Roles of Inorganic Oxide Based HTMs towards Highly Efficient and Long-Term Stable PSC—A Review
Abstract
:1. Introduction
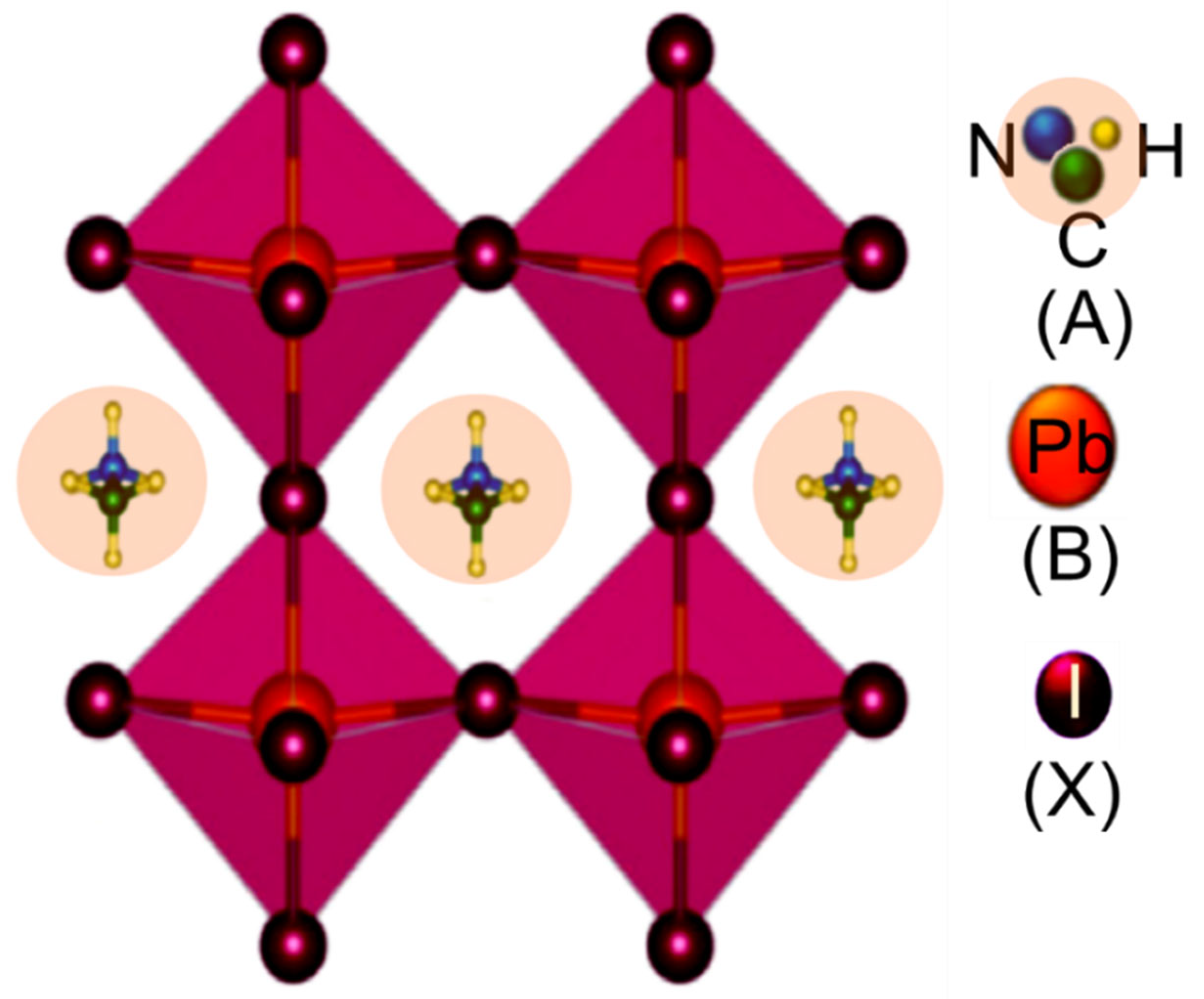
2. Perovskite Solar Cell
3. HTM in PSC
4. Major Fabrication Techniques for IHTM
4.1. Spin Coating
4.2. Sputtering
4.3. Spray Pyrolysis
4.4. Solution Combustion Process
4.5. Other Methods
5. Inorganic Oxide-Based HTM in PSCs
5.1. NiOx
5.2. CuxO
5.3. Other Metal Oxides Such as HTL
Graphene Oxide
5.4. CoO
5.5. CrO
| Device Structure | Fabrication Processes | Efficiency (%) | FF | VOC | JSC | Year | References |
|---|---|---|---|---|---|---|---|
| ITO/GO/perovskite/C60/BCP/Au | Solution process | 16.5 | 0.762 | 1.00 | 21.6 | 2017 | [143] |
| FTO/PSK/GO | Spin coating | 15.1 | 0.730 | 1.03 | 20.2 | 2014 | [144] |
| ITO/(mixed with organic HTM) | Spin coating | 11.90 | 0.705 | 0.88 | 19.18 | 2014 | [154] |
| ITO/graphene oxide/PVK/PCBM/ZnO/Al | Spin coating | 11.11 | 0.720 | 0.99 | 15.59 | 2014 | [155] |
| ITO/reduced graphene oxide/PCBM/PCB/Ag | Spin coating | 10.8 | 0.716 | 0.98 | 15.4 | 2015 | [145] |
| CoOx/Glass/ITO/CoOx/Psk/PCBM/Ag | Solution process | 14.5 | 0.755 | 0.949 | 20.28 | 2016 | [149] |
| Co3O4 Glass/FTO/cl-TiO2/mp-TiO2/mp ZrO2/Psk/mp-Co3O4/carbon | Skin printing | 13.27 | 0.64 | 0.88 | 23.43 | 2018 | [150] |
| Co1-yCuyOx/Glass/FTO/Co1-yCuyOx/Psk/PCBM/Ag | Sputtering | 9.98 | 0.599 | 0.925 | 17.98 | 2017 | [149] |
| CH3NH3PbI3/Cu:CrOx | RF sputtering | 14.76 | 0.71 | 1.03 | 20.17 | 2018 | [153] |
| Cu:CrOx/Glass/FTO/Cu:CrOx/Psk/ PCBM/Ag | RF sputtering | 10.99 | 0.7 | 0.98 | 16.02 | 2016 | [152] |
| CuyCrzO2/Glass/FTO/CuyCrzO2/Psk/ PCBM/Ag | Solution process | 15.3 | 0.7 | 1.07 | 20.48 | 2017 | [151] |
6. Inorganic HTL and Interface Engineering
7. Efficiency and Stability Issues in Oxide-Based PSC
8. Future Outlook
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Im, J.-H.; Lee, C.-R.; Lee, J.-W.; Park, S.-W.; Park, N.-G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Hishikawa, Y.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Ho-Baillie, A.W. Solar cell efficiency tables (version 52). Prog. Photovolt. Res. Appl. 2018, 26, 427–436. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef]
- Hao, F.; Stoumpos, C.C.; Cao, D.H.; Chang, R.P.; Kanatzidis, M.G. Lead-free solid-state organic–inorganic halide perovskite solar cells. Nat. Photon. 2014, 8, 489–494. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths >175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Miyata, A.; Mitioglu, A.; Plochocka, P.; Portugall, O.; Wang, J.T.-W.; Stranks, S.D.; Snaith, H.J.; Nicholas, R.J. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic–inorganic tri-halide perovskites. Nat. Phys. 2015, 11, 582–587. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Armin, A.; Nagiri, R.C.R.; Burn, P.L.; Meredith, P. Electro-optics of perovskite solar cells. Nat. Photon. 2015, 9, 106–112. [Google Scholar] [CrossRef]
- Brenner, T.M.; Egger, D.A.; Rappe, A.M.; Kronik, L.; Hodes, G.; Cahen, D. Are mobilities in hybrid organic–inorganic halide perovskites actually “high”? J. Phys. Chem. Lett. 2015, 6, 4754–4757. [Google Scholar] [CrossRef]
- Motta, C.; El-Mellouhi, F.; Sanvito, S. Charge carrier mobility in hybrid halide perovskites. Sci. Rep. 2015, 5, 12746. [Google Scholar] [CrossRef]
- Park, N.-G. Methodologies for high efficiency perovskite solar cells. Nano Converg. 2016, 3, 15. [Google Scholar] [CrossRef]
- Paek, S. Novel Anthracene HTM Containing TIPs for Perovskite Solar Cells. Processes 2021, 9, 2249. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, W.; Meng, Y.S. Spectrum-dependent spiro-OMeTAD oxidization mechanism in perovskite solar cells. ACS Appl. Mater. Interfaces 2015, 7, 24791–24798. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Wu, C.-G. Bulk heterojunction perovskite–PCBM solar cells with high fill factor. Nat. Photon. 2016, 10, 196–200. [Google Scholar] [CrossRef]
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.-S.; Chang, J.A.; Lee, Y.H.; Kim, H.-j.; Sarkar, A.; Nazeeruddin, M.K. Efficient inorganic–organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photon. 2013, 7, 486–491. [Google Scholar] [CrossRef]
- Seo, J.; Noh, J.H.; Seok, S.I. Rational strategies for efficient perovskite solar cells. Acc. Chem. Res. 2016, 49, 562–572. [Google Scholar] [CrossRef]
- Chen, H.-W.; Huang, T.-Y.; Chang, T.-H.; Sanehira, Y.; Kung, C.-W.; Chu, C.-W.; Ikegami, M.; Miyasaka, T.; Ho, K.-C. Efficiency enhancement of hybrid perovskite solar cells with MEH-PPV hole-transporting layers. Sci. Rep. 2016, 6, 34319. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Shi, J.; Liu, H.; Xu, Y.; Lv, S.; Luo, Y.; Li, D.; Meng, Q.; Li, Y. Efficient CH3NH3PbI3 Perovskite Solar Cells Based on Graphdiyne (GD)-Modified P3HT Hole-Transporting Material. Adv. Energy Mater. 2015, 5, 1401943. [Google Scholar] [CrossRef]
- Bi, D.; Yang, L.; Boschloo, G.; Hagfeldt, A.; Johansson, E.M. Effect of different hole transport materials on recombination in CH3NH3PbI3 perovskite-sensitized mesoscopic solar cells. J. Phys. Chem. Lett. 2013, 4, 1532–1536. [Google Scholar] [CrossRef] [PubMed]
- Son, D.-Y.; Im, J.-H.; Kim, H.-S.; Park, N.-G. 11% efficient perovskite solar cell based on ZnO nanorods: An effective charge collection system. J. Phys. Chem. C 2014, 118, 16567–16573. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, L.; Han, J.; Fu, Y.Q.; Shen, Y.; Zhang, Z.; Li, Y.; Wang, M. New generation perovskite solar cells with solution-processed amino-substituted perylene diimide derivative as electron-transport layer. J. Mater. Chem. A 2016, 4, 8724–8733. [Google Scholar] [CrossRef]
- Xiao, Y.; Han, G.; Chang, Y.; Zhou, H.; Li, M.; Li, Y. An all-solid-state perovskite-sensitized solar cell based on the dual function polyaniline as the sensitizer and p-type hole-transporting material. J. Power Sources 2014, 267, 1–8. [Google Scholar] [CrossRef]
- Arora, N.; Dar, M.I.; Hinderhofer, A.; Pellet, N.; Schreiber, F.; Zakeeruddin, S.M.; Grätzel, M. Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%. Science 2017, 358, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Chueh, C.C.; Jen, A.K.Y. A low-temperature, solution-processable, Cu-doped nickel oxide hole-transporting layer via the combustion method for high-performance thin-film perovskite solar cells. Adv. Mater. 2015, 27, 7874–7880. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, Y.; Ye, S.; Rao, H.; Yan, W.; Peng, H.; Li, Y.; Liu, Z.; Wang, S.; Chen, Z. High-performance inverted planar heterojunction perovskite solar cells based on a solution-processed CuOx hole transport layer. Nanoscale 2016, 8, 10806–10813. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, M.; Shen, Y.; Zhu, G.; Xu, K.; Cao, M.; Gu, F.; Wang, L. Copper iodide as inorganic hole conductor for perovskite solar cells with different thickness of mesoporous layer and hole transport layer. Appl. Surf. Sci. 2015, 357, 2234–2240. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Chen, W.; Jen, A.K.Y. CuGaO2: A promising inorganic hole-transporting material for highly efficient and stable perovskite solar cells. Adv. Mater. 2017, 29, 1604984. [Google Scholar] [CrossRef] [PubMed]
