Evaluating the Growth of Ceria-Modified N-Doped Carbon-Based Materials and Their Performance in the Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
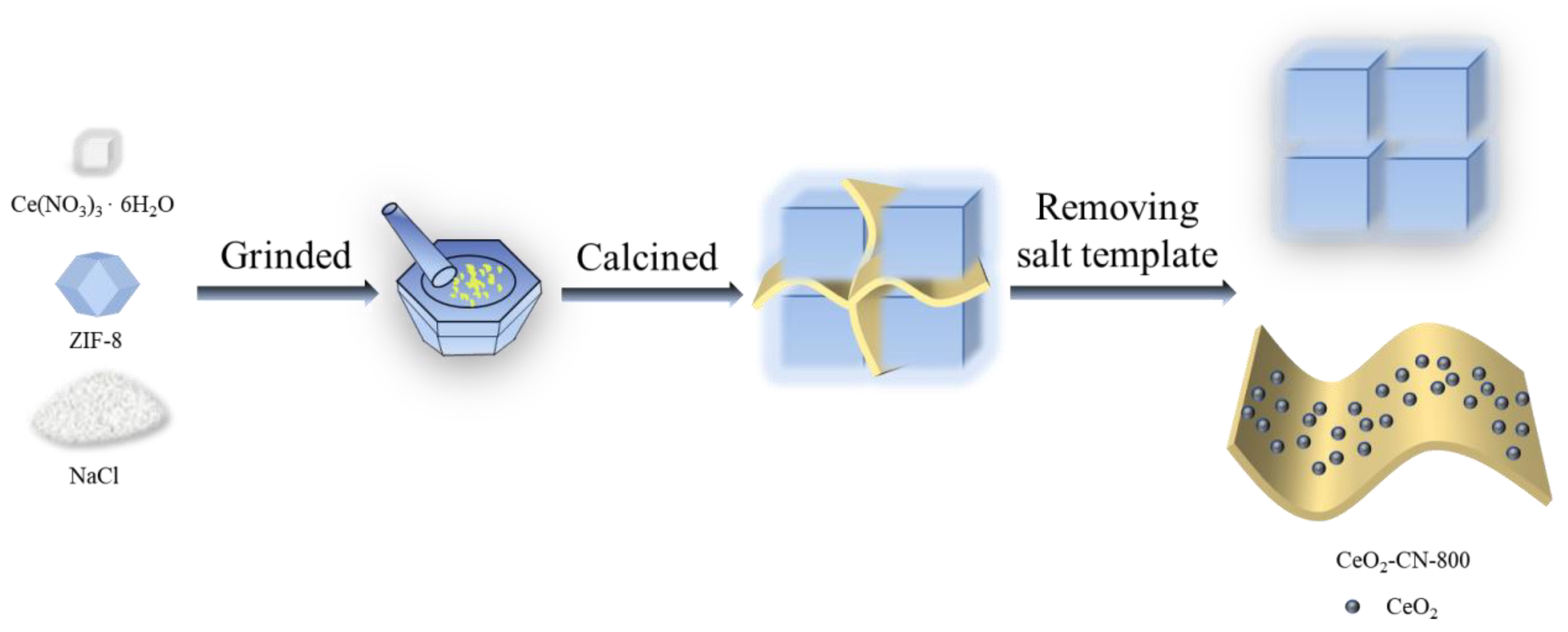
2.2. Synthesis of Materials
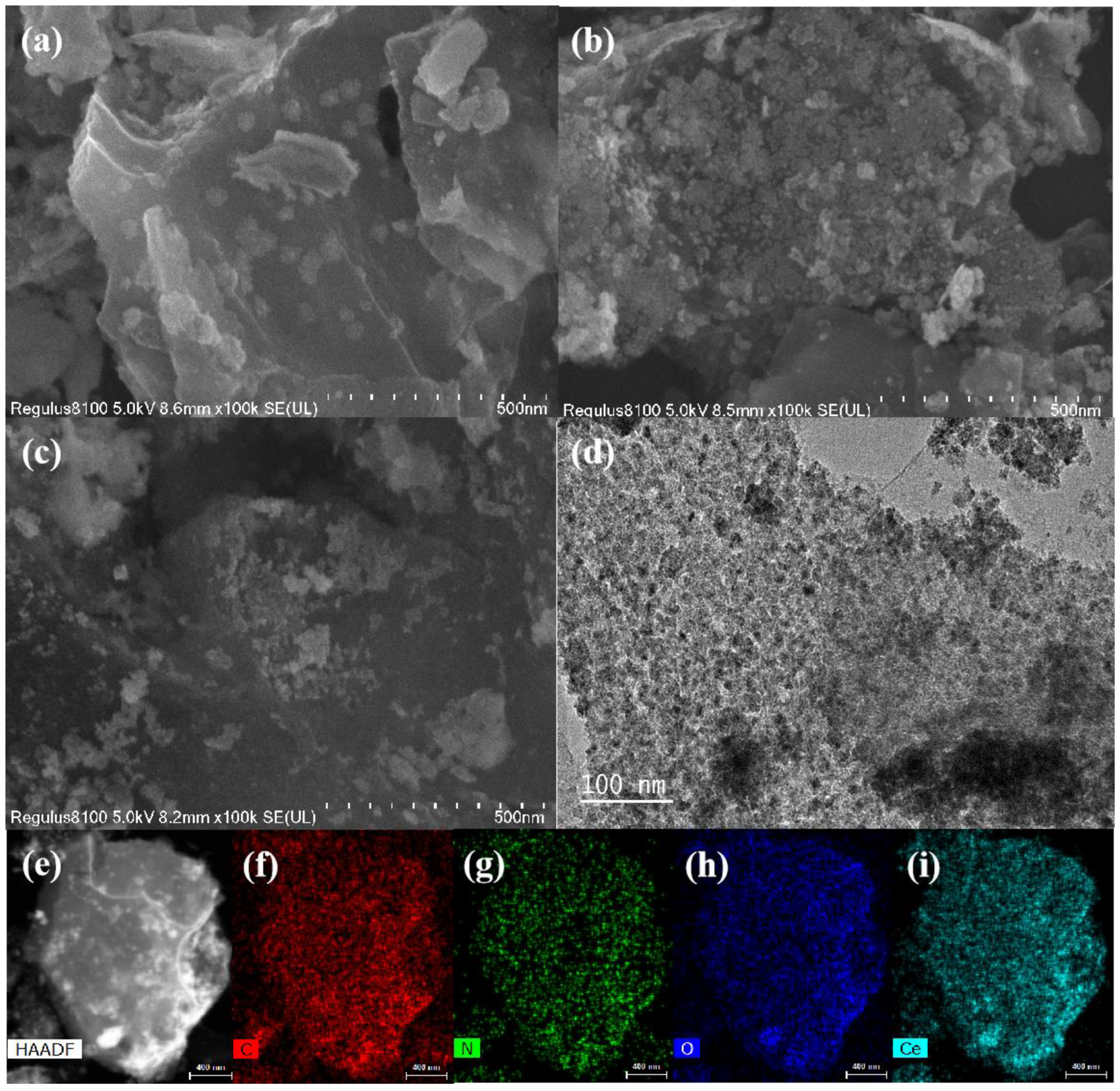
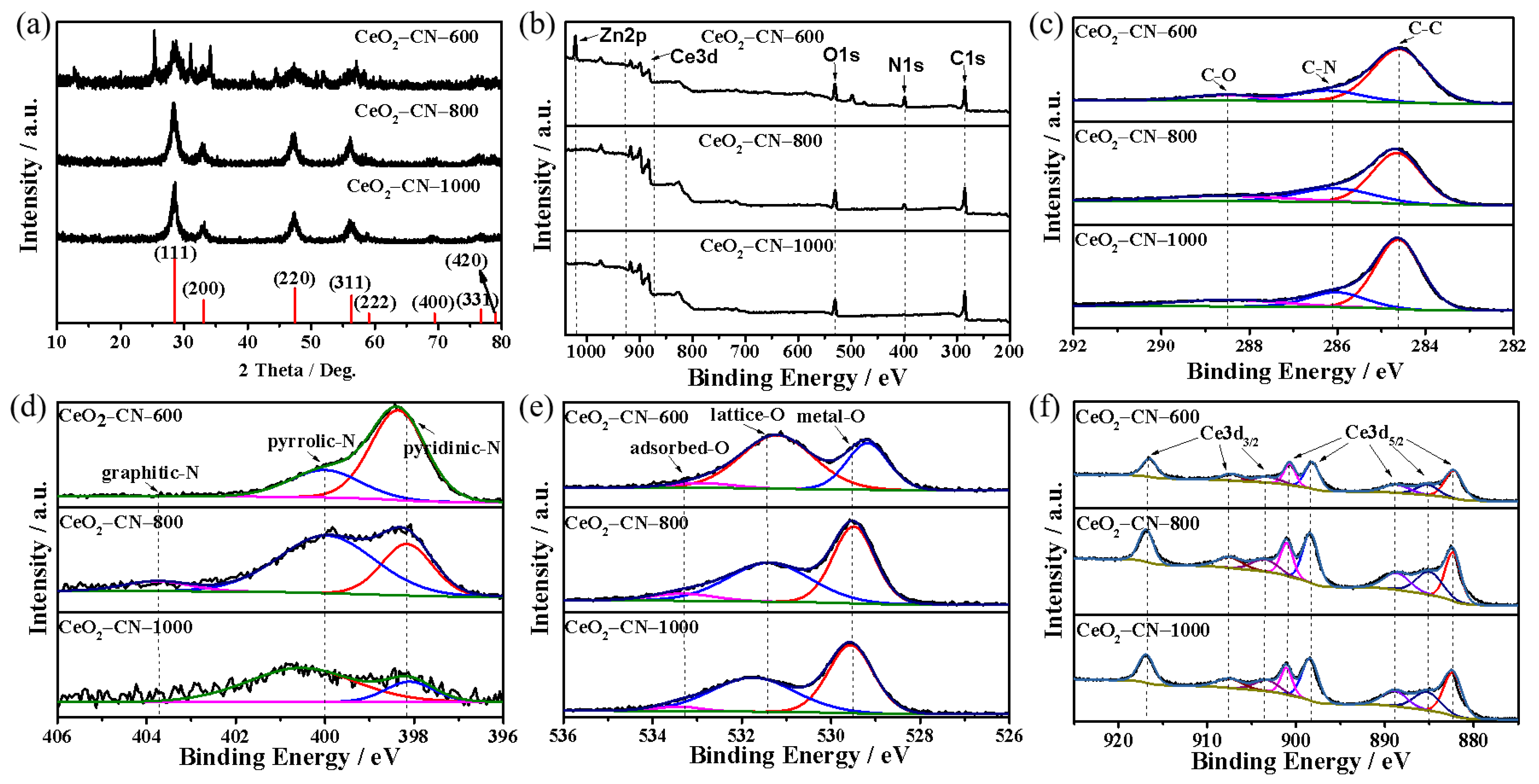
2.3. Characterization of Physical Properties
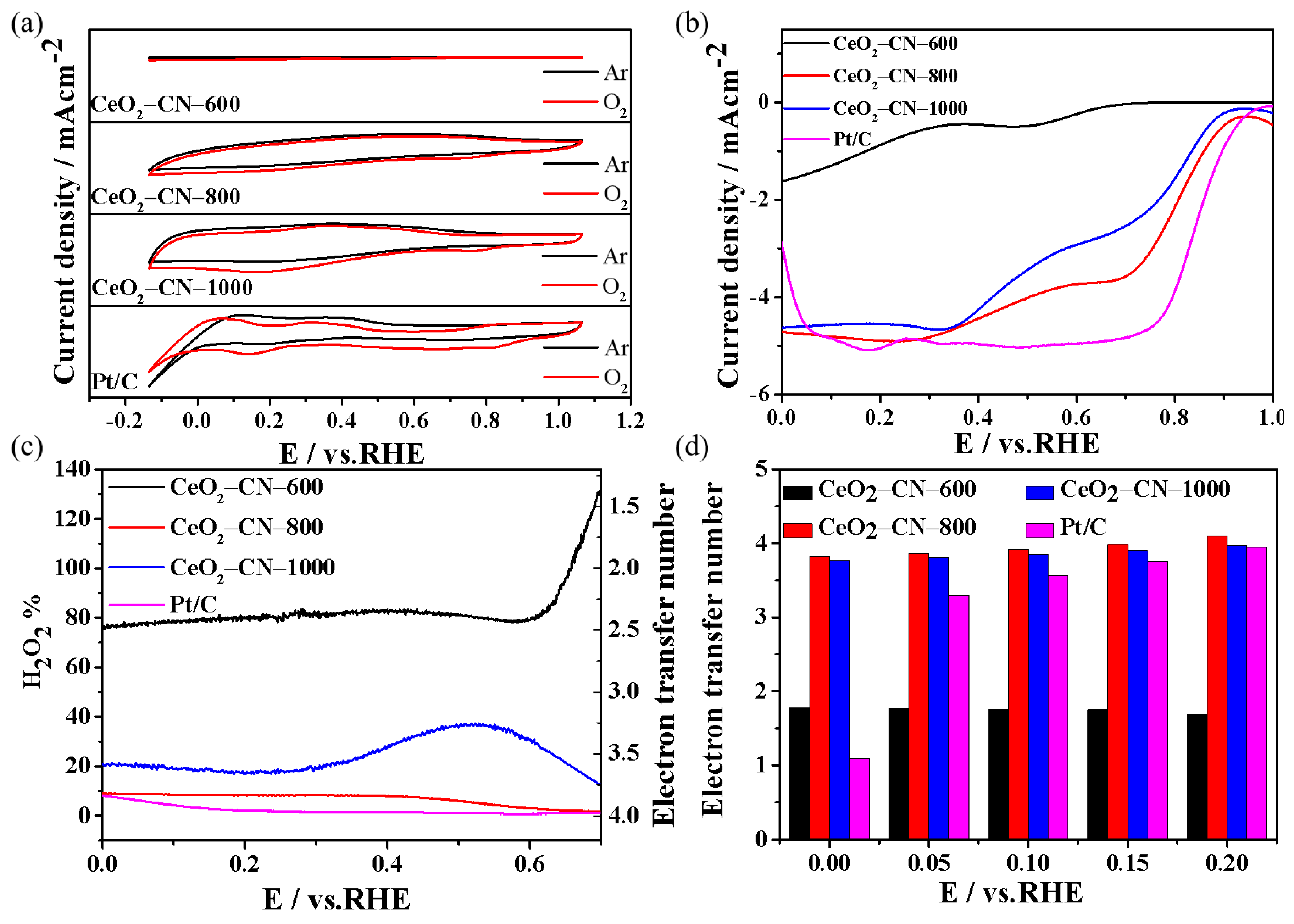
2.4. Electrochemical Characterization
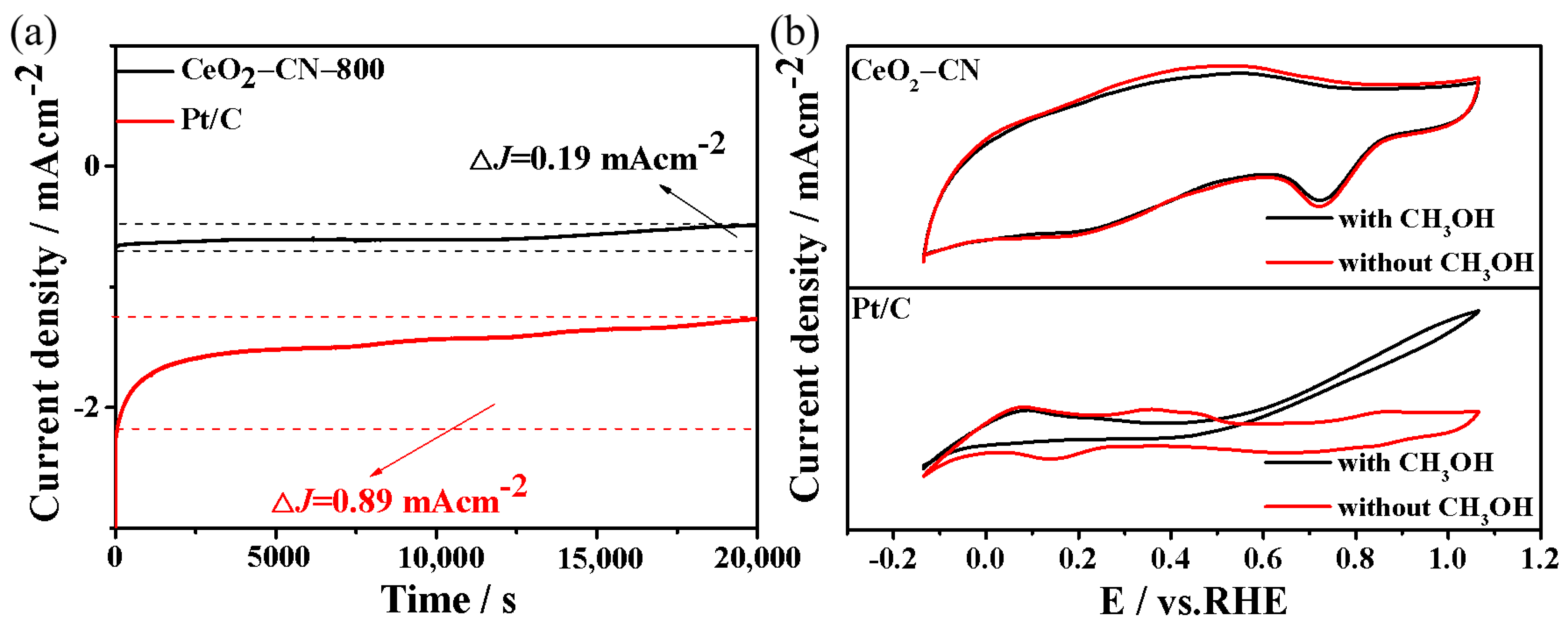
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghosh, A.; Ghosh, S.; Seshadhri, G.M.; Ramaprabhu, S. Green synthesis of nitrogen-doped self-assembled porous carbon-metal oxide composite towards energy and environmental applications. Sci. Rep. 2019, 9, 5187. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Sarkar, S.; Hassan, M.; Hossain, M.S.; Minett, A.I.; Dou, S.X.; Gomes, V.G. Tuning graphene for energy and environmental applications: Oxygen reduction reaction and greenhouse gas mitigation. J. Power Sources 2016, 328, 472–481. [Google Scholar] [CrossRef]
- Xiao, M.J.; Ma, B.; Zhang, Z.-Q.; Xiao, Q.; Li, X.-Y.; Zhang, Z.-T.; Wang, Q.; Peng, Y.; Zhang, H.-L. Carbon nano-onion encapsulated cobalt nanoparticles for oxygen reduction and lithium-ion batteries. J. Mater. Chem. A 2021, 9, 7227–7237. [Google Scholar] [CrossRef]
- Xu, H.; Ci, S.; Ding, Y.; Wang, G.; Wen, Z. Recent advances in precious metal-free bifunctional catalysts for electrochemical conversion systems. J. Mater. Chem. A 2019, 7, 8006–8029. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; Yang, W.; Pan, Q. ZIFs@chitosan derived efficient bimetallic carbon-based catalyst for oxygen reduction. Ind. Eng. Chem. Res. 2022, 61, 6156–6162. [Google Scholar] [CrossRef]
- Huang, Y.; Mohamed, A.G.A.; Xie, J.; Wang, Y. Surface evolution of electrocatalysts in energy conversion reactions. Nano Energy 2021, 82, 105745. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, G.; Chen, J.; Bi, L.; Dai, L.; Wen, Z. N-doped porous carbon nanosheets as pH-universal ORR electrocatalyst in various fuel cell devices. Nano Energy 2018, 49, 393–402. [Google Scholar] [CrossRef]
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-based metal-free ORR electrocatalysts for fuel cells: Past, present, and future. Adv. Mater. 2019, 31, e1804799. [Google Scholar] [CrossRef]
