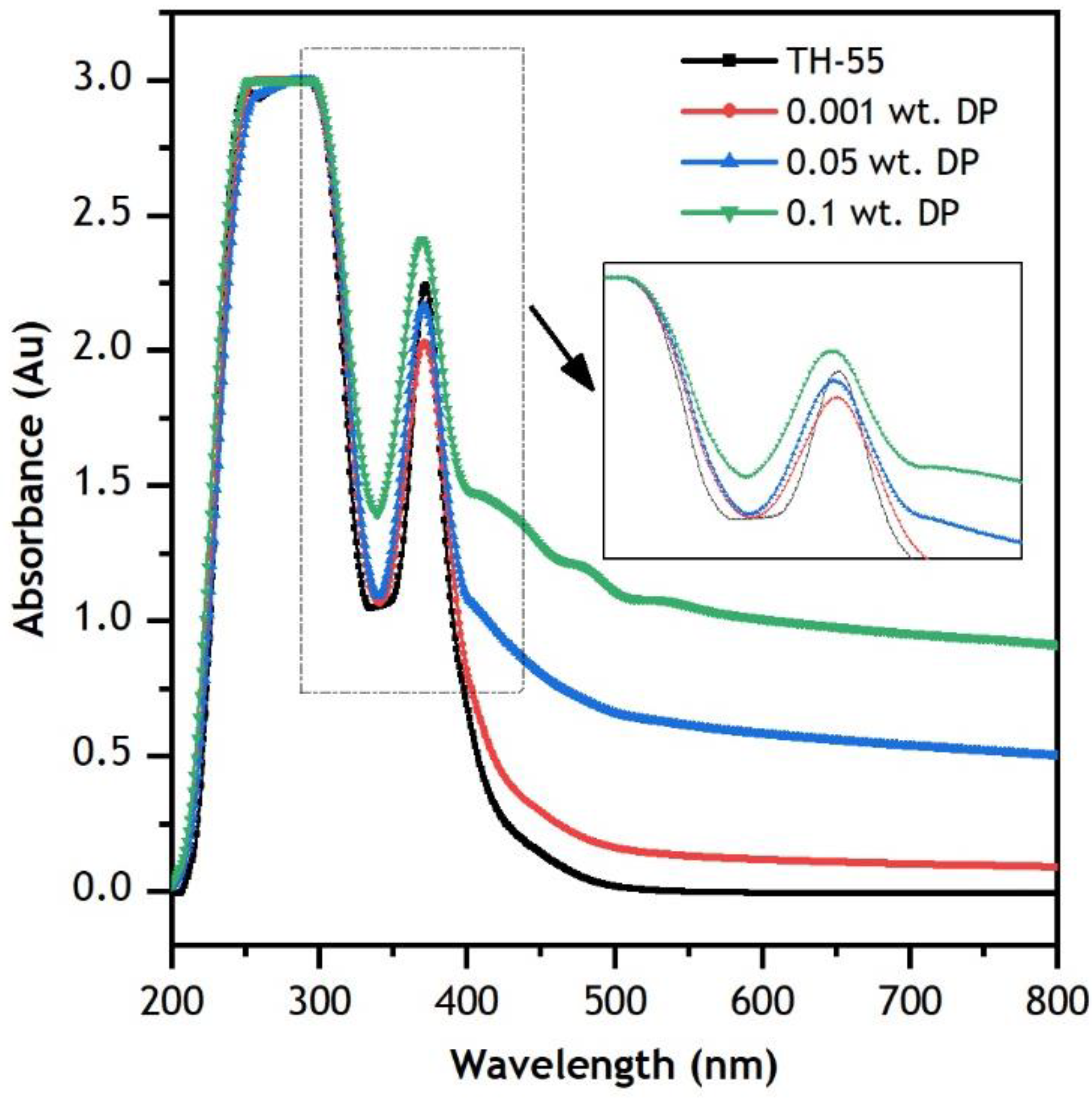
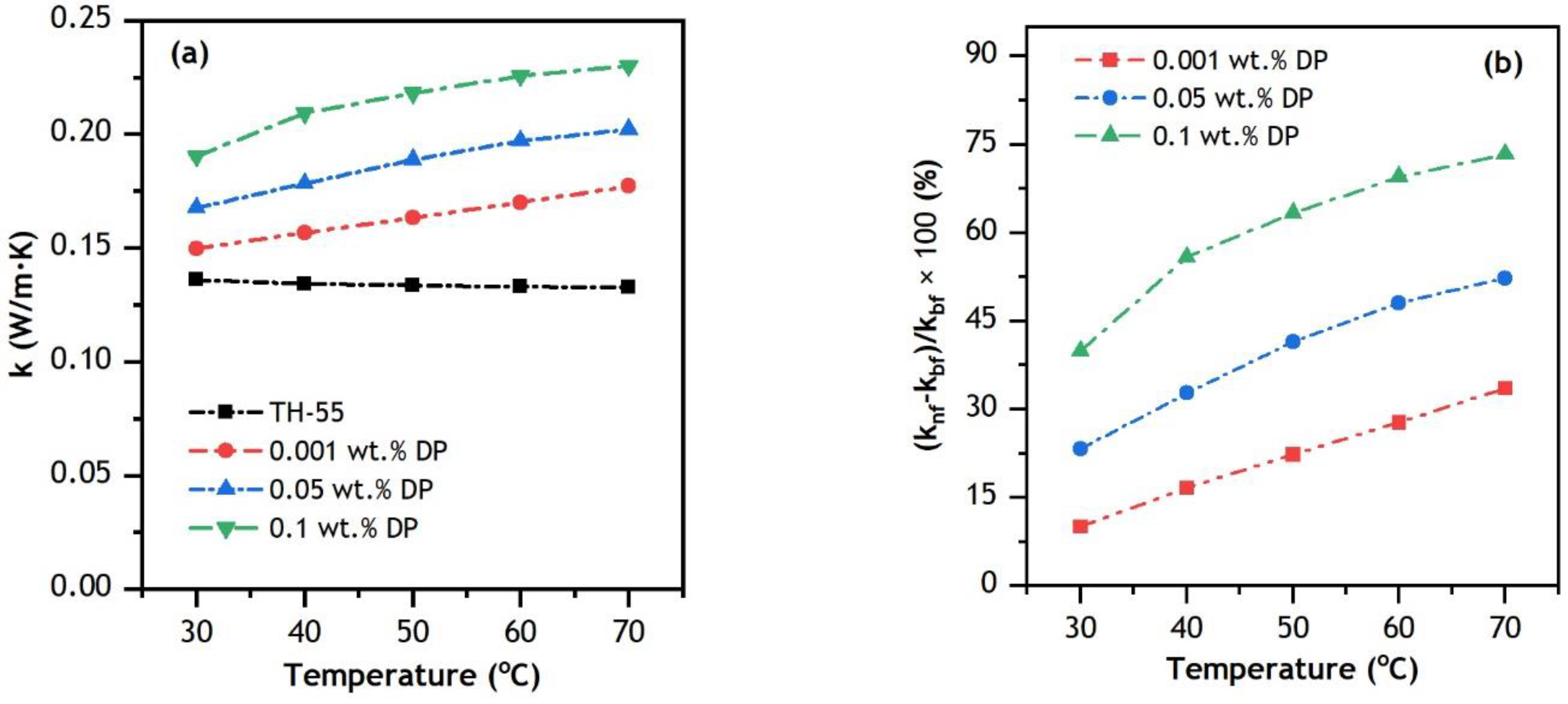
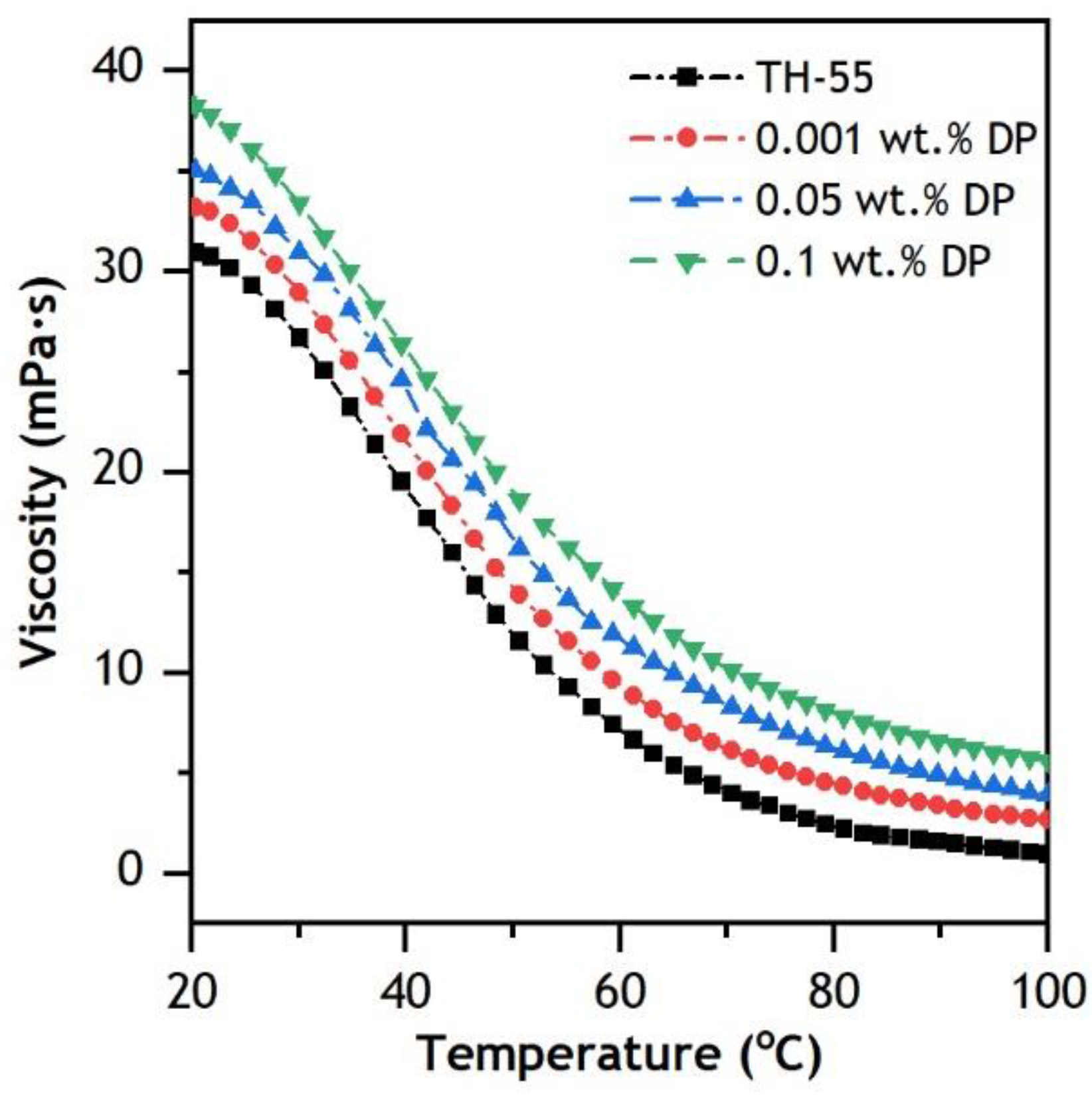

| Study | Nanofluids | Dimension and Concentration (φ) | Solar Irradiance | Temperature Range | Flow Rate | Important Findings |
|---|---|---|---|---|---|---|
| Nasrin, et al. [31] | Water/Cu, Ag and Al | -- Φ = 0.5–3 vol.% | 5000 W/m2 | 20–140 °C | 180 L/h | - The nanofluids exhibited optimized perfor-mance at 2 vol.% concentration. The is augmented by 7.49, 7.08 and 4.97% for PV/T operated by water/Ag, water/Cu, and water/Al NFs, respectively, relative to water. |
| Bellos and Tzivanidis [32] | Syltherm-800/CuO | -- Φ = 5 vol.% | 1000 W/m2 | 25–200 °C | 300–720 L/h | - 1.66, 5.17, 3.05 and 2.08% enhancements were obtained in and , respectively. |
| Alous, et al. [33] | Water/Graphene and MWCNT | 0.55–1.2 nm 18–28 nm respectively. Φ = 0.5 wt.% | Up to 1000 W/m2 | 20–80 °C | 0.5 L/min | - The PV surface temperature was reduced by 14 °C with graphene nanoplatelets and 16 °C with MWCNT NFs. In comparison to PVT-water, the NF-based PV/T had an improved average daily total energetic efficiency of 18.0 and 7% for graphene and MWCNT NFs, respectively. |
| Rubbi, et al. [34] | Soybean oil/ MXene | 1–10 µm × 1 nm Φ = 0.025–0.125 wt.% | 1000 W/m2 | 25–80 °C | 0.01–0.07 kg/s | - and augmented by 15.51 and 15.41% relative to alumina NF. Temperature of the PV unit dropped by 14 °C relative to conventional PV/T. |
