Efficient Exciton Dislocation and Ultrafast Charge Extraction in CsPbI3 Perovskite Quantum Dots by Using Fullerene Derivative as Semiconductor Ligand
Abstract
:1. Introduction
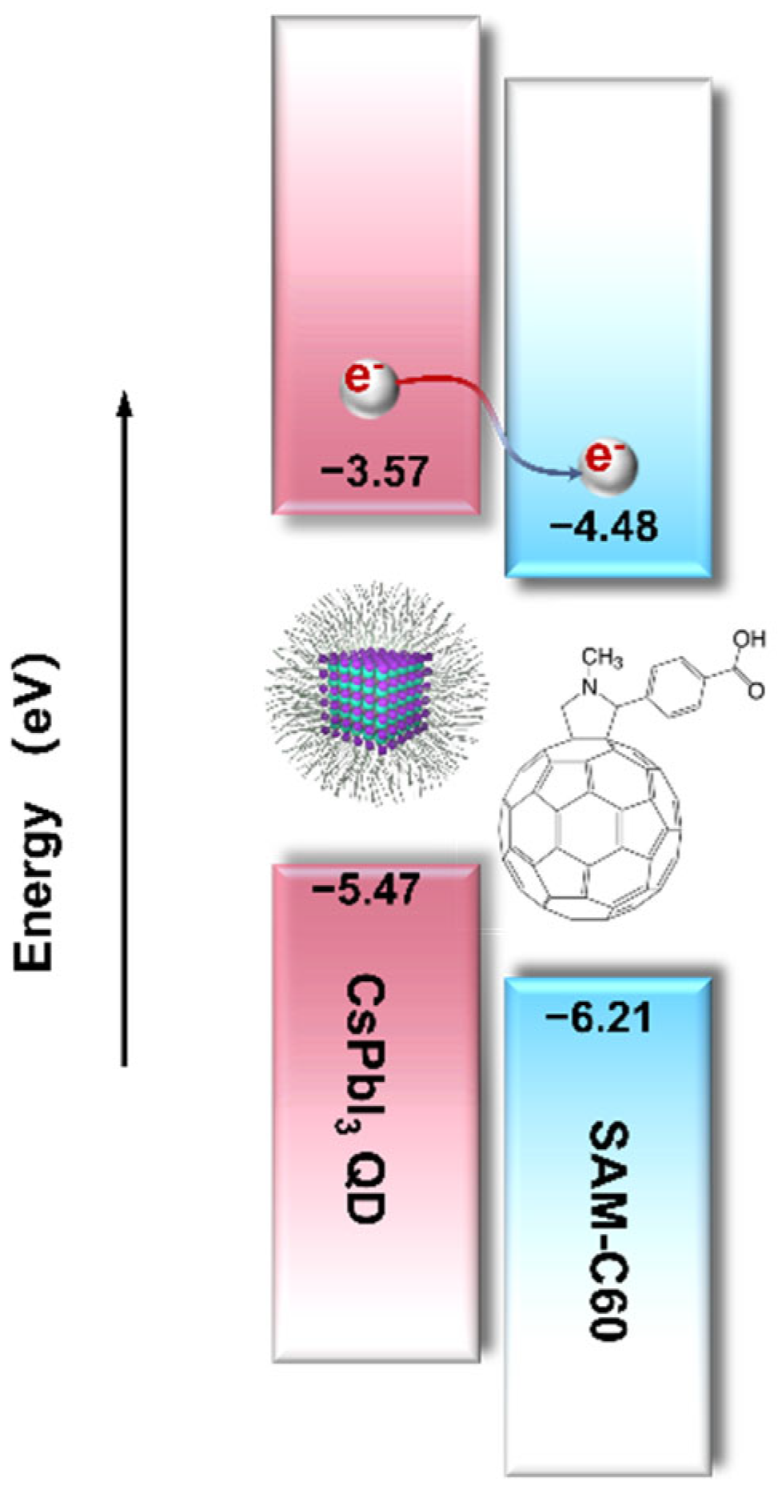
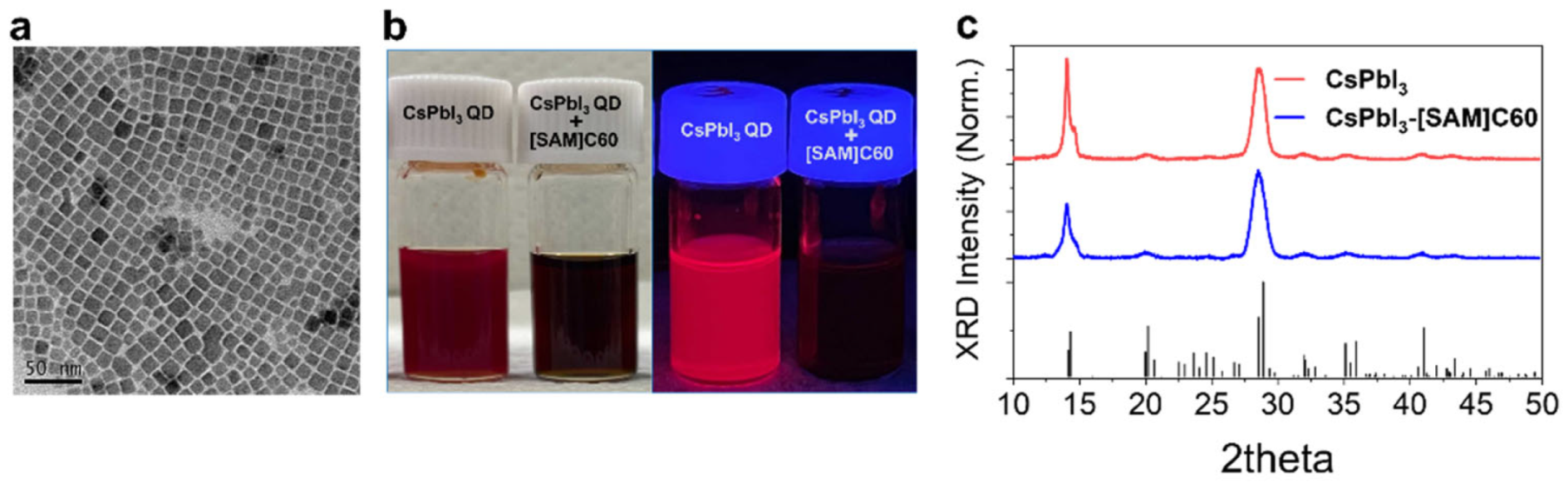
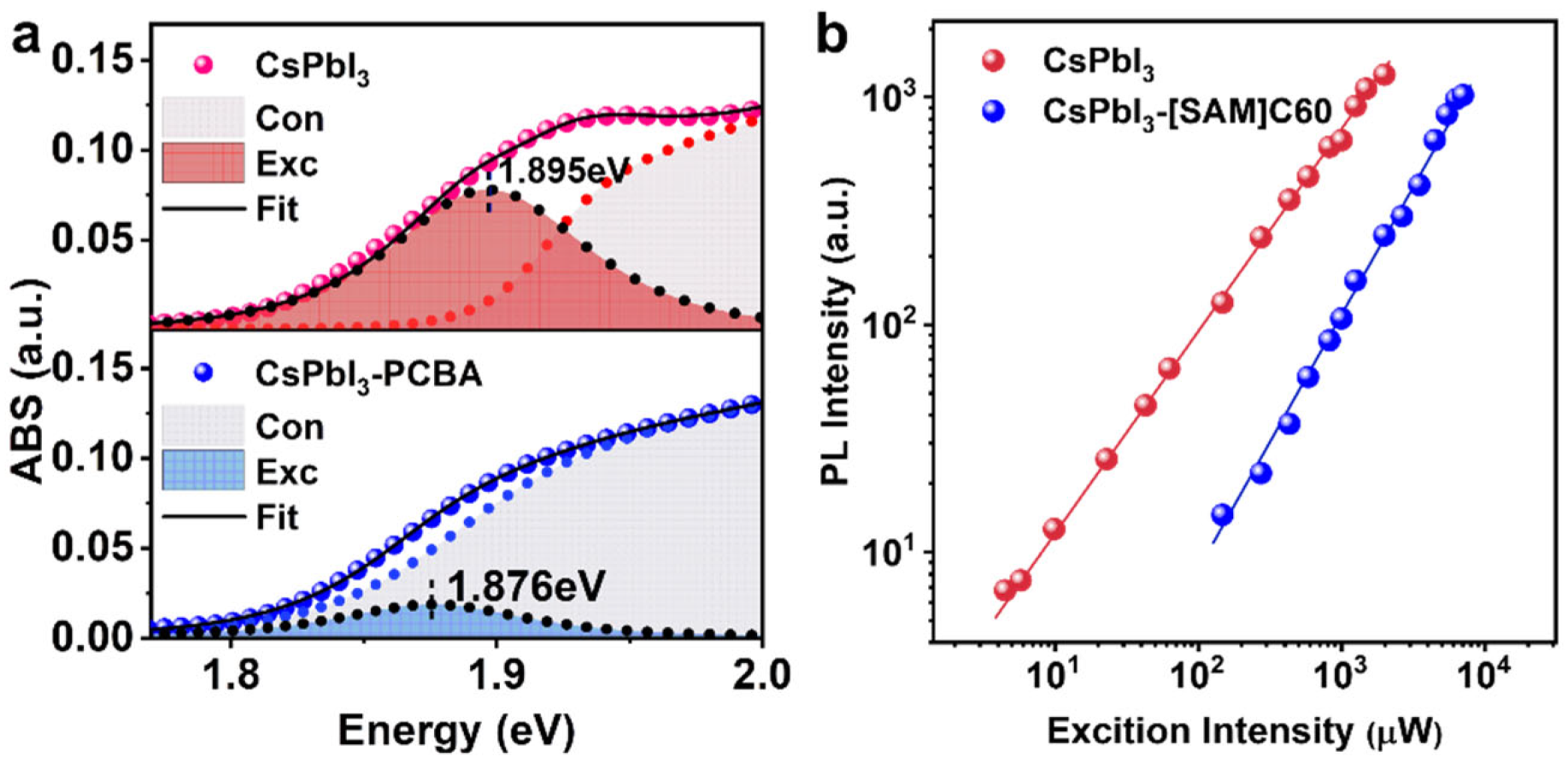
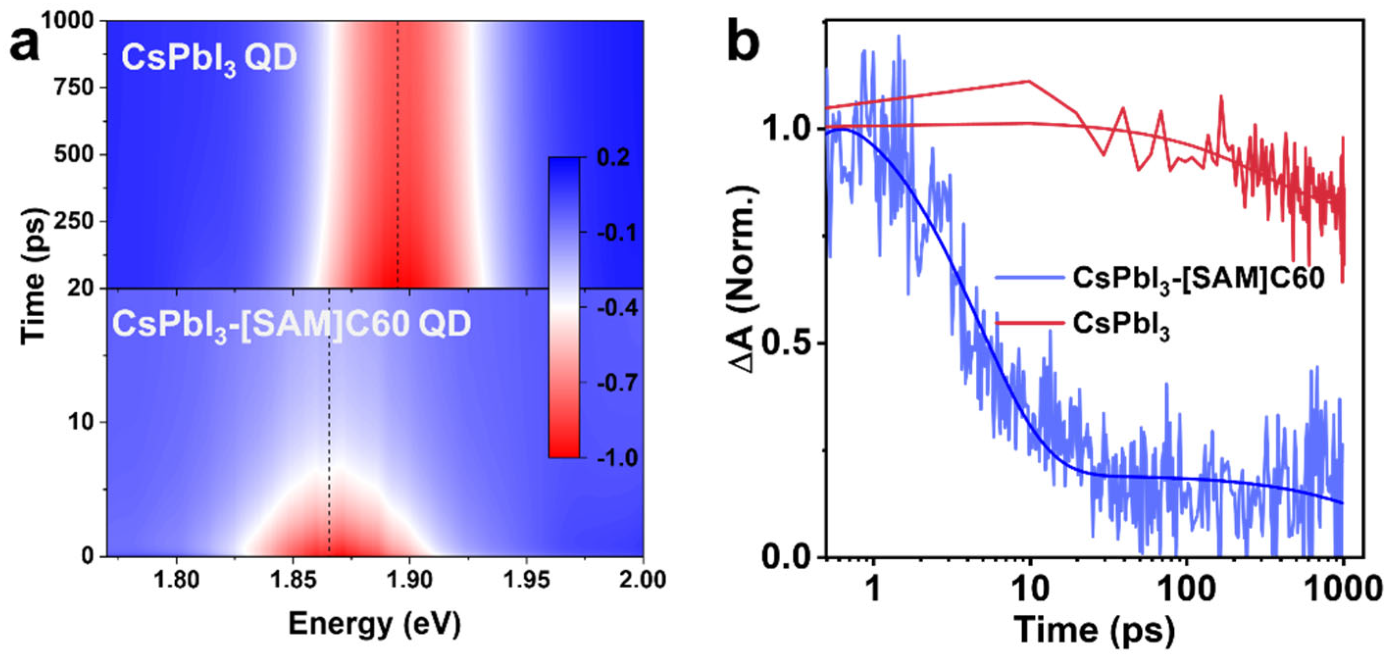
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Colloidal Synthesis of CsPbI3 QDs
4.3. Preparation of CsPbI3 QDs with [SAM]C60 (CsPbI3-[SAM]C60 QDs)
4.4. Characterization
Author Contributions
Funding
Conflicts of Interest
References
- Luther, J.M. Quantum dot—Induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar]
- Liu, F.; Zhang, Y.; Ding, C.; Kobayashi, S.; Izuishi, T.; Nakazawa, N.; Toyoda, T.; Ohta, T.; Hayase, S.; Minemoto, T.; et al. Highly Luminescent Phase-Stable CsPbI3 Perovskite Quantum Dots Achieving Near 100% Absolute Photoluminescence Quantum Yield. ACS Nano 2017, 11, 10373–10383. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Begum, R.; Fu, J.; Xu, Q.; Koh, T.M.; Veldhuis, S.A.; Grätzel, M.; Mathews, N.; Mhaisalkar, S.; Sum, T.C. Low threshold and efficient multiple exciton generation in halide perovskite nanocrystals. Nat. Commun. 2018, 9, 4197. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bhaumik, S.; Goh, T.W.; Kumar, M.S.; Yantara, N.; Gratzel, M.; Mhaisalkar, S.; Mathews, N.; Sum, T.C. Slow cooling and highly efficient extraction of hot carriers in colloidal perovskite nanocrystals. Nat. Commun. 