Scanning Tunneling Microscopy of Biological Structures: An Elusive Goal for Many Years
Abstract
:1. Introduction
2. STM Imaging Formation and Contrast Mechanisms
3. Imaging of Biomolecules by STM
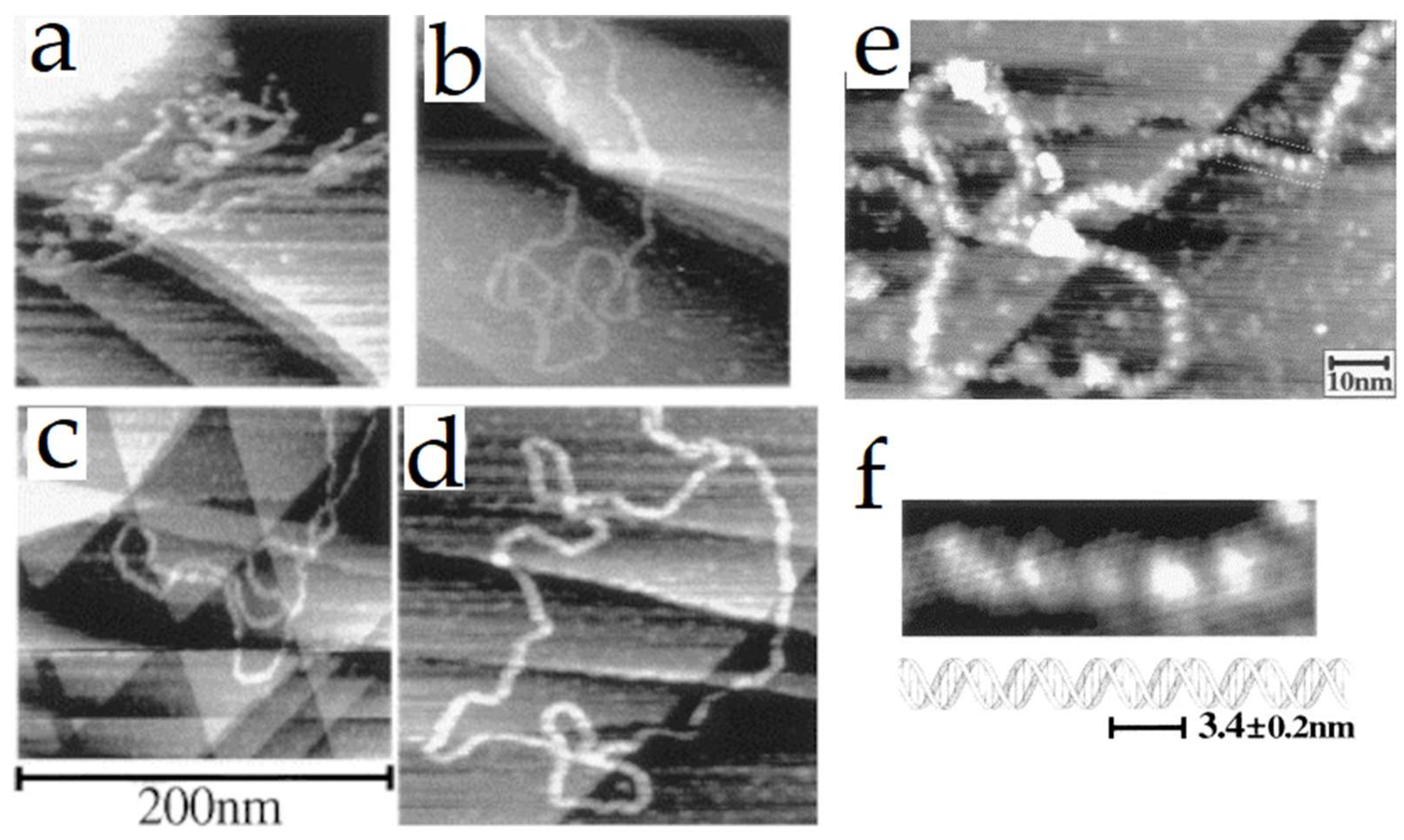
3.1. DNA
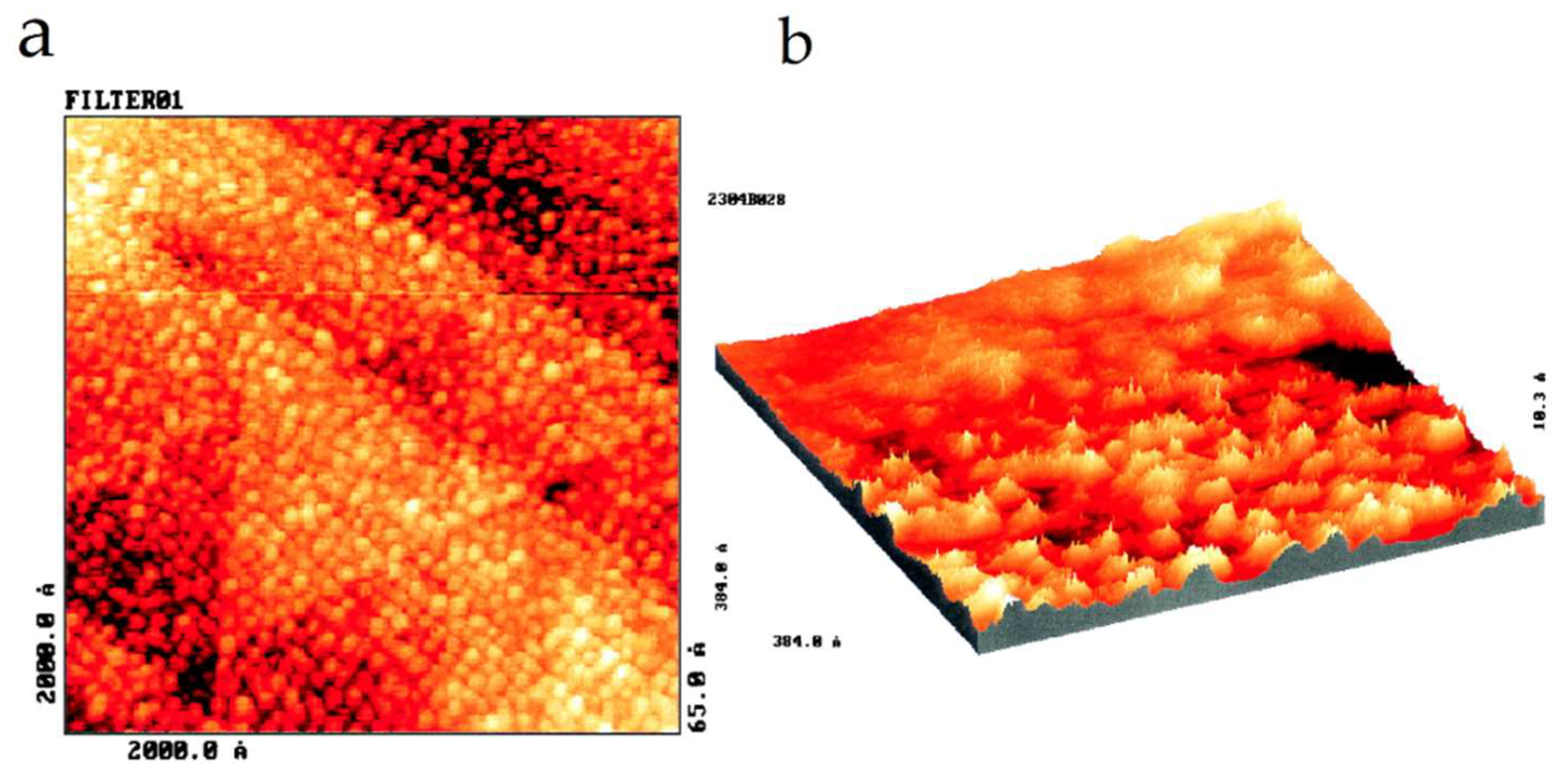
3.2. Lipids
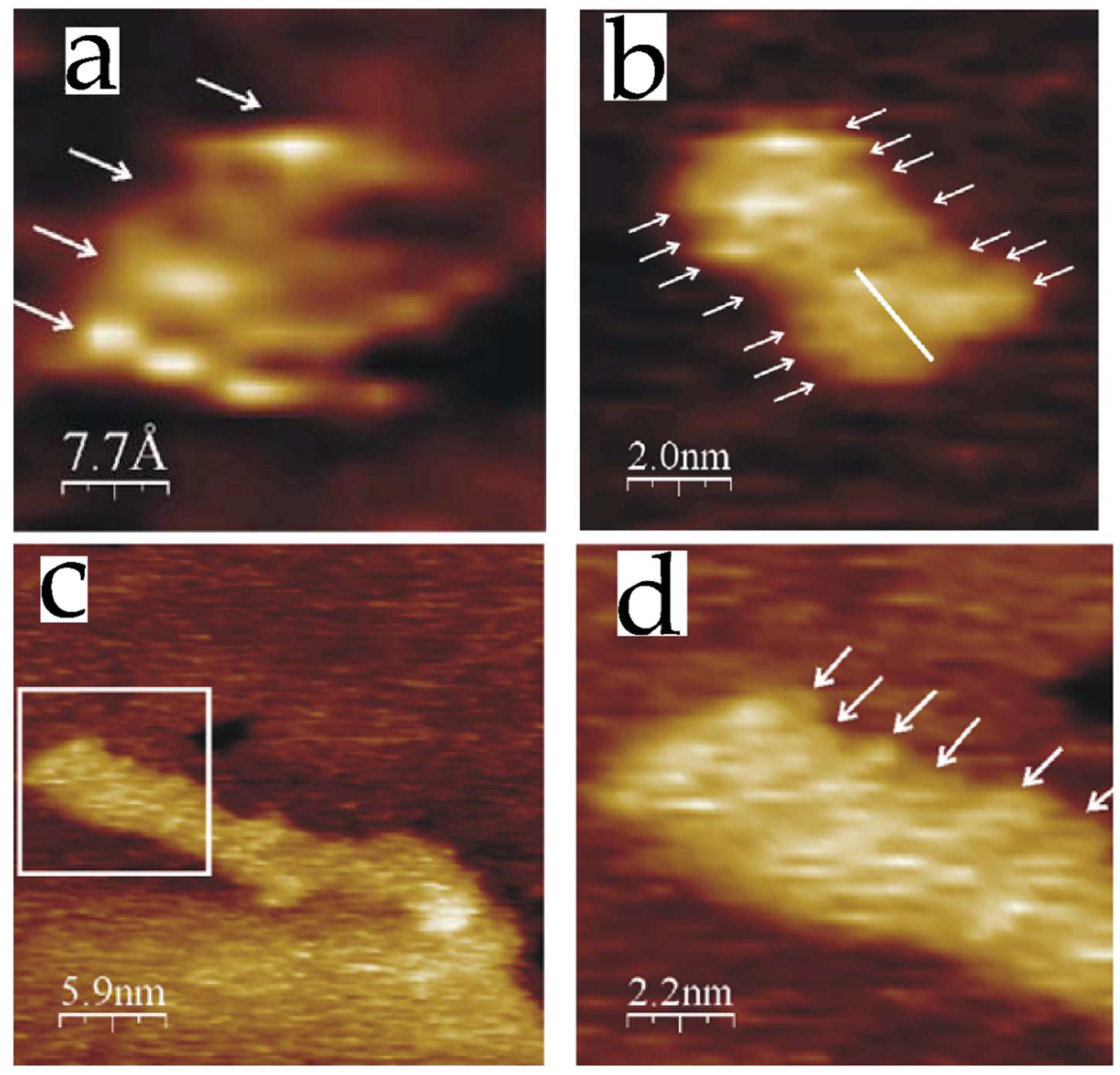
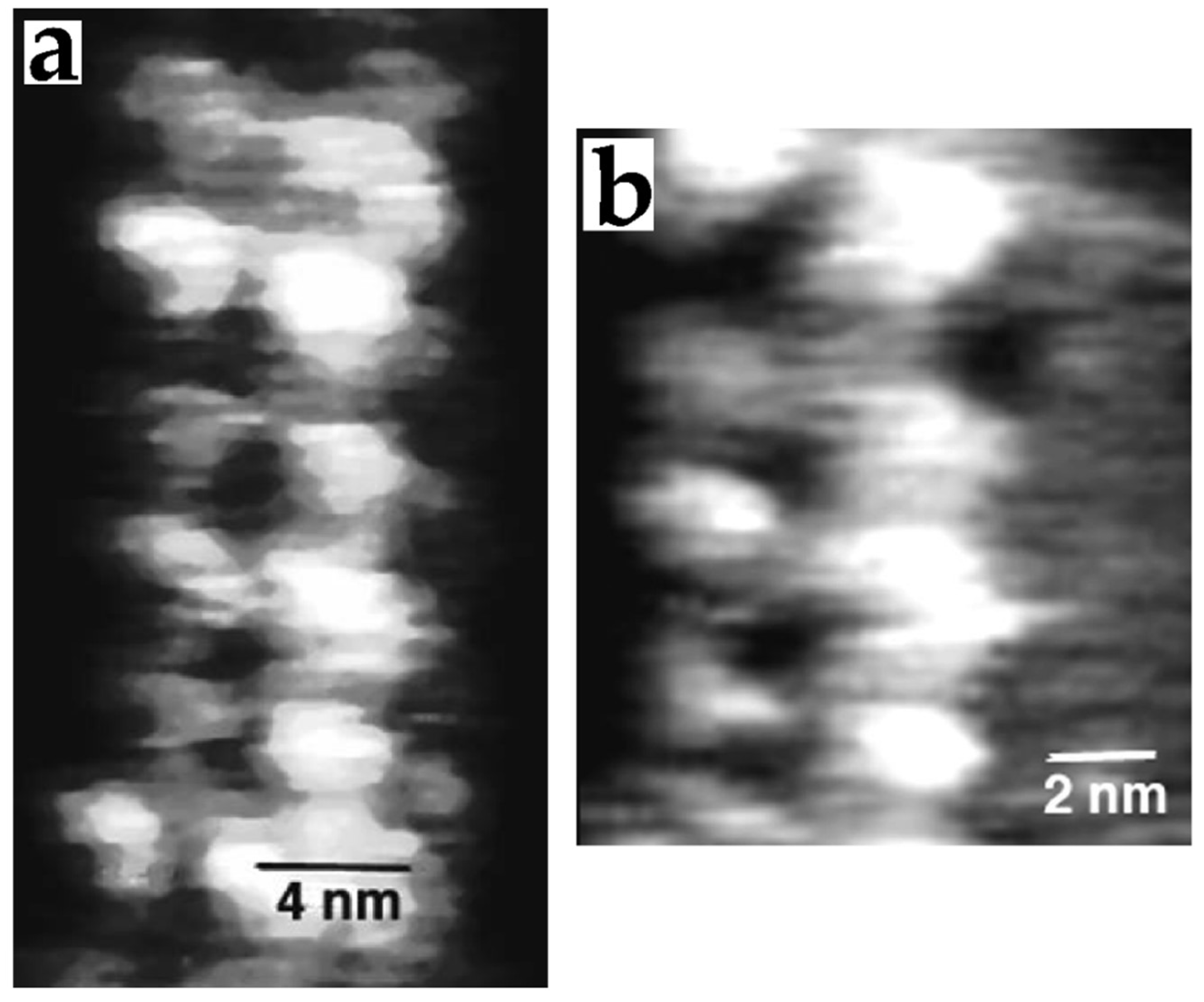
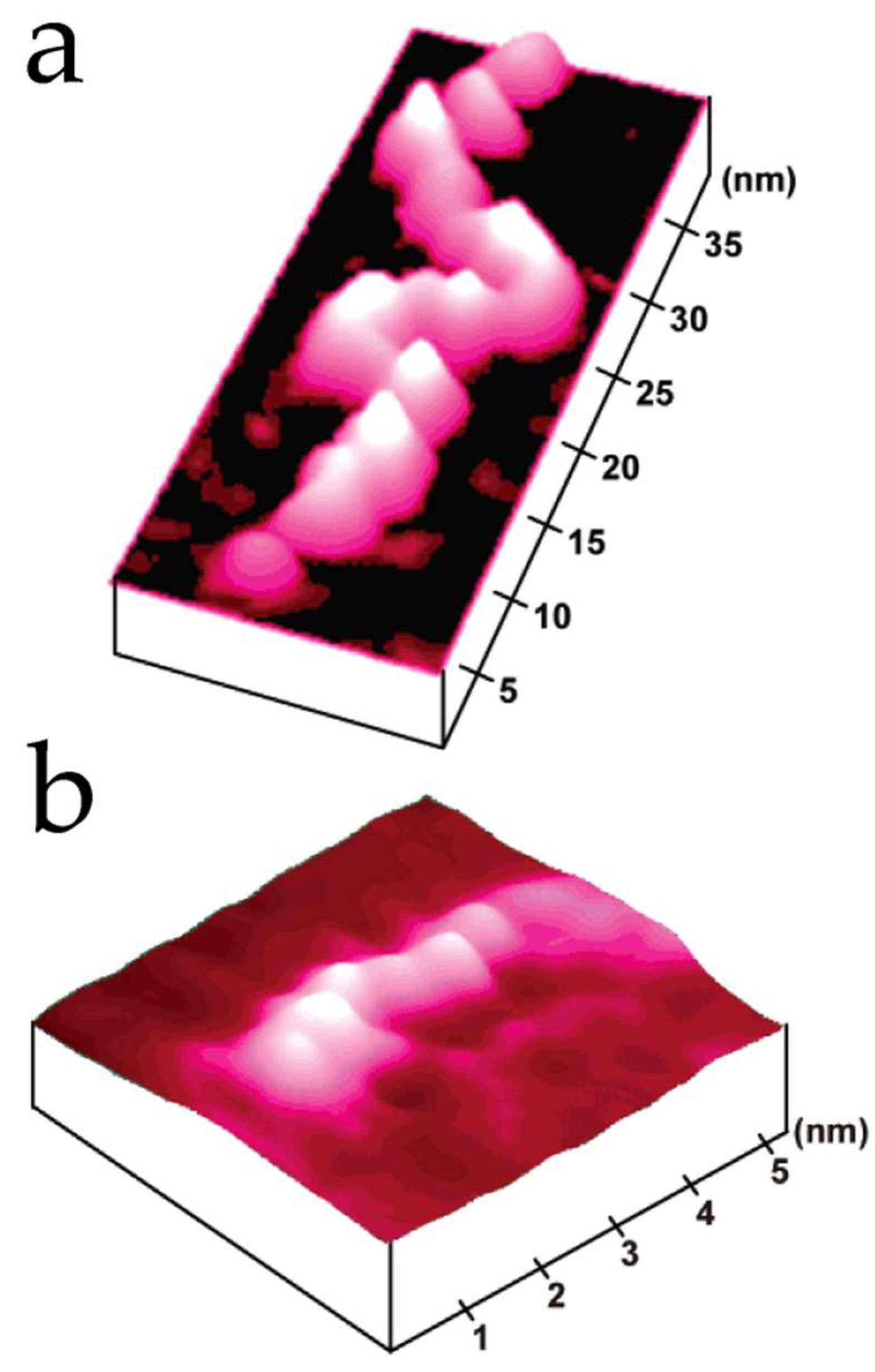
3.3. Proteins
3.4. Carbohydrates
4. Relevance of STM Imaging in Biological Applications
5. Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Dumont, S.; Prakash, M. Emergent Mechanics of Biological Structures. Mol. Biol. Cell 2014, 25, 3461–3465. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.N.; Fromme, P.; Barty, A.; White, T.A.; Kirian, R.A.; Aquila, A.; Hunter, M.S.; Schulz, J.; DePonte, D.P.; Weierstall, U.; et al. Femtosecond X-ray Protein Nanocrystallography. Nature 2011, 470, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Selenko, P. Quo Vadis Biomolecular NMR Spectroscopy? Int. J. Mol. Sci. 2019, 20, 1278. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Falqui, A.; Moretti, M.; Limongi, T.; Allione, M.; Genovese, A.; Lopatin, S.; Tirinato, L.; Das, G.; Torre, B.; et al. The Structure of DNA by Direct Imaging. Sci. Adv. 2015, 1, e1500734. [Google Scholar] [CrossRef] [PubMed]
- Kermani, A.A. A Guide to Membrane Protein X-ray Crystallography. FEBS J. 2021, 288, 5788–5804. [Google Scholar] [CrossRef]
- Frueh, D.P.; Goodrich, A.C.; Mishra, S.H.; Nichols, S.R. NMR methods for structural studies of large monomeric and multimeric proteins. Curr. Opin. Struct. Biol. 2013, 23, 734–739. [Google Scholar] [CrossRef]
- Longchamp, J.-N.; Rauschenbach, S.; Abb, S.; Escher, C.; Latychevskaia, T.; Kern, K.; Fink, H.-W. Imaging Proteins at the Single-Molecule Level. Proc. Natl. Acad. Sci. USA 2017, 114, 1474–1479. [Google Scholar] [CrossRef]
- Bian, K.; Gerber, C.; Heinrich, A.J.; Müller, D.J.; Scheuring, S.; Jiang, Y. Scanning Probe Microscopy. Nat. Rev. Methods Primer 2021, 1, 36. [Google Scholar] [CrossRef]
- Hansma, P.K.; Elings, V.B.; Marti, O.; Bracker, C.E. Scanning Tunneling Microscopy and Atomic Force Microscopy: Application to Biology and Technology. Science 1988, 242, 209–216. [Google Scholar] [CrossRef]
- Sonnenfeld, R.; Hansma, P.K. Atomic-Resolution Microscopy in Water. Science 1986, 232, 211–213. [Google Scholar] [CrossRef]
- Rodríguez-Galván, A.; Heredia, A.; Plascencia-Villa, G.; Ramírez, O.T.; Palomares, L.A.; Basiuk, V.A. Scanning Tunneling Microscopy of Rotavirus VP6 Protein Self-Assembled into Nanotubes and Nanospheres. J. Scanning Probe Microsc. 2008, 3, 25–31. [Google Scholar] [CrossRef]
- Maaloum, M.; Chretien, D.; Karsenti, E.; Horber, J.K. Approaching Microtubule Structure with the Scanning Tunneling Microscope (STM). J. Cell Sci. 1994, 107, 3127–3131. [Google Scholar] [CrossRef]
- Engel, A.; Müller, D.J. Observing Single Biomolecules at Work with the Atomic Force Microscope. Nat. Struct. Biol. 2000, 7, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Gómez-Varela, A.I.; Stylianou, A.; Hirvonen, L.M.; Sánchez, H.; Beule, P.A.A.D. How Did Correlative Atomic Force Microscopy and Super-Resolution Microscopy Evolve in the Quest for Unravelling Enigmas in Biology? Nanoscale 2021, 13, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.J.; Hill, H.A.O. The Scanning Probe Microscopy of Metalloproteins and Metalloenzymes. Chem. Commun. 2002, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Hofer, W.A. Challenges and Errors: Interpreting High Resolution Images in Scanning Tunneling Microscopy. Prog. Surf. Sci. 2003, 71, 147–183. [Google Scholar] [CrossRef]