| Kazem, et al. [35] | Water-EG/SiC | 45–65 nm Φ = 0.1–4 wt.% | 200–1000 W/m2 | 25–70 °C | 10–40 kg/h | - PV-cell temperature dropped by 11.34%, while and improved by 14.3 and 11.7% using the NF as working fluid instead of water. |
| Aslfattahi, et al. [36] | Silicon oil/ MXene | 1–10 µm × 1 nm Φ = 0.05–0.1 wt.% | 1000–8000 W/m2 | 30–200 °C | 0.005 kg/s | - PV panel temperature lessened by 12.45% and 11.92% enhanced energy output was achieved for the collector using NF at 0.1 wt.%. |
| Huaxu, et al. [37] | Glycol/ZnO | -- Φ = 11.2–89.2 ppm | Up to 861 W/m2 | -- | -- | - Photo-thermal efficiency improved by 47% adding ZnO NPs from 11.2 to 89.2 ppm. 3.8% higher energy conversion efficiency is achieved relative to conventional CPV. |
| Khanjari, et al. [38] | Water/Al2O3 | -- Φ = 2 vol.% | 200–800 W/m2 | 30–70 °C | 0.00136 kg/s | - enhanced by 10% using the NF compared to water operated PV/T. However, declined at higher absorbed solar radiation. |
| Nasrin, et al. [31] | Water/Cu, Ag and Al | -- Φ = 0.5–3 vol.% | 5000 W/m2 | 20–140 °C | 180 L/h | - The nanofluids exhibited optimized perfor-mance at 2 vol.% concentration. The is augmented by 7.49, 7.08 and 4.97% for PV/T operated by water/Ag, water/Cu, and water/Al NFs, respectively, relative to water. |
