Synthesis of Porous Hierarchical In2O3 Nanostructures with High Methane Sensing Property at Low Working Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
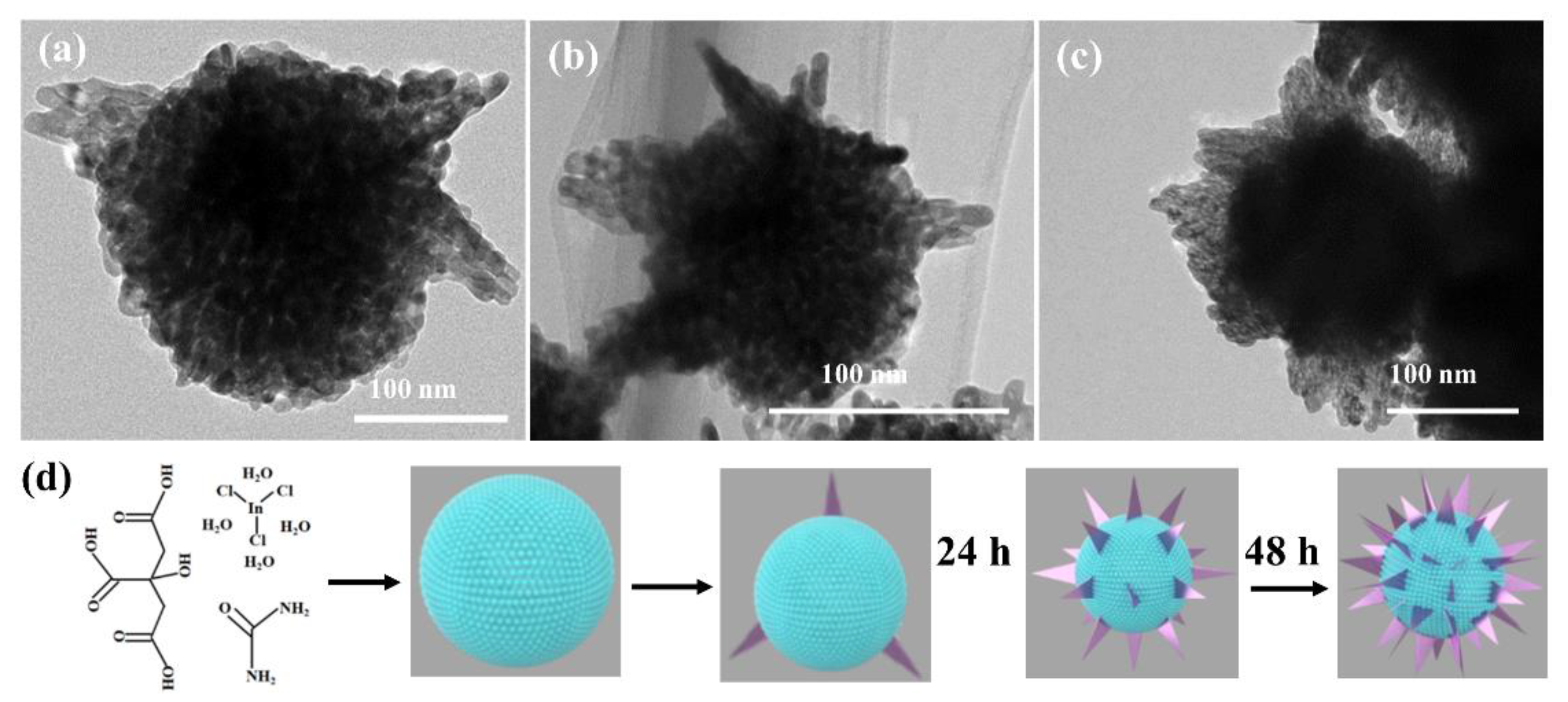
2.2. Material Synthesis
2.3. Material Characterization
2.4. Sensor Fabrication and Measurement
3. Results and Discussion
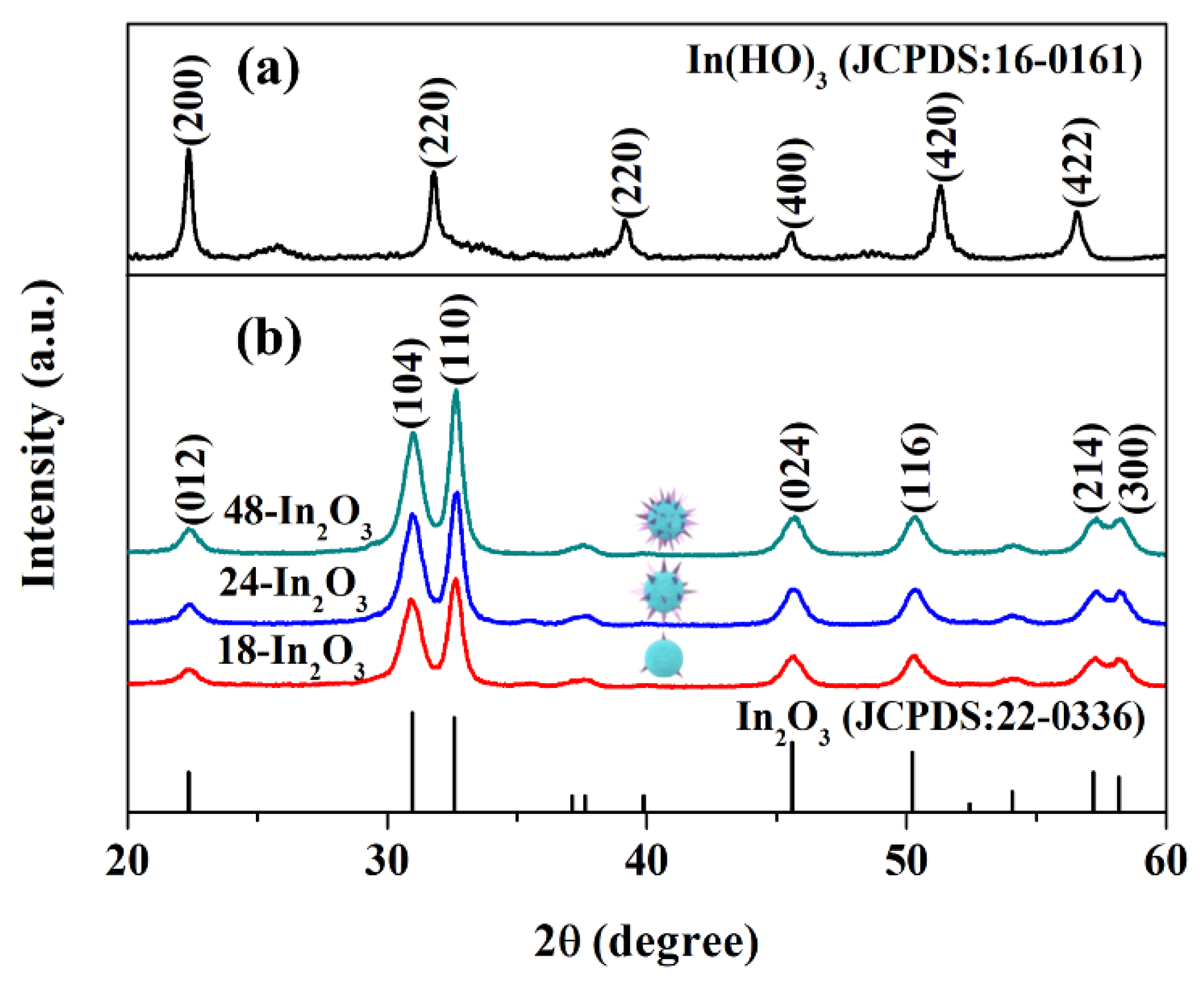
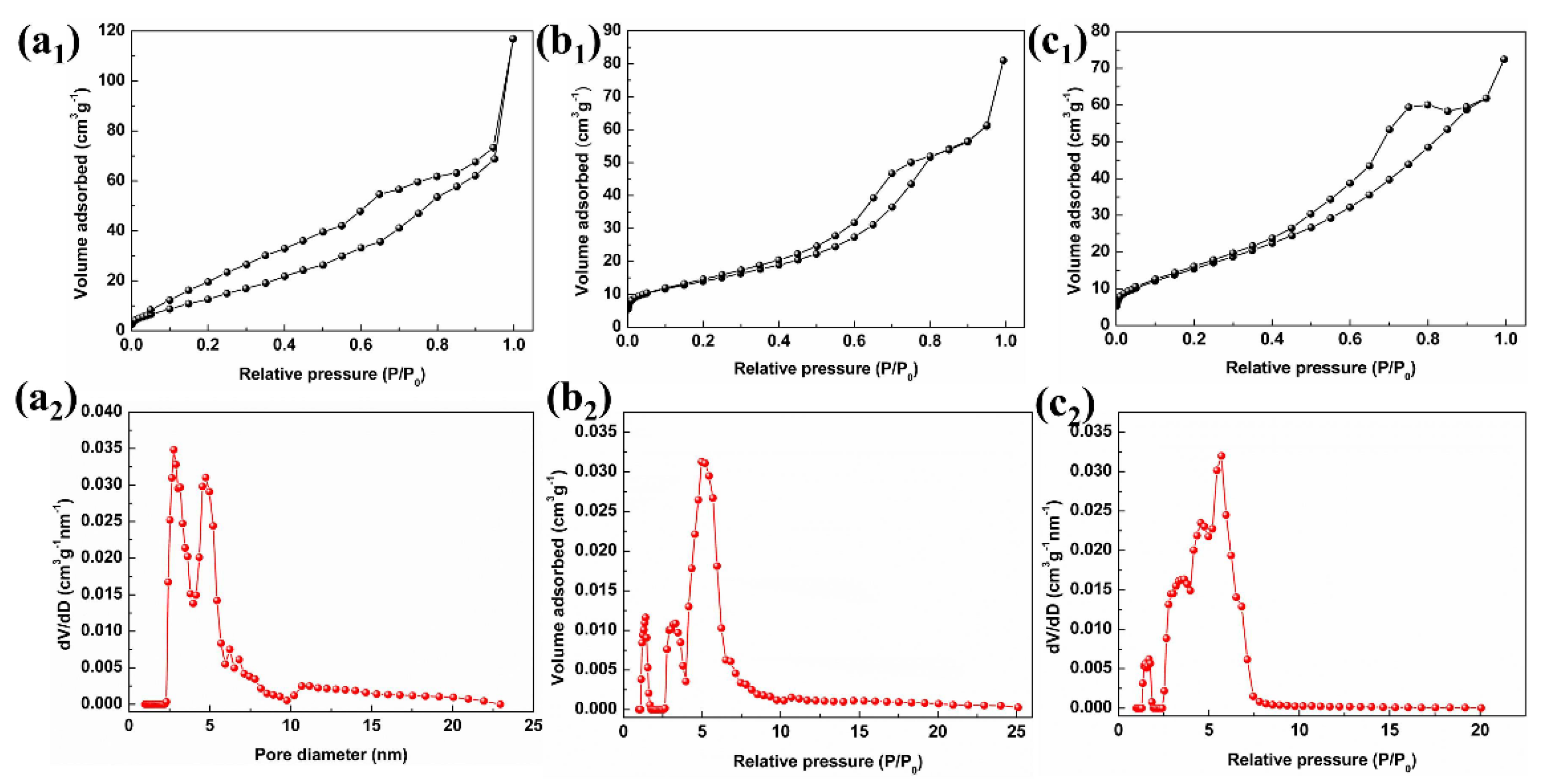
3.1. Sample Characterization
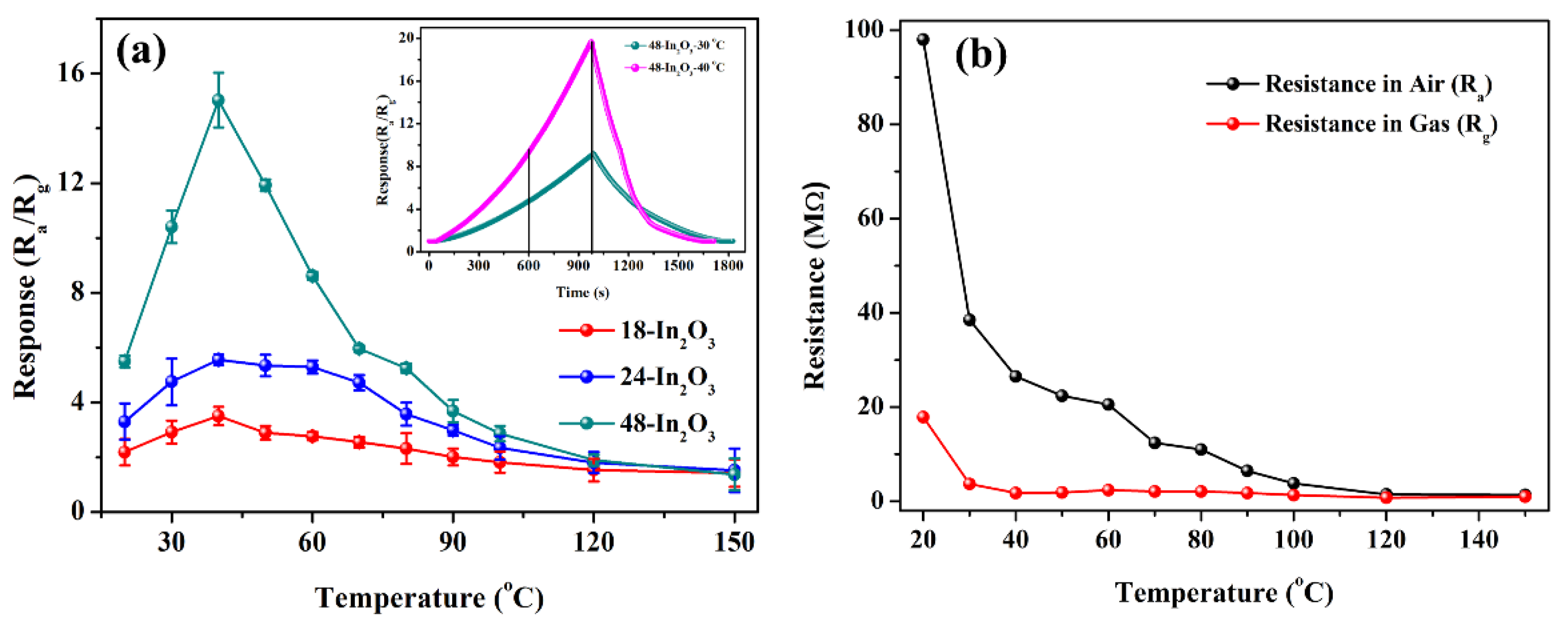
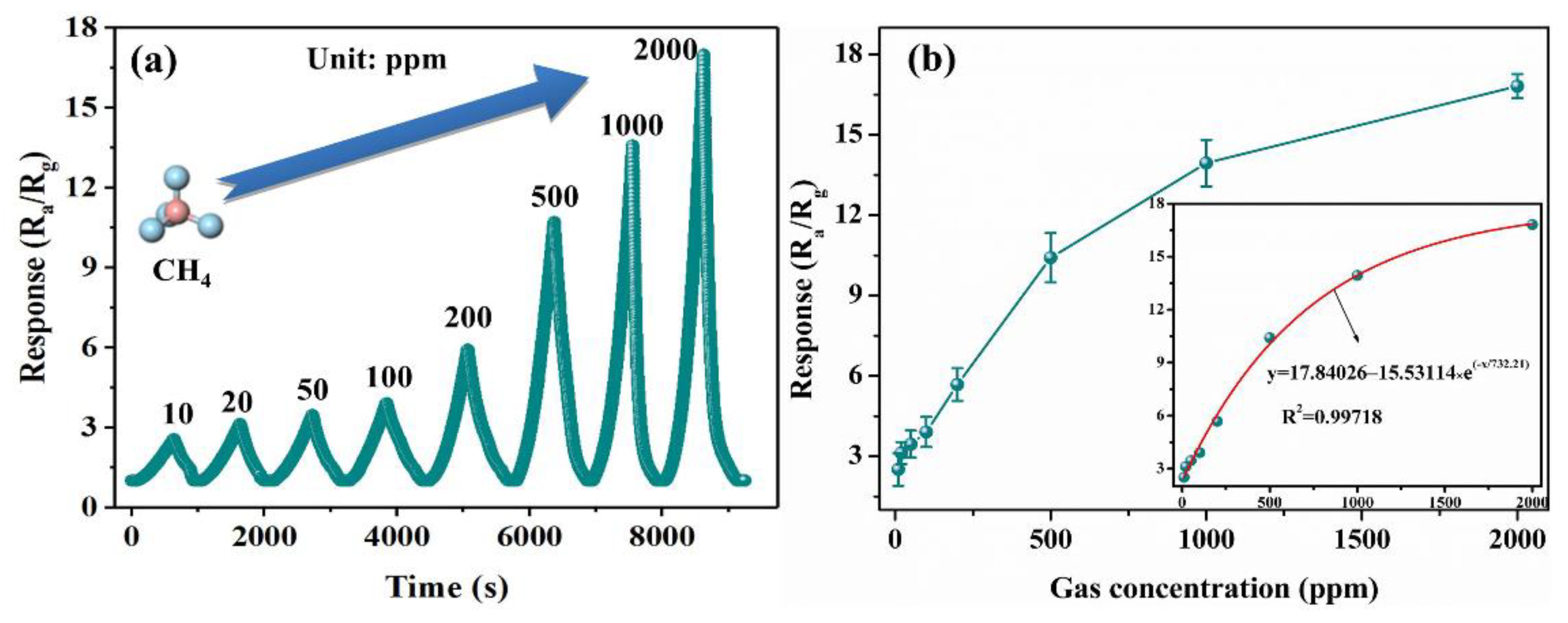
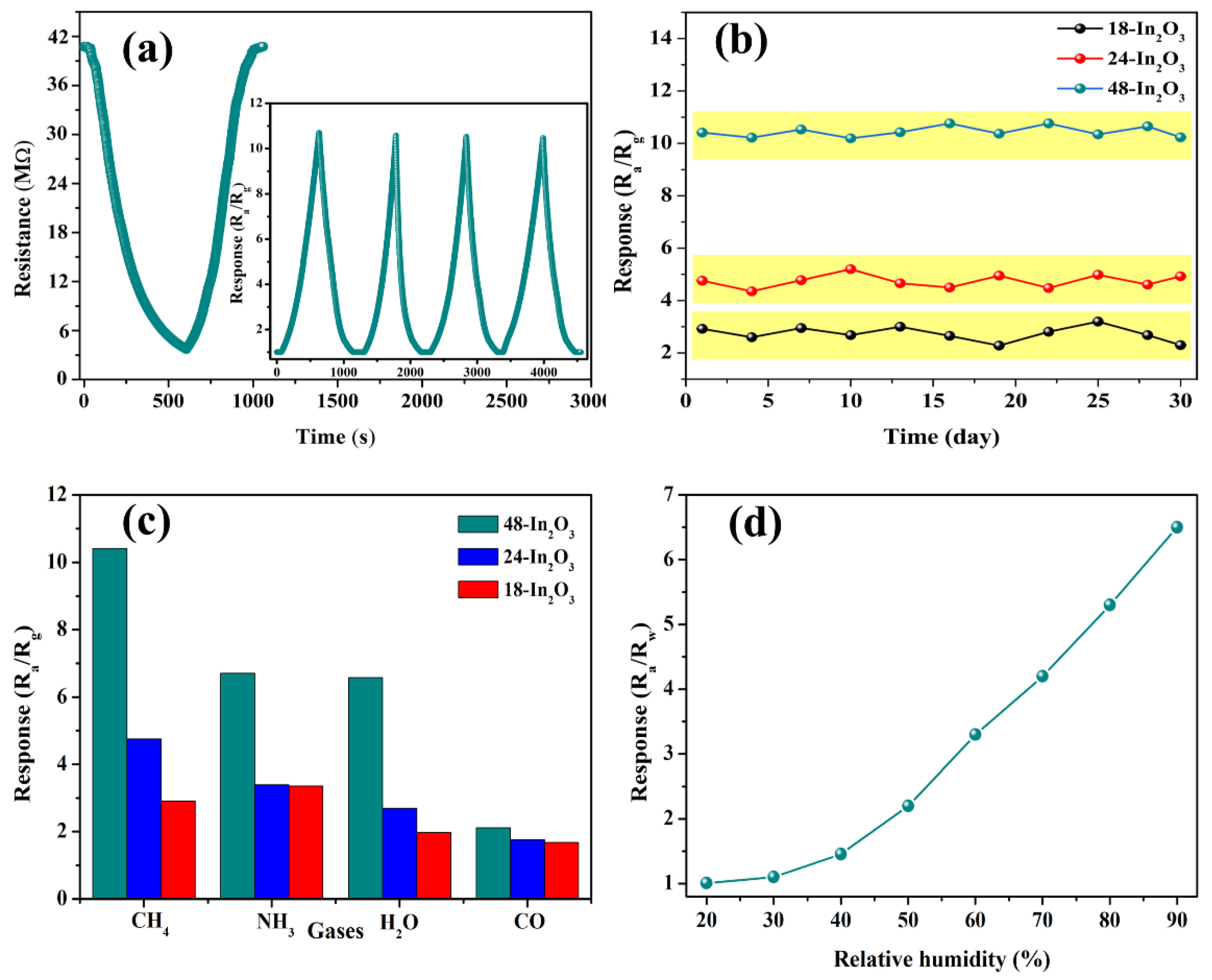
3.2. Gas Sensing Properties Evaluation
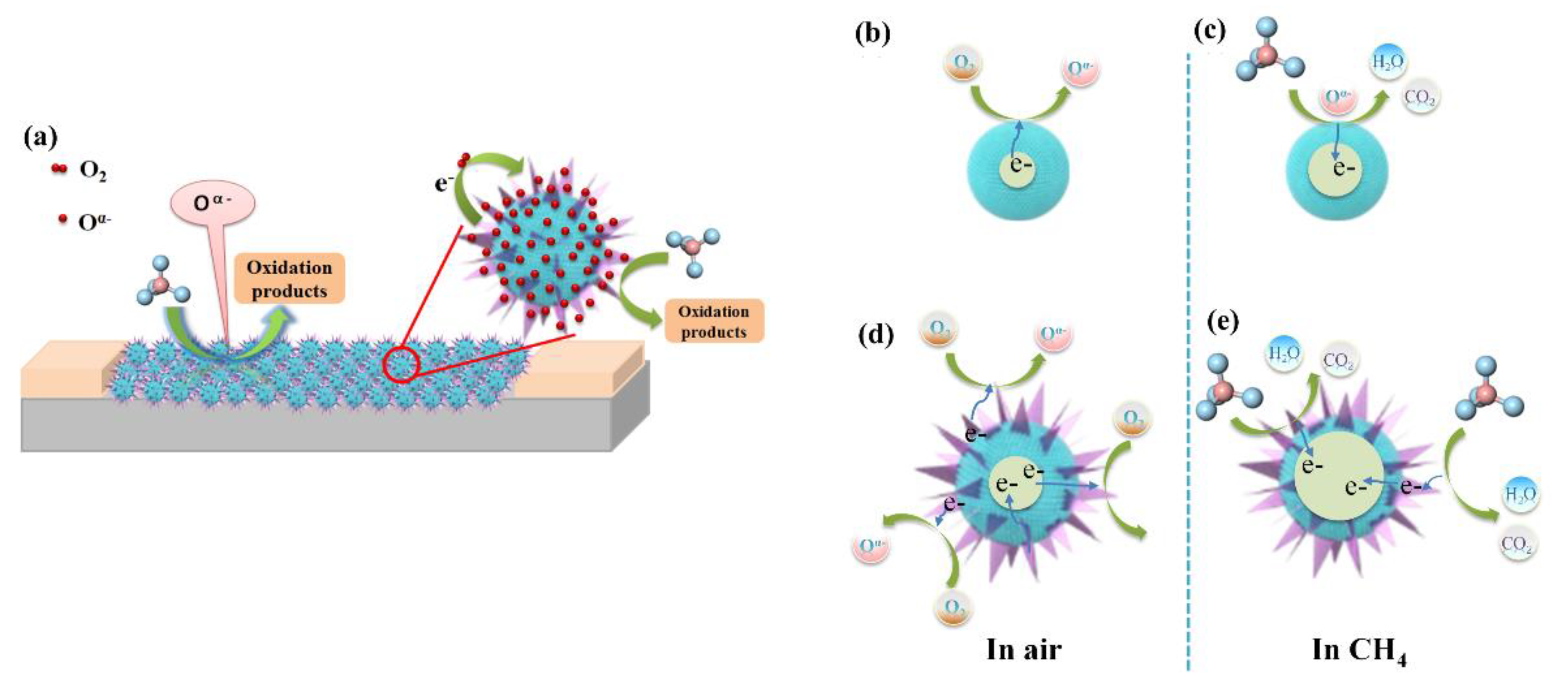
3.3. Gas Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, Y.; Wang, J.; Xu, L.; Li, X.; Huang, S. A room-temperature methane sensor based on Pd-decorated ZnO/rGO hybrids enhanced by visible light photocatalysis. Sens. Actuators B Chem. 2020, 304, 127334. [Google Scholar] [CrossRef]
- Xia, J.; Zhu, F.; Zhang, S.; Kolomenskii, A.; Schuessler, H. A ppb level sensitive sensor for atmospheric methane detection. Infrared Phys. Technol. 2017, 86, 194–201. [Google Scholar] [CrossRef]
- Hu, J.; Gao, F.; Zhao, Z.; Sang, S.; Li, P.; Zhang, W.; Zhou, X.; Chen, Y. Synthesis and characterization of Cobalt-doped ZnO microstructures for methane gas sensing. Appl. Surf. Sci. 2016, 363, 181–188. [Google Scholar] [CrossRef]