- Igbari, F.; Li, M.; Hu, Y.; Wang, Z.-K.; Liao, L.-S. A room-temperature CuAlO2 hole interfacial layer for efficient and stable planar perovskite solar cells. J. Mater. Chem. A 2016, 4, 1326–1335. [Google Scholar] [CrossRef]
- Sung, H.; Ahn, N.; Jang, M.S.; Lee, J.K.; Yoon, H.; Park, N.G.; Choi, M. Transparent conductive oxide-free graphene-based perovskite solar cells with over 17% efficiency. Adv. Energy Mater. 2016, 6, 1501873. [Google Scholar] [CrossRef]
- Rao, H.; Sun, W.; Ye, S.; Yan, W.; Li, Y.; Peng, H.; Liu, Z.; Bian, Z.; Huang, C. Solution-processed CuS NPs as an inorganic hole-selective contact material for inverted planar perovskite solar cells. ACS Appl. Mater. Interfaces 2016, 8, 7800–7805. [Google Scholar] [CrossRef]
- Singh, E.; Kim, K.S.; Yeom, G.Y.; Nalwa, H.S. Atomically thin-layered molybdenum disulfide (MoS2) for bulk-heterojunction solar cells. ACS Appl. Mater. Interfaces 2017, 9, 3223–3245. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Singh, P.K.; Singh, R.; Khan, Z.H. Perovskite sensitized solar cell using solid polymer electrolyte. Int. J. Hydrog. Energy 2016, 41, 2847–2852. [Google Scholar]
- Yu, Z.; Sun, L. Recent progress on hole-transporting materials for emerging organometal halide perovskite solar cells. Adv. Energy Mater. 2015, 5, 1500213. [Google Scholar] [CrossRef]
- Al Mamun, A.; Ava, T.T.; Zhang, K.; Baumgart, H.; Namkoong, G. New PCBM/carbon based electron transport layer for perovskite solar cells. Phys. Chem. Chem. Phys. 2017, 19, 17960–17966. [Google Scholar] [CrossRef]
- Aitola, K.; Sveinbjörnsson, K.; Correa-Baena, J.-P.; Kaskela, A.; Abate, A.; Tian, Y.; Johansson, E.M.; Grätzel, M.; Kauppinen, E.I.; Hagfeldt, A. Carbon nanotube-based hybrid hole-transporting material and selective contact for high efficiency perovskite solar cells. Energy Environ. Sci. 2016, 9, 461–466. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Y.-M.; Jing, X.; Yin, J.; Li, J.; Xu, B.; Tan, Y.-Z.; Zheng, N. Well-defined thiolated nanographene as hole-transporting material for efficient and stable perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 10914–10917. [Google Scholar] [CrossRef]
- Singh, R.; Jun, H.; Arof, A.K. Activated carbon as back contact for HTM-free mixed cation perovskite solar cell. Phase Transit. 2018, 91, 1268–1276. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, Z.; Su, J.; Hu, Z.; Chang, J.; Hao, Y. Recent progress of inorganic hole transport materials for efficient and stable perovskite solar cells. Nano Sel. 2021, 2, 1055–1080. [Google Scholar] [CrossRef]
- Li, S.; Cao, Y.-L.; Li, W.-H.; Bo, Z.-S. A brief review of hole transporting materials commonly used in perovskite solar cells. Rare Met. 2021, 40, 2712–2729. [Google Scholar] [CrossRef]
- Park, H.; Chaurasiya, R.; Ho Jeong, B.; Sakthivel, P.; Joon Park, H. Nickel Oxide for Perovskite Photovoltaic Cells. Adv. Photon. Res. 2021, 2, 2000178. [Google Scholar] [CrossRef]
- Cai, L.; Zhu, F. Toward efficient and stable operation of perovskite solar cells: Impact of sputtered metal oxide interlayers. Nano Sel. 2021, 2, 1417–1436. [Google Scholar] [CrossRef]
- Arumugam, G.M.; Karunakaran, S.K.; Liu, C.; Zhang, C.; Guo, F.; Wu, S.; Mai, Y. Inorganic hole transport layers in inverted perovskite solar cells: A review. Nano Sel. 2021, 2, 1081–1116. [Google Scholar] [CrossRef]
- Di Girolamo, D.; Di Giacomo, F.; Matteocci, F.; Marrani, A.G.; Dini, D.; Abate, A. Progress, highlights and perspectives on NiO in perovskite photovoltaics. Chem. Sci. 2020, 11, 7746–7759. [Google Scholar] [CrossRef] [PubMed]
- Pitchaiya, S.; Natarajan, M.; Santhanam, A.; Asokan, V.; Yuvapragasam, A.; Ramakrishnan, V.M.; Palanisamy, S.E.; Sundaram, S.; Velauthapillai, D. A review on the classification of organic/inorganic/carbonaceous hole transporting materials for perovskite solar cell application. Arab. J. Chem. 2020, 13, 2526–2557. [Google Scholar] [CrossRef]
- Singh, R.; Singh, P.K.; Bhattacharya, B.; Rhee, H.-W. Review of current progress in inorganic hole-transport materials for perovskite solar cells. Appl. Mater. Today 2019, 14, 175–200. [Google Scholar] [CrossRef]
- Tai, Q.; Tang, K.-C.; Yan, F. Recent progress of inorganic perovskite solar cells. Energy Environ. Sci. 2019, 12, 2375–2405. [Google Scholar] [CrossRef]
- Chen, J.; Park, N.-G. Inorganic hole transporting materials for stable and high efficiency perovskite solar cells. J. Phys. Chem. C 2018, 122, 14039–14063. [Google Scholar] [CrossRef]
- Bhat, A.; Dhamaniya, B.P.; Chhillar, P.; Korukonda, T.B.; Rawat, G.; Pathak, S.K. Analysing the Prospects of Perovskite Solar Cells within the Purview of Recent Scientific Advancements. Crystals 2018, 8, 242. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Zhao, Z.; Wang, Y.; Wu, J.; Jiang, Q.; You, J. Recent progress in stability of perovskite solar cells. J. Semicond. 2017, 38, 011002. [Google Scholar] [CrossRef]
- Rajeswari, R.; Mrinalini, M.; Prasanthkumar, S.; Giribabu, L. Emerging of inorganic hole transporting materials for perovskite solar cells. Chem. Rec. 2017, 17, 681–699. [Google Scholar] [CrossRef]
- Meng, L.; You, J.; Guo, T.-F.; Yang, Y. Recent advances in the inverted planar structure of perovskite solar cells. Acc. Chem. Res. 2016, 49, 155–165. [Google Scholar] [CrossRef]
- Fan, R.; Huang, Y.; Wang, L.; Li, L.; Zheng, G.; Zhou, H. The progress of interface design in perovskite-based solar cells. Adv. Energy Mater. 2016, 6, 1600460. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Ibn-Mohammed, T.; Koh, S.; Reaney, I.; Acquaye, A.; Schileo, G.; Mustapha, K.; Greenough, R. Perovskite solar cells: An integrated hybrid lifecycle assessment and review in comparison with other photovoltaic technologies. Renew. Sustain. Energy Rev. 2017, 80, 1321–1344. [Google Scholar] [CrossRef]
- Ball, J.M.; Stranks, S.D.; Hörantner, M.T.; Hüttner, S.; Zhang, W.; Crossland, E.J.; Ramirez, I.; Riede, M.; Johnston, M.B.; Friend, R.H. Optical properties and limiting photocurrent of thin-film perovskite solar cells. Energy Environ. Sci. 2015, 8, 602–609. [Google Scholar] [CrossRef]
- Guo, T.; Lin, M.; Huang, J.; Zhou, C.; Tian, W.; Yu, H.; Jiang, X.; Ye, J.; Shi, Y.; Xiao, Y. The recent advances of magnetic nanoparticles in medicine. J. Nanomater. 2018, 2018, 7805147. [Google Scholar] [CrossRef]
