Copper-Modified Titania-Based Photocatalysts for the Efficient Hydrogen Production under UV and Visible Light from Aqueous Solutions of Glycerol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Pretreatment of TiO2 Samples
2.3. Synthesis of Photocatalysts CuOx/TiO2 and Pt/TiO2
2.4. Sample Testing Methods
2.4.1. Physical Methods
2.4.2. Catalytic Tests
3. Results and Discussion
3.1. Physical Methods
3.1.1. XRD and BET Methods
3.1.2. UV–Vis Spectroscopy
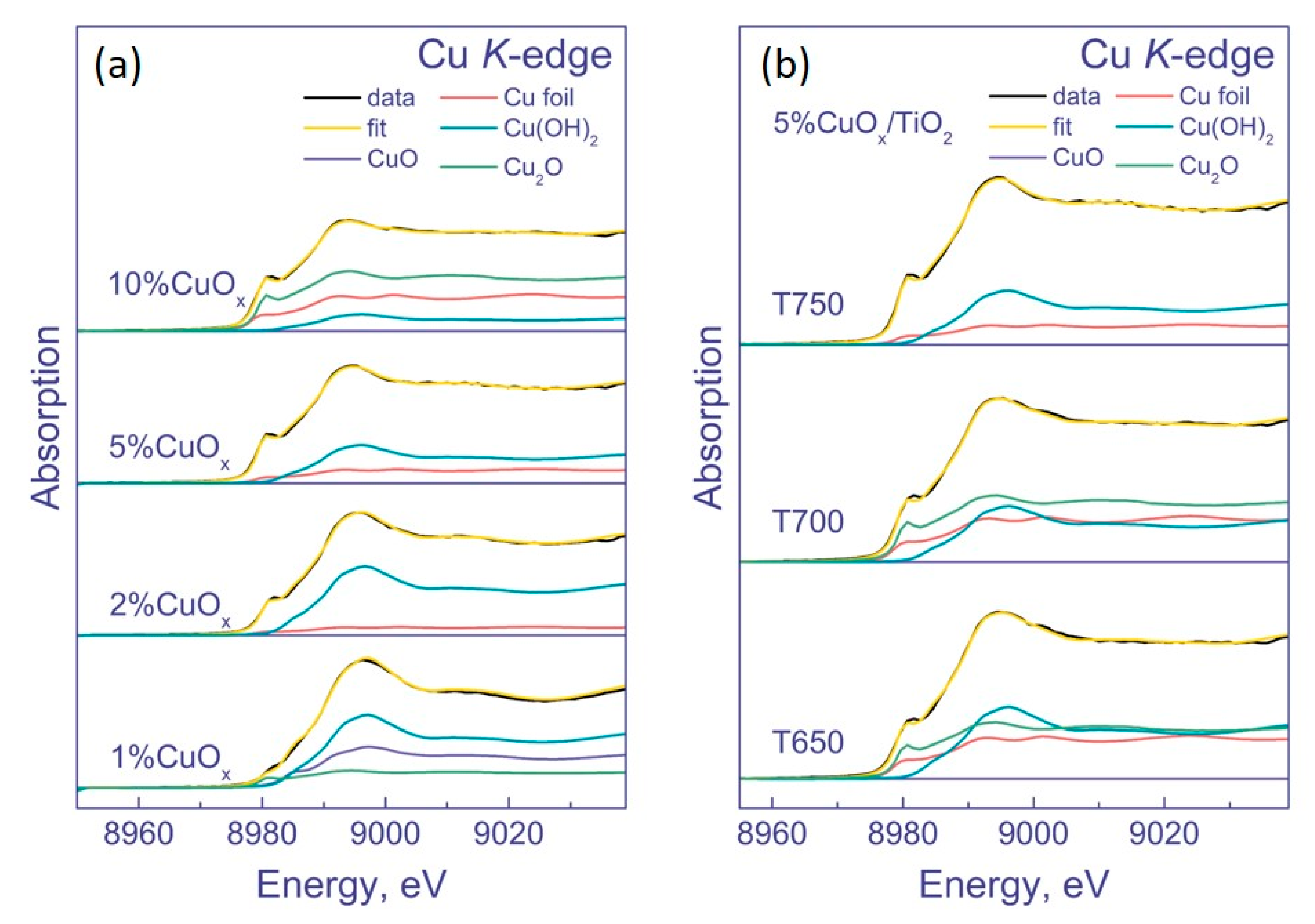
3.1.3. XPS and XANES Methods
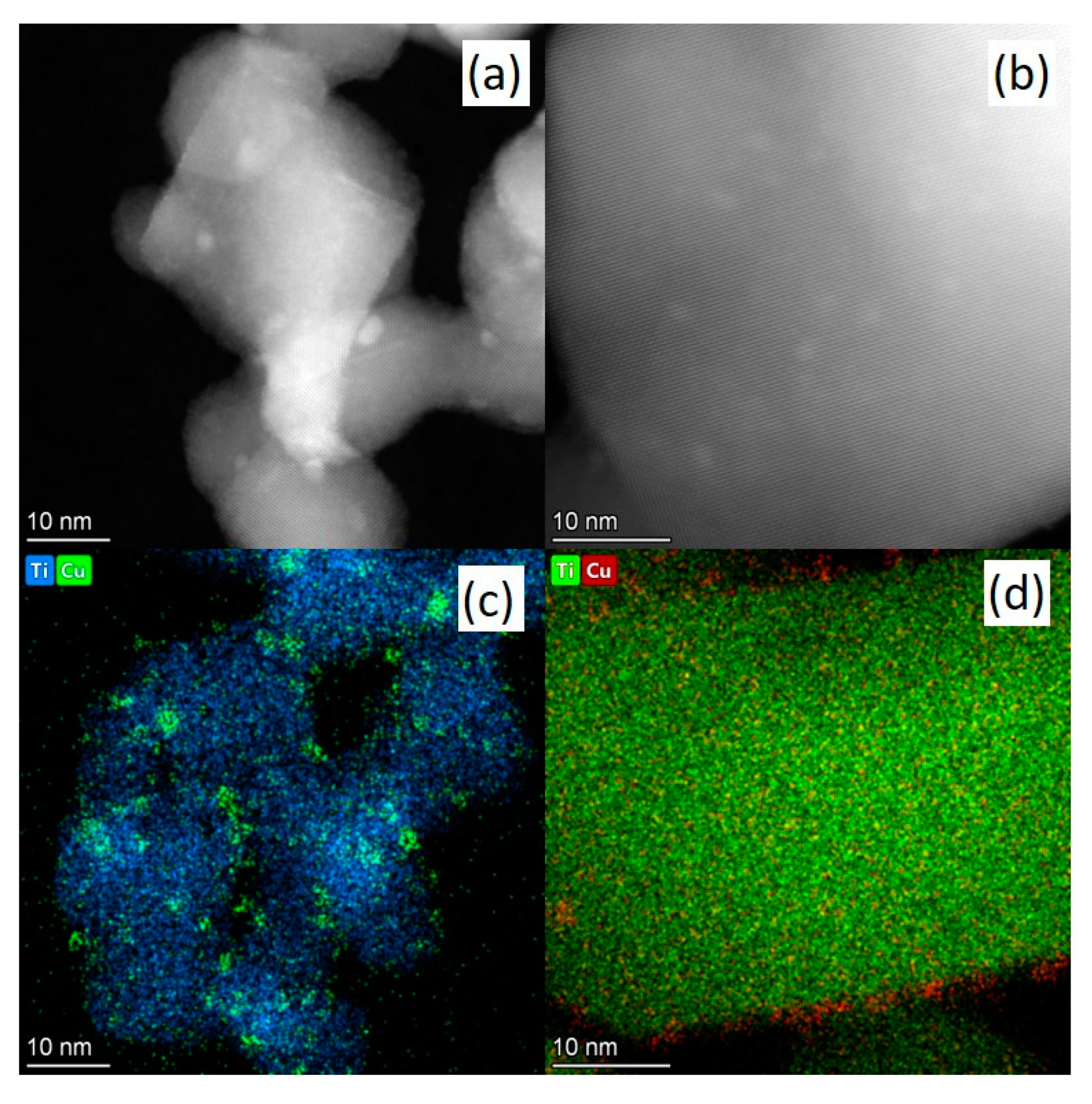
3.1.4. HR TEM Method
3.2. Kinetic Experiments
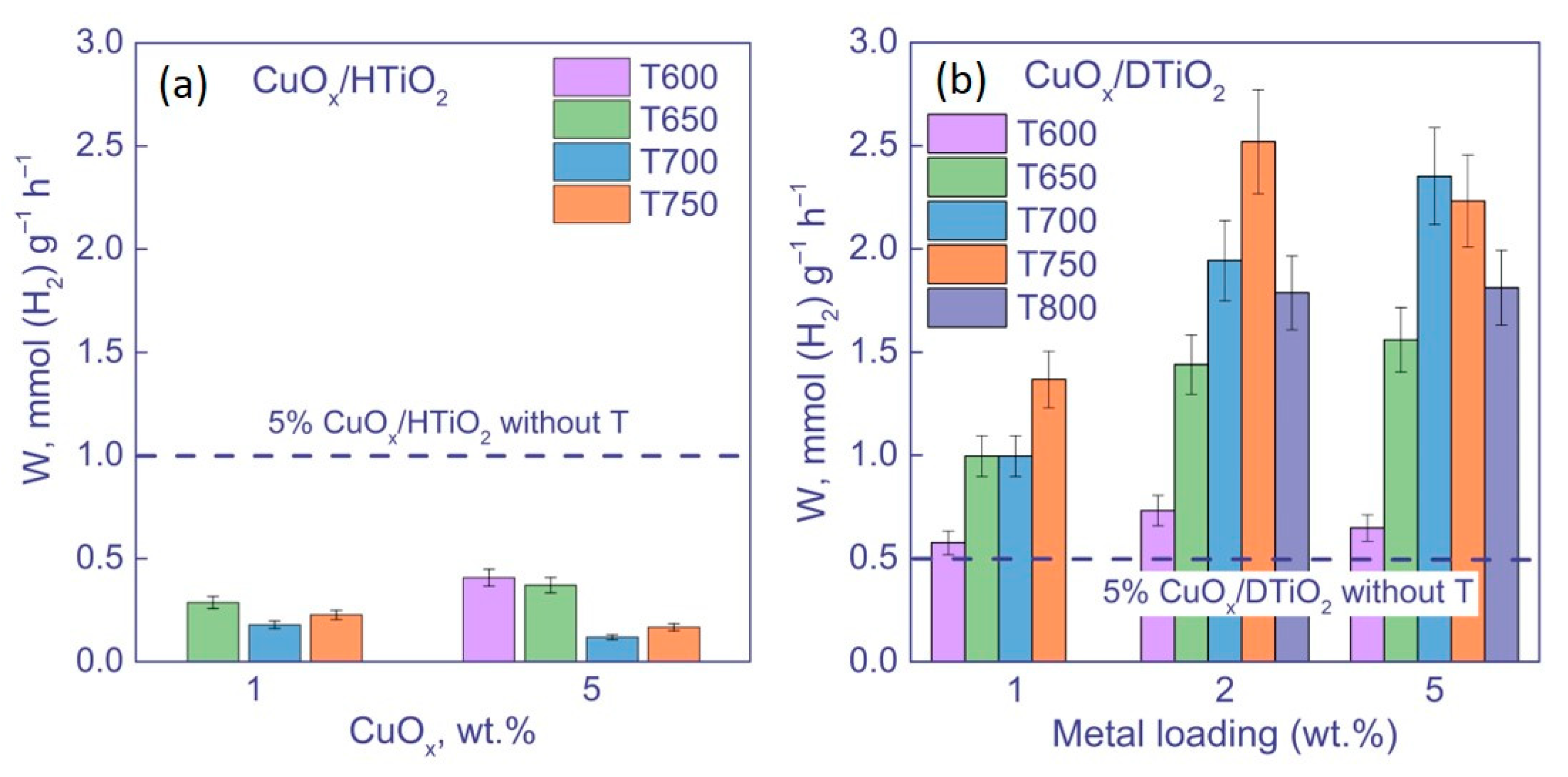
3.2.1. Activity of Photocatalysts under UV Irradiation
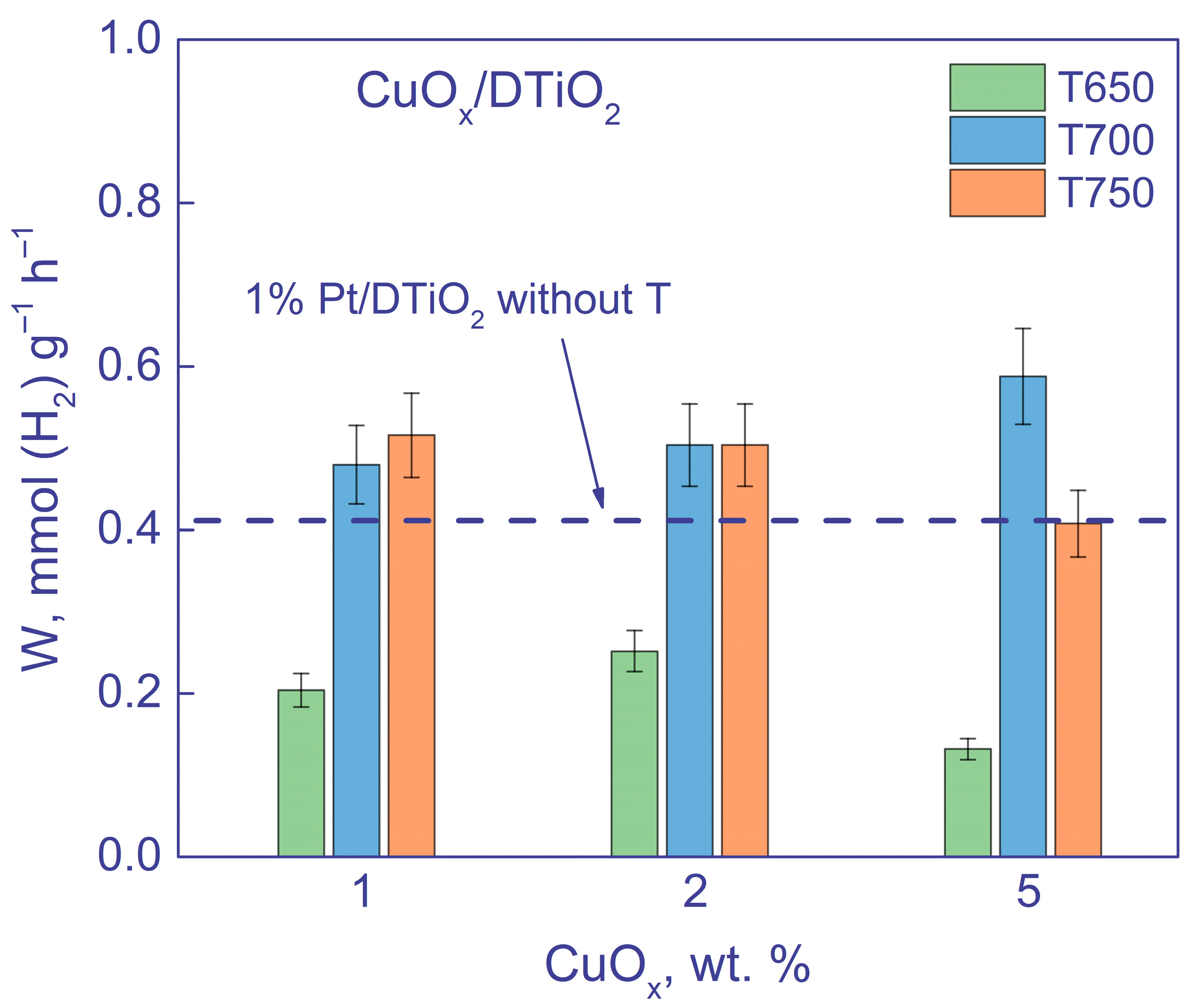
3.2.2. Activity of Photocatalysts under Visible Light Irradiation
- An increase in the absorption of the photocatalyst due to the shift in the absorption edge of DTiO2 and the deposition of copper compounds as a cocatalyst;
- The optimal ratio of anatase and rutile phases for the formation of heterojunctions;
- The size of DTiO2 particles after calcination at 700 °C does not increase as much as after calcination at higher temperatures;
- Deposition of Cu/CuOx on the surface of calcined DTiO2 leads to a uniform distribution of small cocatalyst particles;
- The presence of copper in the composition of various compounds (Cu, Cu2O, Cu(OH)2) facilitates the formation of a complex set of photogenerated charge heterojunctions, which significantly increases the electron lifetime and process efficiency.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Clarizia, L.; Vitiello, G.; Pallotti, D.K.; Silvestri, B.; Nadagouda, M.; Lettieri, S.; Luciani, G.; Andreozzi, R.; Maddalena, P.; Marotta, R. Effect of surface properties of copper-modified commercial titanium dioxide photocatalysts on hydrogen production through photoreforming of alcohols. Int. J. Hydrogen Energy 2017, 42, 28349–28362. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Kozlova, E.A.; Besov, A.S.; Kozlov, D.V.; Kiselev, S.A.; Safatov, A.S. Photocatalysis: Light energy conversion for the oxidation, disinfection, and decomposition of water. Kinet. Catal. 2010, 51, 801–808. [Google Scholar] [CrossRef]
- Valeeva, A.A.; Dorosheva, I.B.; Kozlova, E.A.; Kamalov, R.V.; Vokhmintsev, A.S.; Selishchev, D.S.; Saraev, A.A.; Gerasimov, E.Y.; Weinstein, I.A.; Rempel, A.A. Influence of Calcination on Photocatalytic Properties of Nonstoichiometric Titanium Dioxide Nanotubes. J. Alloys Compnd. 2019, 796, 293–299. [Google Scholar] [CrossRef]
- Rioja-Cabanillas, A.; Valdesueiro, D.; Fernández-Ibáñez, P.; Byrne, J.A. Hydrogen from wastewater by photocatalytic and photoelectrochemical treatment. J. Phys. Energy 2020, 3, 012006. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Vorontsov, A.V. Photocatalytic hydrogen evolution from aqueous solutions of organophosphorous compounds. Int. J. Hydrogen Energy 2010, 35, 7337–7343. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Liu, C.; Luo, J.; Crittenden, J.; Liu, X.; Cai, T.; Yuan, J.; Pei, Y.; Liu, Y. Photocatalytic wastewater purification with simultaneous hydrogen production using MoS2 QD-decorated hierarchical assembly of ZnIn2S4 on reduced graphene oxide photocatalyst. Water Res. 2017, 121, 11–19. [Google Scholar] [CrossRef]
- Sang, H.X.; Wang, X.T.; Fan, C.C.; Wang, F. Enhanced photocatalytic H2 production from glycerol solution over ZnO/ZnS core/shell nanorods prepared by a low temperature route. Int. J. Hydrogen Energy 2012, 37, 1348–1355. [Google Scholar] [CrossRef]
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Kurenkova, A.Y.; Gerasimov, E.Y.; Gromov, N.V.; Medvedeva, T.B.; Saraev, A.A.; Kaichev, V.V. Comparative study of photoreforming of glycerol on Pt/TiO2 and CuOx/TiO2 photocatalysts under UV light. Mater. Lett. 2021, 283, 128901. [Google Scholar] [CrossRef]