- Khan, I.A.; Qian, Y.; Badshah, A.; Nadeem, M.A.; Zhao, D. Highly porous carbon derived from MOF-5 as a support of ORR electrocatalysts for fuel cells. ACS Appl. Mater. Interfaces 2016, 8, 17268–17275. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Y.-C.; Chen, C.; Yang, X.-D.; Zhou, Z.-Y.; Sun, S.-G. A mesoporous Fe/N/C ORR catalyst for polymer electrolyte membrane fuel cells. Chin. J. Catal. 2016, 37, 1103–1108. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, P.; Wang, Y.; Wang, Y.; Zaghib, K.; Zhou, Z. ZIF-derived Co–N–C ORR catalyst with high performance in proton exchange membrane fuel cells. Prog. Nat. Sci. Mater. Int. 2020, 30, 855–860. [Google Scholar] [CrossRef]
- Yu, J.; Li, B.-Q.; Zhao, C.-X.; Zhang, Q. Seawater electrolyte-based metal–air batteries: From strategies to applications. Energy Environ. Sci. 2020, 13, 3253–3268. [Google Scholar] [CrossRef]
- Zhu, B.; Liang, Z.; Xia, D.; Zou, R. Metal-organic frameworks and their derivatives for metal-air batteries. Energy Storage Mater. 2019, 23, 757–771. [Google Scholar] [CrossRef]
- Tang, W.; Li, B.; Teng, K.; Wang, X.; Liu, R.; Wu, M.; Zhang, L.; Ren, P.; Zhang, J.; Feng, M. Advanced noble-metal-free bifunctional electrocatalysts for metal-air batteries. J. Mater. 2022, 8, 454–474. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, Q.; Guan, J. Applications of metal–organic framework-derived materials in fuel cells and metal-air batteries. Coord. Chem. Rev. 2020, 307, 106–129. [Google Scholar] [CrossRef]
- Cai, P.; Hong, Y.; Ci, S.; Wen, Z. In situ integration of Co-Fe alloy nanoparticles with nitrogen-doped carbon nanotubes as advanced bifunctional cathode catalysts for Zn-air batteries. Nanoscale 2016, 8, 20048–20055. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhu, X.; Yang, W. Micro-nanostructural designs of bifunctional electrocatalysts for metal-air batteries. Chin. J. Catal. 2020, 41, 390–403. [Google Scholar] [CrossRef]
- Allendorf, M.D. Oxygen reduction reaction: A framework for success. Nat. Energy 2016, 1, 16058. [Google Scholar] [CrossRef]