- Grancini, G.; Roldán-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8, 15684. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for commercializing perovskite solar cells. Science 2018, 361, eaat8235. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photon. 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Kazim, S.; Nazeeruddin, M.K.; Grätzel, M.; Ahmad, S. Perovskite as light harvester: A game changer in photovoltaics. Angew. Chem. Int. Ed. 2014, 53, 2812–2824. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, S.A.; Weickert, J.; Dorman, J.A.; Schmidt-Mende, L. Research update: Physical and electrical characteristics of lead halide perovskites for solar cell applications. APL Mater. 2014, 2, 155204. [Google Scholar] [CrossRef]
- Mitzi, D.B. Synthesis, structure, and properties of organic-inorganic perovskites and related materials. Prog. Inorg. Chem. 1999, 48, 1–121. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Abate, A.; Saliba, M.; Tress, W.; Jacobsson, T.J.; Grätzel, M.; Hagfeldt, A. The rapid evolution of highly efficient perovskite solar cells. Energy Environ. Sci. 2017, 10, 710–727. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, Z.; Lai, J.; Zhang, Y.; Lei, N.; Wang, D.; Sun, L. Efficient perovskite solar cells employing a solution-processable copper phthalocyanine as a hole-transporting material. Sci. China Chem. 2017, 60, 423–430. [Google Scholar] [CrossRef]
- Tzounis, L.; Stergiopoulos, T.; Zachariadis, A.; Gravalidis, C.; Laskarakis, A.; Logothetidis, S. Perovskite solar cells from small scale spin coating process towards roll-to-roll printing: Optical and morphological studies. Mater. Today Proc. 2017, 4, 5082–5089. [Google Scholar] [CrossRef]
- Wang, G.; Wu, F.; Wu, R.; Chen, T.; Ding, B.F.; Song, Q.L. Crystallization process of perovskite modified by adding lead acetate in precursor solution for better morphology and higher device efficiency. Org. Electron. 2017, 43, 189–195. [Google Scholar]
- Guo, F.; He, W.; Qiu, S.; Wang, C.; Liu, X.; Forberich, K.; Brabec, C.J.; Mai, Y. Sequential Deposition of High-Quality Photovoltaic Perovskite Layers via Scalable Printing Methods. Adv. Funct. Mater. 2019, 29, 1900964. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Wang, Y.; Sarvari, H.; Zhang, P.; Wang, M.; Chen, Z. A modified sequential deposition method for fabrication of perovskite solar cells. Sol. Energy 2016, 126, 243–251. [Google Scholar] [CrossRef]
- Reinoso, M.Á.; Otálora, C.A.; Gordillo, G. Improvement Properties of Hybrid Halide Perovskite Thin Films Prepared by Sequential Evaporation for Planar Solar Cells. Materials 2019, 12, 1394. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Huang, S.; Wen, X.; Green, M.A.; Ho-Baillie, A.W. Hole transport layer free inorganic CsPbIBr2 perovskite solar cell by dual source thermal evaporation. Adv. Energy Mater. 2016, 6, 1502202. [Google Scholar] [CrossRef]
- Yang, M.; Li, Z.; Reese, M.O.; Reid, O.G.; Kim, D.H.; Siol, S.; Klein, T.R.; Yan, Y.; Berry, J.J.; Van Hest, M.F. Perovskite ink with wide processing window for scalable high-efficiency solar cells. Nat. Energy 2017, 2, 17038. [Google Scholar] [CrossRef]
- Jung, M.; Ji, S.-G.; Kim, G.; Seok, S.I. Perovskite precursor solution chemistry: From fundamentals to photovoltaic applications. Chem. Soc. Rev. 2019, 48, 2011–2038. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei, H.; Li, B.; Wan, J. Low-temperature solution-processed tin oxide as an alternative electron transporting layer for efficient perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 6730–6733. [Google Scholar] [CrossRef]
- Etgar, L.; Gao, P.; Xue, Z.; Peng, Q.; Chandiran, A.K.; Liu, B.; Nazeeruddin, M.K.; Grätzel, M. Mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells. J. Am. Chem. Soc. 2012, 134, 17396–17399. [Google Scholar] [CrossRef]
- Wang, K.-C.; Jeng, J.-Y.; Shen, P.-S.; Chang, Y.-C.; Diau, E.W.-G.; Tsai, C.-H.; Chao, T.-Y.; Hsu, H.-C.; Lin, P.-Y.; Chen, P. P-type mesoscopic nickel oxide/organometallic perovskite heterojunction solar cells. Sci. Rep. 2014, 4, 4756. [Google Scholar] [CrossRef]
- Jung, H.S.; Park, N.G. Perovskite solar cells: From materials to devices. Small 2015, 11, 10–25. [Google Scholar] [CrossRef]
- Jeon, N.J.; Na, H.; Jung, E.H.; Yang, T.-Y.; Lee, Y.G.; Kim, G.; Shin, H.-W.; Seok, S.I.; Lee, J.; Seo, J. A fluorene-terminated hole-transporting material for highly efficient and stable perovskite solar cells. Nat. Energy 2018, 3, 682–689. [Google Scholar] [CrossRef]
- Luo, D.; Yang, W.; Wang, Z.; Sadhanala, A.; Hu, Q.; Su, R.; Shivanna, R.; Trindade, G.F.; Watts, J.F.; Xu, Z. Enhanced photovoltage for inverted planar heterojunction perovskite solar cells. Science 2018, 360, 1442–1446. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Yuan, J.; Hong, Q.; Shi, G.; Yuan, D.; Wei, J.; Huang, C.; Tang, J.; Fung, M.-K. Improved performance of inverted planar perovskite solar cells with F4-TCNQ doped PEDOT: PSS hole transport layers. J. Mater. Chem. A 2017, 5, 5701–5708. [Google Scholar] [CrossRef]
- Xie, F.; Chen, C.-C.; Wu, Y.; Li, X.; Cai, M.; Liu, X.; Yang, X.; Han, L. Vertical recrystallization for highly efficient and stable formamidinium-based inverted-structure perovskite solar cells. Energy Environ. Sci. 2017, 10, 1942–1949. [Google Scholar] [CrossRef]
- Duan, C.; Zhao, M.; Zhao, C.; Wang, Y.; Li, J.; Han, W.; Hu, Q.; Yao, L.; Jian, H.; Lu, F. Inverted CH3NH3PbI3 perovskite solar cells based on solution-processed V2O5 film combined with P3CT salt as hole transport layer. Mater. Today Energy 2018, 9, 487–495. [Google Scholar] [CrossRef]
- Li, Z. Stable perovskite solar cells based on WO3 nanocrystals as hole transport layer. Chem. Lett. 2015, 44, 1140–1141. [Google Scholar] [CrossRef]
- Nazari, P.; Ansari, F.; Abdollahi Nejand, B.; Ahmadi, V.; Payandeh, M.; Salavati-Niasari, M. Physicochemical interface engineering of CuI/Cu as advanced potential hole-transporting materials/metal contact couples in hysteresis-free ultralow-cost and large-area perovskite solar cells. J. Phys. Chem. C 2017, 121, 21935–21944. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Deng, L.-L.; Dai, S.-M.; Wang, X.; Tian, C.-B.; Zhan, X.-X.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. Low-cost solution-processed copper iodide as an alternative to PEDOT: PSS hole transport layer for efficient and stable inverted planar heterojunction perovskite solar cells. J. Mater. Chem. A 2015, 3, 19353–19359. [Google Scholar] [CrossRef]
- Özütok, F.; Demiri, S.; Özbek, E. Electrochromic NiO thin films prepared by spin coating. In Proceedings of the AIP Conference Proceedings, Bucharest, Romania, 19–22 September 2017; p. 050011. [Google Scholar]
- Zhang, B.; Su, J.; Guo, X.; Zhou, L.; Lin, Z.; Feng, L.; Zhang, J.; Chang, J.; Hao, Y. NiO/perovskite heterojunction contact engineering for highly efficient and stable perovskite solar cells. Adv. Sci. 2020, 7, 1903044. [Google Scholar] [CrossRef]