- Biaggi-Labiosa, A.; Solá, F.; Lebrón-Colón, M.; Evans, L.J.; Xu, J.C.; Hunter, G.; Berger, G.M.; Gonzalez, J.M. A novel methane sensor based on porous SnO2 nanorods: Room temperature to high temperature detection. Nanotechnology 2012, 23, 455501. [Google Scholar] [CrossRef]
- Thundat, T. Methane sensing at room temperature using photo thermal cantilevered flection spectroscopy. Sens. Actuators B Chem. 2015, 221, 564–569. [Google Scholar]
- Liu, F.; Zhang, Y.; Yu, Y.; Xu, J.; Sun, J.; Lu, G. Enhanced sensing performance of catalytic combustion methane sensor by using Pd nanorod/γ-Al2O3. Sens. Actuators B Chem. 2011, 160, 1091–1097. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba, R.G.V.; Chowdari, B.V.R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, X.; Li, F.; Lu, G.; Zhang, T.; Barsan, N. Pt-In2O3 mesoporous nanofibers with enhanced gas sensing performance towards ppb-level NO2 at room temperature. Sens. Actuators B Chem. 2018, 260, 927–936. [Google Scholar] [CrossRef]
- Elouali, S.; Bloor, L.G.; Binions, R.; Parkin, I.P.; Carmalt, C.J.; Darr, J.A. Gas sensing with nano-indium oxides (In2O3) prepared via continuous hydrothermal flow synthesis. Langmuir 2012, 28, 1879–1885. [Google Scholar] [CrossRef]
- Huang, X.; Sun, B.; Su, D.; Zhao, D.; Wang, G. Soft-template synthesis of 3D porous graphene foams with tunable architectures for lithium-O2 batteries and oil adsorption applications. J. Mater. Chem. A 2014, 2, 7973–7979. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, D.; Margolese, D.I.; Chmelka, B.F.; Stucky, G.D. Generalized syntheses of large-pore meosporous metal oxides with semicrystalline frameworks. Nature 1998, 396, 152–155. [Google Scholar] [CrossRef]