- Tong, X.; Zhan, X.; Rawach, D.; Chen, Z.; Zhang, G.; Sun, S. Low-dimensional catalysts for oxygen reduction reaction. Prog. Nat. Sci. Mater. Int. 2020, 30, 787–795. [Google Scholar] [CrossRef]
- Risch, M. Perovskite electrocatalysts for the oxygen reduction reaction in alkaline media. Catalysts 2017, 7, 154. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhang, J.; Liu, D.; Li, T.; Yan, Y.; Wei, X.; Yang, Y.; Yan, S.; Zou, Z. N-doped graphene-coated commercial Pt/C catalysts toward high-stability and antipoisoning in oxygen reduction reaction. J. Phys. Chem. Lett. 2022, 13, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Kim, S.-K.; Kim, K.-H.; Lee, E.; Park, G.-G. Enhanced durability of Pt/C catalyst by coating carbon black with silica for oxygen reduction reaction. Int. J. Hydrog. Energy 2021, 46, 1133–1143. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, S.; Wang, Z.; Liao, W.; Chen, M.; Wang, Q. Pt/C decorated with N-doped carbon layers as a highly durable electrocatalyst for the oxygen reduction reaction. Energy Fuels 2021, 35, 20300–20308. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, D.; Feng, C.; Wu, H.; Zhang, G. Synthesis and properties towards oxygen reduction reaction of transition metal selenides. Ionics 2019, 26, 1337–1345. [Google Scholar] [CrossRef]
- An, L.; Xia, Z.; Chen, P.; Xia, D. Layered transition metal oxynitride Co3Mo2OxN6-x/C catalyst for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2016, 8, 29536–29542. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Hu, L.; Li, J.; Wang, E. Recent advancements in transition metal-nitrogen-carbon catalysts for oxygen reduction reaction. Electroanalysis 2018, 30, 1217–1228. [Google Scholar] [CrossRef]
- Park, J.; Nabae, Y.; Hayakawa, T.; Kakimoto, M.-A. Highly selective two-electron oxygen reduction catalyzed by mesoporous nitrogen-doped carbon. ACS Catal. 2014, 4, 3749–3754. [Google Scholar] [CrossRef]
- Yang, Y.; He, F.; Shen, Y.; Chen, X.; Mei, H.; Liu, S.; Zhang, Y. A biomass derived N/C-catalyst for the electrochemical production of hydrogen peroxide. Chem. Commun. 2017, 53, 9994–9997. [Google Scholar] [CrossRef]