- Markovskaya, D.V.; Zhurenok, A.V.; Kurenkova, A.Y.; Kremneva, A.M.; Saraev, A.A.; Zharkov, S.M.; Kozlova, E.A.; Kaichev, V.V. New titania-based photocatalysts for hydrogen production from aqueous-alcoholic solutions of methylene blue. RSC Adv. 2020, 10, 34137–34148. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Fu, X.; Wang, C.; Ni, M.; Leung, M.K.H.; Wang, X.; Fu, X. Hydrogen production over titania-based photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Cai, Y.; Li, L.; Wang, X.; Gao, J. Amino-Functionalized Titanium Based Metal-Organic Framework for Photocatalytic Hydrogen Production. Molecules 2022, 27, 4241. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liu, F.; Wang, Q.; Song, H.; Luo, S.; Li, S.; Yang, G.; Deng, B.; Huang, Z.; Wang, X.S.; et al. Engineering interfacial charge transfer channel for efficient photocatalytic H2 evolution: The interplay of CoPx and Ca2+ dopant. Appl. Catal. B Environ. 2022, 303, 120887. [Google Scholar] [CrossRef]
- Wang, X.S.; Li, L.; Li, D.; Ye, J. Recent Progress on Exploring Stable Metal–Organic Frameworks for Photocatalytic Solar Fuel Production. Sol. RRL 2020, 4, 1900547. [Google Scholar] [CrossRef]
- Lee, A.C.; Lin, R.H.; Yang, C.Y.; Lin, M.H.; Wang, W.Y. Preparations and characterization of novel photocatalysts with mesoporous titanium dioxide (TiO2) via a sol–gel method. Mater. Chem. Phys. 2008, 109, 275–280. [Google Scholar] [CrossRef]
- Amano, F.; Nakata, M.; Yamamoto, A.; Tanaka, T. Rutile titanium dioxide prepared by hydrogen reduction of Degussa P25 for highly efficient photocatalytic hydrogen evolution. Catal. Sci. Technol. 2016, 6, 5693–5699. [Google Scholar] [CrossRef]