- Lee, C.C.; Chen, C.I.; Fang, C.T.; Huang, P.Y.; Wu, Y.T.; Chueh, C.C. Improving Performance of Perovskite Solar Cells Using [7] Helicenes with Stable Partial Biradical Characters as the Hole-Extraction Layers. Adv. Funct. Mater. 2019, 29, 1808625. [Google Scholar] [CrossRef]
- Ferdaous, M.T.; Shahahmadi, S.A.; Sapeli, M.M.I.; Chelvanathan, P.; Akhtaruzzaman, M.; Tiong, S.K.; Amin, N. Interplay between variable direct current sputtering deposition process parameters and properties of ZnO: Ga thin films. Thin Solid Films 2018, 660, 538–545. [Google Scholar] [CrossRef]
- Ferdaous, M.; Chelvanathan, P.; Shahahmadi, S.; Sapeli, M.; Sopian, K.; Amin, N. Compositional disparity in Cu2ZnSnS4 (CZTS) thin film deposited by RF-sputtering from a single quaternary compound target. Mater. Lett. 2018, 221, 201–205. [Google Scholar] [CrossRef]
- Patil, P.; Kadam, L. Preparation and characterization of spray pyrolyzed nickel oxide (NiO) thin films. Appl. Surf. Sci. 2002, 199, 211–221. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, B.; Feng, D.; Lin, Z.; Zhang, J.; Hao, Y.; Fan, X.; Chang, J. Achieving high performance and stable inverted planar perovskite solar cells using lithium and cobalt co-doped nickel oxide as hole transport layers. J. Mater. Chem. C 2019, 7, 9270–9277. [Google Scholar] [CrossRef]
- Ge, B.; Qiao, H.W.; Lin, Z.Q.; Zhou, Z.R.; Chen, A.P.; Yang, S.; Hou, Y.; Yang, H.G. Deepening the Valance Band Edges of NiOx Contacts by Alkaline Earth Metal Doping for Efficient Perovskite Photovoltaics with High Open-Circuit Voltage. Sol. RRL 2019, 3, 1900192. [Google Scholar] [CrossRef]
- Yin, X.; Han, J.; Zhou, Y.; Gu, Y.; Tai, M.; Nan, H.; Zhou, Y.; Li, J.; Lin, H. Critical roles of potassium in charge-carrier balance and diffusion induced defect passivation for efficient inverted perovskite solar cells. J. Mater. Chem. A 2019, 7, 5666–5676. [Google Scholar] [CrossRef]
- Seo, S.; Jeong, S.; Bae, C.; Park, N.G.; Shin, H. Perovskite Solar Cells with Inorganic Electron-and Hole-Transport Layers Exhibiting Long-Term (≈ 500 h) Stability at 85 °C under Continuous 1 Sun Illumination in Ambient Air. Adv. Mater. 2018, 30, 1801010. [Google Scholar] [CrossRef]
- Xiao, M.; Gao, M.; Huang, F.; Pascoe, A.R.; Qin, T.; Cheng, Y.B.; Bach, U.; Spiccia, L. Efficient perovskite solar cells employing inorganic interlayers. ChemNanoMat 2016, 2, 182–188. [Google Scholar] [CrossRef]
- Park, I.J.; Kang, G.; Park, M.A.; Kim, J.S.; Seo, S.W.; Kim, D.H.; Zhu, K.; Park, T.; Kim, J.Y. Highly Efficient and Uniform 1 cm2 Perovskite Solar Cells with an Electrochemically Deposited NiOx Hole-Extraction Layer. ChemSusChem 2017, 10, 2660–2667. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, H.; Zheng, X.; Meng, X.; Zhang, T.; Hu, C.; Bai, Y.; Xiao, S.; Yang, S. Ultrasound-spray deposition of multi-walled carbon nanotubes on NiO nanoparticles-embedded perovskite layers for high-performance carbon-based perovskite solar cells. Nano Energy 2017, 42, 322–333. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, H.; Xiao, S.; Xue, Q.; Zhang, T.; Zhu, Z.; Li, Q.; Hu, C.; Yang, Y.; Hu, Z. Effects of a molecular monolayer modification of NiO nanocrystal layer surfaces on perovskite crystallization and interface contact toward faster hole extraction and higher photovoltaic performance. Adv. Funct. Mater. 2016, 26, 2950–2958. [Google Scholar] [CrossRef]
- Xue, Q.; Bai, Y.; Liu, M.; Xia, R.; Hu, Z.; Chen, Z.; Jiang, X.F.; Huang, F.; Yang, S.; Matsuo, Y. Dual interfacial modifications enable high performance semitransparent perovskite solar cells with large open circuit voltage and fill factor. Adv. Energy Mater. 2017, 7, 1602333. [Google Scholar] [CrossRef]
- Wang, Q.; Chueh, C.C.; Zhao, T.; Cheng, J.; Eslamian, M.; Choy, W.C.; Jen, A.K. Effects of Selfâ Assembled Monolayer Modification of Nickel Oxide Nanoparticles Layer on the Performance and Application of Inverted Perovskite Solar Cells. ChemSusChem 2017, 10, 3794–3803. [Google Scholar] [CrossRef]
- Li, R.; Wang, P.; Chen, B.; Cui, X.; Ding, Y.; Li, Y.; Zhang, D.; Zhao, Y.; Zhang, X. NiOx/Spiro Hole Transport Bilayers for Stable Perovskite Solar Cells with Efficiency Exceeding 21%. ACS Energy Lett. 2019, 5, 79–86. [Google Scholar] [CrossRef]
- Ru, P.; Bi, E.; Zhang, Y.; Wang, Y.; Kong, W.; Sha, Y.; Tang, W.; Zhang, P.; Wu, Y.; Chen, W. High electron affinity enables fast hole extraction for efficient flexible inverted perovskite solar cells. Adv. Energy Mater. 2020, 10, 1903487. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, Z.; Ning, Z.; Li, T.; Guo, X.; Ma, J.; Su, J.; Zhang, C.; Zhang, J.; Liu, S. Highly efficient and stable planar perovskite solar cells with modulated diffusion passivation toward high power conversion efficiency and ultrahigh fill factor. Sol. RRL 2019, 3, 1900293. [Google Scholar] [CrossRef]
- Yue, S.; Liu, K.; Xu, R.; Li, M.; Azam, M.; Ren, K.; Liu, J.; Sun, Y.; Wang, Z.; Cao, D. Efficacious engineering on charge extraction for realizing highly efficient perovskite solar cells. Energy Environ. Sci. 2017, 10, 2570–2578. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.Z.; Feng, X.Y.; Djurišić, A.B.; Chan, W.K.; He, Z.B. Cesium doped NiOx as an efficient hole extraction layer for inverted planar perovskite solar cells. Adv. Energy Mater. 2017, 7, 1700722. [Google Scholar] [CrossRef]
- Aydin, E.; Troughton, J.; De Bastiani, M.; Ugur, E.; Sajjad, M.; Alzahrani, A.; Neophytou, M.; Schwingenschlögl, U.; Laquai, F.; Baran, D. Room-temperature-sputtered nanocrystalline nickel oxide as hole transport layer for p–i–n perovskite solar cells. ACS Appl. Energy Mater. 2018, 1, 6227–6233. [Google Scholar] [CrossRef]
- Chang, Y.-M.; Li, C.-W.; Lu, Y.-L.; Wu, M.-S.; Li, H.; Lin, Y.-S.; Lu, C.-W.; Chen, C.-P.; Chang, Y.J. Spherical Hole-Transporting Interfacial Layer Passivated Defect for Inverted NiOx-Based Planar Perovskite Solar Cells with High Efficiency of over 20%. ACS Appl. Mater. Interfaces 2021, 13, 6450–6460. [Google Scholar] [CrossRef]
- Mann, D.S.; Patil, P.; Kwon, S.-N.; Na, S.-I. Enhanced performance of pin perovskite solar cell via defect passivation of nickel oxide/perovskite interface with self-assembled monolayer. Appl. Surf. Sci. 2021, 560, 149973. [Google Scholar] [CrossRef]
- Chen, W.; Xu, L.; Feng, X.; Jie, J.; He, Z. Metal acetylacetonate series in interface engineering for full low-temperature-processed, high-performance, and stable planar perovskite solar cells with conversion efficiency over 16% on 1 cm2 scale. Adv. Mater. 2017, 29, 1603923. [Google Scholar] [CrossRef]
- He, Q.; Yao, K.; Wang, X.; Xia, X.; Leng, S.; Li, F. Room-temperature and solution-processable Cu-doped nickel oxide nanoparticles for efficient hole-transport layers of flexible large-area perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 41887–41897. [Google Scholar] [CrossRef]