- Li, J.C.; Hou, P.X.; Liu, C. Heteroatom-doped carbon nanotube and graphene-based electrocatalysts for oxygen reduction reaction. Small 2017, 13, 1702002. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, H.; Lu, X.F.; Tong, Y.X.; Li, G.R. Recent progress on MOF-derived heteroatom-doped carbon-based electrocatalysts for oxygen reduction reaction. Adv. Sci. 2018, 5, 1700515. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; An, T.; Birgersson, K.E.; Liu, Z.; Zhao, D. Web-like interconnected carbon networks from NaCl-assisted pyrolysis of ZIF-8 for highly efficient oxygen reduction catalysis. Small 2018, 14, e1704169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liang, Z.; Zou, R. Metal-organic frameworks for electrochemical energy conversion: Status and challenges. Sci. China Chem. 2019, 63, 7–10. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Q.; Fang, H.; Wang, H.; Han, J.; Ge, Q.; Zhu, X. Ce–UiO-66 derived CeO2 octahedron catalysts for efficient ketonization of propionic acid. Ind. Eng. Chem. Res. 2020, 59, 17269–17278. [Google Scholar] [CrossRef]
- Saraci, F.; Quezada-Novoa, V.; Donnarumma, P.R.; Howarth, A.J. Rare-earth metal-organic frameworks: From structure to applications. Chem. Soc. Rev. 2020, 49, 7949–7977. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, L.; Xiao, Z.; Chen, R.; Jiang, Z.; Wang, S. 3D carbon electrocatalysts in situ constructed by defect-rich nanosheets and polyhedrons from NaCl-sealed zeolitic imidazolate frameworks. Adv. Funct. Mater. 2018, 28, 1705356. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, Y.; Ma, T.; Yang, C.; Peng, Y.; Zhou, Z.; Zhou, M.; Li, S.; Wang, Y.; Cheng, C. Designing MOF nanoarchitectures for electrochemical water splitting. Adv. Mater. 2021, 33, e2006042. [Google Scholar] [CrossRef]
- Zhu, C.; Ding, T.; Gao, W.; Ma, K.; Tian, Y.; Li, X. CuO/CeO2 catalysts synthesized from Ce-UiO-66 metal-organic framework for preferential CO oxidation. Int. J. Hydrog. Energy 2017, 42, 17457–17465. [Google Scholar] [CrossRef]
- Chhetri, K.; Muthurasu, A.; Dahal, B.; Kim, T.; Mukhiya, T.; Chae, S.H.; Ko, T.H.; Choi, Y.C.; Kim, H.Y. Engineering the abundant heterointerfaces of integrated bimetallic sulfide-coupled 2D MOF-derived mesoporous CoS2 nanoarray hybrids for electrocatalytic water splitting. Mater. Today Nano 2022, 17, 100146. [Google Scholar] [CrossRef]