- Spong, J.K.; Mizes, H.A.; LaComb Jr, L.J.; Dovek, M.M.; Frommer, J.E.; Foster, J.S. Contrast Mechanism for Resolving Organic Molecules with Tunnelling Microscopy. Nature 1989, 338, 137–139. [Google Scholar] [CrossRef]
- Miles, M.J.; McMaster, T.; Carr, H.J.; Tatham, A.S.; Shewry, P.R.; Field, J.M.; Belton, P.S.; Jeenes, D.; Hanley, B.; Whittam, M.; et al. Scanning Tunneling Microscopy of Biomolecules. J. Vac. Sci. Technol. A 1990, 8, 698–702. [Google Scholar] [CrossRef]
- Guckenberger, R.; Heim, M.; Cevc, G.; Knapp, H.; Wiegrabe, W.; Hillebrand, A. Scanning Tunneling Microscopy of Insulators and Biological Specimens Based on Lateral Conductivity of Ultrathin Water Films. Science 1994, 266, 1538–1540. [Google Scholar] [CrossRef]
- Baxter, S. Electrical Conduction of Textiles. Trans. Faraday Soc. 1943, 39, 207–214. [Google Scholar] [CrossRef]
- Rosenberg, B. Electrical Conductivity of Proteins. II. Semiconduction in Crystalline Bovine Hemoglobin. J. Chem. Phys. 1962, 36, 816–823. [Google Scholar] [CrossRef]
- Yuan, J.-Y.; Shao, Z.; Gao, C. Alternative Method of Imaging Surface Topologies of Nonconducting Bulk Specimens by Scanning Tunneling Microscopy. Phys. Rev. Lett. 1991, 67, 863–866. [Google Scholar] [CrossRef]
- Cross, G.; Schirmeisen, A.; Stalder, A.; Grütter, P.; Tschudy, M.; Dürig, U. Adhesion Interaction between Atomically Defined Tip and Sample. Phys. Rev. Lett. 1998, 80, 4685–4688. [Google Scholar] [CrossRef]
- Dunlap, D.D.; García, R.; Schabtach, E.; Bustamante, C. Masking Generates Contiguous Segments of Metal-Coated and Bare DNA for Scanning Tunneling Microscope Imaging. Proc. Natl. Acad. Sci. USA 1993, 90, 7652–7655. [Google Scholar] [CrossRef]
- Chaika, A.N.; Orlova, N.N.; Semenov, V.N.; Postnova, E.Y.; Krasnikov, S.A.; Lazarev, M.G.; Chekmazov, S.V.; Aristov, V.Y.; Glebovsky, V.G.; Bozhko, S.I.; et al. Fabrication of [001]-Oriented Tungsten Tips for High Resolution Scanning Tunneling Microscopy. Sci. Rep. 2014, 4, 3742. [Google Scholar] [CrossRef] [PubMed]
- Barinov, N.A.; Prokhorov, V.V.; Dubrovin, E.V.; Klinov, D.V. AFM Visualization at a Single-Molecule Level of Denaturated States of Proteins on Graphite. Colloids Surf. B Biointerfaces 2016, 146, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Kaliyaraj Selva Kumar, A.; Zhang, Y.; Li, D.; Compton, R.G. A Mini-Review: How Reliable Is the Drop Casting Technique? Electrochem. Commun. 2020, 121, 106867. [Google Scholar] [CrossRef]
- Frommer, J. Scanning Tunneling Microscopy and Atomic Force Microscopy in Organic Chemistry. Angew. Chem. Int. Ed. 1992, 31, 1298–1328. [Google Scholar] [CrossRef]
- Rauschenbach, S.; Ternes, M.; Harnau, L.; Kern, K. Mass Spectrometry as a Preparative Tool for the Surface Science of Large Molecules. Annu. Rev. Anal. Chem. 2016, 9, 473–498. [Google Scholar] [CrossRef]
- Dürig, U.; Züger, O.; Pohl, D.W. Force Sensing in Scanning Tunnelling Microscopy: Observation of Adhesion Forces on Clean Metal Surfaces. J. Microsc. 1988, 152, 259–267. [Google Scholar] [CrossRef]
- Dürig, U.; Züger, O.; Pohl, D.W. Observation of Metallic Adhesion Using the Scanning Tunneling Microscope. Phys. Rev. Lett. 1990, 65, 349–352. [Google Scholar] [CrossRef]
- Chen, C.J. Introduction to Scanning Tunneling Microscopy, 2nd ed.; Monographs on the Physics and Chemistry of Materials; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Loppacher, C.; Bammerlin, M.; Guggisberg, M.; Schär, S.; Bennewitz, R.; Baratoff, A.; Meyer, E.; Güntherodt, H.-J. Dynamic Force Microscopy of Copper Surfaces: Atomic Resolution and Distance Dependence of Tip-Sample Interaction and Tunneling Current. Phys. Rev. B 2000, 62, 16944–16949. [Google Scholar] [CrossRef]