2017, 8, 14350. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Cai, B.; Song, J.; Zhang, F.; Fang, T.; Zeng, H. A bilateral interfacial passivation strategy promoting efficiency and stability of perovskite quantum dot light-emitting diodes. Nat. Commun. 2020, 11, 3902. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Kershaw, S.V.; Rogach, A.L.; Tian, J. Improved stability and photodetector performance of CsPbI3 perovskite quantum dots by ligand exchange with aminoethanethiol. Adv. Funct. Mater. 2019, 29, 1902446. [Google Scholar] [CrossRef]
- Hao, M.; Bai, Y.; Zeiske, S.; Ren, L.; Liu, J.; Yuan, Y.; Zarrabi, N.; Cheng, N.; Ghasemi, M.; Chen, P.; et al. Ligand-assisted cation-exchange engineering for high-efficiency colloidal Cs1−xFAxPbI3 quantum dot solar cells with reduced phase segregation. Nat. Energy 2020, 5, 79–88. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Zou, C.; Mao, C.; Corp, K.L.; Yao, Y.-C.; Lee, Y.-J.; Schlenker, C.W.; Jen, A.K.; Lin, L.Y. CsPbBr3 perovskite quantum dot vertical cavity lasers with low threshold and high stability. Acs Photonics 2017, 4, 2281–2289. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, Z.; Chen, D.; Bai, D.; Bian, H.; Sun, J.; Zhu, G.; Wang, G.; Liu, S. µ-Graphene Crosslinked CsPbI3 Quantum Dots for High Efficiency Solar Cells with Much Improved Stability. Adv. Energy Mater. 2018, 8, 1800007. [Google Scholar] [CrossRef]