- Nair, R.V.; Gummaluri, V.S.; Matham, M.V.; Vijayan, C. A review on optical bandgap engineering in TiO2 nanostructures via doping and intrinsic vacancy modulation towards visible light applications. J. Phys. D. Appl. Phys. 2022, 55, 313003. [Google Scholar] [CrossRef]
- Puga, A.V. Photocatalytic production of hydrogen from biomass-derived feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Sun, T.; Liu, E.; Liang, X.; Hu, X.; Fan, J. Enhanced hydrogen evolution from water splitting using Fe-Ni codoped and Ag deposited anatase TiO2 synthesized by solvothermal method. Appl. Surf. Sci. 2015, 347, 696–705. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Wang, D.; Gao, Y.; Shen, J. Cu2O/Cu/TiO2 nanotube Ohmic heterojunction arrays with enhanced photocatalytic hydrogen production activity. Int. J. Hydrogen Energy 2012, 37, 6431–6437. [Google Scholar] [CrossRef]
- Taylor, S.; Mehta, M.; Samokhvalov, A. Production of Hydrogen by Glycerol Photoreforming Using Binary Nitrogen–Metal-Promoted N-M-TiO2 Photocatalysts. ChemPhysChem 2014, 15, 942–949. [Google Scholar] [CrossRef]
- Clarizia, L.; Vitiello, G.; Luciani, G.; Di Somma, I.; Andreozzi, R.; Marotta, R. In situ photodeposited nanoCu on TiO2 as a catalyst for hydrogen production under UV/visible radiation. Appl. Catal. A Gen. 2016, 518, 142–149. [Google Scholar] [CrossRef]
- Yu, J.; Hai, Y.; Jaroniec, M. Photocatalytic hydrogen production over CuO-modified titania. J. Colloid Interface Sci. 2011, 357, 223–228. [Google Scholar] [CrossRef]
- Nishikiori, H.; Harata, N.; Yamaguchi, S.; Ishikawa, T.; Kondo, H.; Kikuchi, A.; Yamakami, T.; Teshima, K. Formation of CuO on TiO2 Surface Using its Photocatalytic Activity. Catalysts 2019, 9, 383. [Google Scholar] [CrossRef]
- Yin, M.; Wu, C.K.; Lou, Y.; Burda, C.; Koberstein, J.T.; Zhu, Y.; O’Brien, S. Copper oxide nanocrystals. J. Am. Chem. Soc. 2005, 127, 9506–9511. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.P.; Wei, L.W.; Wang, H.P. Photocatalytic Reduction of CO2 to Methanol by Cu2O/TiO2 Heterojunctions. Sustainability 2021, 14, 374. [Google Scholar] [CrossRef]