- Hu, C.; Bai, Y.; Xiao, S.; Zhang, T.; Meng, X.; Ng, W.K.; Yang, Y.; Wong, K.S.; Chen, H.; Yang, S. Tuning the A-site cation composition of FA perovskites for efficient and stable NiO-based p–i–n perovskite solar cells. J. Mater. Chem. A 2017, 5, 21858–21865. [Google Scholar] [CrossRef]
- Li, E.; Guo, Y.; Liu, T.; Hu, W.; Wang, N.; He, H.; Lin, H. Preheating-assisted deposition of solution-processed perovskite layer for an efficiency-improved inverted planar composite heterojunction solar cell. RSC Adv. 2016, 6, 30978–30985. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, D.; Chueh, C.-C.; Shi, X.; Li, Z.; Jen, A.K.-Y. Highly efficient and stable perovskite solar cells enabled by all-crosslinked charge-transporting layers. Joule 2018, 2, 168–183. [Google Scholar] [CrossRef]
- You, J.; Meng, L.; Song, T.-B.; Guo, T.-F.; Yang, Y.M.; Chang, W.-H.; Hong, Z.; Chen, H.; Zhou, H.; Chen, Q. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotechnol. 2016, 11, 75–81. [Google Scholar] [CrossRef]
- Nie, W.; Tsai, H.; Blancon, J.C.; Liu, F.; Stoumpos, C.C.; Traore, B.; Kepenekian, M.; Durand, O.; Katan, C.; Tretiak, S. Critical role of interface and crystallinity on the performance and photostability of perovskite solar cell on nickel oxide. Adv. Mater. 2018, 30, 1703879. [Google Scholar] [CrossRef]
- Kim, H.-S.; Seo, J.-Y.; Xie, H.; Lira-Cantu, M.; Zakeeruddin, S.M.; Grätzel, M.; Hagfeldt, A. Effect of Cs-incorporated NiOx on the performance of perovskite solar cells. ACS Omega 2017, 2, 9074–9079. [Google Scholar] [CrossRef]
- Ciro, J.; Ramírez, D.; Mejía Escobar, M.A.; Montoya, J.F.; Mesa, S.; Betancur, R.; Jaramillo, F. Self-Functionalization Behind a Solution-Processed NiOx Film Used As Hole Transporting Layer for Efficient Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 12348–12354. [Google Scholar] [CrossRef]
- Yin, X.; Chen, P.; Que, M.; Xing, Y.; Que, W.; Niu, C.; Shao, J. Highly efficient flexible perovskite solar cells using solution-derived NiOx hole contacts. ACS Nano 2016, 10, 3630–3636. [Google Scholar] [CrossRef] [PubMed]
- Kwon, U.; Kim, B.-G.; Nguyen, D.C.; Park, J.-H.; Ha, N.Y.; Kim, S.-J.; Ko, S.H.; Lee, S.; Lee, D.; Park, H.J. Solution-processible crystalline NiO nanoparticles for high-performance planar perovskite photovoltaic cells. Sci. Rep. 2016, 6, 30759. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.M.; Bae, I.-G.; Aggarwal, Y.; Park, J.; Jeong, H.-R.; Choi, E.H.; Park, B. Highly efficient self-powered perovskite photodiode with an electron-blocking hole-transport NiOx layer. Sci. Rep. 2021, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Xu, X.; Bu, L.; Zhang, W.; Li, W.; Zhao, Z.; Wang, M.; Cheng, Y.-B.; He, H. p-Type mesoscopic NiO as an active interfacial layer for carbon counter electrode based perovskite solar cells. Dalton Trans. 2015, 44, 3967–3973. [Google Scholar] [CrossRef]
- Huang, A.; Zhu, J.; Zheng, J.; Yu, Y.; Liu, Y.; Yang, S.; Bao, S.; Lei, L.; Jin, P. Achieving high-performance planar perovskite solar cells with co-sputtered Co-doping NiOx hole transport layers by efficient extraction and enhanced mobility. J. Mater. Chem. C 2016, 4, 10839–10846. [Google Scholar] [CrossRef]
- Park, J.H.; Seo, J.; Park, S.; Shin, S.S.; Kim, Y.C.; Jeon, N.J.; Shin, H.W.; Ahn, T.K.; Noh, J.H.; Yoon, S.C. Efficient CH3NH3PbI3 perovskite solar cells employing nanostructured p-type NiO electrode formed by a pulsed laser deposition. Adv. Mater. 2015, 27, 4013–4019. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, F.; Chen, H.; Yang, X.; Su, H.; Cai, M.; Zhou, Z.; Noda, T.; Han, L. Thermally stable MAPbI3 perovskite solar cells with efficiency of 19.19% and area over 1 cm2 achieved by additive engineering. Adv. Mater. 2017, 29, 1701073. [Google Scholar] [CrossRef]
- Koushik, D.; Jošt, M.; Dučinskas, A.; Burgess, C.; Zardetto, V.; Weijtens, C.; Verheijen, M.A.; Kessels, W.M.; Albrecht, S.; Creatore, M. Plasma-assisted atomic layer deposition of nickel oxide as hole transport layer for hybrid perovskite solar cells. J. Mater. Chem. C 2019, 7, 12532–12543. [Google Scholar] [CrossRef]
- Seo, S.; Park, I.J.; Kim, M.; Lee, S.; Bae, C.; Jung, H.S.; Park, N.-G.; Kim, J.Y.; Shin, H. An ultra-thin, un-doped NiO hole transporting layer of highly efficient (16.4%) organic–inorganic hybrid perovskite solar cells. Nanoscale 2016, 8, 11403–11412. [Google Scholar] [CrossRef]
- Pae, S.R.; Byun, S.; Kim, J.; Kim, M.; Gereige, I.; Shin, B. Improving Uniformity and Reproducibility of Hybrid Perovskite Solar Cells via a Low-Temperature Vacuum Deposition Process for NiOx Hole Transport Layers. ACS Appl. Mater. Interfaces 2018, 10, 534–540. [Google Scholar] [CrossRef]
- Rao, H.; Ye, S.; Sun, W.; Yan, W.; Li, Y.; Peng, H.; Liu, Z.; Bian, Z.; Li, Y.; Huang, C. A 19.0% efficiency achieved in CuOx-based inverted CH3NH3PbI3−xClx solar cells by an effective Cl doping method. Nano Energy 2016, 27, 51–57. [Google Scholar] [CrossRef]
- Guo, Y.; Lei, H.; Xiong, L.; Li, B.; Fang, G. An integrated organic–inorganic hole transport layer for efficient and stable perovskite solar cells. J. Mater. Chem. A 2018, 6, 2157–2165. [Google Scholar] [CrossRef]
- Zuo, C.; Ding, L. Solution-processed Cu2O and CuO as hole transport materials for efficient perovskite solar cells. Small 2015, 11, 5528–5532. [Google Scholar] [CrossRef]
- Yu, Z.-K.; Fu, W.-F.; Liu, W.-Q.; Zhang, Z.-Q.; Liu, Y.-J.; Yan, J.-L.; Ye, T.; Yang, W.-T.; Li, H.-Y.; Chen, H.-Z. Solution-processed CuOx as an efficient hole-extraction layer for inverted planar heterojunction perovskite solar cells. Chin. Chem. Lett. 2017, 28, 13–18. [Google Scholar] [CrossRef]
- Yu, W.; Li, F.; Wang, H.; Alarousu, E.; Chen, Y.; Lin, B.; Wang, L.; Hedhili, M.N.; Li, Y.; Wu, K. Ultrathin Cu2O as an efficient inorganic hole transporting material for perovskite solar cells. Nanoscale 2016, 8, 6173–6179. [Google Scholar] [CrossRef]
- Liu, L.; Xi, Q.; Gao, G.; Yang, W.; Zhou, H.; Zhao, Y.; Wu, C.; Wang, L.; Xu, J. Cu2O particles mediated growth of perovskite for high efficient hole-transporting-layer free solar cells in ambient conditions. Sol. Energy Mater. Sol. Cells 2016, 157, 937–942. [Google Scholar] [CrossRef]
- Nejand, B.A.; Ahmadi, V.; Gharibzadeh, S.; Shahverdi, H.R. Cuprous oxide as a potential low-cost hole-transport material for stable perovskite solar cells. ChemSusChem 2016, 9, 302–313. [Google Scholar] [CrossRef]
- Chatterjee, S.; Pal, A.J. Introducing Cu2O thin films as a hole-transport layer in efficient planar perovskite solar cell structures. J. Phys. Chem. C 2016, 120, 1428–1437. [Google Scholar] [CrossRef]
- Chen, L.-C.; Chen, C.-C.; Liang, K.-C.; Chang, S.H.; Tseng, Z.-L.; Yeh, S.-C.; Chen, C.-T.; Wu, W.-T.; Wu, C.-G. Nano-structured CuO-Cu2O complex thin film for application in CH3NH3 PbI3 perovskite solar cells. Nanoscale Res. Lett. 2016, 11, 402. [Google Scholar] [CrossRef]