- Thomas, C.J.; Zhang, Y.; Guillaussier, A.; Bdeir, K.; Aly, O.F.; Kim, H.G.; Noh, J.; Reimnitz, L.C.; Li, J.; Deepak, F.L.; et al. Thermal Stability of the Black Perovskite Phase in Cesium Lead Iodide Nanocrystals Under Humid Conditions. Chem. Mater. 2019, 31, 9750–9758. [Google Scholar] [CrossRef]
- Jia, D.; Chen, J.; Mei, X.; Fan, W.; Luo, S.; Yu, M.; Liu, J.; Zhang, X. Surface matrix curing of inorganic CsPbI3 perovskite quantum dots for solar cells with efficiency over 16%. Energ. Environ. Sci. 2021, 14, 4599–4609. [Google Scholar] [CrossRef]
- Yoon, S.M.; Min, H.; Kim, J.B.; Kim, G.; Lee, K.S.; Seok, S.I. Surface Engineering of Ambient-Air-Processed Cesium Lead Triiodide Layers for Efficient Solar Cells. Joule 2021, 5, 183–196. [Google Scholar] [CrossRef]
- Ling, X.; Yuan, J.; Zhang, X.; Qian, Y.; Zakeeruddin, S.M.; Larson, B.W.; Zhao, Q.; Shi, J.; Yang, J.; Ji, K.; et al. Guanidinium-Assisted Surface Matrix Engineering for Highly Efficient Perovskite Quantum Dot Photovoltaics. Adv. Mater. 2020, 32, 2001906. [Google Scholar] [CrossRef] [PubMed]
- Masi, S.; Gualdrón-Reyes, A.F.; Mora-Seró, I. Stabilization of Black Perovskite Phase in FAPbI3 and CsPbI3. ACS Energy Lett. 2020, 5, 1974–1985. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef]
- Wheeler, L.M.; Sanehira, E.M.; Marshall, A.R.; Schulz, P.; Suri, M.; Anderson, N.C.; Christians, J.A.; Nordlund, D.; Sokaras, D.; Kroll, T.; et al. Targeted Ligand-Exchange Chemistry on Cesium Lead Halide Perovskite Quantum Dots for High-Efficiency Photovoltaics. J. Am. Chem. Soc. 2018, 140, 10504–10513. [Google Scholar] [CrossRef]