- Gombac, V.; Sordelli, L.; Montini, T.; Delgado, J.J.; Adamski, A.; Adami, G.; Cargnello, M.; Bernai, S.; Fornasiero, P. CuOx-TiO2 Photocatalysts for H2 production from ethanol and glycerol solutions. J. Phys. Chem. A 2010, 114, 3916–3925. [Google Scholar] [CrossRef]
- Sharma, A.; Varshney, M.; Park, J.; Ha, T.K.; Chae, K.H.; Shin, H.J. XANES, EXAFS and photocatalytic investigations on copper oxide nanoparticles and nanocomposites. RSC Adv. 2015, 5, 21762–21771. [Google Scholar] [CrossRef]
- Sekine, R.; Marzouk, E.R.; Khaksar, M.; Scheckel, K.G.; Stegemeier, J.P.; Lowry, G.V.; Donner, E.; Lombi, E. Aging of Dissolved Copper and Copper-based Nanoparticles in Five Different Soils: Short-term Kinetics vs. Long-term Fate. J. Environ. Qual. 2017, 46, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, L.; Du, L.; Li, X. Photocatalytic Water Splitting Towards Hydrogen Production on Gold Nanoparticles (NPs) Entrapped in TiO2 Nanotubes. Catal. Lett. 2015, 145, 1771. [Google Scholar] [CrossRef]
- Chen, J.L.; Liu, M.M.; Xie, S.Y.; Yue, L.J.; Gong, F.L.; Chai, K.M.; Zhang, Y.H. Cu2O-loaded TiO2 heterojunction composites for enhanced photocatalytic H2 production. J. Mol. Struct. 2022, 1247, 131294. [Google Scholar] [CrossRef]
- Díaz, L.; Rodríguez, V.D.; González-Rodríguez, M.; Rodríguez-Castellón, E.; Algarra, M.; Núñez, P.; Moretti, E. M/TiO2 (M = Fe, Co, Ni, Cu, Zn) catalysts for photocatalytic hydrogen production under UV and visible light irradiation. Inorg. Chem. Front. 2021, 8, 3491–3500. [Google Scholar] [CrossRef]
- Yao, G.Y.; Zhao, Z.Y. Unraveling the role of cuprous oxide and boosting solar energy conversion: Via interface engineering in a Cu/TiO2 plasmonic photocatalyst. J. Mater. Chem. C 2020, 8, 8567–8578. [Google Scholar] [CrossRef]