- Schirmeisen, A.; Cross, G.; Stalder, A.; Grütter, P.; Dürig, U. Metallic Adhesion and Tunnelling at the Atomic Scale. New J. Phys. 2000, 2, 329. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Ondráček, M.; Abe, M.; Pou, P.; Morita, S.; Perez, R.; Flores, F.; Jelínek, P. Quantum Degeneracy in Atomic Point Contacts Revealed by Chemical Force and Conductance. Phys. Rev. Lett. 2013, 111, 106803. [Google Scholar] [CrossRef]
- Ashino, M.; Obergfell, D.; Haluška, M.; Yang, S.; Khlobystov, A.N.; Roth, S.; Wiesendanger, R. Atomically Resolved Mechanical Response of Individual Metallofullerene Molecules Confined inside Carbon Nanotubes. Nat. Nanotechnol. 2008, 3, 337–341. [Google Scholar] [CrossRef]
- Gross, L.; Mohn, F.; Moll, N.; Liljeroth, P.; Meyer, G. The Chemical Structure of a Molecule Resolved by Atomic Force Microscopy. Science 2009, 325, 1110–1114. [Google Scholar] [CrossRef]
- Church, G.M.; Gao, Y.; Kosuri, S. Next-Generation Digital Information Storage in DNA. Science 2012, 337, 1628. [Google Scholar] [CrossRef]
- Travaglini, G.; Rohrer, H.; Amrein, M. Scanning Tunneling Microscopy on Biological Matter. Surf. Sci. 1987, 181, 380–390. [Google Scholar] [CrossRef]
- Driscoll, R.J.; Youngquist, M.G.; Baldeschwieler, J.D. Atomic-Scale Imaging of DNA Using Scanning Tunnelling Microscopy. Nature 1990, 346, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Bard, A.J. Observation and Characterization by Scanning Tunneling Microscopy of Structures Generated by Cleaving Highly Oriented Pyrolytic Graphite. Langmuir 1991, 7, 1143–1153. [Google Scholar] [CrossRef]
- Tanaka, H.; Hamai, C.; Kanno, T.; Kawai, T. High-Resolution Scanning Tunneling Microscopy Imaging of DNA Molecules on Cu(111) Surfaces. Surf. Sci. 1999, 432, L611–L616. [Google Scholar] [CrossRef]
- Tanaka, H.; Kawai, T. Visualization of Detailed Structures within DNA. Surf. Sci. 2003, 539, L531–L536. [Google Scholar] [CrossRef]
- Jing, T.W.; Jeffrey, A.M.; DeRose, J.A.; Lyubchenko, Y.L.; Shlyakhtenko, L.S.; Harrington, R.E.; Appella, E.; Larsen, J.; Vaught, A.; Rekesh, D. Structure of Hydrated Oligonucleotides Studied by in Situ Scanning Tunneling Microscopy. Proc. Natl. Acad. Sci. USA 1993, 90, 8934–8938. [Google Scholar] [CrossRef]
- Wang, H.; Tang, Z.; Li, Z.; Wang, E. Self-Assembled Monolayer of SsDNA on Au(111) Substrate. Surf. Sci. 2001, 480, L389–L394. [Google Scholar] [CrossRef]
- Shapir, E.; Cohen, H.; Borovok, N.; Kotlyar, A.B.; Porath, D. High-Resolution STM Imaging of Novel Poly(G)−Poly(C) DNA Molecules. J. Phys. Chem. B 2006, 110, 4430–4433. [Google Scholar] [CrossRef]
- Shapir, E.; Cohen, H.; Calzolari, A.; Cavazzoni, C.; Ryndyk, D.A.; Cuniberti, G.; Kotlyar, A.; Di Felice, R.; Porath, D. Electronic Structure of Single DNA Molecules Resolved by Transverse Scanning Tunnelling Spectroscopy. Nat. Mater. 2008, 7, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Mielke, S.P.; Benham, C.J.; Kawai, T. Visualization of the Detailed Structure of Plasmid DNA. J. Phys. Chem. B 2008, 112, 16788–16792. [Google Scholar] [CrossRef]
- Tanaka, H.; Kawai, T. Partial Sequencing of a Single DNA Molecule with a Scanning Tunnelling Microscope. Nat. Nanotechnol. 2009, 4, 518–522. [Google Scholar] [CrossRef]
- Abel, G.R.; Korshoj, L.E.; Otoupal, P.B.; Khan, S.; Chatterjee, A.; Nagpal, P. Nucleotide and Structural Label Identification in Single RNA Molecules with Quantum Tunneling Spectroscopy. Chem. Sci. 2019, 10, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Fardian-Melamed, N.; Eidelshtein, G.; Rotem, D.; Kotlyar, A.; Porath, D. Scanning Tunneling Microscopy and Spectroscopy of Novel Silver–Containing DNA Molecules. Adv. Mater. 2019, 31, 1902816. [Google Scholar] [CrossRef]