| Material | Parameter | Value |
|---|---|---|
| Therminol®55 (EASTMAN) | Appearance | Clear, yellow liquid |
| Normal boiling point | 351 (°C) | |
| Liquid density (20 °C) | 872 (kg/m3) | |
| Thermal conductivity (20 °C) | 0.1284 W/(m·K) | |
| Viscosity (20 °C) | 41.6 (mPa·s) | |
| Diamond (US Research Nanomaterials, Inc) | Color | Grey |
| Purity | 98.3% | |
| Size | 3–10 (nm) | |
| Morphology | Spherical | |
| Density | 0.16–0.18 (g/cm3) |
| Parameter | Values |
|---|---|
| Power | 300 W |
| Dimensions | 1955 mm × 982 mm × 36 mm |
| Weight of PV panel | 20.5 kg |
| 150 W/m2K | |
| 77 W/m2K | |
| 66 W/m2K | |
| 0.9 | |
| 0.5 | |
| 0.99 | |
| 0.311 W/m·K | |
| 148 W/m·K | |
| 0.15 W/m·K |
| S. No. | Mesh Size (Elements) | PV Temp. (°C) | Deviation (%) | Outlet Temp. (°C) | Deviation (%) |
|---|---|---|---|---|---|
| 1 | 42.341 | -- | 41.213 | -- | |
| 2 | 43.872 | 1.2 | 40.751 | −1.13 | |
| 3 | 44.003 | 0.29 | 40.254 | −1.23 | |
| 4 | 44.118 | 0.26 | 39.104 | −2.94 | |
| 5 | 45.200 | 2.3 | 38.889 | −0.55 | |
| 6 | 45.201 | 0.002 | 38.801 | −0.22 |
| Concentration (wt.%) | Absolute Zeta Potential (mV) | |||
|---|---|---|---|---|
| At 25 °C | Uncertainty (%) | At 80 °C | Uncertainty (%) | |
| 0.001 | 45.13 | <5 | 48.25 | <5 |
| 0.05 | 41.21 | 44.98 | ||