- Lv, S.; Wang, Y.; Zhou, Y.; Liu, Q.; Song, C.; Wang, D. Oxygen vacancy stimulated direct Z-scheme of mesoporous Cu2O/TiO2 for enhanced photocatalytic hydrogen production from water and seawater. J. Alloys Compd. 2021, 868, 159144. [Google Scholar] [CrossRef]
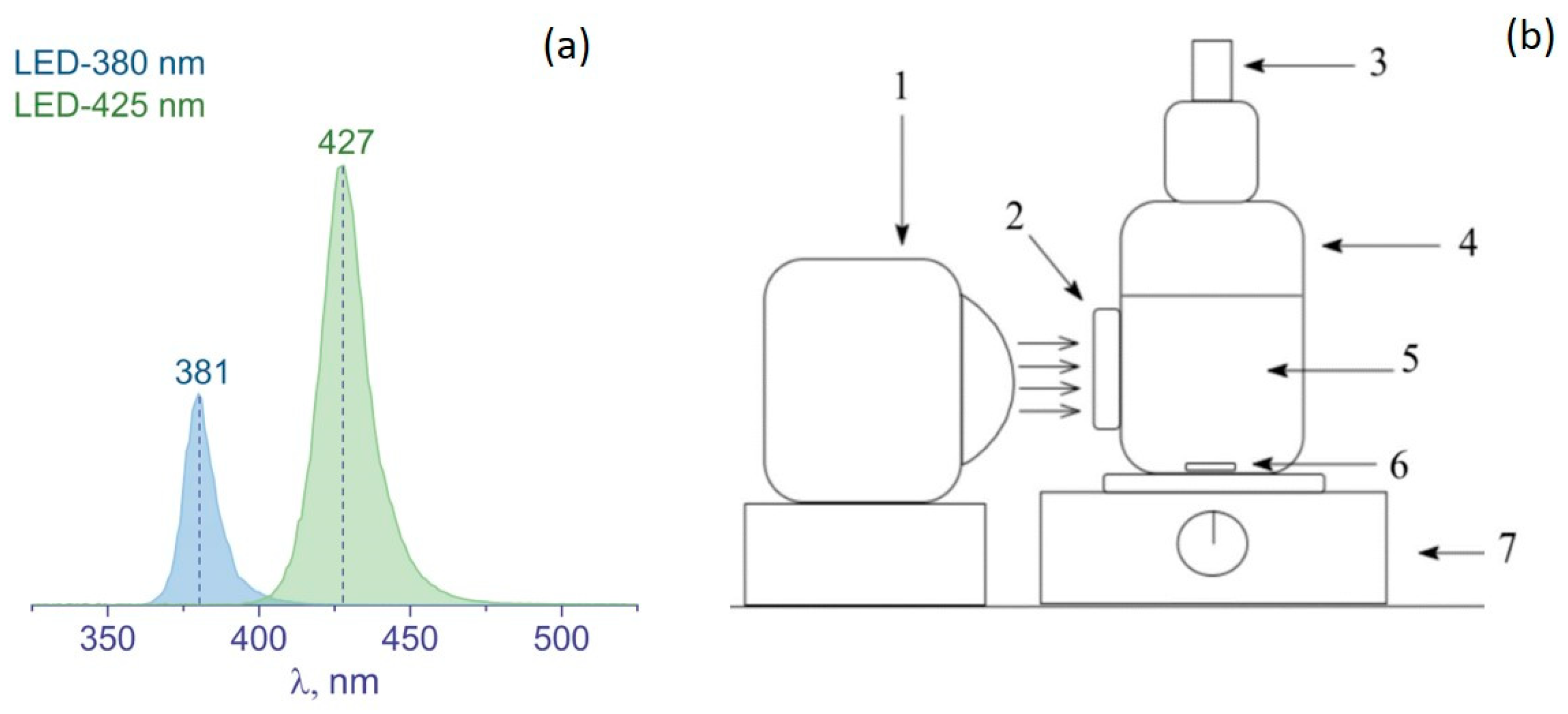

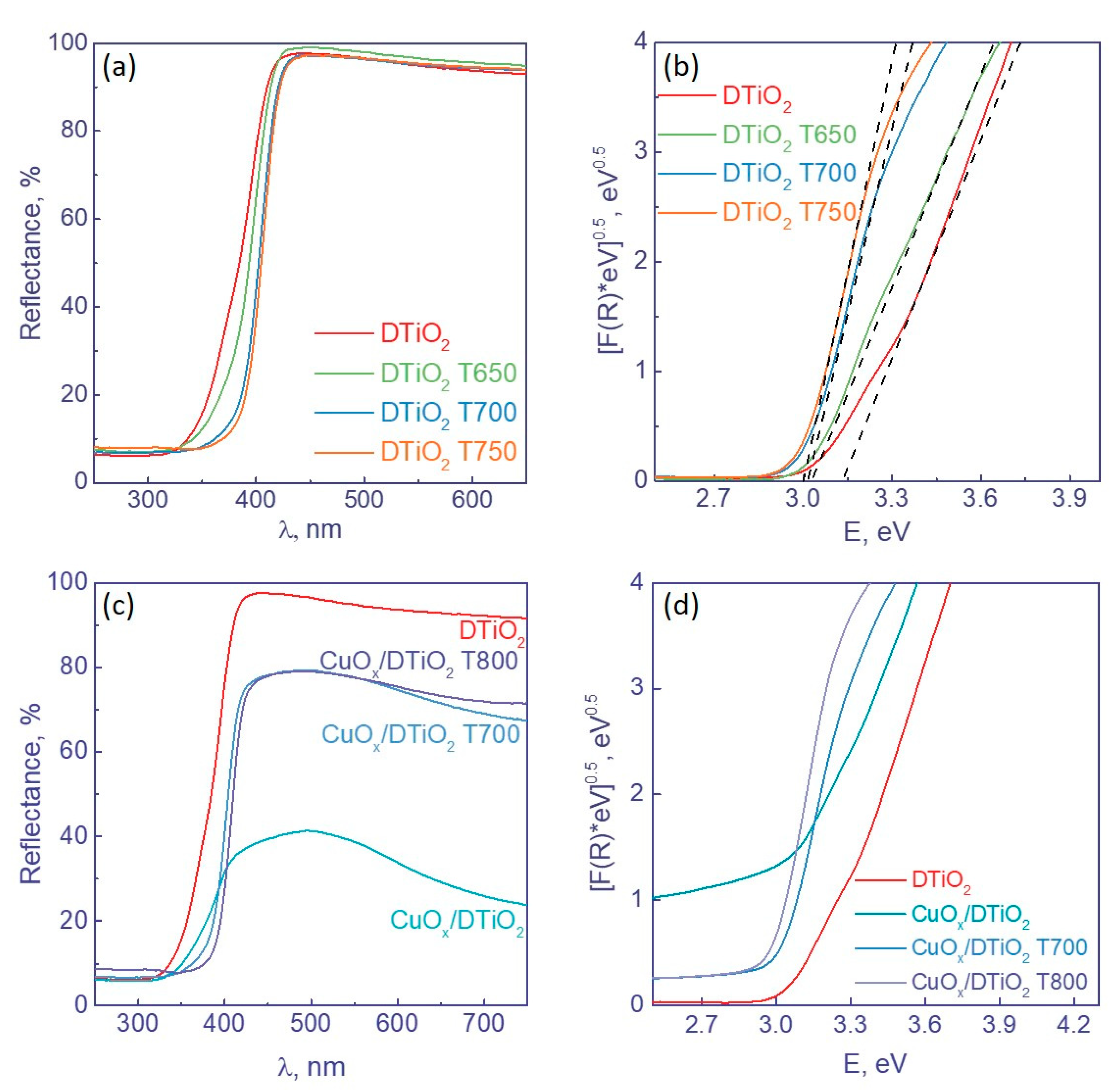
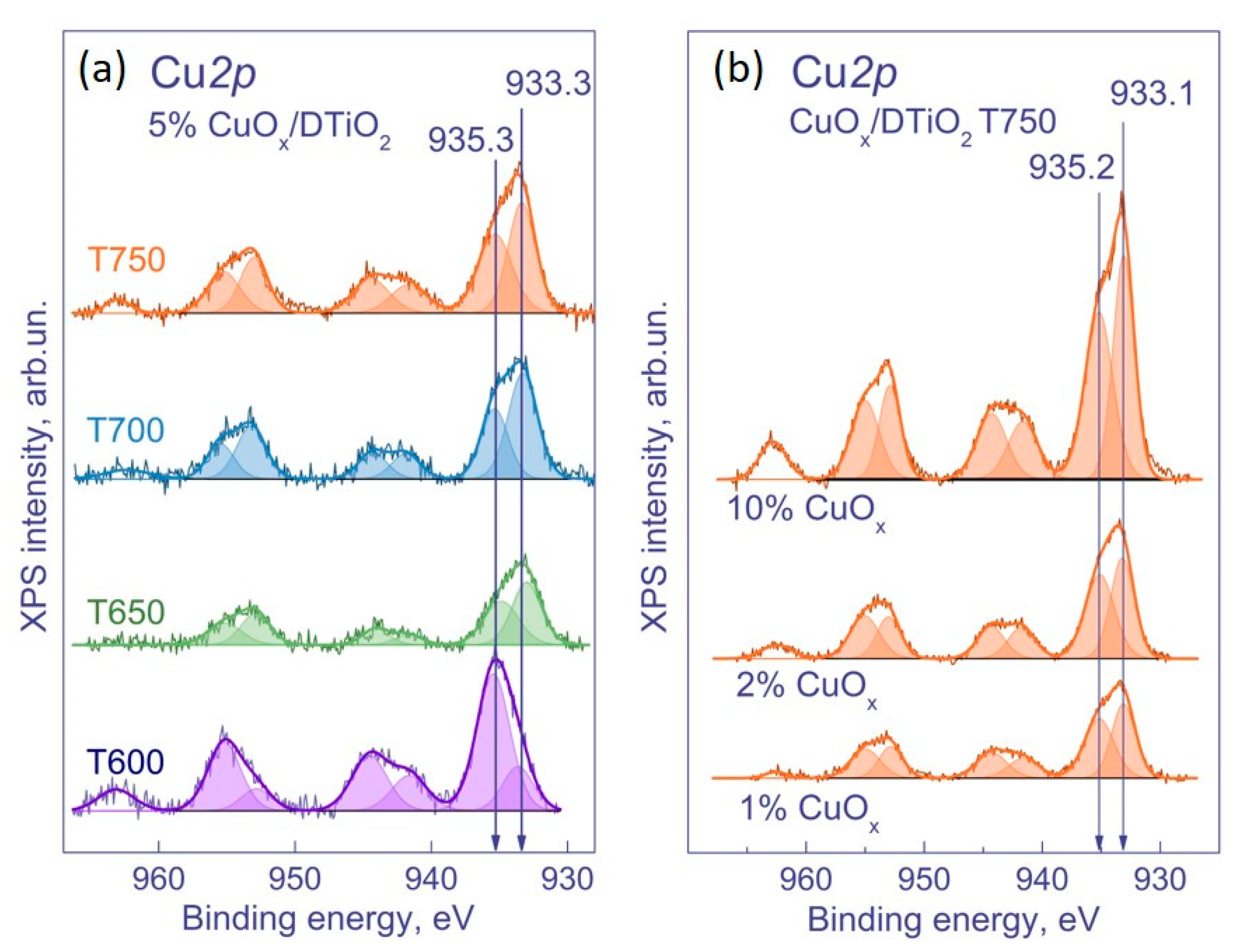
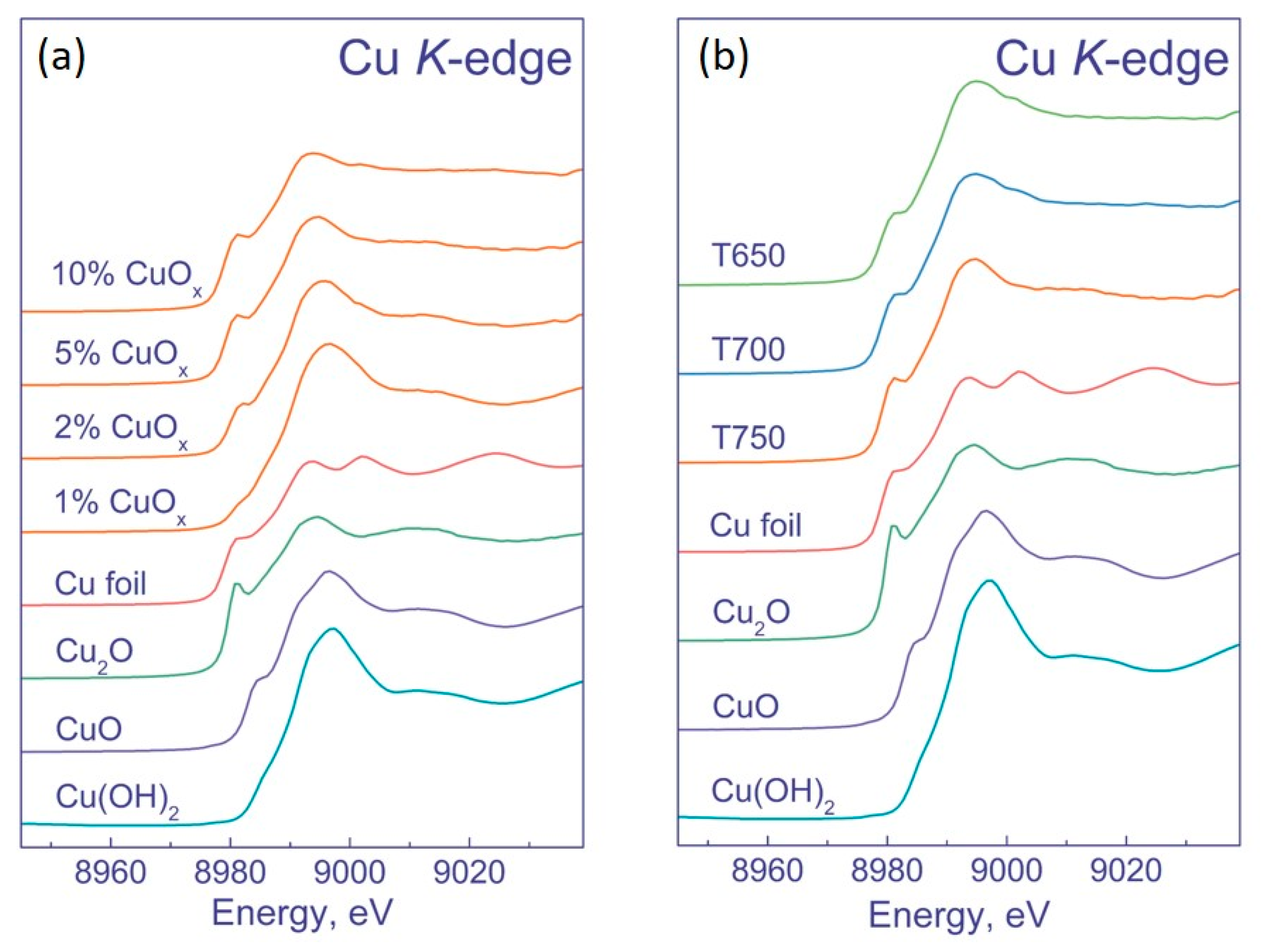
| No. | Sample | Phase Composition, wt. % | CS, nm | SSA, m2 g−1 | V, cm3 g−1 |
|---|---|---|---|---|---|
| 1 | DTiO2 | 85% (Anatase)/15% (Rutile) | 19/30 | 55 | 0.48 |
| 2 | DTiO2 600 | 75% (Anatase)/25% (Rutile) | 21/40 | 55 | 0.52 |
| 3 | DTiO2 650 | 68% (Anatase)/32% (Rutile) | 35/65 | 41 | 0.16 |
| 4 | DTiO2 700 | 36% (Anatase)/63% (Rutile) | 45/85 | 19 | 0.074 |
| 5 | DTiO2 750 | 5% (Anatase)/95% (Rutile) | 62/>100 | 12 | 0.046 |
| 6 | DTiO2 800 | 100% (Rutile) | >100 | 10 | 0.035 |
| No. | Sample | [Cu]/[Ti] | [O]/[Ti] | Cu2+, % |
|---|---|---|---|---|
| 1 | 5% CuOx/DTiO2 T600 | 0.26 | 4.8 | 85 |
| 2 | 5% CuOx/DTiO2 T650 | 0.14 | 3.3 | 52 |
| 3 | 5% CuOx/DTiO2 T700 | 0.22 | 3.2 | 51 |
| 4 | 5% CuOx/DTiO2 T750 | 0.24 | 3.4 | 60 |
| 5 | 1% CuOx/DTiO2 T750 | 0.17 | 3.1 | 63 |
| 6 | 2% CuOx/DTiO2 T750 | 0.25 | 3.2 | 64 |
| 7 | 10% CuOx/DTiO2 T750 | 0.48 | 3.7 | 64 |
| No. | Sample | Cu2+, % | Cu1+, % | Cu0, % |
|---|---|---|---|---|
| Weight content of copper | ||||
| 1 | 1% CuOx/DTiO2 T750 | 85 | 15 | – |
| 2 | 2% CuOx/DTiO2 T750 | 49 | 42 | 8 |
| 3 | 5% CuOx/DTiO2 T750 | 27 | 59 | 13 |
| 4 | 10% CuOx/DTiO2 T750 | 12 | 54 | 35 |
| Temperature of TiO2 Degussa P25 calcination | ||||
| 1 | 5% CuOx/DTiO2 T650 | 36 | 36 | 28 |
| 2 | 5% CuOx/DTiO2 T700 | 28 | 42 | 30 |
| 3 | 5% CuOx/DTiO2 T750 | 27 | 59 | 13 |