- Fardian-Melamed, N.; Eidelshtein, G.; Rotem, D.; Kotlyar, A.; Porath, D. Temperature Dependence of the STM Morphology and Electronic Level Structure of Silver-Containing DNA. Small 2020, 16, 1905901. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, C.; Cao, F.; Ren, J.; Qu, X. DNA Metallization: Principles, Methods, Structures, and Applications. Chem. Soc. Rev. 2018, 47, 4017–4072. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Smith, D.P.; Bryant, A.; Quate, C.F.; Rabe, J.P.; Gerber, C.; Swalen, J.D. Images of a Lipid Bilayer at Molecular Resolution by Scanning Tunneling Microscopy. Proc. Natl. Acad. Sci. USA 1987, 84, 969–972. [Google Scholar] [CrossRef]
- Hörber, J.K.H.; Lang, C.A.; Hänsch, T.W.; Heckl, W.M.; Möhwald, H. Scanning Tunneling Microscopy of Lipid Films and Embedded Biomolecules. Chem. Phys. Lett. 1988, 145, 151–158. [Google Scholar] [CrossRef]
- Matsuda, H.; Kishi, E.; Kuroda, R.; Albrecht, O.; Eguchi, K.; Hatanaka, K.; Nakagiri, T. Parallel Arrangement of Fatty Acid Molecules in Films Deposited at a Lower Surface Pressure. Thin Solid Films 1993, 224, 248–252. [Google Scholar] [CrossRef]
- Woodward IV, J.T.; Zasadzinski, J.A. High-Resolution Scanning Tunneling Microscopy of Fully Hydrated Ripple- Phase Bilayers. Biophys. J. 1997, 72, 964–976. [Google Scholar] [CrossRef]
- Zareýe, M.H.; Mozafarý, M.R.; Hasirci, V.; Pýkýn, E. Scanning Tunnelling Microscopy Investigation of Liposome-DNA-Ca2+ Complexes. J. Liposome Res. 1997, 7, 491–502. [Google Scholar] [CrossRef]
- Xu, S.; Szymanski, G.; Lipkowski, J. Self-Assembly of Phospholipid Molecules at a Au(111) Electrode Surface. J. Am. Chem. Soc. 2004, 126, 12276–12277. [Google Scholar] [CrossRef] [PubMed]
- Sek, S.; Xu, S.; Chen, M.; Szymanski, G.; Lipkowski, J. STM Studies of Fusion of Cholesterol Suspensions and Mixed 1,2-Dimyristoyl-Sn-Glycero-3-Phosphocholine (DMPC)/Cholesterol Vesicles onto a Au(111) Electrode Surface. J. Am. Chem. Soc. 2008, 130, 5736–5743. [Google Scholar] [CrossRef]
- Pawłowski, J.; Juhaniewicz, J.; Güzeloğlu, A.; Sęk, S. Mechanism of Lipid Vesicles Spreading and Bilayer Formation on a Au(111) Surface. Langmuir 2015, 31, 11012–11019. [Google Scholar] [CrossRef] [PubMed]
- Sek, S.; Laredo, T.; Dutcher, J.R.; Lipkowski, J. Molecular Resolution Imaging of an Antibiotic Peptide in a Lipid Matrix. J. Am. Chem. Soc. 2009, 131, 6439–6444. [Google Scholar] [CrossRef]
- Pieta, P.; Mirza, J.; Lipkowski, J. Direct Visualization of the Alamethicin Pore Formed in a Planar Phospholipid Matrix. Proc. Natl. Acad. Sci. USA 2012, 109, 21223–21227. [Google Scholar] [CrossRef]
- Smetanin, M.; Sek, S.; Maran, F.; Lipkowski, J. Molecular Resolution Visualization of a Pore Formed by Trichogin, an Antimicrobial Peptide, in a Phospholipid Matrix. Biochim. Biophys. Acta BBA-Biomembr. 2014, 1838, 3130–3136. [Google Scholar] [CrossRef]
- Matsunaga, S.; Shimizu, H.; Yamada, T.; Kobayashi, T.; Kawai, M. In Situ STM and Vibrational Study of Nanometer-Scale Reorganization of a Phospholipid Monolayer Accompanied by Potential-Driven Headgroup Digestion. Langmuir 2017, 33, 13157–13167. [Google Scholar] [CrossRef] [PubMed]
- Doerr, A. High-Speed Protein Crystallography. Nat. Methods 2018, 15, 855. [Google Scholar] [CrossRef]
- Bonanni, B.; Andolfi, L.; Bizzarri, A.R.; Cannistraro, S. Functional Metalloproteins Integrated with Conductive Substrates: Detecting Single Molecules and Sensing Individual Recognition Events. J. Phys. Chem. B 2007, 111, 5062–5075. [Google Scholar] [CrossRef]
- Edstrom, R.D.; Meinke, M.H.; Yang, X.; Yang, R.; Evans, D.F. Direct Observation of Phosphorylase Kinase and Phosphorylase b by Scanning Tunneling Microscopy. Biochemistry 1989, 28, 4939–4942. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.J.; Carr, H.J.; McMaster, T.C.; I’Anson, K.J.; Belton, P.S.; Morris, V.J.; Field, J.M.; Shewry, P.R.; Tatham, A.S. Scanning Tunneling Microscopy of a Wheat Seed Storage Protein Reveals Details of an Unusual Supersecondary Structure. Proc. Natl. Acad. Sci. USA 1991, 88, 68–71. [Google Scholar] [CrossRef]