- Bu, I.Y.; Fu, Y.-S.; Li, J.-F.; Guo, T.-F. Large-area electrospray-deposited nanocrystalline CuXO hole transport layer for perovskite solar cells. RSC Adv. 2017, 7, 46651–46656. [Google Scholar] [CrossRef]
- Yang, Q.-D.; Li, J.; Cheng, Y.; Li, H.-W.; Guan, Z.; Yu, B.; Tsang, S.-W. Graphene oxide as an efficient hole-transporting material for high-performance perovskite solar cells with enhanced stability. J. Mater. Chem. A 2017, 5, 9852–9858. [Google Scholar] [CrossRef]
- Li, W.; Dong, H.; Guo, X.; Li, N.; Li, J.; Niu, G.; Wang, L. Graphene oxide as dual functional interface modifier for improving wettability and retarding recombination in hybrid perovskite solar cells. J. Mater. Chem. A 2014, 2, 20105–20111. [Google Scholar] [CrossRef]
- Yeo, J.-S.; Kang, R.; Lee, S.; Jeon, Y.-J.; Myoung, N.; Lee, C.-L.; Kim, D.-Y.; Yun, J.-M.; Seo, Y.-H.; Kim, S.-S. Highly efficient and stable planar perovskite solar cells with reduced graphene oxide nanosheets as electrode interlayer. Nano Energy 2015, 12, 96–104. [Google Scholar] [CrossRef]
- Palma, A.L.; Cinà, L.; Busby, Y.; Marsella, A.; Agresti, A.; Pescetelli, S.; Pireaux, J.-J.; Di Carlo, A. Mesoscopic perovskite light-emitting diodes. ACS Appl. Mater. Interfaces 2016, 8, 26989–26997. [Google Scholar] [CrossRef] [PubMed]
- Suragtkhuu, S.; Tserendavag, O.; Vandandoo, U.; Bati, A.S.; Bat-Erdene, M.; Shapter, J.G.; Batmunkh, M.; Davaasambuu, S. Efficiency and stability enhancement of perovskite solar cells using reduced graphene oxide derived from earth-abundant natural graphite. RSC Adv. 2020, 10, 9133–9139. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Lei, L.; Zhu, J.; Yu, Y.; Liu, Y.; Yang, S.; Bao, S.; Cao, X.; Jin, P. Fast fabrication of a stable perovskite solar cell with an ultrathin effective novel inorganic hole transport layer. Langmuir 2017, 33, 3624–3634. [Google Scholar] [CrossRef] [PubMed]
- Shalan, A.E.; Oshikiri, T.; Narra, S.; Elshanawany, M.M.; Ueno, K.; Wu, H.-P.; Nakamura, K.; Shi, X.; Diau, E.W.-G.; Misawa, H. Cobalt oxide (CoOx) as an efficient hole-extracting layer for high-performance inverted planar perovskite solar cells. ACS Appl. Mater. Interfaces 2016, 8, 33592–33600. [Google Scholar] [CrossRef]
- Bashir, A.; Shukla, S.; Lew, J.H.; Shukla, S.; Bruno, A.; Gupta, D.; Baikie, T.; Patidar, R.; Akhter, Z.; Priyadarshi, A. Spinel Co3O4 nanomaterials for efficient and stable large area carbon-based printed perovskite solar cells. Nanoscale 2018, 10, 2341–2350. [Google Scholar] [CrossRef]
- Qin, P.L.; He, Q.; Chen, C.; Zheng, X.L.; Yang, G.; Tao, H.; Xiong, L.B.; Xiong, L.; Li, G.; Fang, G.J. High-Performance Rigid and Flexible Perovskite Solar Cells with Low-Temperature Solution-Processable Binary Metal Oxide Hole-Transporting Materials. Sol. RRL 2017, 1, 1700058. [Google Scholar] [CrossRef]
- Qin, P.L.; Lei, H.W.; Zheng, X.L.; Liu, Q.; Tao, H.; Yang, G.; Ke, W.J.; Xiong, L.B.; Qin, M.C.; Zhao, X.Z. Copper-Doped Chromium Oxide Hole-Transporting Layer for Perovskite Solar Cells: Interface Engineering and Performance Improvement. Adv. Mater. Interfaces 2016, 3, 1500799. [Google Scholar] [CrossRef]
- Qin, P.; He, Q.; Yang, G.; Yu, X.; Xiong, L.; Fang, G. Metal ions diffusion at heterojunction chromium Oxide/CH3NH3PbI3 interface on the stability of perovskite solar cells. Surf. Interfaces 2018, 10, 93–99. [Google Scholar] [CrossRef]
- Liu, T.; Kim, D.; Han, H.; bin Mohd Yusoff, A.R.; Jang, J. Fine-tuning optical and electronic properties of graphene oxide for highly efficient perovskite solar cells. Nanoscale 2015, 7, 10708–10718. [Google Scholar] [CrossRef]
- Wu, Z.; Bai, S.; Xiang, J.; Yuan, Z.; Yang, Y.; Cui, W.; Gao, X.; Liu, Z.; Jin, Y.; Sun, B. Efficient planar heterojunction perovskite solar cells employing graphene oxide as hole conductor. Nanoscale 2014, 6, 10505–10510. [Google Scholar] [CrossRef]
- Lee, J.W.; Seol, D.J.; Cho, A.N.; Park, N.G. High-efficiency perovskite solar cells based on the black polymorph of HC (NH2)2PbI3. Adv. Mater. 2014, 26, 4991–4998. [Google Scholar] [CrossRef]
- Cho, K.T.; Paek, S.; Grancini, G.; Roldán-Carmona, C.; Gao, P.; Lee, Y.; Nazeeruddin, M.K. Highly efficient perovskite solar cells with a compositionally engineered perovskite/hole transporting material interface. Energy Environ. Sci. 2017, 10, 621–627. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, T.; Schulz, P.; Li, Z.; Li, G.; Kim, D.H.; Guo, N.; Berry, J.J.; Zhu, K.; Zhao, Y. Facile fabrication of large-grain CH3NH3PbI3−xBrx films for high-efficiency solar cells via CH3NH3Br-selective Ostwald ripening. Nat. Commun. 2016, 7, 12305. [Google Scholar] [CrossRef]
- Cha, M.; Da, P.; Wang, J.; Wang, W.; Chen, Z.; Xiu, F.; Zheng, G.; Wang, Z.-S. Enhancing perovskite solar cell performance by interface engineering using CH3NH3PbBr0.9I2.1 quantum dots. J. Am. Chem. Soc. 2016, 138, 8581–8587. [Google Scholar] [CrossRef]
- Sicot, L.; Fiorini, C.; Lorin, A.; Raimond, P.; Sentein, C.; Nunzi, J.-M. Improvement of the photovoltaic properties of polythiophene-based cells. Sol. Energy Mater. Sol. Cells 2000, 63, 49–60. [Google Scholar] [CrossRef]
- Chen, J.; Park, N.-G. Materials and methods for interface engineering toward stable and efficient perovskite solar cells. ACS Energy Lett. 2020, 5, 2742–2786. [Google Scholar] [CrossRef]
- Feng, S.; Yang, Y.; Li, M.; Wang, J.; Cheng, Z.; Li, J.; Ji, G.; Yin, G.; Song, F.; Wang, Z. High-performance perovskite solar cells engineered by an ammonia modified graphene oxide interfacial layer. ACS Appl. Mater. Interfaces 2016, 8, 14503–14512. [Google Scholar] [CrossRef]
- Wang, Z.-K.; Li, M.; Yuan, D.-X.; Shi, X.-B.; Ma, H.; Liao, L.-S. Improved hole interfacial layer for planar perovskite solar cells with efficiency exceeding 15%. ACS Appl. Mater. Interfaces 2015, 7, 9645–9651. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Mandal, R.; Mandal, D. Impact of HTM on lead-free perovskite solar cell with high efficiency. Opt. Quantum Electron. 2022, 54, 455. [Google Scholar] [CrossRef]
- Shalan, A.E.; Oshikiri, T.; Sawayanagi, H.; Nakamura, K.; Ueno, K.; Sun, Q.; Wu, H.-P.; Diau, E.W.-G.; Misawa, H. Versatile plasmonic-effects at the interface of inverted perovskite solar cells. Nanoscale 2017, 9, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.; Wu, T.; Sun, W.; Wu, J.; Lan, Z. Interface engineering with NiO nanocrystals for highly efficient and stable planar perovskite solar cells. Electrochim. Acta 2019, 293, 211–219. [Google Scholar] [CrossRef]
- Marin-Beloqui, J.M.; Lanzetta, L.; Palomares, E. Decreasing charge losses in perovskite solar cells through mp-TiO2/MAPI interface engineering. Chem. Mater. 2016, 28, 207–213. [Google Scholar] [CrossRef]
- Wang, Y.; Mahmoudi, T.; Rho, W.-Y.; Yang, H.-Y.; Seo, S.; Bhat, K.S.; Ahmad, R.; Hahn, Y.-B. Ambient-air-solution-processed efficient and highly stable perovskite solar cells based on CH3NH3PbI3−xClx-NiO composite with Al2O3/NiO interfacial engineering. Nano Energy 2017, 40, 408–417. [Google Scholar] [CrossRef]