- Liu, J.; Xue, D. Thermal oxidation strategy towards porous metal oxide. Adv. Mater. 2008, 20, 2622–2627. [Google Scholar] [CrossRef]
- Xue, D.; Wang, Y.; Cao, J.; Sun, G.; Zhang, Z. Improving methane gas sensing performance of flower-like SnO2 decorated by WO3 nanoplates. Talanta 2019, 199, 603–611. [Google Scholar] [CrossRef]
- Xue, D.; Wang, P.; Zhang, Z.; Wang, Y. Enhanced methane sensing property of flower-like SnO2 doped by Pt nanoparticles: A combined experimental and first-principle study. Sens. Actuators B Chem. 2019, 296, 126710. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, X.; Wang, Y.Z.; Bing, Y.; Qiao, L.; Liang, Z.; Yu, S.; Zeng, Y.; Zheng, W. Pd-loaded SnO2 ultrathin nanorod-assembled hollow microspheres with the significant improvement for toluene detection. Sens. Actuators B Chem. 2017, 243, 465–474. [Google Scholar] [CrossRef]
- Chava, R.K.; Cho, H.Y.; Yoon, J.M.; Yu, Y.T. Fabrication of aggregated In2O3 nanospheres for highly sensitive acetaldehyde gas sensors. J. Alloy Compd. 2019, 772, 834–842. [Google Scholar] [CrossRef]
- Haridas, D.; Gupta, V. Enhanced response characteristics of SnO2 thin film based sensors loaded with Pd clusters for methane detection. Sens. Actuators B Chem. 2012, 166–167, 156–164. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Tomar, M.; Gupt, V. Reduced graphene oxide-SnO2 nanocomposite thin film based CNG/PNG sensor. Sens. Actuators B Chem. 2017, 245, 590–598. [Google Scholar] [CrossRef]