- Jingxuan Chen, X.Z. Review—Emerging Perovskite Quantum Dot Solar Cells: Feasible Approaches to Boost Performance. Energy Environ. Sci. 2020, 14, 224–261. [Google Scholar] [CrossRef]
- Chi, W.; Banerjee, S.K. Application of Perovskite Quantum Dots as an Absorber in Perovskite Solar Cells. Angew. Chem. Int. Ed. Engl. 2022, 61, e202112412. [Google Scholar] [CrossRef]
- Suh, Y.-H.; Kim, T.; Choi, J.W.; Lee, C.-L.; Park, J. High-Performance CsPbX3 Perovskite Quantum-Dot Light-Emitting Devices via Solid-State Ligand Exchange. ACS Appl. Nano Mater. 2018, 1, 488–496. [Google Scholar] [CrossRef]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Wu, C.-G. Bulk heterojunction perovskite—PCBM solar cells with high fill factor. Nat. Photonics 2016, 10, 196–200. [Google Scholar] [CrossRef]
- Mandal, S.; George, L.; Tkachenko, N.V. Charge transfer dynamics in CsPbBr3 perovskite quantum dots-anthraquinone/fullerene (C 60) hybrids. Nanoscale 2019, 11, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, M.; Li, Q.; Chen, R.; Liu, X. Hybrid photodetector based on CsPbBr3 perovskite nanocrystals and PC71BM fullerene derivative. Chem. Phys. Lett. 2018, 699, 208–211. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.-C.; Li, X.; Song, C.; Wan, L.; Usman, K.; Fang, J. Recent Advance in Solution-Processed Organic Interlayers for High-Performance Planar Perovskite Solar Cells. Adv. Sci. 2018, 5, 1800159. [Google Scholar] [CrossRef]