- Du, Y.; Cai, H.; Xing, Z.; Wu, Y.; Xu, J.; Li, Z.; Huang, L.; Ni, J.; Li, J.; Zhang, J. Propelling efficiency and stability of planar perovskite solar cells via Al2O3 interface modification to compact TiO2 layer. Org. Electron. 2017, 51, 249–256. [Google Scholar] [CrossRef]
- Ma, J.; Yang, G.; Qin, M.; Zheng, X.; Lei, H.; Chen, C.; Chen, Z.; Guo, Y.; Han, H.; Zhao, X. MgO nanoparticle modified anode for highly efficient SnO2-based planar perovskite solar cells. Adv. Sci. 2017, 4, 1700031. [Google Scholar] [CrossRef]
- Hou, Y.; Du, X.; Scheiner, S.; McMeekin, D.P.; Wang, Z.; Li, N.; Killian, M.S.; Chen, H.; Richter, M.; Levchuk, I. A generic interface to reduce the efficiency-stability-cost gap of perovskite solar cells. Science 2017, 358, 1192–1197. [Google Scholar] [CrossRef]
- Ciro, J.; Mesa, S.; Uribe, J.I.; Mejía-Escobar, M.A.; Ramirez, D.; Montoya, J.F.; Betancur, R.; Yoo, H.-S.; Park, N.-G.; Jaramillo, F. Optimization of the Ag/PCBM interface by a rhodamine interlayer to enhance the efficiency and stability of perovskite solar cells. Nanoscale 2017, 9, 9440–9446. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, Q.; Li, T.; Gruverman, A.; Huang, J. Thin insulating tunneling contacts for efficient and water-resistant perovskite solar cells. Adv. Mater. 2016, 28, 6734–6739. [Google Scholar] [CrossRef]
- Wolff, C.M.; Zu, F.; Paulke, A.; Toro, L.P.; Koch, N.; Neher, D. Reduced interface-mediated recombination for high open-circuit voltages in CH3NH3PbI3 solar cells. Adv. Mater. 2017, 29, 1700159. [Google Scholar] [CrossRef]
- Li, M.; Yan, X.; Kang, Z.; Huan, Y.; Li, Y.; Zhang, R.; Zhang, Y. Hydrophobic polystyrene passivation layer for simultaneously improved efficiency and stability in perovskite solar cells. ACS Appl. Mater. Interfaces 2018, 10, 18787–18795. [Google Scholar] [CrossRef]
- Koushik, D.; Verhees, W.J.; Kuang, Y.; Veenstra, S.; Zhang, D.; Verheijen, M.A.; Creatore, M.; Schropp, R.E. High-efficiency humidity-stable planar perovskite solar cells based on atomic layer architecture. Energy Environ. Sci. 2017, 10, 91–100. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Liu, J.; Qin, C.; Yang, X.; Islam, A.; Cheng, Y.-B.; Han, L. Hybrid interfacial layer leads to solid performance improvement of inverted perovskite solar cells. Energy Environ. Sci. 2015, 8, 629–640. [Google Scholar] [CrossRef]
- Hamukwaya, S.L.; Hao, H.; Zhao, Z.; Dong, J.; Zhong, T.; Xing, J.; Hao, L.; Mashingaidze, M.M. A Review of Recent Developments in Preparation Methods for Large-Area Perovskite Solar Cells. Coatings 2022, 12, 252. [Google Scholar] [CrossRef]
- Kano, N. Attractive quality and must-be quality. Hinshitsu (Qual. J. Jpn. Soc. Qual. Control) 1984, 14, 39–48. [Google Scholar]
- Onwubiko, I.; Khan, W.S.; Subeshan, B.; Asmatulu, R. Investigating the effects of carbon-based counter electrode layers on the efficiency of hole-transporter-free perovskite solar cells. Energy Ecol. Environ. 2020, 5, 141–152. [Google Scholar] [CrossRef]
- Que, M.; Zhang, B.; Chen, J.; Yin, X.; Yun, S. Carbon-based electrodes for perovskite solar cells. Mater. Adv. 2021, 2, 5560–5579. [Google Scholar] [CrossRef]
- Cai, Y.; Liang, L.; Gao, P. Promise of commercialization: Carbon materials for low-cost perovskite solar cells. Chin. Phys. B 2018, 27, 018805. [Google Scholar] [CrossRef]
- Tsarev, S.; Boldyreva, A.G.; Luchkin, S.Y.; Elshobaki, M.; Afanasov, M.I.; Stevenson, K.J.; Troshin, P.A. Hydrazinium-assisted stabilisation of methylammonium tin iodide for lead-free perovskite solar cells. J. Mater. Chem. A 2018, 6, 21389–21395. [Google Scholar] [CrossRef]
- Zuo, C.; Ding, L. Lead-free Perovskite Materials (NH4)3Sb2IxBr9−x. Angew. Chem. 2017, 129, 6628–6632. [Google Scholar] [CrossRef]
- Lyu, M.; Yun, J.-H.; Cai, M.; Jiao, Y.; Bernhardt, P.V.; Zhang, M.; Wang, Q.; Du, A.; Wang, H.; Liu, G. Organic–inorganic bismuth (III)-based material: A lead-free, air-stable and solution-processable light-absorber beyond organolead perovskites. Nano Res. 2016, 9, 692–702. [Google Scholar] [CrossRef] [Green Version]












| No. | Title | Journal | Year | References |
|---|---|---|---|---|
| 01 | Recent progress of inorganic hole transport materials for efficient and stable perovskite solar cells | Nano Select | 2021 | [41] |
| 02 | A brief review of hole transporting materials commonly used in perovskite solar cells | Rare Metals | 2021 | [42] |
| 03 | Nickel Oxide for Perovskite Photovoltaic Cells | Advanced Photonics Research | 2021 | [43] |
| 04 | Toward efficient and stable operation of perovskite solar cells: Impact of sputtered metal oxide interlayers | Nano Select | 2021 | [44] |
| 05 | Inorganic hole transport layers in inverted perovskite solar cells: A review | Nano Select | 2021 | [45] |
| 06 | Progress, highlights, and perspectives on NiO in perovskite photovoltaics | Chemical Science | 2020 | [46] |
| 07 | A review on the classification of organic/inorganic/carbonaceous hole-transporting materials for perovskite solar cell application | Arabian Journal of Chemistry | 2020 | [47] |
| 08 | Review of current progress in inorganic hole-transport materials for perovskite solar cells | Applied Materials Today | 2019 | [48] |
| 09 | Recent progress of inorganic perovskite solar cells | Energy & Environmental Science | 2019 | [49] |
| 10 | Inorganic hole transporting materials for stable and high efficiency perovskite solar cells | The Journal of Physical Chemistry C | 2018 | [50] |
| 11 | Analysing the prospects of perovskite solar cells within the purview of recent scientific advancements | Crystals | 2018 | [51] |
| 12 | Recent progress in stability of perovskite solar cells | Journal of Semiconductors | 2017 | [52] |
| 13 | Emerging of inorganic hole transporting materials for perovskite solar cells | The Chemical Record | 2017 | [53] |
| 14 | Recent advances in the inverted planar structure of perovskite solar cells | Accounts of chemical research | 2016 | [54] |
| 15 | The progress of interface design in perovskite-based solar cells | Advanced Energy Materials | 2016 | [55] |
| 16 | Recent progress on hole-transporting materials for emerging organometal halide perovskite solar cells | Advanced Energy Materials | 2015 | [36] |
| HTMs | Mobility (cm2/V-S) | Price (per Gram, USD) | Reference |
|---|---|---|---|
| Spiro-OMeTAD | 4 × 10−5 | 422 | [82] |
| PTAA:poly(triarylamine) | 10−2–10−3 | 1145 | [83] |
| PEDOT:PSS | 1 × 10−2 | 166 | [84] |
| NiOx | 0.14 | 14 | [85] |
| V2O5 | 0.23 | 49 | [86] |
| MoO3 | 0.4 | 22 | [32] |
| WO3 | 0.25 | 15.6 | [87] |
| Cu2O | 100 | 2.96 | [53] |
| CuO | 0.129 | 2.96 | [53] |
| CuSCN | 0.01–0.1 | 3 | [53] |
| Cu2ZnSnS4 | 6.0–30 | - | [53] |
| CuAlO2 | 3.6 | 5.16 | [88] |
| CuCrO2 | 7.6 | 22.4 | [89] |
| CuGaO2 | 0.01–10 | 3.49 | [30] |