- Navazani, S.; Shokuhfar, A.; Hassanisadi, M.; Askarieh, M.; Carlo, A.D.; Agresti, A. Facile synthesis of a SnO2@rGO nanohybrid and optimization of its methane-sensing parameters. Talanta 2018, 181, 422–430. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, N.; Xia, B. Facile fabrication of ZnO nanocrystalline-modified graphene hybrid nanocomposite toward methane gas sensing application. J. Mater. Sci. Mater. Electron. 2015, 26, 5937–5945. [Google Scholar] [CrossRef]
- Vuong, N.M.; Hieu, N.M.; Hieu, H.N.; Yi, H.; Kim, D.; Han, Y.S.; Kim, M. Ni2O3-decorated SnO2 particulate films for methane gas sensors. Sens. Actuators B Chem. 2014, 192, 327–333. [Google Scholar] [CrossRef]
- Keshtkar, S.; Rashidi, A.; Kooti, M. Development of tin dioxide quantum dots/multiwalled carbon nanotubes and tin dioxide quantum dots/carbon nanohorns nanohybrids as low temperatures natural gas sensors. Ceram. Int. 2017, 43, 14326–14333. [Google Scholar] [CrossRef]
- Amutha, A.; Amirthapandian, S.; Prasad, A.K.; Panigrahi, B.K.; Thangadurai, P. Methane gas sensing at relatively low operating temperature by hydrothermally prepared SnO2 nanorods. J. Nanopart. Res. 2015, 17, 289. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Kholmanov, I.; Faglia, G.; Sberveglieri, G. Reduced graphene oxide/ZnO nanocomposite for application in chemical gas sensors. RSC Adv. 2016, 6, 34225–34232. [Google Scholar] [CrossRef]