| 0.1 | 34.81 | 39.67 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, L.; Rubbi, F.; Habib, K.; Aslfattahi, N.; Rahman, S.; Yahya, S.M.; Kadirgama, K. Insight into the Investigation of Diamond Nanoparticles Suspended Therminol®55 Nanofluids on Concentrated Photovoltaic/Thermal Solar Collector. Nanomaterials 2022, 12, 2975. https://doi.org/10.3390/nano12172975
Das L, Rubbi F, Habib K, Aslfattahi N, Rahman S, Yahya SM, Kadirgama K. Insight into the Investigation of Diamond Nanoparticles Suspended Therminol®55 Nanofluids on Concentrated Photovoltaic/Thermal Solar Collector. Nanomaterials. 2022; 12(17):2975. https://doi.org/10.3390/nano12172975
Chicago/Turabian StyleDas, Likhan, Fazlay Rubbi, Khairul Habib, Navid Aslfattahi, Saidur Rahman, Syed Mohd Yahya, and Kumaran Kadirgama. 2022. "Insight into the Investigation of Diamond Nanoparticles Suspended Therminol®55 Nanofluids on Concentrated Photovoltaic/Thermal Solar Collector" Nanomaterials 12, no. 17: 2975. https://doi.org/10.3390/nano12172975
APA StyleDas, L., Rubbi, F., Habib, K., Aslfattahi, N., Rahman, S., Yahya, S. M., & Kadirgama, K. (2022). Insight into the Investigation of Diamond Nanoparticles Suspended Therminol®55 Nanofluids on Concentrated Photovoltaic/Thermal Solar Collector. Nanomaterials, 12(17), 2975. https://doi.org/10.3390/nano12172975