- Arakawa, H.; Umemura, K.; Ikai, A. Protein Images Obtained by STM, AFM and TEM. Nature 1992, 358, 171–173. [Google Scholar] [CrossRef]
- Liu, Z.F.; Manivannan, A.; Yanagi, H.; Ashida, M.; Fujishima, A.; Inokuchi, H. Direct Observation of the Secondary Structure of Unfolded Pseudomonas-Cytochrome C551 by Scanning Tunneling Microscopy. Surf. Sci. 1993, 284, L411–L415. [Google Scholar] [CrossRef]
- Chi, Q.; Zhang, J.; Dong, S.; Wang, E. Direct Observation of Native and Unfolded Glucose Oxidase Structures by Scanning Tunnelling Microscopy. J. Chem. Soc. Faraday Trans. 1994, 90, 2057–2060. [Google Scholar] [CrossRef]
- Zhang, J.-D.; Chi, Q.; Dong, S.-J.; Wang, E. High Resolution Imaging of Native Hemoglobin by Scanning Tunneling Microscopy. J. Electroanal. Chem. 1994, 379, 535–539. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, E. Tip-Induced Dynamic Characterization of Multilayers Andvariable Morphologies of Cytochrome c Molecules on Highlyoriented Pyrolytic Graphite Revealed by in-Situscanning Tunnelling Microscopy. J. Chem. Soc. Faraday Trans. 1997, 93, 327–331. [Google Scholar] [CrossRef]
- Davis, J.J.; Halliwell, C.M.; Hill, H.A.O.; Canters, G.W.; van Amsterdam, M.C.; Verbeet, M.P. Protein Adsorption at a Gold Electrode Studied by Insitu Scanning Tunnelling Microscopy. New J. Chem. 1998, 22, 1119–1123. [Google Scholar] [CrossRef]
- Friis, E.P.; Andersen, J.E.T.; Kharkats, Y.I.; Kuznetsov, A.M.; Nichols, R.J.; Zhang, J.-D.; Ulstrup, J. An Approach to Long-Range Electron Transfer Mechanisms in Metalloproteins: In Situ Scanning Tunneling Microscopy with Submolecular Resolution. Proc. Natl. Acad. Sci. USA 1999, 96, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Davis, J.J.; Kyritsis, P.; Hill, H.A.O.; Meyer, J. A Scanning Tunnelling Microscopy Study of Clostridium Pasteurianum Rubredoxin. J. Inorg. Biochem. 2000, 78, 251–254. [Google Scholar] [CrossRef]
- Rodríguez-Galván, A.; Contreras-Torres, F.F.; Basiuk, E.V.; Alvarez-Zauco, E.; Heredia, A.; Basiuk, V.A. Aggregation of Human Serum Albumin on Graphite and Single-Walled Carbon Nanotubes as Studied by Scanning Probe Microscopies. J. Nanosci. Nanotechnol. 2011, 11, 5491–5498. [Google Scholar] [CrossRef]
- Riordan, J.F. The Role of Metals in Enzyme Activity. Ann. Clin. Lab. Sci. 1977, 7, 119–129. [Google Scholar]
- Contera, S.A.; Iwasaki, H.; Suzuki, S. Ambient STM and in Situ AFM Study of Nitrite Reductase Proteins Adsorbed on Gold and Graphite: Influence of the Substrate on Protein Interactions. Ultramicroscopy 2003, 97, 65–72. [Google Scholar] [CrossRef]
- Contera, S.A.; Iwasaki, H. Imaging the Proteins Pseudoazurin and Apo-Pseudoazurin on Gold by STM in Air: Effect of the Bias Voltage. Ultramicroscopy 2002, 91, 231–243. [Google Scholar] [CrossRef]
- Contera, S.A.; Okajima, T.; Iwasaki, H. Scanning Tunnelling Microscopy Images of the Copper-Containing Amine Oxidase from Arthrobacter Globiformis in the Holo and Apo Forms Adsorbed on Gold under Ambient Conditions. Jpn. J. Appl. Phys. 2002, 41, 3916. [Google Scholar] [CrossRef]
- Parker, M.; Davies, M.C.; Tendler, S.J.B. Hydrogen Bonding Molecules and Their Effect on Scanning Tunneling Microscope Image Contrast of Covalently Immobilized Protein Molecules. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1996, 14, 1432–1437. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Hu, C.; Liu, Q.; Hou, Y.; Zhang, X.; Lu, Q. Sub-Molecular Features of Single Proteins in Solution Resolved with Scanning Tunneling Microscopy. Nano Res. 2016, 9, 2551–2560. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Wang, H.; Wang, W.; Li, Z.; Liu, J.; Yang, X.; Ji, X.; Luo, Y.; Hu, C.; et al. Moderate and Strong Static Magnetic Fields Directly Affect EGFR Kinase Domain Orientation to Inhibit Cancer Cell Proliferation. Oncotarget 2016, 7, 41527–41539. [Google Scholar] [CrossRef]