- Cabot, A.; Arbiol, J.; Morante, J.R.; Weimar, U.; Barsan, N.; Gopel, W. Analysis of the noble metal catalytic additives introduced by impregnation of as obtained SnO2 sol-gel nanocrystals for gas sensors. Sens. Actuators B Chem. 2000, 70, 87–100. [Google Scholar] [CrossRef]
- Tshabalala, Z.P.; Shingange, K.; Dhonge, B.P.; Ntwaeaborwa, O.M.; Mhlongo, G.H.; Motaung, D.E. Fabrication of ultra-high sensitive and selective CH4 room temperature gas sensing of TiO2 nanorods: Detailed study on the annealing temperature. Sen. Actuators B Chem. 2017, 238, 402–419. [Google Scholar] [CrossRef]
- Nasresfahani, S.; Sheikhi, M.H.; Tohidi, M.; Zarifkar, A. Methane gas sensing properties of Pd-doped SnO2/reduced graphene oxide synthesized by a facile hydrothermal route. Mater. Res. Bull. 2017, 89, 161–169. [Google Scholar] [CrossRef]
- Wang, C.X.; Yin, L.W.; Zhang, L.Y.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Shankar, P.; Rayappan, J.B.B. Gas sensing mechanism of metal oxides: The role of ambient atmosphere, type of semiconductor and gases-A Review. Sci. Lett. J. 2015, 4, 126. [Google Scholar]
- Chen, H.; Sun, L.; Li, G.D.; Zou, X. Well-tuned surface oxygen chemistry of cation off-stoichiometric spinel oxides for highly selective and sensitive formaldehyde detection. Chem. Mater. 2018, 30, 2018–2027. [Google Scholar] [CrossRef]