- Chhetri, K.; Dahal, B.; Mukhiya, T.; Tiwari, A.P.; Muthurasu, A.; Kim, T.; Kim, H.; Kim, H.Y. Integrated hybrid of graphitic carbon-encapsulated CuxO on multilayered mesoporous carbon from copper MOFs and polyaniline for asymmetric supercapacitor and oxygen reduction reactions. Carbon 2021, 179, 89–99. [Google Scholar] [CrossRef]
- Hu, Y.; Jensen, J.O.; Norby, P.; Cleemann, L.N.; Yang, F.; Li, Q. Mechanistic insights into the synthesis of platinum–rare earth metal nanoalloys by a solid-state chemical route. Chem. Mater. 2021, 33, 535–546. [Google Scholar] [CrossRef]
- Kuang, C.; Xu, Y.; Xie, G.; Pan, Z.; Zheng, L.; Lai, W.; Ling, J.; Talawar, M.; Zhou, X. Preparation of CeO2-doped carbon nanotubes cathode and its mechanism for advanced treatment of pig farm wastewater. Chemosphere 2021, 262, 128215. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Qin, X.; Xiao, F.; Liang, C.; Xu, M.; Meng, Y.; Sarnello, E.; Fang, L.; Li, T.; Ding, S.; et al. Highly dispersive cerium atoms on carbon nanowires as oxygen reduction reaction electrocatalysts for Zn-air batteries. Nano Lett. 2021, 21, 4508–4515. [Google Scholar] [CrossRef] [PubMed]
- Trevelin, L.C.; Valim, R.B.; Carneiro, J.F.; De Siervo, A.; Rocha, R.S.; Lanza, M.R.V. Using black carbon modified with NbMo and NbPd oxide nanoparticles for the improvement of H2O2 electrosynthesis. J. Electroanal. Chem. 2020, 877, 114746. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Jin, C.; Wang, N.; Wang, F. Hexagonal La2O3 nanocrystals chemically coupled with nitrogen-doped porous carbon as efficient catalysts for the oxygen reduction reaction. Chemistry 2020, 26, 12606–12614. [Google Scholar] [CrossRef] [PubMed]
- Hota, I.; Debnath, A.K.; Muthe, K.P.; Varadwaj, K.S.K.; Parhi, P. Electrocatalytic production of hydrogen-peroxide from molecular oxygen by rare earth (Pr, Nd, Sm or Gd) oxide nanorods. Electroanalysis 2020, 32, 2521–2527. [Google Scholar] [CrossRef]
- Bai, L.; Liu, J.; Gu, W.; Song, Y.; Wang, F. Carbon-based nanostructures vertically arrayed on layered lanthanum oxycarbonate as highly efficient catalysts for oxygen reduction reactions. ACS Appl. Mater. Interfaces 2019, 11, 16452–16460. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sun, L.; Zhu, J.; Hu, L.; Guo, D. Enhanced oxygen reduction reaction performance of ReOx/NC (Re = La, Ce, Pr, Sm, Eu, Tb, Er, Tm and Yb)-especially Pr6O11/NC via accommodating oxygen vacancies and its application for Zn-air battery. Appl. Surf. Sci. 2021, 535, 147689. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, W.; Li, J.; Mi, Y.; Gong, H.; Lei, Z. 3D rosa centifolia-like CeO2 encapsulated with N-doped carbon as an enhanced electrocatalyst for Zn-air batteries. J. Colloid Interface Sci. 2020, 578, 796–804. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, L.; Liu, X.; Wang, Y.; Xing, S. Enhancing the catalytic activity of zeolitic imidazolate framework-8-derived N-doped carbon with incorporated CeO2 nanoparticles in the oxygen reduction reaction. Chemistry 2017, 23, 10690–10697. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; He, C.; Zhao, N.; Shi, C.; Liu, E.; Li, J. Porous graphitic carbon nanosheets as a high-rate anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 9537–9545. [Google Scholar] [CrossRef]
- Fu, G.; Cui, Z.; Chen, Y.; Li, Y.; Tang, Y.; Goodenough, J.B. Ni3Fe-N doped carbon sheets as a bifunctional electrocatalyst for air cathodes. Adv. Energy Mater. 2017, 7, 1601172. [Google Scholar] [CrossRef]
- He, Y.; Zhuang, X.; Lei, C.; Lei, L.; Hou, Y.; Mai, Y.; Feng, X. Porous carbon nanosheets: Synthetic strategies and electrochemical energy related applications. Nano Today 2019, 24, 103–119. [Google Scholar] [CrossRef]
- Tang, Q.; Niu, R.; Gong, J. Salt-templated synthesis of 3D porous foam-like C3N4 towards high-performance photodegradation of tetracyclines. New J. Chem. 2020, 44, 17405–17412. [Google Scholar] [CrossRef]
- Xu, Y.; Li, W.; Zhang, F.; Zhang, X.; Zhang, W.; Lee, C.-S.; Tang, Y. In situ incorporation of FeS nanoparticles/carbon nanosheets composite with an interconnected porous structure as a high-performance anode for lithium-ion batteries. J. Mater. Chem. A 2016, 4, 3697–3703. [Google Scholar] [CrossRef]