- Shivji, A.P.; Brown, F.; Davies, M.C.; Jennings, K.H.; Roberts, C.J.; Tendler, S.J.B.; Wilkinson, M.J.; Williams, P.M. Scanning Tunnelling Microscopy Studies of β-Amyloid Fibril Structure and Assembly. FEBS Lett. 1995, 371, 25–28. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, C.; Wang, C.; Wan, L.; Fang, X.; Bai, C. AFM and STM Study of β-Amyloid Aggregation on Graphite. Ultramicroscopy 2003, 97, 73–79. [Google Scholar] [CrossRef]
- Losic, D.; Martin, L.L.; Mechler, A.; Aguilar, M.-I.; Small, D.H. High Resolution Scanning Tunnelling Microscopy of the β-Amyloid Protein (Aβ1–40) of Alzheimer’s Disease Suggests a Novel Mechanism of Oligomer Assembly. J. Struct. Biol. 2006, 155, 104–110. [Google Scholar] [CrossRef]
- Naruse, N.; Satooka, H.; Todo, K.; Nakanishi, A.; Taguchi, H.; Mera, Y. Oligomers Imaging of Amyloid-Β1-42 by Scanning Tunneling Microscopy. Jpn. J. Appl. Phys. 2019, 58, SIIB30. [Google Scholar] [CrossRef]
- Forman, C.J.; Wang, N.; Yang, Z.Y.; Mowat, C.G.; Jarvis, S.; Durkan, C.; Barker, P.D. Probing the Location of Displayed Cytochrome B562on Amyloid by Scanning Tunnelling Microscopy. Nanotechnology 2013, 24, 175102. [Google Scholar] [CrossRef]
- Gathercole, L.J.; Miles, M.J.; McMaster, T.J.; Holmes, D.F. Scanning Probe Microscopy of Collagen I and PN-Collagen I Assemblies and the Relevance to Scanning Tunnelling Microscopy Contrast Generation in Proteins. J. Chem. Soc. Faraday Trans. 1993, 89, 2589–2594. [Google Scholar] [CrossRef]
- Deng, Z.; Thontasen, N.; Malinowski, N.; Rinke, G.; Harnau, L.; Rauschenbach, S.; Kern, K. A Close Look at Proteins: Submolecular Resolution of Two- and Three-Dimensionally Folded Cytochrome c at Surfaces. Nano Lett. 2012, 12, 2452–2458. [Google Scholar] [CrossRef] [PubMed]
- Perez, S. X-ray Diffraction and Crystallography of Oligosaccharides and Polysaccharides. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2767–2777. [Google Scholar]
- Cummings, J.H.; Stephen, A.M. Carbohydrate Terminology and Classification. Eur. J. Clin. Nutr. 2007, 61, S5–S18. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-Based Pharmaceutics: Past, Present and Future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Rabe, J.P.; Buchholz, S.; Ritcey, A.M. Reactive Graphite Etch and the Structure of an Adsorbed Organic Monolayer—A Scanning Tunneling Microscopy Study. J. Vac. Sci. Technol. A 1990, 8, 679–683. [Google Scholar] [CrossRef]
- Yang, X.; Miller, M.A.; Yang, R.; Evans, D.F.; Edstrom, R.D. Scanning Tunneling Microscopic Images Show a Laminated Structure for Glycogen Molecules. FASEB J. 1990, 4, 3140–3143. [Google Scholar] [CrossRef]
- Lee, I.; Atkins, E.D.T.; Miles, M.J. Visualization of the Algal Polysaccharide Carrageenan by Scanning Tunnelling Microscopy. Ultramicroscopy 1992, 42–44, 1107–1112. [Google Scholar] [CrossRef]
- Miyake, K.; Yasuda, S.; Harada, A.; Sumaoka, J.; Komiyama, M.; Shigekawa, H. Formation Process of Cyclodextrin Necklace−Analysis of Hydrogen Bonding on a Molecular Level. J. Am. Chem. Soc. 2003, 125, 5080–5085. [Google Scholar] [CrossRef]
- Jia, Y.-G.; Malveau, C.; Mezour, M.A.; Perepichka, D.F.; Zhu, X.X. A Molecular Necklace: Threading β-Cyclodextrins onto Polymers Derived from Bile Acids. Angew. Chem. Int. Ed. 2016, 55, 11979–11983. [Google Scholar] [CrossRef]
- Abb, S.; Tarrat, N.; Cortés, J.; Andriyevsky, B.; Harnau, L.; Schön, J.C.; Rauschenbach, S.; Kern, K. Carbohydrate Self-Assembly at Surfaces: STM Imaging of Sucrose Conformation and Ordering on Cu(100). Angew. Chem. Int. Ed. 2019, 58, 8336–8340. [Google Scholar] [CrossRef]