- Zhu, Z.; Zheng, L.; Zheng, S.; Chen, J.; Liang, M.; Tian, Y.; Yang, D. Cr doped WO3 nanofibers enriched with surface oxygen vacancies for highly sensitive detection of the 3-hydroxy-2-butanone biomarker. J. Mater. Chem. A 2018, 6, 21419–21427. [Google Scholar] [CrossRef]
- Gao, L.; Cheng, Z.; Xiang, Q.; Zhang, Y.; Xu, J. Porous corundum-type In2O3 nanosheets: Synthesis and NO2 sensing properties. Sens. Actuators B Chem. 2015, 208, 436–443. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Z.; Han, D.; Gu, F. Highly sensitive and selective ethanol sensor fabricated with In-doped 3DOM ZnO. ACS Appl. Mater. Interfaces 2016, 8, 5466–5474. [Google Scholar] [CrossRef] [PubMed]
- Sakai, G.; Matsunaga, N.; Shimanoe, K.; Yamazoe, N. Theory of gas-diffusion controlled sensitivity for thin film semiconductor gas sensor. Sens. Actuators B Chem. 2001, 80, 125–131. [Google Scholar] [CrossRef]
- Nayak, A.K.; Ghosh, R.; Santra, S.; Guha, P.K.; Pradhan, D. Hierarchical nanostructured WO3-SnO2 for selective sensing of volatile organic compounds. Nanoscale 2015, 7, 12460–12473. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.C.; Teter, M.P.; Allan, D.C.; Arias, T.; Joannopoulos, J. Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045–1097. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.; Vosko, S.; Jackson, K.A.; Pederson, M.R.; Singh, D.