- Zhu, J.; Sakaushi, K.; Clavel, G.; Shalom, M.; Antonietti, M.; Fellinger, T.P. A general salt-templating method to fabricate vertically aligned graphitic carbon nanosheets and their metal carbide hybrids for superior lithium-ion batteries and water splitting. J. Am. Chem. Soc. 2015, 137, 5480–5485. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; Ma, L.; Guo, L.; Li, Q.; He, C.; Liu, E.; He, F.; Shi, C.; Zhao, N. Three-dimensional network of N-doped carbon ultrathin nanosheets with closely packed mesopores: Controllable synthesis and application in electrochemical energy storage. ACS Appl. Mater. Interfaces 2016, 8, 11720–11728. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, G.; Siahrostami, S.; Chen, Z.; Liu, K.; Xie, J.; Liao, L.; Wu, T.; Lin, D.; Liu, Y.; et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 2018, 1, 156–162. [Google Scholar] [CrossRef]
- Huang, H.; Chang, Y.; Jia, J.; Jia, M.; Wen, Z. Understand the Fe3C nanocrystalline grown on rGO and its performance for oxygen reduction reaction. Int. J. Hydrog. Energy 2020, 45, 28764–28773. [Google Scholar] [CrossRef]
- Liu, K.; Huang, X.; Wang, H.; Li, F.; Tang, Y.; Li, J.; Shao, M. Co3O4-CeO2/C as a highly active electrocatalyst for oxygen reduction reaction in Al-air batteries. ACS Appl. Mater. Interfaces 2016, 8, 34422–34430. [Google Scholar] [CrossRef]
- Li, X.; You, S.; Du, J.; Dai, Y.; Chen, H.; Cai, Z.; Ren, N.; Zou, J. ZIF-67-derived Co3O4@carbon protected by oxygen-buffering CeO2 as an efficient catalyst for boosting oxygen reduction/evolution reactions. J. Mater. Chem. A 2019, 7, 25853–25864. [Google Scholar] [CrossRef]
- Wang, J.; Dong, X.; Yang, J.; Zhu, L.; Zeng, W.; Wang, J. Facile hydrothermal synthesis of 3D flower-like NiCo2O4/CeO2 composite as effective oxygen reduction reaction catalyst. J. Mater. Sci. Mater. Electron. 2020, 31, 16600–16608. [Google Scholar] [CrossRef]
- Ghosh, D.; Parwaiz, S.; Mohanty, P.; Pradhan, D. Tuning the morphology of CeO2 nanostructures using a template-free solvothermal process and their oxygen reduction reaction activity. Dalton Trans. 2020, 49, 17594–17604. [Google Scholar] [CrossRef]
- Jiang, L.; Yao, M.; Liu, B.; Li, Q.; Liu, R.; Lv, H.; Lu, S.; Gong, C.; Zou, B.; Cui, T.; et al. Controlled synthesis of CeO2/graphene nanocomposites with highly enhanced optical and catalytic properties. J. Phys. Chem. C 2012, 116, 11741–11745. [Google Scholar] [CrossRef]