- Abb, S.; Tarrat, N.; Cortés, J.; Andriyevsky, B.; Harnau, L.; Schön, J.C.; Rauschenbach, S.; Kern, K. Polymorphism in Carbohydrate Self-Assembly at Surfaces: STM Imaging and Theoretical Modelling of Trehalose on Cu(100). RSC Adv. 2019, 9, 35813–35819. [Google Scholar] [CrossRef]
- Wu, X.; Delbianco, M.; Anggara, K.; Michnowicz, T.; Pardo-Vargas, A.; Bharate, P.; Sen, S.; Pristl, M.; Rauschenbach, S.; Schlickum, U.; et al. Imaging Single Glycans. Nature 2020, 582, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Anggara, K.; Zhu, Y.; Delbianco, M.; Rauschenbach, S.; Abb, S.; Seeberger, P.H.; Kern, K. Exploring the Molecular Conformation Space by Soft Molecule–Surface Collision. J. Am. Chem. Soc. 2020, 142, 21420–21427. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Imaging Single Glycan Molecules. Nat. Methods 2020, 17, 757. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Baldacchini, C.; Bizzarri, A.R.; Cannistraro, S. Electron Transfer, Conduction and Biorecognition Properties of the Redox Metalloprotein Azurin Assembled onto Inorganic Substrates. Eur. Polym. J. 2016, 83, 407–427. [Google Scholar] [CrossRef]
- Elliott, M.; Jones, D.D. Approaches to Single-Molecule Studies of Metalloprotein Electron Transfer Using Scanning Probe-Based Techniques. Biochem. Soc. Trans. 2017, 46, 1–9. [Google Scholar] [CrossRef]
- De Morais, M.G.; Martins, V.G.; Steffens, D.; Pranke, P.; da Costa, J.A.V. Biological Applications of Nanobiotechnology. J. Nanosci. Nanotechnol. 2014, 14, 1007–1017. [Google Scholar] [CrossRef]
- Rodríguez-Galván, A.; Heredia, A.; Amelines-Sarria, O.; Rivera, M.; Medina, L.A.; Basiuk, V.A. Non-Covalent Attachment of Silver Nanoclusters onto Single-Walled Carbon Nanotubes with Human Serum Albumin as Linking Molecule. Appl. Surf. Sci. 2015, 331, 271–277. [Google Scholar] [CrossRef]
- Shu, Y.; Cinier, M.; Shu, D.; Guo, P. Assembly of Multifunctional Phi29 PRNA Nanoparticles for Specific Delivery of SiRNA and Other Therapeutics to Targeted Cells. Methods 2011, 54, 204–214. [Google Scholar] [CrossRef]
- Cai, Y.; Sheetz, M.P. Force Propagation across Cells: Mechanical Coherence of Dynamic Cytoskeletons. Curr. Opin. Cell Biol. 2009, 21, 47–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, S.; Kanchanawong, P. Nanoscale Mechanobiology of Cell Adhesions. Semin. Cell Dev. Biol. 2017, 71, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Shtengel, G.; Wang, Y.; Zhang, Z.; Goh, W.I.; Hess, H.F.; Kanchanawong, P. Chapter 15—Imaging Cellular Ultrastructure by PALM, IPALM, and Correlative IPALM-EM. In Methods in Cell Biology; Waters, J.C., Wittman, T., Eds.; Quantitative Imaging in Cell Biology; Academic Press: Cambridge, MA, USA, 2014; Volume 123, pp. 273–294. [Google Scholar]
- Peng, W.; Wang, H.; Lu, H.; Yin, L.; Wang, Y.; Grandidier, B.; Yang, D.; Pi, X. Recent Progress on the Scanning Tunneling Microscopy and Spectroscopy Study of Semiconductor Heterojunctions. Small 2021, 17, 2100655. [Google Scholar] [CrossRef] [PubMed]
- Willner, I. Biomaterials for Sensors, Fuel Cells, and Circuitry. Science 2002, 298, 2407–2408. [Google Scholar] [CrossRef]
- Homola, J. Surface Plasmon Resonance Sensors for Detection of Chemical and Biological Species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Rusmini, F.; Zhong, Z.; Feijen, J. Protein Immobilization Strategies for Protein Biochips. Biomacromolecules 2007, 8, 1775–1789. [Google Scholar] [CrossRef]
- Prabowo, B.A.; Purwidyantri, A.; Liu, K.-C. Surface Plasmon Resonance Optical Sensor: A Review on Light Source Technology. Biosensors 2018, 8, 80. [Google Scholar] [CrossRef]