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- White, J.; Bird, D. Implementation of gradient-corrected exchange-correlation potentials in Car-Parrinello total-energy calculations. Phys. Rev. B 1994, 50, 4954–4957. [Google Scholar] [CrossRef]
- Park, S.; Ahn, H.-S.; Lee, C.-K.; Kim, H.; Jin, H.; Lee, H.-S.; Seo, S.; Yu, J.; Han, S. Interaction and ordering of vacancy defects in NiO. Phys. Rev. B 2008, 77, 134103. [Google Scholar] [CrossRef]
- Wu, G.X.; Zhang, J.; Wu, Y.; Li, Q.; Chou, K.; Bao, X. Adsorption and dissociation of hydrogen on MgO surface: A first-principles study. J. Alloys Compd. 2009, 480, 788–793. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, T.M.; Liu, D.J.; Han, E.J. Hydrogen sensing and mechanism of M-doped SnO2 (M = Cr3+, Cu2+ and Pd2+) nanocomposite. Sens. Actuators B 2011, 160, 455–462. [Google Scholar] [CrossRef]


| Materials | Hydrothermal Time (h) | D (nm) | Lattice Strain |
|---|---|---|---|
| 18-In2O3 | 18 | 14.7 | 0.0029 |
| 24-In2O3 | 24 | 14.8 | 0.0023 |
| 48-In2O3 | 48 | 15.7 | 0.0022 |
| Samples | Response (Ra/Rg) | Temperature (°C) | Concentration (ppm) | Specific Surface Area (m2·g−1) | Response/Recovery Time (s) |
|---|---|---|---|---|---|
| SnO2-Pd [18] | 4.4 | 180 | 200 | - | 90/90 |
| rGO-SnO2 [19] | 1.6 | 200 | 1000 | - | 32/19 |
| SnO2@rGO [20] | 1.11 | 150 | 1000 | 110.55 | 61/330 |
| ZnO–rGO [21] | 1.047 | 190 | 4000 | - | 30/40 |
| Ni2O3-SnO2 [22] | 2.27 | 400 | 200 | - | - |