| Photocatalyst | Modification Method | Electron Donor | Radiation Source | W, μmol (H2) g−1 h−1 | AQE, % | Ref |
|---|---|---|---|---|---|---|
| Fe/TiO2 | Doping with metal atoms and deposition of metals | 10% Ethanol | Xenon lamp, 500 W (λ > 400 nm) | 248 | 0.1 | [22] |
| Ni/TiO2 | 205 | |||||
| Ag/TiO2 | 265 | |||||
| Fe-Ni/TiO2 | 348 | |||||
| Fe/Ag/TiO2 | 512 | |||||
| Ni/Ag/TiO2 | 336 | |||||
| Fe-Ni/Ag/TiO2 | 793 | 0.2 | ||||
| Cr-N@TiO2 | Doping with non-metal atoms and metal cations | 10% Glycerol | Medium pressure mercury lamp, 450 W | 126 | - | [24] |
| Co-N@TiO2 | 313 | |||||
| Ni-N@TiO2 | 506 | |||||
| Cu-N@TiO2 | 1615 | |||||
| 1.5% Au@TiO2 (nanotubes) | Vacuum-assisted-impregnation route | 25% Methanol | Xenon lamp, 100 mW·cm−1 (λ > 400 nm) | 482 | 0.1 | [33] |
| 1.5% Au/TiO2 (nanotubes) | 223 | |||||
| CuOx/TiO2 | Surface deposition of metals | 0.5% Glycerol | LED source, 30 W (λ = 380 nm) | 550 | - | [12] |
| Pt/TiO2 | 1350 | |||||
| 80% Cu2O/TiO2 | Microemulsion method | 20% Methanol | Xenon lamp | 215 | - | [34] |
| 60% Cu2O/TiO2 | 892 | |||||
| 40% Cu2O/TiO2 | 1242 | |||||
| 30% Cu2O/TiO2 | 1388 | |||||
| 20% Cu2O/TiO2 | 1345 | |||||
| TiO2 | 28 | |||||
| Cu2O | 3 | |||||
| 2% Cu/TiO2 | Impregnation reduction | 25% Methanol | UV lamp, 300 W (λ = 340 nm) | 5000 | - | [35] |
| 2% Ni/TiO2 | 2300 | |||||
| 2% Co/TiO2 | 2250 | |||||
| 2% Zn/TiO2 | 200 | |||||
| 2% Cu/TiO2 | Halogen lamp, 500 W (λ > 420 nm) | 220 | - | |||
| 2% Ni/TiO2 | 10 | |||||
| 2% Co/TiO2 | 20 | |||||
| 2% Zn/TiO2 | 11 |
| No. | Sample | Catalytic Activity, mmol (H2) g−1 h−1 | |
|---|---|---|---|
| 381 nm | 427 nm | ||
| Cu/TiO2 (Degussa P25) | |||
| 1 | 1% CuOx/DTiO2 | 0.5 ± 0.05 | 0 |
| 2 | 1% CuOx/DTiO2 T600 | 0.6 ± 0.06 | - |
| 3 | 1% CuOx/DTiO2 T650 | 1.0 ± 0.1 | 0.2 ± 0.02 |
| 4 | 1% CuOx/DTiO2 T700 | 1.0 ± 0.1 | 0.5 ± 0.05 |
| 5 | 1% CuOx/DTiO2 T750 | 1.3 ± 0.1 | 0.5 ± 0.05 |