- Meléndez-González, P.C.; Sánchez-Castro, E.; Alonso-Lemus, I.L.; Pérez-Hernández, R.; Escobar-Morales, B.; Garay-Tapia, A.M.; Pech-Rodríguez, W.J.; Rodríguez-Varela, J. Bifunctional Pd-CeO2 nanorods/C nanocatalyst with high electrochemical stability and catalytic activity for the ORR and EOR in alkaline media. ChemistrySelect 2020, 5, 14032–14040. [Google Scholar] [CrossRef]
- Sun, S.; Xue, Y.; Wang, Q.; Huang, H.; Miao, H.; Liu, Z. Cerium ion intercalated MnO2 nanospheres with high catalytic activity toward oxygen reduction reaction for aluminum-air batteries. Electrochim. Acta 2018, 263, 544–554. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Wu, Z.; Zhi, M.; Hong, Z.; Huang, F. Complexing-coprecipitation method to synthesize catalysts of cobalt, nitrogen-doped carbon, and CeO2 nanosheets for highly efficient oxygen reduction. ChemNanoMat 2019, 5, 831–837. [Google Scholar] [CrossRef]
- Sridharan, M.; Maiyalagan, T. Enhanced oxygen reduction activity of bimetallic Pd–Ag alloy-supported on mesoporous cerium oxide electrocatalysts in alkaline media. New J. Chem. 2021, 45, 22181–22192. [Google Scholar] [CrossRef]
- Hota, I.; Debnath, A.K.; Muthe, K.P.; Varadwaj, K.S.K.; Parhi, P. A synergistic approach of Vulcan carbon and CeO2 in their composite as an efficient oxygen reduction reaction catalyst. J. Appl. Electrochem. 2020, 50, 1069–1077. [Google Scholar] [CrossRef]
- Sridharan, M.; Maiyalagan, T. Synergistically enhanced electrocatalytic activity of cerium oxide/manganese tungstate composite for oxygen reduction reaction. J. Mater. Sci. Mater. Electron. 2022, 33, 9538–9548. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Chang, Y.; Jia, J. Evaluating the Growth of Ceria-Modified N-Doped Carbon-Based Materials and Their Performance in the Oxygen Reduction Reaction. Nanomaterials 2022, 12, 3057. https://doi.org/10.3390/nano12173057
Wen X, Chang Y, Jia J. Evaluating the Growth of Ceria-Modified N-Doped Carbon-Based Materials and Their Performance in the Oxygen Reduction Reaction. Nanomaterials. 2022; 12(17):3057. https://doi.org/10.3390/nano12173057
Chicago/Turabian StyleWen, Xin, Ying Chang, and Jingchun Jia. 2022. "Evaluating the Growth of Ceria-Modified N-Doped Carbon-Based Materials and Their Performance in the Oxygen Reduction Reaction" Nanomaterials 12, no. 17: 3057. https://doi.org/10.3390/nano12173057