- Zhao, Q.; Hazarika, A.; Chen, X.; Harvey, S.P.; Larson, B.W.; Teeter, G.R.; Liu, J.; Song, T.; Xiao, C.; Shaw, L.; et al. High efficiency perovskite quantum dot solar cells with charge separating heterostructure. Nat. Commun. 2019, 10, 2842. [Google Scholar] [CrossRef] [PubMed]
- Saba, M.; Cadelano, M.; Marongiu, D.; Chen, F.; Sarritzu, V.; Sestu, N.; Figus, C.; Aresti, M.; Piras, R.; Lehmann, A.G.; et al. Correlated electron-hole plasma in organometal perovskites. Nat. Commun. 2014, 5, 5049. [Google Scholar] [CrossRef]
- Elliott, R.J. Intensity of optical absorption by excitons. Phys. Rev. 1957, 108, 1384. [Google Scholar] [CrossRef]
- Schmidt, T.; Lischka, K.; Zulehner, W. Excitation-power dependence of the near-band-edge photoluminescence of semiconductors. Phys. Rev. B Condens. Matter 1992, 45, 8989–8994. [Google Scholar] [CrossRef]
- Shi, J.; Li, Y.; Wu, J.; Wu, H.; Luo, Y.; Li, D.; Jasieniak, J.J.; Meng, Q. Exciton Character and High-Performance Stimulated Emission of Hybrid Lead Bromide Perovskite Polycrystalline Film. Adv. Opt. Mater. 2020, 8, 1902026. [Google Scholar] [CrossRef]
- Giansante, C.; Infante, I.; Fabiano, E.; Grisorio, R.; Suranna, G.P.; Gigli, G. “Darker-than-Black” PbS Quantum Dots: Enhancing Optical Absorption of Colloidal Semiconductor Nanocrystals via Short Conjugated Ligands. J. Am. Chem. Soc. 2015, 137, 1875–1886. [Google Scholar] [CrossRef]