| 6 | 2% CuOx/DTiO2 T600 | 0.7 ± 0.07 | — |
| 7 | 2% CuOx/DTiO2 T650 | 1.4 ± 0.1 | 0.3 ± 0.03 |
| 8 | 2% CuOx/DTiO2 T700 | 2.0 ± 0.1 | 0.5 ± 0.05 |
| 9 | 2% CuOx/DTiO2 T750 | 2.5 ± 0.25 | 0.5 ± 0.05 |
| 10 | 5% CuOx/DTiO2 T600 | 0.6 ± 0.06 | — |
| 11 | 5% CuOx/DTiO2 T650 | 1.6 ± 0.2 | 0.1 ± 0.01 |
| 12 | 5% CuOx/DTiO2 T700 | 2.4 ± 0,2 | 0.6 ± 0.06 |
| 13 | 5% CuOx/DTiO2 T750 | 2.2 ± 0.2 | 0.4 ± 0.04 |
| 14 | 10% CuOx/DTiO2 T700 | 1.4 ± 0.1 | — |
| 15 | 10% CuOx/DTiO2 T750 | 1.0 ± 0.1 | — |
| Cu/TiO2 (Hombifine N) | |||
| 16 | 5% CuOx/HTiO2 T600 | 0.4 ± 0.04 | — |
| 17 | 5% CuOx/HTiO2 T650 | 0.4 ± 0.04 | — |
| 18 | 5% CuOx/HTiO2 T700 | 0.1 ± 0.01 | — |
| 19 | 5% CuOx/HTiO2 T750 | 0.2 ± 0.02 | — |
| 20 | 1% CuOx/HTiO2 T650 | 0.3 ± 0.03 | — |
| 21 | 1% CuOx/HTiO2 T700 | 0.2 ± 0.02 | — |
| 22 | 1% CuOx/HTiO2 T750 | 0.2 ± 0.02 | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurenkova, A.Y.; Yakovleva, A.Y.; Saraev, A.A.; Gerasimov, E.Y.; Kozlova, E.A.; Kaichev, V.V. Copper-Modified Titania-Based Photocatalysts for the Efficient Hydrogen Production under UV and Visible Light from Aqueous Solutions of Glycerol. Nanomaterials 2022, 12, 3106. https://doi.org/10.3390/nano12183106
Kurenkova AY, Yakovleva AY, Saraev AA, Gerasimov EY, Kozlova EA, Kaichev VV. Copper-Modified Titania-Based Photocatalysts for the Efficient Hydrogen Production under UV and Visible Light from Aqueous Solutions of Glycerol. Nanomaterials. 2022; 12(18):3106. https://doi.org/10.3390/nano12183106
Chicago/Turabian StyleKurenkova, Anna Yu., Anastasiya Yu. Yakovleva, Andrey A. Saraev, Evgeny Yu. Gerasimov, Ekaterina A. Kozlova, and Vasily V. Kaichev. 2022. "Copper-Modified Titania-Based Photocatalysts for the Efficient Hydrogen Production under UV and Visible Light from Aqueous Solutions of Glycerol" Nanomaterials 12, no. 18: 3106. https://doi.org/10.3390/nano12183106