- Rabia, A.; Tumino, F.; Milani, A.; Russo, V.; Bassi, A.L.; Achilli, S.; Fratesi, G.; Onida, G.; Manini, N.; Sun, Q.; et al. Scanning Tunneling Microscopy and Raman Spectroscopy of Polymeric Sp–Sp2 Carbon Atomic Wires Synthesized on the Au(111) Surface. Nanoscale 2019, 11, 18191–18200. [Google Scholar] [CrossRef]
- Hla, S.-W.; Bartels, L.; Meyer, G.; Rieder, K.-H. Inducing All Steps of a Chemical Reaction with the Scanning Tunneling Microscope Tip: Towards Single Molecule Engineering. Phys. Rev. Lett. 2000, 85, 2777–2780. [Google Scholar] [CrossRef]
- Reimers, J.R.; Yang, J.; Darwish, N.; Kosov, D.S. Silicon-Single Molecule-Silicon Circuits. Chem. Sci. 2021, 12, 15870–15881. [Google Scholar] [CrossRef]
- Deng, X.; Xiong, F.; Li, X.; Xiang, B.; Li, Z.; Wu, X.; Guo, C.; Li, X.; Li, Y.; Li, G.; et al. Application of Atomic Force Microscopy in Cancer Research. J. Nanobiotechnol. 2018, 16, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-C.; Zhou, Y.; Baker, L.A. Scanning Ion Conductance Microscopy. Annu. Rev. Anal. Chem. 2012, 5, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Odom, T.W.; Huang, J.-L.; Kim, P.; Lieber, C.M. Atomic Structure and Electronic Properties of Single-Walled Carbon Nanotubes. Nature 1998, 391, 62–64. [Google Scholar] [CrossRef]
- Binnig, G.; Rohrer, H. Scanning Tunneling Microscopy—From Birth to Adolescence. Rev. Mod. Phys. 1987, 59, 615–625. [Google Scholar] [CrossRef]
- Binnig, G.; Rohrer, H. Scanning Tunneling Microscopy. Surf. Sci. 1985, 152–153, 17–26. [Google Scholar] [CrossRef]
- Drexler, K.E. Molecular Engineering: An Approach to the Development of General Capabilities for Molecular Manipulation. Proc. Natl. Acad. Sci. USA 1981, 78, 5275–5278. [Google Scholar] [CrossRef]
- Potorochin, D.V.; Chaika, A.N.; Molodtsova, O.V.; Aristov, V.Y.; Marchenko, D.E.; Smirnov, D.A.; Makarova, A.A.; Walls, B.; Zhussupbekov, K.; Walshe, K.; et al. Surface Functionalization of Few-Layer Graphene on β-SiC(001) by Neutral Red Dye. Appl. Surf. Sci. 2022, 585, 152542. [Google Scholar] [CrossRef]
- Jäckel, F.; Perera, U.G.E.; Iancu, V.; Braun, K.-F.; Koch, N.; Rabe, J.P.; Hla, S.-W. Investigating Molecular Charge Transfer Complexes with a Low Temperature Scanning Tunneling Microscope. Phys. Rev. Lett. 2008, 100, 126102. [Google Scholar] [CrossRef]
- Baird, D.; Nordmann, A.; Schummer, J. Discovering the Nanoscale, 3rd ed.; IOS Press: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Abb, S.; Harnau, L.; Gutzler, R.; Rauschenbach, S.; Kern, K. Two-Dimensional Honeycomb Network through Sequence-Controlled Self-Assembly of Oligopeptides. Nat. Commun. 2016, 7, 10335. [Google Scholar] [CrossRef]
- Anand, G.; Sharma, S.; Dutta, A.K.; Kumar, S.K.; Belfort, G. Conformational Transitions of Adsorbed Proteins on Surfaces of Varying Polarity. Langmuir 2010, 26, 10803–10811. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Galván, A.; Contreras-Torres, F.F. Scanning Tunneling Microscopy of Biological Structures: An Elusive Goal for Many Years. Nanomaterials 2022, 12, 3013. https://doi.org/10.3390/nano12173013
Rodríguez-Galván A, Contreras-Torres FF. Scanning Tunneling Microscopy of Biological Structures: An Elusive Goal for Many Years. Nanomaterials. 2022; 12(17):3013. https://doi.org/10.3390/nano12173013
Chicago/Turabian StyleRodríguez-Galván, Andrés, and Flavio F. Contreras-Torres. 2022. "Scanning Tunneling Microscopy of Biological Structures: An Elusive Goal for Many Years" Nanomaterials 12, no. 17: 3013. https://doi.org/10.3390/nano12173013
APA StyleRodríguez-Galván, A., & Contreras-Torres, F. F. (2022). Scanning Tunneling Microscopy of Biological Structures: An Elusive Goal for Many Years. Nanomaterials, 12(17), 3013. https://doi.org/10.3390/nano12173013