- Debellis, D.; Gigli, G.; ten Brinck, S.; Infante, I.; Giansante, C. Quantum-Confined and Enhanced Optical Absorption of Colloidal PbS Quantum Dots at Wavelengths with Expected Bulk Behavior. Nano Lett. 2017, 17, 1248–1254. [Google Scholar] [CrossRef] [PubMed]

| CsPbI3 | CsPbI3-[SAM]C60 | |
|---|---|---|
| Eg (eV) | 1.92 | 1.90 |
| Eb (meV) | 25 | 7 |
| T (meV) | 32 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, D.; Hayase, S.; Yang, Y.; Ding, C.; Shen, Q. Efficient Exciton Dislocation and Ultrafast Charge Extraction in CsPbI3 Perovskite Quantum Dots by Using Fullerene Derivative as Semiconductor Ligand. Nanomaterials 2022, 12, 3101. https://doi.org/10.3390/nano12183101
Li Y, Wang D, Hayase S, Yang Y, Ding C, Shen Q. Efficient Exciton Dislocation and Ultrafast Charge Extraction in CsPbI3 Perovskite Quantum Dots by Using Fullerene Derivative as Semiconductor Ligand. Nanomaterials. 2022; 12(18):3101. https://doi.org/10.3390/nano12183101
Chicago/Turabian StyleLi, Yusheng, Dandan Wang, Shuzi Hayase, Yongge Yang, Chao Ding, and Qing Shen. 2022. "Efficient Exciton Dislocation and Ultrafast Charge Extraction in CsPbI3 Perovskite Quantum Dots by Using Fullerene Derivative as Semiconductor Ligand" Nanomaterials 12, no. 18: 3101. https://doi.org/10.3390/nano12183101
APA StyleLi, Y., Wang, D., Hayase, S., Yang, Y., Ding, C., & Shen, Q. (2022). Efficient Exciton Dislocation and Ultrafast Charge Extraction in CsPbI3 Perovskite Quantum Dots by Using Fullerene Derivative as Semiconductor Ligand. Nanomaterials, 12(18), 3101. https://doi.org/10.3390/nano12183101