| Device Structure | Deposition Technique | Efficiency (%) | FF | VOC [V] | JOC (J-V) [mAcm−2] | Year | References |
|---|---|---|---|---|---|---|---|
| NiOx/F2HCNQ | Spin coating | 22.13 | 0.82 | 1.14 | 23.44 | 2020 | [107] |
| ITO/SnO2/(FAPbI3)x(MAPbBr3)1−x/NiOx/spiro-OMeTAD/Au | Spin coating | 21.66 | 0.79 | 1.14 | 23.82 | 2020 | [106] |
| ITO/NiOx/MA1-yFAyPbI3-xClx/2D-3D perovskite/PCBM/BCP/Ag | Spin coating | 21.4 | 0.83 | 1.12 | 23.1 | 2019 | [108] |
| FTO/NiOx/PVK/PCBM/ZrAcac/Al | Spin coating | 20.5 | 0.77 | 1.12 | 23.07 | 2017 | [109] |
| ITO/NiOx/MSs/perovskite/PC61BM/BCP/Ag | - | 20.34 | 0.80 | 1.12 | 22.34 | 2021 | [112] |
| NiO/TSPA(p-i-n) | Spin coating | 20.21 | - | - | - | 2021 | [113] |
| FTO/Cs:NiOx/PVK/PCBM/ZrAcac/Ag | Spin coating | 19.35 | 0.79 | 1.12 | 21.77 | 2017 | [110] |
| p-i-n | Spin coated | 19.0 | 0.77 | 1.05 | 23.17 | 2019 | [92] |
| ITO/NiOx/PVK/PCBM/ZrAcac/Al | Spin coating | 18.69 | 0.78 | 1.079 | 22.17 | 2017 | [114] |
| ITO/Cu:NiOx/PVK/PCBM/BCP/Ag | Spin coating | 18.66 | 0.81 | 1.11 | 20.76 | 2017 | [115] |
| FTO/NiOx/PVK/PCBM/Ag | Spin coating | 18.6 | 0.75 | 1.09 | 22.8 | 2017 | [116] |
| ITO/NiOx/Psk/PCBM/Au | Spin coating | 18.23 | 0.47 | 0.79 | 6.4 | 2016 | [117] |
| ITO/NiOx/PVK/PCBM/c-HATNA/Bis-C60/Ag | Spin coating | 18.21 | 0.79 | 1.09 | 21.25 | 2018 | [118] |
| FTO/cp-TiO2/mp-TiO2/mp-NiO/Psk/carbon (n-i-p) | Spin coating | 18.2 | 0.71 | 0.89 | 11.4 | 2016 | [119] |
| ITO/NiOx/Psk/PCBM/C60/BCP/Al | Spin coating | 18 | 0.56 | 1.06 | 10.6 | 2016 | [100] |
| ITO/NiOx/PVK/PCBM/Al | Spin coating | 18.0 | 0.74 | 1.12 | 21.79 | 2018 | [120] |
| ITO/NiOx/PVK/PCBM/Ag | Spin coating | 17.2 | 0.78 | 1.03 | 21.4 | 2017 | [121] |
| ITO/NiOx/PVK/PCBM/Ag | Spin coating | 16.55 | 0.75 | 1.04 | 21.22 | 2017 | [122] |
| ITO/NiOx/Psk/PCBM/Ag | Spin coating | 16.47 | 0.75 | 1.07 | 20.58 | 2016 | [123] |
| - | Spin coating | 16.4 | 0.67 | 1.12 | 21.8 | 2020 | [123] |
| ITO/NiOx/PVK/ZnO/Al | Spin coating | 16.1 | 0.76 | 1.01 | 21.01 | 2016 | [119] |
| ITO/NiOx/PVK/PCBM/LiF/Al | Spin coating | 13.4 | 0.69 | 1.03 | 19 | 2016 | [124] |
| ITO/NiOx/CH3NH3PbI3/PCBM60/ZnO NPs/BCP/Al | Spin coating | 13 | 0.61 | 1.03 | 21 | 2021 | [125] |
| FTO/cp-TiO2/mp-TiO2/mp-NiO/Psk/carbon | Spin coating | 11.4 | 0.71 | 0.89 | 18.2 | 2016 | [126] |
| ITO/NiOx/MAPbI3/PCBM/BCP/Ag | Sputtering | 17.6 | 0.79 | 1.07 | 20.65 | 2018 | [111] |
| FTO/Co:NiOx/MAPbI3/PCBM/Ag | Sputtering | 12.63 | 0.63 | 1.01 | 20.02 | 2016 | [127] |
| ITO/Li and Co:NiOx/MA1-yFAyPbI3-xClx/PCBM/BCP/Ag | Solution combustion process | 20.1 | 0.78 | 1.09 | 23.8 | 2019 | [96] |
| ITO/NiOx/CsBr/MA1-yFAyPbI3-x Clx/PCBM/BCP/Ag | Solution combustion process | 19.7 | 0.75 | 1.09 | 23.5 | 2020 | [91] |
| FTO/Sr:NiOx/MAPbI3/PCBM/BCP/Ag | Solution process | 19.49 | 0.75 | 1.14 | 22.66 | 2019 | [97] |
| FTO/K:NiOx/MAPbIxBr3-x/PCBM:C60/BCP/Ag | Solution process | 18.05 | 0.78 | 1.01 | 22.77 | 2019 | [98] |
| ITO/PLD-NiOx/Psk/PCBM/LiF/Al | e-beam evaporation | 17.3 | 0.81 | 1.06 | 20.2 | 2015 | [128] |
| Planar p-i-n FTO/NiOx/FAPbI3/PCBM/TiOx/Ag | Spraying | 20.65 | 0.81 | 1.10 | 23.09 | 2017 | [85] |
| FTO/NiOx/PVK/PCBM/Ag | Spray pyrolysis | 19.58 | 0.77 | 1.12 | 22.68 | 2017 | [129] |
| ITO/NiOx/Cs0.05MA0.95PbI3/PCBM/ BCP/AZO/Ag | Atomic layer deposition | 18.4 | 0.78 | 1.05 | 22.56 | 2018 | [99] |
| ITO/NiOx/CsMAFAPbI3-xBrx/C60/BCP/Cu | Atomic layer Deposition | 17.07 | 0.73 | 1.07 | 21.75 | 2019 | [130] |
| ITO/NiOx/MAPbI3/PCBM/Ag | Atomic layer deposition | 16.4 | 0.72 | 1.04 | 21.9 | 2016 | [131] |
| ITO/NiOx/PVK/PCBM/BCP/Ag | Vacuum deposition | 15.4 | 0.78 | 1.06 | 18.6 | 2018 | [132] |
| Planar p-i-n ITO/NiOx/PVK/PCBM/C60/BCP/Al | Vacuum thermal evaporation | 10.6 | 0.56 | 1.06 | 18 | 2016 | [100] |
| ITO/NiOx/PVK/PCBM/Ag | Electrodeposition | 17.1 | 0.72 | 1.05 | 22.6 | 2017 | [101] |
| Mesoscopic n-i-p FTO/c-TiO2/m-TiO2/PVK:NiO-MWCNTs | Drop casting | 15.38 | 0.76 | 0.91 | 22.38 | 2017 | [102] |
| Device Structure | Fabrication Processes | Efficiency (%) | FF | VOC | JSC | Year | References |
|---|---|---|---|---|---|---|---|
| ITO/CuOx/Psk/PCBM/C60/ BCP/Ag | Spin coating | 19.0 | 0.758 | 1.11 | 22.5 | 2016 | [133] |
| FTO/SnO2/ PCBM/MAPbI3/FBT-Th4/CuxO/Au | Thermal evaporation | 18.85 | 0.75 | 1.12 | 22.35 | 2018 | [134] |
| ITO/CuOx/Psk/PC61BM/ZnO/Al | Vapor deposition | 17.43 | 0.76 | 1.03 | 22.42 | 2017 | [136] |
| ITO/CuOx/Psk/C60/BCP/Ag | Spin coating | 17.1 | 0.744 | 0.99 | 23.2 | 2016 | [28] |
| ITO/Cu2O/Psk/PCBM/Ag | Sputtering | 11.03 | 0.662 | 0.95 | 17.5 | 2016 | [137] |
| ITO/Cu2O/Psk/C60/Bphen/Ag | Electrodeposition | 9.64 | 0.61 | 0.88 | 18.03 | 2016 | [138] |
| FTO/TiO2/Psk/Cu2O/Au | Sputtering | 8.93 | 0.59 | 0.96 | 15.8 | 2016 | [139] |
| ITO/Cu2O/Psk/PCBM/Al | SILAR | 8.23 | 0.56 | 0.89 | 16.52 | 2016 | [140] |
| ITO/CuO–Cu2O/Psk/C60/BCP/Ag | Sputtering | 8.1 | 0.586 | 0.96 | 14.4 | 2016 | [141] |
| ITO/CuOx/Psk/C60/BCP/Al | Electrospray | 5.83 | 0.48 | 0.7 | 17.22 | 2017 | [142] |
| ITO/CuOx/Psk/PCBM/C60/BCP/Ag | Spin coating | 19.0 | 0.758 | 1.11 | 22.5 | 2016 | [133] |
| IHTMs | Deposition Technique | Highest Efficiency (%) | Highest Stability | References |
|---|---|---|---|---|
| NiOx | Spin-coating | 21.66 | 90% over 1200 h | [91] |
| Sputtering | 17.6 | - | [111] | |
| Spray pyrolysis | 20.65 | 90% over 500 h | [91] | |
| Solution combustion process | 20.1 | - | [96] | |
| Atomic layer deposition | 18.4 | 86.7% over 500 h | [99] | |
| Others | 17.1 | - | [101] | |
| CuO | Spin-coating | 19.0 | - | [133] |
| Sputtering | 11.03 | 40% over 500 h | [137] | |
| Vapor deposition | 17.43 | 90% over 500 h | [136] | |
| Thermal evaporation | 18.85 | 90% over 500 h | [133] | |
| Electrodeposition | 9.64 | - | [138] | |
| Graphene oxide | Solution process | 16.5 | 80% over 20,000 h | [143] |
| CoO | Solution process | 145 | 80% over 1000 h | [149] |
| Skin printing | 13.27 | 2500 h | [150] | |
| CrO | Solution process | 15.3 | - | [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahinuzzaman, M.; Afroz, S.; Mohafez, H.; Jamal, M.S.; Khandaker, M.U.; Sulieman, A.; Tamam, N.; Islam, M.A. Roles of Inorganic Oxide Based HTMs towards Highly Efficient and Long-Term Stable PSC—A Review. Nanomaterials 2022, 12, 3003. https://doi.org/10.3390/nano12173003
Shahinuzzaman M, Afroz S, Mohafez H, Jamal MS, Khandaker MU, Sulieman A, Tamam N, Islam MA. Roles of Inorganic Oxide Based HTMs towards Highly Efficient and Long-Term Stable PSC—A Review. Nanomaterials. 2022; 12(17):3003. https://doi.org/10.3390/nano12173003
Chicago/Turabian StyleShahinuzzaman, M., Sanjida Afroz, Hamidreza Mohafez, M. S. Jamal, Mayeen Uddin Khandaker, Abdelmoneim Sulieman, Nissren Tamam, and Mohammad Aminul Islam. 2022. "Roles of Inorganic Oxide Based HTMs towards Highly Efficient and Long-Term Stable PSC—A Review" Nanomaterials 12, no. 17: 3003. https://doi.org/10.3390/nano12173003