| SnO2 QDs/CNHs [23] | 2.5 | 90 | 1000 | - | 1074/1080 |
| SnO2 nanorods [24] | 1.05 | 100 | 500 | - | 13/- |
| RGO/ZnO [25] | 1.67 | 250 | 500 | - | - |
| SnO2 nanocrystals [26] | 1.21 | 350 | 500 | - | - |
| 48-In2O3 (this work) | 10.4 | 30 | 500 | 59.635 | 600/280 |
| Materials | Adsorbed Oxygen | Operating Temperature (°C) | Response (Rg/Ra) |
|---|---|---|---|
| 18-In2O3 | 43.1% | 30 | 2.92 |
| 24-In2O3 | 46.7% | 30 | 4.8 |
| 48-In2O3 | 50.5% | 30 | 10.4 |
| Adsorption Energy (eV) | C-H Bond Length (Å) | ||
|---|---|---|---|
| Before Adsorption | After Adsorption | ||
| Absence of adsorption oxygen | 6.751 | 1.097 | 1.179 |
| Presence of adsorption oxygen | −3.079 | 3.394 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Chang, J.; Wang, Y.; Cao, J. Synthesis of Porous Hierarchical In2O3 Nanostructures with High Methane Sensing Property at Low Working Temperature. Nanomaterials 2022, 12, 3081. https://doi.org/10.3390/nano12173081
Zhang H, Chang J, Wang Y, Cao J. Synthesis of Porous Hierarchical In2O3 Nanostructures with High Methane Sensing Property at Low Working Temperature. Nanomaterials. 2022; 12(17):3081. https://doi.org/10.3390/nano12173081
Chicago/Turabian StyleZhang, Huiju, Jiangnan Chang, Yan Wang, and Jianliang Cao. 2022. "Synthesis of Porous Hierarchical In2O3 Nanostructures with High Methane Sensing Property at Low Working Temperature" Nanomaterials 12, no. 17: 3081. https://doi.org/10.3390/nano12173081




